Abstract
Direct-to-physician opioid marketing by pharmaceutical companies is widespread and may contribute to opioid overprescribing, an important driver of the US opioid crisis. Using a difference-indifferences approach and Medicare Part D prescriber data, we examined the effects of academic medical centers’ conflict-of-interest policies that restrict direct-to-physician marketing of all drugs on opioid prescribing by physicians at eighty-five centers in the period 2013–16. We examined restrictions on gifts and meals, speaking and consulting engagements, and industry representatives’ access to academic medical centers, as well as rules requiring conflict-of-interest disclosures. Bans on sales representatives were associated with a 4.7 percent reduction in the total volume of opioids prescribed and disclosure requirements with a 2.5 percent reduction, while having all four marketing restriction policies was associated with an 8.8 percent reduction. Policies that restrict direct-to-physician pharmaceutical marketing may curb opioid prescribing, but additional patient-level research is needed to understand how such policies affect the delivery of evidence-based treatment for chronic pain.
In the US, 47,600 people died of opioid overdoses in 2017.1 High rates of opioid prescribing by physicians, which increased fourfold between the late 1990s and early 2010s,2 are an important driver of the opioid overdose crisis. The increase in opioid prescribing that began in the late 1990s was driven by US clinicians’ growing recognition of the high prevalence of poorly managed pain, coupled with aggressive pharmaceutical marketing of opioids as safe and effective for long-term use—claims later shown to be misleading.3–5 While the overall volume of opioid prescribing has begun to decline since peaking in 20126 and the majority of opioid overdose deaths in the US are now due to nonprescription opioids such as heroin and illicit fentanyl, the opioid prescribing rate is still three times higher than it was in 1999.7 More than 35 percent of opioid overdose deaths in 2017 involved prescription opioids,8 and about 70 percent of the people who use nonprescription opioids such as heroin initiated their use with a prescription opioid.9
Over 400 US jurisdictions are suing pharmaceutical companies based on claims that their misleading direct-to-physician marketing materials and presentations have contributed to the opioid crisis.10–12 In August 2019 one of the first cases was decided when a judge ordered Johnson & Johnson to pay the State of Oklahoma $572 million.13 Pharmaceutical firms primarily market new brand-name drugs; generic drugs are rarely marketed. Federal law grants firms the right to sell new medications with no competition from generics for five years, and much marketing is concentrated in this period of exclusivity—though some firms continue to market brand-name products after generic versions become available. Despite the now widespread recognition of the role that direct-to-physician marketing of opioids has played in driving opioid prescribing, such marketing remains common.
Federal law does not prohibit direct-to-physician marketing but requires pharmaceutical companies to report the monetary value of each activity of such marketing, including educational visits (called “detailing”), meals, gifts, and payments for nonresearch activities such as consulting or public speaking.14 In 2017 pharmaceutical companies spent $10.7 million on 89,466 individual opioid marketing activities to physicians (authors’ calculation based on data from the Centers for Medicare and Medicaid Services [CMS]).14 Scott Hadland and colleagues found that in the period 2013–15, approximately one in twelve US physicians overall and one in five family medicine physicians received an opioid marketing payment.15
A large literature has shown that direct-to-physician marketing increases physicians’ prescribing of marketed drugs.16–20 Two recent studies have shown a positive correlation between physicians’ receipt of direct-to-physician opioid marketing and their rates of opioid prescribing21 and opioid overdose deaths, respectively.22 However, the study methods did not allow the authors to identify a causal relationship, and the positive correlations observed may be due to the fact that pharmaceutical companies target direct-to-physician marketing to the physicians most likely to prescribe their products.20
There is growing interest in restricting direct-to-physician marketing, but the effects that such restrictions have on opioid prescribing are unclear. Since 2017, legislation to restrict direct-to-physician pharmaceutical marketing has been considered at the federal,23 state,24–26 and city27 levels, with bill sponsors citing the opioid crisis to support their proposals. While prior studies have shown that marketing restrictions are associated with small reductions in the prescribing of marketed drugs,28–31 marketed drugs are typically newly released brand-name medications. In the case of opioids, many nonmarketed generic options exist.32
In the face of widespread generic availability, it is unclear whether reducing physicians’ exposure to marketing for new prescription opioids would influence overall opioid prescribing behavior. Our study addressed this question by evaluating the effects of restrictions on direct-to-physician pharmaceutical marketing on overall opioid prescribing as well as on the prescribing of brand-name and marketed, brand-name but not marketed, and generic opioids.
Study Overview
We conducted a difference-in-differences analysis to examine how changes in restrictions on direct-to-physician pharmaceutical marketing included in academic medical centers’ conflict-of-interest policies have influenced opioid prescribing in Medicare Part D. Specifically, we compared prescribers at centers that added a new marketing restriction policy to prescribers at centers that made no change in their policies. In response to recommendations in the late 2000s from the Institute of Medicine,33 Association of American Medical Colleges,34 and American Medical Association,35 academic medical centers have increasingly imposed various types of restrictions—including restrictions on gifts and meals, speaking and consulting engagements, and industry representatives’ access to the centers, as well as requirements to disclose conflicts of interest.36 The fact that centers have implemented these policies at different times creates an ideal natural experiment in which to examine the effects on opioid prescribing patterns of specific types of restrictions on direct-to-physician marketing, as well as the cumulative effects of multiple restrictions.
Medicare Part D beneficiaries are an ideal population in which to evaluate the effects of such policies, given that adults ages sixty-five and older receive the majority of opioid prescriptions in the US6,37 and that nearly half of disabled Medicare beneficiaries younger than age sixty-five use prescription opioids in a given year.37 Rates of opioid misuse and overdose are rising among Medicare beneficiaries,38 with a more than sixfold increase in opioid overdose deaths among adults ages sixty-five and older from 1999 to 2016—a trend that mirrors the almost sevenfold increase in the younger population.39
We hypothesized that we would see the largest percentage reduction in the prescribing of opioids that were both brand-name and currently marketed to prescribers. We also examined whether restrictions on direct-to-physician marketing had spillover effects that influenced the volume of prescribing of nonmarketed generic opioids.
Study Data And Methods
STUDY SAMPLE
Our study sample included all physicians who prescribed medications covered by Medicare Part D at any of eighty-five US academic medical centers. There are 103 integrated allopathic medical schools in the US. However, following previous research,28–31 we included only the integrated allopathic academic medical centers that had a minimum of 100 physicians. This definition included centers that shared their administration with schools of medicine (that is, they were under common ownership, administrative leadership, or both) and excluded medical schools with teaching hospitals that were under separate administrative leadership. This was necessary, as marketing restriction policies apply to all physicians in integrated academic medical centers but only to residents and medical school faculty members in nonintegrated academic medical centers.
OPIOID PRESCRIBING DATA
We used two data sources to create an analytic data set of opioids prescribed to Medicare Part D beneficiaries by physicians practicing at the eighty-five academic medical centers in our sample. First, we used the Medicare Part D Prescriber Public Use File for the period 2013–16 to construct a data set of all opioids prescribed to Part D beneficiaries during that four-year period. The file includes information on all medications prescribed to enrollees in Medicare Part D plans, including Medicare Advantage Part D plans. We identified all opioid prescriptions in these data (for a list of included prescription opioids, see online appendix 1)40 and used the Open Payments data from CMS14 to identify which drugs were marketed in each year. The Medicare Part D prescriber data are constructed at the prescriber level: Prescriptions can be linked to individual prescribers using their National Provider Identifier (NPI), but not to individual patients. While prescribers’ NPIs are available in the data, information about which medical system a prescriber is affiliated with is not included. To address this issue, we linked the Medicare Part D Prescriber Public Use File to the CMS Physician Compare National Downloadable File41 to identify physicians affiliated with one of the eighty-five academic medical centers. The Physician Compare data include both NPIs and information about physicians’ primary hospital affiliation.
DATA ON MARKETING RESTRICTION POLICIES
We created a unique longitudinal database of all restrictions on direct-to-physician marketing included in academic medical centers’ conflict-of-interest policies in the period 2013–16. We began with publicly available data reported by the American Medical Student Association42 and Columbia University’s Institute on Medicine as a Profession.43 Neither of these sources tracks changes in centers’ restrictions on direct-to-physician marketing on an annual basis. In addition, the association’s definitions of the restrictions—for example, the definition of what constitutes a gift—have changed over time. To overcome these limitations and fill in the gaps, we conducted reviews of documents and made email or telephone inquiries. If centers’ conflict-of-interest policies were publicly available, we reviewed them to clarify their content. If a center’s policy was unavailable or we could not determine the effective date or specific restrictions on direct-to-physician marketing included in the policy, we contacted the center. Through document review, direct contact, or both, we were able to verify 2013–16 policies for all eighty-five centers included in the study sample. While we were able to verify written policies, we did not have information on the degree to which they were enforced.
We identified affiliations between medical schools and hospitals using the Organizational Characteristics Database, a data product of the Association of American Medical Colleges with information on various dimensions of accredited medical colleges.44
OUTCOME MEASURES
We estimated the effect of changes in marketing restriction policies on four measures: volume of prescriptions for opioids overall, for generic opioids, for brand-name and marketed opioids, and for brand-name and nonmarketed opioids. Volumes were calculated by summing the days supplied across each prescription that a prescriber wrote in a given year.
POLICY MEASURES
Our main independent variables were a series of restrictions on direct-to-physician marketing included in academic medical centers’ conflict-of-interest policies, defined at the center-year level. Our primary measures included four core restrictions: gift and meal restrictions that banned physicians from receiving any industry-funded gifts or meals, regardless of their value; speaking and consulting restrictions that prohibited clinicians from being paid by industry for promotional speaking engagements or consulting relationships designed for marketing purposes; sales representative restrictions that prohibited industry sales representatives from accessing the academic medical center; and conflict-of-interest disclosure restrictions that required clinicians at the center to disclose any industry relationship to their institution and trainees. These definitions aligned with those used by the Association of American Medical Colleges.45 We also constructed measures that indicated whether in a given year each center had at least one, two, or three restrictions or all four.
COVARIATES
Covariates were drawn from the Physician Compare data as well as the Part D Prescriber Public Use File. We adjusted for the share of beneficiaries who were female and the average age and Hierarchical Condition Categories risk score (higher scores indicate increased patient complexity) of all beneficiaries treated by each prescriber, as well as the prescriber’s sex, specialty (anesthesiology, behavioral health, dentistry, emergency medicine, general medicine, oncology, surgery, or other), and credentials (MD; DO; registered nurse, nurse practitioner, or physician assistant; DDS).
STATISTICAL ANALYSES
We analyzed the effects of changes in academic medical centers’ marketing restrictions at the prescriber-year level on the outcomes above, using a difference-in-differences, two-way fixed effects study design. Using the first year of data (baseline) for each prescriber before any changes in marketing restrictions occurred allowed us to control for differences in individual characteristics that varied across academic medical center populations but might have been correlated with our outcome measures.
To capture secular trends, as a comparison group we also included prescribers who prescribed opioids and were affiliated with academic medical centers that did not change their marketing restriction policies during the study period. This comparison group consisted of two types of providers: those at centers that never had a restriction policy during our study period and those at centers that always had a policy during the period. Of all centers, 97.8 percent had at least one restriction policy at the end of the study period. By including academic medical center and calendar year fixed effects, our empirical approach compared changes in the quantity of opioid prescriptions over time between treatment-group prescribers (those who prescribed opioids at centers that changed their marketing restriction policies during the study period) and comparison-group prescribers who practiced at centers that did not change their marketing restriction policies. By comparing changes rather than level differences, our analysis differences out any unobservable baseline differences between academic medical centers. Such difference-in-differences models are often used to analyze the effect of policy changes when randomized controlled trials are infeasible.46
We estimated a variety of two-way fixed effects (for academic medical center and calendar year) two-part models, in which the first part was a logistic regression and the second part was a generalized linear model with gamma distributed errors and log link. To interpret the total effects, we report marginal effect semi-elasticities that can be interpreted as the percentage difference in opioid prescribing for physicians in the treatment group relative to the comparison group. Our models controlled for the covariates listed above, and we clustered standard errors at the academic medical center level.
LIMITATIONS
Several limitations are of note. First, a key limitation of our analysis was a lack of a long pre period to test for common trends. While the underlying counterfactual assumption of a difference-in-differences analysis is untestable, similar pre-period trends in outcomes across the treatment and comparison groups could have given us additional confidence in our empirical approach. Because of data availability, we had only one year of baseline data and were unable to formally test for common pre-period trends. Given that the policies we analyzed are not specific to opioids (that is, they affect all pharmaceuticals), there is strong reason to believe that treatment assignment was orthogonal to opioid prescribing and that, given this strong a priori reasoning, testing for common trends might have introduced additional bias.47
Second, we examined the effects of restrictions on direct-to-physician marketing within a dynamic opioid prescribing policy environment. During 2013–16, the study period, some states enacted laws designed to curb high-risk opioid prescribing, including laws that require providers to check their state’s prescription drug monitoring program before prescribing an opioid; pill-mill laws that place strict regulations on pain management clinics; and opioid prescribing cap laws that restrict the days’ supply, dose, or both of opioids prescribed. Our fixed-effects approach accounted for state-level variation in opioid prescribing due to state-level policy changes.
Third, while academic medical centers might also have changed opioid prescribing policies during our study period, our models included center fixed effects. These differenced out any time-invariant baseline differences in opioid prescribing across centers. To the extent that center-specific policies on opioid prescribing were changing over time, they would have biased our results only if they were highly correlated with restrictions on direct-to-physician marketing across the entire treatment group. This unobservable correlation was unlikely, as the restrictions are not specific to opioids—as noted above, they apply to all pharmaceuticals.
Fourth, data constraints limited the alternative counterfactual analyses that we were able to estimate. The comparison group consisted of prescribers affiliated with academic medical centers that either never had any marketing restriction policy or always had consistent policies throughout the study period. While it would be interesting to estimate analyses that used only the centers with no restriction policy, as noted above, 97.8 percent of academic medical centers had at least one policy at the end of our study period.
Fifth, while we were able to control in our statistical models for the types of patients seen by each prescriber overall (percentage female, average age, and average risk score—information included in the Part D data), we were unable to measure the characteristics of individual patients who were prescribed opioids.
Sixth, our use of prescriber-level administrative claims data prevented us from determining the clinical appropriateness of prescribing opioids for individual patients in the sample.
Finally, since our data came from Medicare Part D files, our results might not be generalizable to younger, privately insured, or uninsured populations.
Study Results
SAMPLE DESCRIPTION
In 2013, 87 percent of the eighty-five academic medical centers had at least one of the four restrictions on direct-to-physician marketing, compared to 98 percent in 2016 (exhibit 1). Similarly, 61 percent had at least two restrictions in 2013, compared to 79 percent in 2016, and the shares for those with at least three restrictions and those with all four also increased (from 26 percent to 39 percent and from 2 percent to 4 percent, respectively). The increased adoption of policies occurred over several domains. Details on the specific restrictions adopted by academic medical centers during the study period are in appendix 2.40
EXHIBIT 1. Percent of academic medical center conflict-of-interest policies with one or more restrictions on direct-to-physician pharmaceutical marketing, 2013–16.
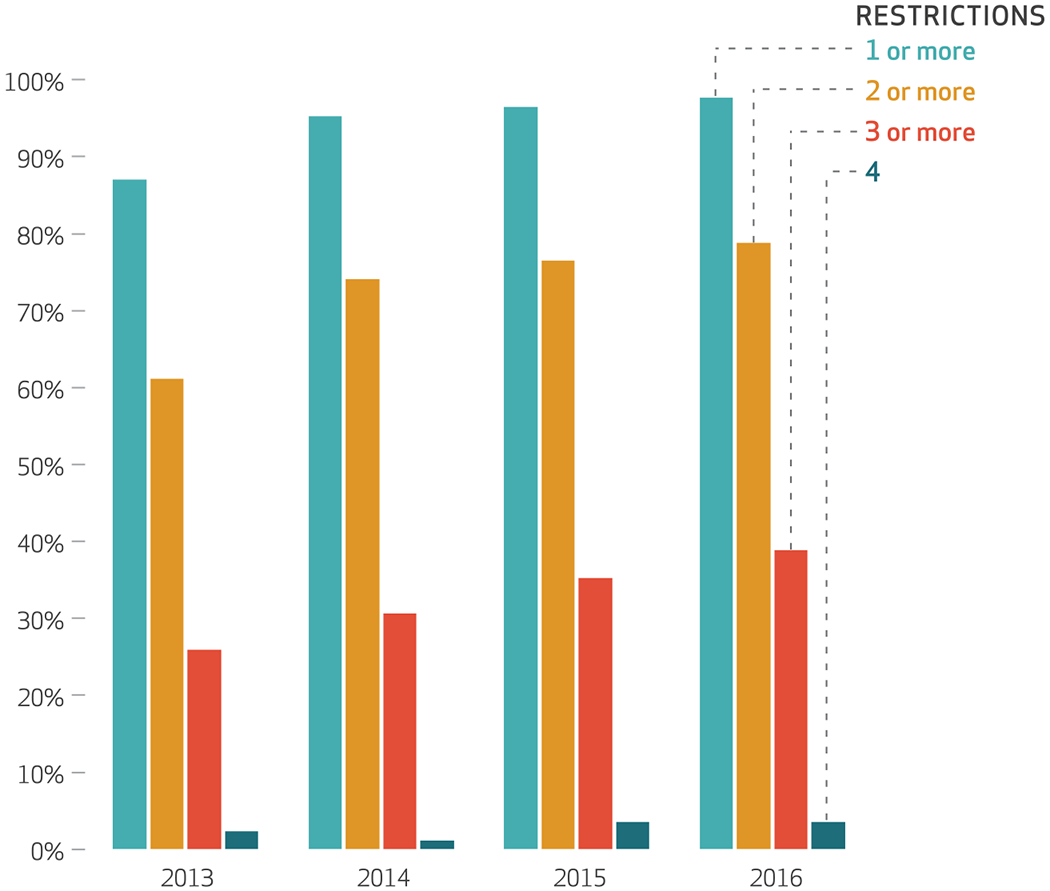
source Authors’ analysis of data for 2013–16 from a longitudinal database of academic medical center direct-to-physician marketing policies. notes There were eighty-five academic medical centers. The restrictions are explained in the text.
There were 47,190 prescribers and 188,644 prescriber-years in the sample (EXHIBIT 2). Most prescribers were male (63 percent) and had an MD credential (84 percent). The most common specialty was general medicine (45 percent). On average, prescribers had a large number of days of opioids prescribed (mean: 1,323), though there was significant variation across prescribers. Most of the opioids prescribed were generic opioids (mean days: 1,277). Much smaller amounts of brand-name and marketed opioids and brand-name but not marketed opioids (mean days: 39 and 7, respectively) were prescribed.
EXHIBIT 2.
Characteristics of opioid prescribers and numbers of days opioids were prescribed in the study sample, 2013–16
| Prescriber characteristics | Percent |
|---|---|
| Female | 37 |
| Specialty | |
| General medicine | 45 |
| Othera | 41 |
| Oncology | 5 |
| Surgery | 5 |
| Emergency medicine | 2 |
| Behavioral health | 2 |
| Anesthesiology | <1 |
| Dentistry | <1 |
| Credential | |
| MD | 84 |
| DO | 4 |
| Registered nurse, nurse practitioner, or physician assistant | 7 |
| Other | 4 |
| DDS | <1 |
| Census region | |
| Midwest | 27.05 |
| Northeast | 29.01 |
| South | 23.96 |
| West | 19.98 |
| Mean days opioids prescribed, per prescriber-year | Number (SD) |
| All opioids | 1,323 (2,949) |
| Generic opioids | 1,277 (2,849) |
| Brand-name and not marketed opioids | 7 (76) |
| Brand-name and marketed opioids | 39 (190) |
source Authors’ analysis of data for 2013–16 from the Medicare Part D Prescriber Public Use File.
notes There were 47,190 prescribers and 188,644 prescriber-years. SD is standard deviation.
Includes immunology, cardiology, dermatology, endocrinology, gastroenterology, gynecology, infectious disease, nephrology, neurology, ophthalmology, otolaryngology, physical medicine, podiatry, pulmonary disease, rheumatology, and urology.
IMPACT OF POLICIES RESTRICTING DIRECT-TO-PHYSICIAN MARKETING
Exhibit 3 presents results from our models examining the impact of each specific marketing restriction on opioid prescribing. Bans on sales representatives resulted in 4.7 percent fewer days of total opioids prescribed, and disclosure requirements resulted in 2.5 percent fewer days. Given that on average, prescribers prescribed 1,323 total days of opioids (exhibit 2), the sales representative ban was associated with 62 fewer days of opioid prescriptions, and the disclosure policy was associated with 33 fewer days of opioid prescriptions. We did not observe significant effects for bans on gifts and meals or bans on speaking and consulting relationships (exhibit 3). Decreases in prescribed opioids that were both brand-name and marketed were significant for both sales representative bans (11.0 percent fewer days) and disclosure requirements (6.3 percent fewer days). For these two categories, we also observed significant spillover effects, with decreases in days of generic opioids prescribed of 4.9 percent for sales representative bans and 2.6 percent for disclosure requirements. No other estimates were significant, except for a large effect for speaking and consulting bans for brand-name and not marketed opioids: a decline of 24.3 percent. However, given the large standard error, the true effect might be much smaller. Full regression results are in appendix 3.40
EXHIBIT 3:
Effects of specific conflict-of-interest policy restrictions on opioid prescribing volume at academic medical centers, 2013–16
| Brand-name opioids |
||||||||
|---|---|---|---|---|---|---|---|---|
| All opioids |
Generic opioids |
Not marketed |
Marketed |
|||||
| Direct-to-physician marketing restriction | % diff. | SE | % diff. | SE | % diff. | SE | % diff. | SE |
| Gift and meal ban | ||||||||
| Marginal effect | 0.06 | (1.46) | −0.26 | (1.57) | −1.83 | (9.87) | 1.23 | (3.32) |
| Speaking or consulting ban | ||||||||
| Marginal effect | −1.51 | (1.88) | −1.55 | (1.90) | −24.30** | (11.20) | −0.77 | (3.26) |
| Sales representative ban | ||||||||
| Marginal effect | −4.73* | (2.78) | −4.87* | (2.71) | 21.50 | (17.1) | −11.00* | (5.75) |
| Disclosure requirement | ||||||||
| Marginal effect | −2.46** | (1.18) | −2.56** | (1.21) | −0.41 | (9.24) | −6.28** | (2.68) |
source Authors’ analysis of data for 2013–16 from the Medicare Part D Prescriber Public Use File. notes Numbers of prescribers and prescriber-years are in the notes to exhibit 2. The restrictions are explained in the text.The exhibit presents semi-elasticity marginal effects from two-part models (explained in the text) that were estimated at the physician-year level. Standard errors (SEs) are clustered at the level of the academic medical center. Covariates include average beneficiary age, share of female beneficiaries, average beneficiary Hierarchical Condition Categories risk score, prescriber credential, and prescriber specialty. Fixed effects for year and academic calendar year are included in every model.
p < 0.10
p < 0.05
Exhibit 4 presents results from models that estimated the impact of having various numbers of marketing restrictions on opioid prescribing. Other outcome measures are in appendix 4.40 We observed no significant effects for models that examined the effect of having at least one or at least two marketing restrictions (exhibit 4). Having at least three restrictions was associated with 3.0 percent fewer days of opioids prescribed, relative to having fewer than three (p < 0.01). Similarly, having all four restrictions, relative to having fewer than four, was associated with an 8.8 percent reduction in days (p < 0.01). The findings were similar for days of generic opioids prescribed (at least three restrictions: 3.1 percent, p = 0.01; four restrictions: 9.1 percent, p < 0.01) (appendix 4). For brand-name opioids that were not marketed, we observed no significant effects and no clear trend in the effects as the number of marketing restrictions increased. We also found no significant effects on the prescribing of brand-name opioids marketed to physicians of the number of restrictions on direct-to-physician marketing. However, the point estimates monotonically increased in absolute value (that is, they became more negative) as we moved from a model that estimated the effect of having at least one restriction (0.1 percent; p = 0.90) to models using at least two restrictions (−3.6 percent; p = 0.23), at least three restrictions (−4.8 percent; p = 0.11), and all four restrictions (−8.2 percent; p = 0.38).
Exhibit 4. Percent differences in total days of opioid prescribing of one or more restrictions on direct-to-physician pharmaceutical marketing, 2013–16.
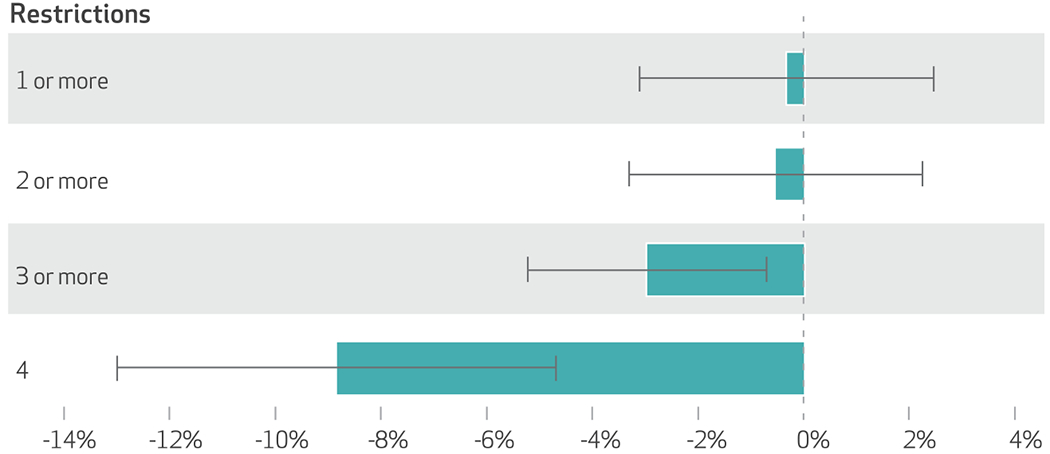
source Authors’ analysis of data for 2013–16 from the Medicare Part D Prescriber Public Use File. notes Numbers of prescribers and prescriber-years are in the notes to exhibit 2. The four restrictions are explained in the text. The exhibit presents semi-elasticity marginal effects from two-part models (explained in the text) that were estimated at the physician-year level. The error bars represent 95%confidence intervals. Standard errors and covariates are explained in the notes to exhibit 3. Year and academic calendar year fixed effects are included in every model.
Discussion
In this study we examined the effects on opioid prescribing of academic medical centers’ conflict-of-interest policies that restricted direct-to-physician pharmaceutical marketing. Overall, we found evidence that the presence of restrictions—specifically, bans on sales representatives and disclosure requirements—was associated with reduced volume of opioid prescribing. We found the largest percentage reductions in the prescribing of opioids that were both brand-name and marketed to prescribers during the period 2013–16. However, we also found substantial spillover effects in the reductions of generic opioids prescribed—which, in turn, drove significant reductions in the total number of opioids prescribed.
Though many academic medical centers had incorporated some restrictions on direct-to-physician pharmaceutical marketing in their conflict-of-interest policies by the end of the study period, only 3.5 percent of the centers had all four marketing restrictions in place. The President’s Commission on Combating Drug Addiction and the Opioid Crisis acknowledged the role of direct-to-physician marketing in fueling the opioid crisis, but it made no recommendations directed at such marketing.48 Our results suggest that even in a market saturated by generics and an era in which rates of opioid prescribing are trending downward across the US, direct-to-physician marketing contributes to increases in opioid prescribing.
While our study focused on policies that restrict direct-to-physician marketing of all types of medications, future research should consider the effects of policies that restrict opioid marketing specifically. Partly in response to lawsuits, Endo Pharmaceuticals halted direct-to-physician opioid marketing in 2017,49,50 and Purdue Pharma did the same in 2018.51 The effects of these policies are uncertain, given that many other firms (sixteen in 2017, according to the authors’ calculations of CMS data)14 continued marketing opioids to physicians. Future research should also prioritize the examination of restrictions on direct-to-physician marketing using patient-level data, which can be used to examine marketing restrictions’ effects not only on measures of overall volume of opioid prescribing but also on measures of high-risk prescribing shown to be associated with elevated risk of opioid overdose, such as long-term and high-dose prescribing.52
Conclusion
Our results suggest that the presence of conflict-of-interest policies at academic medical centers can reduce opioid prescribing. Leaders at the centers that do not have such policies may want to consider adopting them. Similarly, policy makers should consider additional regulations of direct-to-physician marketing.
Supplementary Material
Footnotes
Earlier versions of this research were presented at the Annual Conference of the Western Economic Association International in Vancouver, British Columbia, June 28, 2018, and at the Fall Research Conference of the Association for Policy Analysis and Management in Washington, D.C., November 9, 2018.
Contributor Information
Matthew D. Eisenberg, Assistant professor, Department of Health Policy and Management and core faculty member of the Center for Mental Health and Addiction Policy Research, Johns Hopkins Bloomberg School of Public Health, in Baltimore, Maryland
Elizabeth M. Stone, Doctoral student, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health
Harlan Pittell, Doctoral student, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health.
Emma E. McGinty, Associate professor, Department of Health Policy and Management, deputy director of the Center for Mental Health and Addiction Policy Research, and core faculty member of the Center for Gun Policy and Research, Johns Hopkins Bloomberg School of Public Health
NOTES
- 1.Centers for Disease Control and Prevention. Drug overdose deaths [Internet]. Atlanta (GA): CDC; [last reviewed 2019 Jun 27; cited 2020 Jan 7]. Available from: https://www.cdc.gov/drugoverdose/data/statedeaths.html [Google Scholar]
- 2.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015; 372(3):241–8. [DOI] [PubMed] [Google Scholar]
- 3.Saloner B, McGinty EE, Beletsky L, Bluthenthal R, Beyrer C, Botticelli M, et al. A public health strategy for the opioid crisis. Public Health Rep. 2018;133(1 suppl):24S–34S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Zee A The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.deShazo RD, Johnson M, Eriator I, Rodenmeyer K. Backstories on the US opioid epidemic. Good intentions gone bad, an industry gone rogue and watch dogs gone to sleep. Am J Med. 2018;131(6):595–601. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Prescription opioid data [Internet]. Atlanta (GA): CDC; [last reviewed 2019 Jun 27; cited 2020 Jan 7]. Available from: https://www.cdc.gov/drugoverdose/data/prescribing.html [Google Scholar]
- 7.Guy GP Jr, Zhang K, Bohm MK, Losby J, Lewis B, Young R, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Overdose death maps: overdose deaths involving prescription opioids [Internet]. Atlanta (GA): CDC; [last reviewed 2019 Aug 13; cited 2020 Jan 7]. Available from: https://www.cdc.gov/drugoverdose/data/prescribing/overdose-death-maps.html [Google Scholar]
- 9.Cicero TJ, Kasper ZA, Ellis MS. Increased use of heroin as an initiating opioid of abuse: further considerations and policy implications. Addict Behav 2018;87:267–71. [DOI] [PubMed] [Google Scholar]
- 10.Associated Press. Justice Dept. says it will seek to join settlement talks in opioid lawsuits STAT [serial on the Internet]. 2018. April 2 [cited 2020 Jan 7]. Available from: https://www.statnews.com/2018/04/02/justice-department-opioids-lawsuits/
- 11.Semuels A Are pharmaceutical companies to blame for the opioid epidemic? The Atlantic [serial on the Internet]. 2017. June 2 [cited 2020 Jan 7]. Available from: https://www.theatlantic.com/business/archive/2017/06/lawsuit-pharmaceutical-companies-opioids/529020/
- 12.Hughes E The pain hustlers New York Times [serial on the Internet]. 2018. May 2 [cited 2020 Jan 6]. Available from: https://www.nytimes.com/interactive/2018/05/02/magazine/money-issue-insys-opioids-kickbacks.html
- 13.Johnson Hoffman J. & Johnson ordered to pay $572 million in land-mark opioid trial. New York Times [serial on the Internet]. [Updated 2018. August 30; cited 2020 Jan 7]. Available from: https://www.nytimes.com/2019/08/26/health/oklahoma-opioids-johnson-and-johnson.html
- 14.CMS.gov. Open payments [Internet]. Baltimore (MD): Centers for Medicare and Medicaid Services; [last modified 2019. November 15; cited 2018 Jan 7].Available from: https://www.cms.gov/openpayments/ [Google Scholar]
- 15.Hadland SE, Krieger MS, Marshall BDL. Industry payments to physicians for opioid products, 2013–2015. Am J Public Health. 2017; 107(9):1493–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berndt ER, Bui L, Reiley DR, Urban GL. Information, marketing, and pricing in the U.S. antiulcer drug market. Am Econ Rev. 1995;85(2): 100–5. [PubMed] [Google Scholar]
- 17.Kalyanaram G The endogenous modeling of the effect of direct advertising to consumers in prescription drugs. Int J Pharm Healthc Mark. 2009;3(2):137–48. [Google Scholar]
- 18.Dave D, Saffer H. Impact of direct to consumer advertising on pharmaceutical prices and demand. South Econ J. 2012;79(1):97–126. [Google Scholar]
- 19.Narayanan S, Desiraju R, Chintagunta PK. Return on investment implications for pharmaceutical promotional expenditures: the role of marketing-mix interactions. J Mark 2004;68(4):90–105. [Google Scholar]
- 20.Datta A, Dave D. Effects of physician-directed pharmaceutical promotion on prescription behaviors: longitudinal evidence. Health Econ. 2017; 26(4):450–68. [DOI] [PubMed] [Google Scholar]
- 21.Hadland SE, Cerdá M, Li Y, Krieger MS, Marshall BDL. Association of pharmaceutical industry marketing of opioid products to physicians with subsequent opioid prescribing. JAMA Intern Med. 2018;178(6): 861–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadland SE, Rivera-Aguirre A, Marshall BDL, Cerda M. Association of pharmaceutical industry marketing of opioid products with mortality from opioid-related overdoses. JAMA Netw Open. 2019;2(1): e186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolan R Ban on direct to consumer opioid advertising and doctor marketing is part of Nolan draft bill [Internet]. Washington (DC): Office of Congressman Rick Nolan; 2018. April 24 [cited 2020 Apr 23]. Available from: https://web.archive.org/web/20180922053203/https://nolan.house.gov/media-center/press-releases/ban-on-direct-to-consumer-opioid-advertising-and-doctor-marketing-is [Google Scholar]
- 24.Sullivan T California Senate passes ban on “gifts” to physicians. Policy and Medicine [serial on the Internet]. [Last updated 2018. May 4; cited 2020 Jan 7]. Available from: https://www.policymed.com/2017/05/california-bans-gifts-to-doctors.html
- 25.Iskowitz M Limits on pharma payments to doctors back on policy menu. MM&M [serial on the Internet]. 2017. November 8 [cited 2020 Jan 7]. Available from: https://www.mmm-online.com/home/channel/regulatory/limits-on-pharma-payments-to-doctors-back-on-policy-menu/
- 26.Sullivan T Maine updates their gift to physicians law online. Policy and Medicine [serial on the Internet]. 2018. May 4 [cited 2020 Jan 7]. Available from: https://www.policymed.com/2018/01/maine-updates-their-gift-to-physicians-law-online.html
- 27.Sullivan T Philly councilman introduces legislation banning pharma gifts to doctors. Policy and Medicine [serial on the Internet] 2018. November 13 [cited 2020 Jan 7]. Available from: https://www.policymed.com/2018/11/philly-councilman-introduces-legislation-banning-pharma-gifts-to-doctors.html
- 28.Larkin I, Ang D, Steinhart J, Chao M, Patterson M, Sah S, et al. Association between academic medical center pharmaceutical detailing policies and physician prescribing. JAMA. 2017;317(17):1785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson TS, Huskamp HA, Epstein AJ, Barry CL, Men A, Berndt ER, et al. Antipsychotic prescribing: do conflict of interest policies make a difference? Med Care. 2015;53(4): 338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein AJ, Busch SH, Busch AB, Asch DA, Barry CL. Does exposure to conflict of interest policies in psychiatry residency affect antidepressant prescribing? Med Care. 2013; 51(2):199–203. [DOI] [PubMed] [Google Scholar]
- 31.Larkin I, Ang D, Avorn J, Kesselheim AS. Restrictions on pharmaceutical detailing reduced off-label prescribing of antidepressants and anti-psychotics in children. Health Aff (Millwood). 2014;33(6):1014–23. [DOI] [PubMed] [Google Scholar]
- 32.Food and Drug Administration. Orange Book: approved drug products with therapeutic equivalence evaluations [Internet]. Silver Spring (MD): FDA; [cited 2020 Jan 6]. Available from: https://www.accessdata.fda.gov/scripts/cder/ob/ [Google Scholar]
- 33.Lo B, Field MJ, editors. Conflict of interest in medical research, education, and practice. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 34.Association of American Medical Colleges. Industry funding of medical education: report of an AAMC task force [Internet]. Washington (DC): AAMC; 2008. June [cited 2020 Jan 7]. Available from: https://www.aamc.org/system/files/c/2/482220-industryfundingofmedicaleducation.pdf [Google Scholar]
- 35.American Medical Association. Conflict of interest guidelines for organized medical staffs. Chicago (IL): AMA; 2007. [Google Scholar]
- 36.Carlat DJ, Fagrelius T, Ramachandran R, Ross JS, Bergh S. The updated AMSA scorecard of conflict-of-interest policies: a survey of U.S. medical schools. BMC Med Educ. 2016;16(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Department of Health and Human Services, Office of Inspector General. Opioids in Medicare Part D: concerns about extreme use and questionable prescribing [Internet]. Washington (DC): HHS; 2017. July [cited 2020 Jan 7]. (HHS OIG Data Brief No. OEI-02-17-00250). Available from: https://oig.hhs.gov/oei/reports/oei-02-17-00250.pdf [Google Scholar]
- 38.McBain R, Rose AJ, LaRochelle MR. The U.S. opioid epidemic: one disease, diverging tales. Prev Med 2018;112:176–8. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. CDC WONDER: multiple cause of death data [Internet]. Atlanta (GA): CDC; [last reviewed 2019. November 19; cited 2020 Jan 7]. Available from: https://wonder.cdc.gov/mcd.html [Google Scholar]
- 40.To access the appendix, click on the Details tab of the article online. [Google Scholar]
- 41.Data.Medicare.gov. Physician Compare National Downloadable File [Internet]. Baltimore (MD): Centers for Medicare and Medicaid Services; [cited 2020 Apr 23]. Available from: https://data.medicare.gov/Physician-Compare/Physician-Compare-National-Downloadable-File/mj5m-pzi6 [Google Scholar]
- 42.American Medical Student Association. AMSA scorecard FAQ [Internet]. Chantilly (VA): AMSA; c 2019. [cited 2020 Jan 7]. Available from: https://www.amsa.org/scorecard/faq/ [Google Scholar]
- 43.Institute on Medicine as a Profession. Conflicts of interest [Internet]. New York (NY): The Institute; [cited 2020 Jan 7]. Available from: http://imapny.org/conflictsofinterest/conflictsofinterest2/ [Google Scholar]
- 44.Association of American Medical Colleges. Organizational Characteristics Database (OCD) [Internet]. Washington (DC): AAMC; c 2019. [cited 2020 Jan 7]. Available from: https://www.aamc.org/data-reports/faculty-institutions/report/organizational-characteristics-database-ocd [Google Scholar]
- 45.Association of American Medical Colleges. Industry funding of medical education: report of an AAMC task force [Internet]. Washington (DC): AAMC; 2008. June [cited 2020 Apr 23]. Available from: https://www.aamc.org/system/files/c/2/482220-industryfundingofmedicaleducation.pdf [Google Scholar]
- 46.Wooldridge JM. Econometric analysis of cross section and panel data. 2nd ed. Cambridge (MA): MIT Press; 2010. [Google Scholar]
- 47.Roth J (Harvard University, Cambridge, MA: ). Should we adjust for the test for pre-trends in difference-in-difference designs? [Internet]. Ithaca (NY): Cornell University; arXiv; 2018. May 2 [cited 2020 Jan 8]. Available from: https://arxiv.org/pdf/1804.01208.pdf [Google Scholar]
- 48.Bernstein L White House opioid commission calls for wide-ranging changes to anti-drug policies. Washington Post [serial on the Internet]. 2017. November 1 [cited 2020 Jan 8]. Available from: https://www.washingtonpost.com/news/to-your-health/wp/2017/11/01/white-house-opioid-commission-calls-for-wide-ranging-changes-to-anti-drug-policies/
- 49.Bell J Endo chops sales force after ditching pain drug. BioPharma Dive [serial on the Internet]. 2016. December 8 [cited 2020 Jan 8]. Available from: https://www.biopharmadive.com/news/endo-biodelivery-xiaflex-sales-belbuca/431988/
- 50.Endo. Endo’s open letter on the opioid abuse crisis [Internet]. Dublin (Ireland): Endo; c 2019. [cited 2020 Jan 8]. Available from: http://www.endo.com/our-responsibility/our-commitment/endos-open-letter-on-the-opioid-abuse-crisis [Google Scholar]
- 51.Purdue Pharma [Internet]. Stamford (CT): Purdue; 2018. Press release, Purdue Pharma L.P. issues statement on opioid promotion; 2018. February 9 [cited 2020 Jan 8]. Available from: https://www.purduepharma.com/news/2018/02/09/purdue-pharmal-p-issues-statement-on-opioid-promotion/ [Google Scholar]
- 52.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315(15): 1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


