Abstract
Hypertension affects approximately 1.13 billion adults worldwide and is the leading global risk factor for cardiovascular, cerebrovascular, and kidney diseases. There is emerging evidence that extracellular vesicles participate in the development and progression of hypertension. Extracellular vesicles are membrane-enclosed structures released from nearly all types of eukaryotic cells. During their formation, extracellular vesicles incorporate various parent cell components, including proteins, lipids, and nucleic acids that can be transferred to recipient cells. Extracellular vesicles mediate cell-to-cell communication in a variety of physiological and pathophysiological processes. Therefore, studying the role of circulating and urinary extracellular vesicles in hypertension has the potential to identify novel noninvasive biomarkers and therapeutic targets of different hypertension phenotypes. This review discusses the classification and biogenesis of three EV subcategories (exosomes, microvesicles, and apoptotic bodies) and provides a summary of recent discoveries in the potential impact of extracellular vesicles on hypertension with a specific focus on their role in the blood pressure regulation by organs—artery and kidney, as well as renin-angiotensin-system.
Keywords: Hypertension, extracellular vesicles, endothelium, vascular smooth muscle cells, sodium transporter, RAS
Impact statement
Hypertension is the leading global risk factor for cardiovascular, cerebrovascular, and kidney diseases. There is emerging evidence that circulating and urinary extracellular vesicles are associated with increased blood pressure, suggesting that extracellular vesicles may be biomarkers and also involved in the pathogenesis and progression of hypertension. However, the reviews about the role of extracellular vesicles on hypertension are relatively limited and need to be updated, thus we highlight and discuss the recent studies on the pathophysiological role of extracellular vesicles in the progression of hypertension, with emphasis on the artery and kidney. These may advance our understanding of the pathophysiology of hypertension, find the suitable biomarkers, which could be used to detect the early onset of hypertension before the development of target organ damage, and develop potential therapeutic method by studying role of extracellular vesicles in hypertension.
Introduction
Hypertension, a silent disease, affects approximately 1.13 billion adults worldwide in 2015 and continues to increase.1,2 According to the American Heart Association, nearly half of U.S. adults have hypertension that is uncontrolled in 45.6%. This scenario is perhaps worse in developing and third world countries. Uncontrolled hypertension is the leading global risk factor for cardiovascular, cerebrovascular, and kidney diseases.3–5 For those patients with truly drug-resistant hypertension, nonpharmacological therapies with excellent tolerability profiles may be needed.6,7 Extracellular vesicles (EVs), one of small particles containing various molecules that mediate cell-to-cell communications, have the potential to be such new therapy. There is emerging evidence that hypertension is associated with increased release of EVs and/or different cargoes sorted into EVs in the urine and blood.8–13 Given this fact, EVs may serve as biomarkers for the diagnosis and potential targets for therapeutic intervention in hypertension. In this review, we briefly introduce EVs classification, recipient cell interactions, and biological functions, then discuss the evolving understanding of the role of EVs in hypertension and summarize the current knowledge of EV-mediated regulatory mechanisms. These may advance our understanding of the pathophysiology of hypertension and provide novel insights into the field of translational medicine.
EVs classification
According to the International Society for Extracellular Vesicles, EV is “the generic term for particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate, i.e. do not contain a functional nucleus”.14 These small membrane-enclosed structures are shed from nearly all types of eukaryotic cells and carry information from their parent cells, including membrane receptors, soluble proteins, metabolites, nucleic acids, and lipids. According to their sizes and site of origins, EVs can be divided into three subcategories: exosomes, microvesicles, and apoptotic bodies.10,15
Exosome
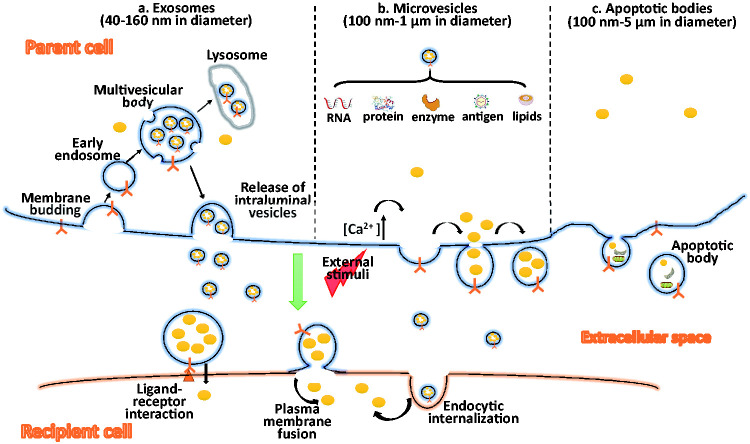
Exosomes are the smallest EVs with diameters from 40 to 160 nm.16,17 The formation of exosomes is multi-step- and multi-mechanism-involved process, including endosomal sorting complex-required transport (ESCRT)-dependent and ESCRT-independent pathways. ESCRT-dependent pathway is the most common pathway regulated by ESCRT-0, -I, -II, and -III. Exosomes are formed when membrane proteins are endocytosed by inward budding of the cell membrane and transferred to early endosomes. Then, the ESCRT-0 complex recruits ubiquitinated proteins and ESCRT-I, -II, -III to invaginate early endosome to form intraluminal vesicles. During the invagination, cytosolic proteins, mRNAs and miRNAs, DNA fragments, and metabolites are incorporated into the intraluminal vesicles. Finally, ESCRT-III induces the fission of intraluminal vesicles, resulting in the formation of multivesicular bodies. When multivesicular bodies fuse with the plasma membrane, intraluminal vesicles are released into the extracellular space and are then referred to as exosomes. Exosome release appears to be controlled by RAB GTPase in the process of vesicular trafficking, endosome recycling, and vesicular plasma membrane fusion.18 If the multivesicular bodies fuse with lysosomes instead of the plasma membrane, then multivesicular bodies undergo degradation (Figure 1).
Figure 1.
EV biogenesis and interaction with recipient cells. (a) Exosome formation starts with inward budding of the plasma membrane and transfer of early endosomes. During early endosome invagination, cytosolic proteins, RNAs, enzyme, antigen, lipids, and other components are incorporated into the intraluminal vesicles. When multivesicular bodies fuse with the lysosome, the protein contents get degraded. When multivesicular bodies fuse with the plasma membrane, exosomes are released. (b) Microvesicles: external stress, e.g. oxidative stress, hypoxia, increases the cytosolic concentration of calcium to induce specific membrane changes and loosen the cytoskeleton, which leads to outward protrusion of the plasma membrane and the formation of microvesicles. (c) Apoptotic bodies: These are released during the late stage of programmed cell death. They contain several intracellular fragments and damaged cellular organelles, as well as other molecules similar to those inside microvesicles. EVs mediate cell-to-cell communication through different mechanisms: (1) ligand-receptor interaction causes the accumulation of EV contents, e.g. inflammatory cytokines, angiogenic factors, growth factors, and extracellular matrix proteins. (2) EV-plasma membrane fusion causes the release of EV content into the recipient cell cytoplasm. 3) Receptor cell internalization causes the endocytosis of EVs to deliver their contents. EVs: extracellular vesicles.
Microvesicles
Unlike exosomes, which are released following the exocytosis of multivesicular bodies, microvesicles, with diameters from 100 nm to 1 μm,16,17 assemble at and are released from the plasma membrane. Cellular stress, which results in an increase in cytosolic calcium, induces specific membrane changes and loosens the cytoskeleton, which leads to the outward protrusion of the plasma membrane and the formation of microvesicles. Lipidic proteins (myristoylated, palmitoylated) in the lumen may play a role by promoting membrane curvature19; some ESCRT subunits, e.g. I/II/III, participate in the assembly and budding of microvesicles.15,20 Microvesicle release is a scission step similar to the final stage of cytokinesis. The activation of acidic sphingomyelinase, leading to ceramide generation, is associated with the plasma membrane release. Plasma membrane protein aggregation is another important factor for microvesicle release. Overexpression leading to higher-order oligomerization in plasma membrane accelerates HIV Gag budding/exosomal sorting and is sufficient to increase protein targeting to microvesicles.21
Apoptotic bodies
Apoptotic bodies (100 nm–5 μm) are released during the late stage of programmed cell death that is controlled by caspase-mediated cleavage, and subsequent activation of Rho-associated protein kinases.15,17,22 They contain several intracellular fragments and damaged cellular organelles, as well as other molecules similar to those inside microvesicles. Apoptotic bodies are smaller than cells and may provide an easier system for phagocytosis. However, the role of apoptotic bodies in intercellular communication is currently unclear. Therefore, the effect of apoptotic bodies in hypertension is not covered here and the term EVs in this review relate to exosomes and microvesicles.
After the release of exosomes and microvesicles into the extracellular space, they bind and fuse similarly to their recipient cells (Figure 1). In general, EVs communicate with recipient cells via three different types of interactions: (1) classical ligand-receptor interaction; (2) EV-plasma membrane fusion; and (3) EV endocytic internalization. EVs selectively bind to specific cell surface receptors located on the plasma membrane of cells.23 Such ligand-receptor interactions likely account for many EV-secreted contents, including EV-carried inflammatory cytokines, angiogenic factors, growth factors, and extracellular matrix proteins that activate immediate responses. EVs can directly fuse with the plasma membrane of cells fusion and release the contents into the cell.24 Exosomes produced by renal proximal tubule cells can be taken up by cells distal to the proximal tubule, such as the distal convoluted and collecting duct cells.25 The receptor cell internalization of EVs by endocytic mechanisms, includes clathrin-/caveola-mediated endocytosis, micropinocytosis, and phagocytosis. The latter two interactions enable the delivery of RNAs or cytoplasmic proteins from EVs to recipient cells26–28 (Figure 1).
Preparation and characteristics of EVs
EVs can be isolated based on their physicochemical properties including buoyant density, shape, size, charge, and surface composition. Therefore, multiple approaches have been developed to isolate and purify EVs, such as ultracentrifugation, sucrose density gradient isolation, size-exclusion chromatography, antibody-based affinity capture, ultrafiltration, and polymer-based precipitation.10,14 More and more investigators realized that each approach has their own advantages and limitations; it is regrettable that there is no technical standard available until now. Researchers choose the appropriate isolation methods mainly based on the type of samples and the forthcoming application of the purified EVs. Generally, if EVs are used as a source of diagnostic biomarker, getting maximal EVs is the major concern. In case of EVs used as therapeutic vehicles, their structure integrity and purification should be the first-priority determinants.
Isolation method
Ultracentrifugation
Among these approaches, differential centrifugation is the most widely accepted method. The sequential centrifugations are initiated with low centrifugal force (g) to remove cells and debris (<1500g), and subsequent higher centrifugal force to remove aggregates of biopolymers and apoptotic bodies (<100,000g), and then ultracentrifuge force to pellet large EVs (10,000–20,000g), and small EVs (100,000–200,000g).29,30 However, contaminants with similar buoyant density, such as fragments of apoptotic cells, lipoproteins, protein aggregates, or proteins, are always presented to prevent the complete purification of EVs and disturb its function.31–33 For example, during the isolation of urinary EVs, contamination of uromodulin is very common. Uromodulin is the most abundant protein in the urine, which could form into polymers to shroud EVs, and influence EV purification and its function, such as the communication to the recipient cells.34 In order to remove the unwanted uromodulin, adding the reducing agent dithiothreitol or the zwitterionic detergent CHAPS to disrupt the polymeric uromodulin is a commonly used method.34
Filtration
According to the varied EV size, different filtration methods are developed in the past years, including ultrafiltration, hydrostatic dialysis, and size-exclusion chromatography. Currently, commercial kits with pores of various diameters are available for EVs extraction process. EVs could be purified by filtration alone or combination of filtration with other methods. As an additional step of ultracentrifugation, it can increase the purity of EVs and save the isolation time. Size-exclusion chromatography uses a gravity flow for separation, which maintains best the vesicle structure, integrity, and biological activity of EVs.35
The combination of ultracentrifugation and filtration might be an ideal way to isolate and purify EVs. First, both methods offer chemical-free handling, which eliminate potential interference due to chemicals. Second, only pipetting and gravity/gravitational force application preserve the EVs’ structure and bioactivity function better. Furthermore, taking the advantage of the ability to deal with large volume, highly diluted samples, e.g. urine, can be centrifugated then concentrated by filtration to a specified EV size. However, adding steps to EV preparation means a loss of EVs and increased preparation time, optimizing each step is necessary in order to obtain the best result.
Other preparation methods
Other EVs isolation methods such as utilizing the EV solubility, aggregation, or affinity are also under intensive investigations. It should be noted that a particular method may influence the activity of EVs. By comparing the impact of different methods on EV preparations, Gámez-Valero et al. found that polyethylene glycol and PRotein Organic Solvent Precipitation-based EV isolation reduced recipient cell viability in vitro,36 probably due to acetone interferes with the functional properties of vesicular membranes. Therefore, the technique using the organic solvent needs further validation.
Characterization
EVs carry different cell type-specific marker proteins from their parent cells, which are used to identify the origin of EVs after isolation of EVs.37 For example, endothelial cell-derived EVs are identified by the presence of CD105, CD144, and CD62e proteins. CD3, CD4, and CD8 are used to identify the lymphocyte-derived EVs. When an enough marker protein is exposed, the cellular origin of EV can be determined by using antibodies directed against such cell-type specific surface antigens. Nevertheless, the assessment of EV purification after isolation is technically complicated because of their heterogeneity and various sizes, as well as the difficulty to distinguish the impurities due to nucleic acids, proteins, and lipid contamination. Nanoparticle tracking analysis and nano-flow cytometry are newly developed methods to characterize the properties of EV size distribution, particle concentration, purity, and phenotype, which might be helpful to determine the purity of isolated EVs.38,39
Biological functions of EVs
Increasing evidence supports the notion that EVs participate in a wide range of biological processes via cell-to-cell communications, which delineates the most critical function of EVs. As stated earlier, EVs can transfer many cellular components from the parent cell to recipient cell, and therefore are able to change the composition and function of the recipient cells. Several databases, such as EVpedia (www.evpedia.info), Vesiclepedia (www.microvesicles.org), and ExoCarta (www.exocarta.org), have online resources that index EV data, including proteomics and transcriptomics. The known cargos of EVs participate in several physiological processes, such as waste management, immune regulation, and cellular homeostasis modulation.
Waste management
EVs were first observed to be released from activated platelets in 1967 and thought to be inert cellular debris and named as “platelet-dust”.40 Since then waste management has become an essential biological function of EVs. EVs can carry redundant intracellular components, thus acting as cellular waste disposal bags by releasing them from the cell. The inhibition of EV secretion results in the accumulation of nuclear DNA fragments in the cytoplasm, which leads to apoptotic cell death in human cells.41 EVs containing cellular waste are especially equipped to facilitate their clearance. For example, apoptotic bodies are easy targets for phagocytosis because of the externalized phosphatidylserine and/or their cargos act as chemotactic signal.22,42,43 The spleen may play a role in accelerating the clearance of EVs. The activity of breast carcinoma- or pancreatic cancer-derived microparticles was detected 5 min after their intravenous injection into mice, but the activity became undetected 2 h after injection. The clearance of the microparticles from circulation is delayed in splenectomized mice.43 However, the underlying mechanisms by which the spleen participates in the clearance of EVs have not been determined.
Immune regulation
The role of EVs in immune regulation is under intense investigation. EVs can impair or enhance immunity and inflammation through their interchange among multiple types of cells. EVs shed from sites of intestinal inflammation in patients with inflammatory bowel disease have increased mRNA and protein levels of anti-inflammatory IL-10, pro- or anti-inflammatory IL-6, and pro-inflammatory IL-8, and TNF-α.44–46 These EVs increase the translation of IL-8 in recipient colonic epithelial cells and induce the migration of macrophages.44 Synovial fluid of rheumatoid arthritis patients contains strong pro-inflammatory and coagulant leukocyte-derived EVs, which trigger autologous fibroblast-like synoviocytes to produce and secrete inflammatory mediators, such as monocyte chemoattractant protein-1, IL-8, IL-6, RANTES, ICAM-1, and VEGF.47 Therefore, EVs can influence the release of chemokines and cytokines and modulate synovial and intestinal inflammation. EVs from TNF-α-induced inflammation of endothelial cells have pro-inflammatory proteins, such as ICAM-1, CCL-2, IL-6, IL-8, CXCL-10, CCL-5, and TNF-α, which mediate inflammation and promote the adhesion and migration of monocytes.48 EVs can also directly modulate various immune cells. Tumor-derived EVs inhibit natural killer cell function by decreasing the activity of CD107a, NKG2D, TNF-α, and INF-γ, and impairing glucose uptake.49 Platelet-derived EVs can also activate monocytes via the RANTES pathway inducing monocyte migration and recruitment to sites of inflammation.50 It should be emphasized that EVs modulate immune responses not only by stimulating the pro-inflammatory cytokines but also triggering the release of anti-inflammatory mediators.48 Human neutrophil-derived EVs have no pro-inflammatory activity on human macrophages but increase the release of transforming growth factor beta 1 (TGF-β1), suggesting that EVs down-modulate macrophage activation and can behave as anti-inflammation effectors.51 However, TGF-β1 can be pro-inflammatory by promoting the secretion of pro-inflammatory cytokines such as IL-17.52 By contrast, the anti-inflammatory interleukin, IL-10, contained in the cargo of EVs derived from adipose tissue-derived autologous mesenchymal stem cells was found to localize and protect renal tubule cells from a porcine model of metabolic syndrome and renal artery stenosis.53
Cellular homeostasis modulation
One aspect of cellular homeostasis is regulated by the balance of EV-associated cell proliferation, apoptosis, and autophagy. EVs from the serum of healthy human volunteers increase the proliferation of H9C2 cardiomyocytes by up-regulating miR-17-3p which inhibits TIMP3 expression.54 TIMP3, aka, tissue inhibitor of metalloproteinases 3, belongs to the TIMP family, which inhibits matrix metalloproteinases. Metalloproteinases promote cell proliferation, as a response to acute kidney injury, for example.55 Mesenchymal stem cell (MSC)-derived EVs enhance the survival of cisplatin-induced acute kidney injury in a mouse model by increasing the expression of anti-apoptotic genes, such as Bcl-xL, Bcl2, and BIRC8, and downregulating the expression of pro-apoptotic genes, such as Casp1, Casp8, and LTA.56 Autophagy induced by EVs also plays a role in cell survival. MSC-derived EVs increase the expression of the autophagy marker LC3 and beclin-1 but decrease the expression of mTOR and fibrotic marker expression in renal tissue, mechanisms that are involved in the improvement renal function and histology of streptozotocin-induced diabetic nephropathy in rats.57 However, the ability of EVs to promote cell survival is not beneficial in cancer because the release of EVs may support tumor cell survival by reducing the chemotherapeutic drug concentration within the tumor cell. Experiments show that after treatment of cultured cancer cell lines with chemotherapeutic agents such as cisplatin and doxorubicin, the cells release EVs which contain the drugs.58,59 The shedding of the EVs is a mechanism for getting rid of the drugs, resulting in drug resistance.
EVs have many other physiological functions which cannot be fully covered in this minireview. In the following sections, we highlight and discuss the recent studies on the pathophysiological role of EVs in the progression of hypertension, with emphasis on the artery and kidney.
EVs and hypertension
Recent clinical studies revealed that circulating and urinary EVs are associated with increased blood pressure, suggesting that EVs may be biomarkers and also involved in the pathogenesis and progression of hypertension.13,60–64 Circulating EVs are derived from the endothelium, platelets, and immune cells, whereas urinary EVs are derived from the kidney and urinary tract. Patient with severe hypertension and even hypertensive patients with well-controlled blood pressure have increased circulating endothelial and platelet microparticles.13,61 Urinary endothelial microparticles are increased in essential and renovascular hypertensive patients, relative to normotensive controls.64 By contrast, endothelial microparticles in renal vein and systemic levels were not different between subjects with essential or renovascular hypertension and normotensive subjects.64 Three months after treatment of renovascular hypertension with or without stenting, the urinary levels of peritubular capillary endothelial microparticles correlated inversely with renal function. The authors suggested that urinary capillary endothelial microparticles may reflect renal microcirculation injury and serve as biomarkers of intrarenal capillary loss.64
In 2018, Otani et al. reported that plasma exosomes can modulate systemic blood pressure in a rat model of hypertension.65 They showed that the intraperitoneal injection of plasma exosomes from spontaneously hypertensive rats (SHRs) increased systolic blood pressure of normotensive Wistar-Kyoto (WKY) rats. By contrast, WKY-derived plasma exosomes decreased the blood pressure of SHRs. Abnormal increase of endothelial EVs in SHRs is associated with endothelial dysfunction and arterial stiffness.66 Good et al. observed that circulating EVs from WKY rats reduced vasodilation of isolated WKY mesenteric arteries but had no effect on SHR mesenteric arteries. However, EVs from hypertensive SHRs failed to reduce vasodilation from both WKY and SHRs.67 These data support the idea that a blood pressure regulating effect of EVs changes after the development of hypertension.
Mechanisms of EV-mediated regulation of blood pressure
EVs and artery
Increased peripheral vascular resistance, a key feature of hypertension, is due to vasoconstriction, impaired vasodilation, and vascular remodeling.68–71 Arteries are classified into: (1) large, elastic, conducting arteries; (2) medium-sized, muscular, distributing arteries; and (3) arterioles. All arteries have three distinct layers: an innermost single layer of endothelial cells; layers of vascular smooth muscle cells (VSMCs); and an outermost layer of connective tissue, primarily comprised of collagen and elastin fibers. Endothelium-dependent vessel dilation and vascular remodeling play important roles in the pathogenesis and maintenance of hypertension.68,71
EVs and endothelium
Endothelial dysfunction, mainly caused by impaired production of endothelial nitric oxide (NO) and increased production of superoxide, is associated with the development and progression of arterial hypertension.71,72 Hypertensive individuals have high levels of endothelial EVs, and endothelial EV levels correlate positively with systolic blood pressure, arterial diameter, and pulse wave velocity and inversely with wall shear stress and microvascular dysfunction.13,61,62,73,74 The arterial vascular endothelium is one of the primary targets of circulating EVs, specifically platelet- and leukocyte-derived EVs. EVs can impair NO release, trigger endothelial inflammation, and alter endothelial cell survival and angiogenesis, to influence arteriolar reactivity.75 EVs from platelets and leukocytes can affect both proinflammatory and anti-inflammatory processes in endothelial cells. In vitro, leukocyte or platelet microparticles release cytokines IL-1β, IL-6, and IL-8, and increase the expression of ICAM-1, VCAM-1, and E-selectin. These factors activate endothelial cell adhesion molecules with/without ERK1/2- and NF-κB-dependent pathways, promoting inflammatory responses in endothelial cells.76,77 However, neutrophil microparticles also contain anti-inflammatory proteins, such as annexin-1, and may attenuate vascular contraction.78 Microparticles from human monocytes cause hypertension and endothelial dysfunction in rats by impairing angiotensin 1–7-mediated vasodilation in mesenteric arteries, which is aggravated by EVs from lipopolysaccharide-treated monocytes. These effects are associated with reduced endothelial NO phosphorylation and Mas receptor expression, via dysregulation of monocyte miRNA-27a.79 Thus, the ability of EVs to increase endothelial inflammation synergizes with the decreased production of NO and endothelial cell proliferation, increasing blood pressure.
EVs and VSMCs
Numerous studies have documented increased thickness of smooth muscle layers and media-to-lumen ratio of arteries in hypertension,68,80,81 which may be the result of maladaptive alterations in VSMCs and the components of the adventitia.82,83 EVs are involved in the thickening of vascular smooth muscle layers and altering of components of the adventitia by different mechanisms that may be related to their different cellular origins.84–89 For example, platelet-derived EVs exert a strong immunomodulatory activity by increasing monocyte adhesion to VSMCs and interacting with increased CD40- and P-selectin, inducing a switch towards a pro-inflammatory phenotype, stimulating VSMC proliferation and migration.89 Tong et al. showed that arterial adventitial fibroblast-derived exosome from SHRs promoted VSMC migration by transferring angiotensin-converting enzyme (ACE) to VSMCs. ACE knockdown in adventitial fibroblasts reduced ACE contents and activity in its exosome and inhibited the migration of VSMCs.90 Recently, Ren et al. found that miR155-5p is involved in the function of aortic adventitial fibroblast-derived EVs. EVs from SHRs, relative to those from WKY rats, had decreased miR155-5p but increased ACE contents. Aortic adventitial fibroblast-derived EVs were able to transfer miR155-5p and ACE at the same time. WKY-EVs or miR155-5p attenuated while SHR-EVs promoted VSMC proliferation, vascular remodeling, and hypertension in both SHRs and WKY rats.84
EVs and kidney
The kidney plays a very important role in the regulation of blood pressure. Besides the role of renal macrovessels and microvessels (afferent and efferent arterioles, vasa recta), renal tubules maintain fluid and electrolyte balance to keep the blood pressure in the normal range.68,83,91,92 After a first discovery of EVs in urine in 2004,93 the interest in urinary EVs in the pathogenesis and diagnosis of hypertension has grown exponentially. In focal segmental glomerulosclerosis and diabetes patients, urinary exosomal Wilms' tumor-1 was significantly increased.94,95 In animal models with podocyte damage (streptozotocin-treated mice, OVE26, and Akita mice), urinary podocyte extracellular vesicles were significantly increased and correlated with the severity of the glomerular injury. The increased biomarkers result from an increased amount of urinary extracellular vesicles and/or increased contents in each vesicle.94,96
Urinary EVs are secreted by various cells of the urinary tract. These EVs contain cell-specific marker proteins from every segment of the nephron. For example, sodium/hydrogen exchanger type 3 (NHE3) and aquaporin-1 from the proximal tubule, sodium potassium chloride cotransporter (NKCC) from the thick ascending limb, sodium chloride cotransporter (NCC) from the distal convoluted tubule, aquaporin-2 from the collecting duct, podocalyxin, podoplanin, and WT-1 from podocytes were all detected in the urinary EVs.9,97 Na+-K+/ATPase is not expressed in intestinal epithelial exosomes.98 However, Na+-K+/ATPase β1 subunit has been reported in urine from healthy human volunteers.93,99 Extracellular vesicles can be filtered in functional nephrons and are found in the urine in healthy subjects due to their small sizes. Since extracellular vesicles circulating in a dynamic process and the lack of effective technology to monitor EV fate, it is difficult to determine to what extent EVs undergo glomerular filtration. Other urinary exosome proteins found in healthy human volunteers can be found in the ESBL urinary exosome protein database (https://hpcwebapps.cit.nih.gov/ESBL/Database/Exosome/).
EVs and sodium transporters
Urinary EVs have been analyzed in various hypertensive disorders and mostly focused on NCC, the sodium chloride cotransporter in the distal convoluted tubule. Urinary total and phosphorylated NCC is increased in EVs of patients with hypertension due to pseudo hypoaldosteronism type II (Gordon’s syndrome) and post-kidney transplant patients taking tacrolimus.100–102 Over-activated NCC leads to the increased renal tubular reabsorption of sodium chloride and subsequently hypertension. Studies have determined whether the abundance of sodium transporters in urinary EVs correlates with sodium reabsorption under physiological conditions. For example, Zachar et al. reported that in healthy humans, total and phosphorylated NCC are present in urinary EVs but not affected by sodium intake.103 Another study also found that the excretions of NCC and NKCC2 in urinary EVs are not associated with the increase in blood pressure in a small number (n = 6) of salt-sensitive humans.104 In rats (strain not given), urinary excretion of exosomal NKCC2 and NCC correlated with their renal abundance. The urinary EVs in women with pre-eclampsia contain NKCC2 with increased phosphorylation at the activating S130 site, and NCC with decreased phosphorylation at the activating T60 site.105 NKCC2 in urinary exosomes has also been reported to be increased in patients with renal dysfunction and American cutaneous leishmaniasis.106
Epithelial sodium channel (ENaC) is responsible for the reabsorption of sodium through the apical membrane of the connecting tubule and the collecting duct. Patients with diabetic nephropathy and hypertension have increased proteolytically cleaved γ-ENaC in their urinary EVs.107 In the aforementioned women with pre-eclampsia, their urinary EVs have decreased phosphorylation of the activating T60 site in NCC.105 Dietary sodium restriction in hypertensive patients or acute aldosterone infusion in healthy humans similarly significantly increased urinary EVs with γENaC, whereas NCC and α-ENaC concentrations were unchanged. Thus, γENaC concentration in EVs may be a useful biomarker of ENaC activation.108 Urinary exosomal miRNA has also been reported to be correlated with an individual’s response to sodium intake.109 Several of these miRNAs, i.e. hsa-miR-4516, are involved in signaling pathways that regulate renal sodium transporters, such as NCC and ENaC.110,111
NHE3 is the major apical sodium exchange in the renal proximal tubule. In Caucasian males and post-menopausal women with type 2 diabetes mellitus, their urinary phosphorylated NHE3 in EVs were higher with glucagon-like peptide receptor agonist, lixisenatide, than insulin glulisine treatment.112 Patients with American cutaneous leishmaniasis, mentioned above, also have increased excretion of urinary exosomes with NHE3.106
EVs and RAS
Abnormalities in sodium and water handling caused by the renin-angiotensin system (RAS) is a common pathophysiology of hypertension.113,114 The RAS is now classified into the classical pathway and the non-classical or counter-regulatory pathway.115,116 The classical pathway starts with angiotensinogen, its conversion to angiotensin I by renin, and conversion of angiotensin I to angiotensin II by angiotensin-converting enzyme. This pathway causes vasoconstriction and stimulation of renal sodium transport. In the non-classical or counter-regulatory pathway, angiotensin-converting enzyme 2 converts angiotensin II from the classical pathway to angiotensin 1–7 or alamandine from angiotensin A and in general, opposes the effects of the classical pathway. There are three angiotensin II receptors: AT1R, AT2R, and AT4R, and two angiotensin 1–7 receptors, MrgD and Mas. AT1R is a vasoconstrictor, while AT2R, AT4R, MrgD, and Mas are vasodilators. Components of the RAS can be transferred by EVs and affect the activity in the recipient cells. As mentioned earlier, ACE contents, but not angiotensin II and AT1R, in adventitial fibroblast EVs are much higher in SHRs than in WKY rats.84,90 The increased ACE in EVs from SHRs increased angiotensin II levels, activated AT1R, and promoted VSMC migration. Biomechanical stress, like osmotic stretch or cardiac pressure overload, induces secretion of AT1R-enriched exosomes.117 AT1R-enriched exosomes traffic to cardiac and skeletal myocytes, and resistance vessels in AT1R knock-out mice to restore Ang II-induced blood pressure response. Other components of the RAS, such as angiotensin 1–7 and Mas receptor, have also been studied. For example, in vivo tail injection of EVs from monocytes to rats impairs angiotensin 1–7-mediated vasodilation in mesenteric arteries, accompanied by decreased Mas receptor expression and eNOS phosphorylation in the endothelium.79 The RAS per se functions as potent stimuli to increase the formation of EVs. Burger et al. found that angiotensin II increased microparticle formation in endothelial cells in vivo and in vitro, accompanied by increased NADPH oxidase-derived ROS generation and Rho kinase activity.118 Endothelial microparticles are enriched in lipid rafts/caveolae, which themselves contribute to generation of new microparticles. These studies indicate that EVs carry the information from RAS into the recipient cells to mediate the known actions of RAS. In turn, the action of RAS increases the formation of EVs. EVs can increase VSMC proliferation, renal sodium reabsorption, vascular remodeling, and finally results in the development of hypertension (Figure 2).
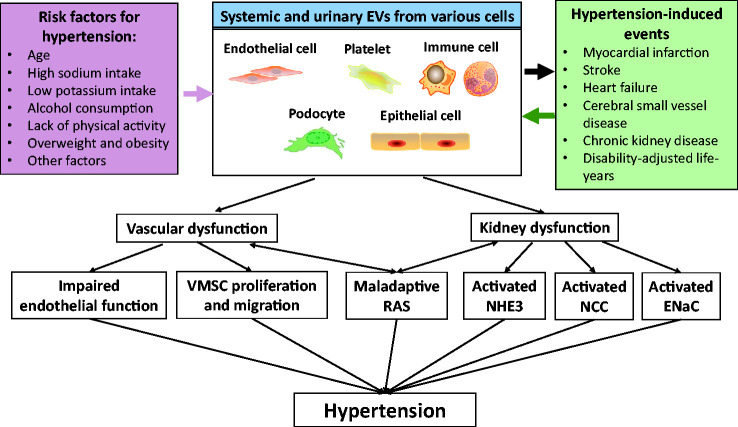
Figure 2.
Role of EVs in the development and progression of hypertension. Risk factors for hypertension-induced events cause the release of systemic and urinary EVs from various cells. EV activation contributes to the pathogenesis of hypertension and hypertension-induced events, such as vascular and kidney dysfunction, including impaired endothelial function, VSMC proliferation and migration, altered NHE3, NCC, ENaC activity, and maladaptive of RAS. These processes collectively contribute to the development and progression of hypertension. EVs: extracellular vesicles; VSMCs: vascular smooth muscle cells; NHE3: sodium/hydrogen exchanger type 3; NCC: sodium chloride cotransporter; ENaC: epithelial sodium channel; RAS: renin-angiotensin-system.
Prognostic and therapeutic potential of EVs in hypertension
The prognostic and therapeutic potential of EVs have gained interest because of their special characteristics. First, as biomarkers for prognosis, EVs can be obtained by noninvasive (urinary EVs) or minimally invasive (circulating EVs) methods and inform the clinician about the possible cause of the hypertension (EVs carry components from their parent cells). EVs are suggested to be surrogate biomarkers for endothelial dysfunction, vascular damage, and increased activity of renal sodium transporters/exchangers in hypertension.9,13,61,62 Moreover, EVs can be used as biomarkers of improved vascular endothelial function. Hypertensive patients on hemodialysis treated with aliskiren, a direct renin inhibitor, for 12 weeks show improved levels of platelet-derived EVs and flow-mediated dilation.119 Subsequent studies aim to analyze the whole spectrum of EVs, including the functional biomolecules within EVs. Proteomic analysis of circulating EVs in albuminuric hypertensive patients showed that two proteins, kalirin and chromodomain-helicase-DNA-binding protein 7, increased with RAS suppression.120 Because expression of these proteins in circulating EVs positively correlates with the endothelial activation marker E-selectin, it is used to monitor the vascular condition of these patients. EV RNA cargo in hypertension is another area of intensive studies. miRNAs are enriched in the urinary EVs of essential hypertensive subjects.109 Kidney nephron-derived exosome miRNAs may be associated with renal sodium transport, and therefore regulation of blood pressure. Low miR-146a expression in EVs is associated with the presence of albuminuria and high miR-27a in EVs may cause hypertension.79,121 Urinary exosome miRNA can be linked to salt sensitivity or inverse salt sensitivity; the latter is a state in which blood pressure is increased by a low salt diet.109
EVs have advantages as therapeutic agents. EV-based therapies have a potential to bypass many hurdles of cell-based therapies because EVs have low immunogenicity.122 EVs are relatively easy to isolate and when frozen are stable over a long period of time, making them available as off-the-shelf products. EVs are able to deliver multiple bioactive molecules that may or may not act synergistically, which allows the tailoring of the method of delivery to optimize their therapeutic effects.123 There are extensive studies reporting the use of EVs in cardiovascular diseases.16,124 In addition, the inhibition of exosome secretion not only has some physiological consequences,41 but also may have some effects to certain diseases. For example, in cancers, tumor cells secrete vast amounts of immune inhibitory exosomes that hinder anti-cancer immune responses. Removal circulating tumor EVs is proposed to inhibit disease progression.125 However, to the best of our knowledge, there is no report that tries to block exosome production in any model of hypertension, which needs to be studied in the future. It should be noted that the therapeutic potential of EVs in hypertension is still in its early phase. An ex vivo study demonstrated plasma-poor circulating EVs from WKY and SHRs can differentially modulate vasoreactivity of isolated mesenteric vessels.67 There is one animal study that demonstrated the potential of plasma EVs to regulate systemic blood pressure with beneficial effect on end-organ damage in hypertension.65 Future studies will shed more light on EV-mediated therapeutics on blood pressure regulation.
Conclusion and prospects
Hypertension is a strong and independent predictor of risk and future incidence of cardiovascular, cerebrovascular, and kidney diseases.3–5 In resistant hypertension, an increasing number of patients are unable to achieve adequate control of their blood pressure despite taking more than three antihypertensive drugs.7 As discussed in this review, EVs play an important role in the development and progression of various hypertensive disorders. The underlying mechanisms include EV-mediated vascular dysfunction, renal sodium and water transporters abnormalities, and RAS disorder. Distinct EVs cause different blood pressure outcomes, which depend upon their origin and stage of disease. Studies are needed to uncover the role of EVs in pathogenesis of hypertension and find EV components that regulate blood pressure, prevent or treat hypertension, and to find out suitable biomarkers, which could be used to detect the early onset of hypertension before the development of target organ damage.
Footnotes
AUTHORS’ CONTRIBUTIONS: ZZL: prepared figures and drafted manuscript; PJ, JY, and CZ: manuscript edition and review.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: These studies were supported, in part, by grants from the National Key R&D Program of China (2018YFC1312700), National Natural Science Foundation of China (31730043, 81930008), Program of Innovative Research Team by National Natural Science Foundation (81721001), Grant from The Third Affiliated Hospital of Chongqing Medical University (KY19024), and the National Institutes of Health, USA (R01 DK039308, DK119652, and P01 HL074940).
ORCID iD: Zhi Z Liu https://orcid.org/0000-0002-7834-4300
References
- 1.(NCD-RisC) NRFC. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017; 389:37–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020; 16:223–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, López-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the lancet commission on hypertension. Lancet 2016; 388:2665–712 [DOI] [PubMed] [Google Scholar]
- 4.Bowe B, Xie Y, Li T, Mokdad AH, Xian H, Yan Y, Maddukuri G, Al-Aly Z. Changes in the US burden of chronic kidney disease from 2002 to 2016: an analysis of the global burden of disease study. JAMA Netw Open 2018; 1:e184412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020; 141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 6.Elliott WJ. Improving outcomes in hypertensive patients: focus on adherence and persistence with antihypertensive therapy. J Clin Hypertens 2009; 11:376–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41:677–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdbrügger U, Le TH. Extracellular vesicles as a novel diagnostic and research tool for patients with HTN and kidney disease. Am J Physiol Renal Physiol 2019; 317:F641–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdbrügger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 2016; 27:12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol 2017; 13:731–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med 2018; 379:958–66 [DOI] [PubMed] [Google Scholar]
- 12.Kwon SH, Woollard JR, Saad A, Garovic VD, Zand L, Jordan KL, Textor SC, Lerman LO. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol Dial Transplant 2017; 32:800–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JM, Su C, Wang Y, Huang YJ, Yang Z, Chen L, Wu F, Xu SY, Tao J. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J Hum Hypertens 2009; 23:307–15 [DOI] [PubMed] [Google Scholar]
- 14.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan M, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ, 2nd, Kornek M, Kosanović MM, Kovács Á F, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG, Jr., Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL, 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ, Jr., Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018; 7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 2019; 21:9–17 [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367:eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of exosome composition. Cell 2019; 177:428–45.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513–25 [DOI] [PubMed] [Google Scholar]
- 19.Kastelowitz N, Yin H. Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. Chembiochem 2014; 15:923–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muralidharan-Chari V, Clancy JW, Sedgwick A, D'Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 2010; 123:1603–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol 2007; 5:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 2016; 311:F844–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature 2015; 527:329–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 2017; 27:172–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gildea JJ, Seaton JE, Victor KG, Reyes CM, Bigler Wang D, Pettigrew AC, Courtner CE, Shah N, Tran HT, Van Sciver RE, Carlson JM, Felder RA. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin Biochem 2014; 47:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, Xiao ZD. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 2014; 289:22258–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010; 11:675–87 [DOI] [PubMed] [Google Scholar]
- 28.Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol 2013; 87:10334–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int 2018; 2018:8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Momen-Heravi F, Balaj L, Alian S, Mantel PY, Halleck AE, Trachtenberg AJ, Soria CE, Oquin S, Bonebreak CM, Saracoglu E, Skog J, Kuo WP. Current methods for the isolation of extracellular vesicles. Biol Chem 2013; 394:1253–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Pol E, Böing AN, Gool EL, Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost 2016; 14:48–56 [DOI] [PubMed] [Google Scholar]
- 32.Jacquillet G, Hoorn EJ, Vilasi A, Unwin RJ. Urinary vesicles: in splendid isolation. Nephrol Dial Transplant 2013; 28:1332–5 [DOI] [PubMed] [Google Scholar]
- 33.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev 2015; 34:474–90 [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Llama P, Khositseth S, Gonzales PA, Star RA, Pisitkun T, Knepper MA. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int 2010; 77:736–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015; 87:3–10 [DOI] [PubMed] [Google Scholar]
- 36.Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borràs FE. Size-Exclusion chromatography-based isolation minimally alters extracellular vesicles' characteristics compared to precipitating agents. Sci Rep 2016; 6:33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci 2013; 124:423–41 [DOI] [PubMed] [Google Scholar]
- 38.Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, Hu X, Aubert D, Zhu S, Wu L, Yan X. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles 2019; 9:1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles 2013; 2:19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967; 13:269–88 [DOI] [PubMed] [Google Scholar]
- 41.Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C, Hara E. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 2017; 8:15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 2014; 14:166–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost 2008; 6:1517–24 [DOI] [PubMed] [Google Scholar]
- 44.Mitsuhashi S, Feldbrügge L, Csizmadia E, Mitsuhashi M, Robson SC, Moss AC. Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm Bowel Dis 2016; 22:1587–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol 2011; 2011:721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulati K, Guhathakurta S, Joshi J, Rai N, Ray A. Cytokines and their role in health and disease: a brief overview. MOJ Immunol 2016; 4:1–9 [Google Scholar]
- 47.Berckmans RJ, Nieuwland R, Kraan MC, Schaap MC, Pots D, Smeets TJ, Sturk A, Tak PP. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res Ther 2005; 7:R536–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosseinkhani B, Kuypers S, van den Akker NMS, Molin DGM, Michiels L. Extracellular vesicles work as a functional inflammatory mediator between vascular endothelial cells and immune cells. Front Immunol 2018; 9:1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao J, Schlößer HA, Wang Z, Qin J, Li J, Popp F, Popp MC, Alakus H, Chon SH, Hansen HP, Neiss WF, Jauch KW, Bruns CJ, Zhao Y. Tumor-derived extracellular vesicles inhibit natural killer cell function in pancreatic cancer. Cancers 2019; 11:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol 2005; 25:1512–8 [DOI] [PubMed] [Google Scholar]
- 51.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004; 104:2543–8 [DOI] [PubMed] [Google Scholar]
- 52.Yang YC, Zhang N, Van Crombruggen K, Hu GH, Hong SL, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy 2012; 67:1193–202 [DOI] [PubMed] [Google Scholar]
- 53.Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 2017; 92:114–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Z, Zhang Z, Yao J, Xie Y, Dai Q, Zhang Y, Zhou L. Serum extracellular vesicles promote proliferation of H9C2 cardiomyocytes by increasing miR-17-3p. Biochem Biophys Res Commun 2018; 499:441–6 [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Tan RJ, Liu Y. The many faces of matrix metalloproteinase-7 in kidney diseases. Biomolecules 2020; 10:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 2012; 7:e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem Y, Hussein AM, Sabry D. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells 2018; 7:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther 2005; 4:1595–604 [DOI] [PubMed] [Google Scholar]
- 59.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res 2003; 63:4331–7 [PubMed] [Google Scholar]
- 60.Sansone R, Baaken M, Horn P, Schuler D, Westenfeld R, Amabile N, Kelm M, Heiss C. Release of endothelial microparticles in patients with arterial hypertension, hypertensive emergencies and catheter-related injury. Atherosclerosis 2018; 273:67–74 [DOI] [PubMed] [Google Scholar]
- 61.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003; 41:211–7 [DOI] [PubMed] [Google Scholar]
- 62.Huang PH, Huang SS, Chen YH, Lin CP, Chiang KH, Chen JS, Tsai HY, Lin FY, Chen JW, Lin SJ. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J Hypertens 2010; 28:1655–65 [DOI] [PubMed] [Google Scholar]
- 63.Gilani SI, Weissgerber TL, Garovic VD, Jayachandran M. Preeclampsia and extracellular vesicles. Curr Hypertens Rep 2016; 18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun IO, Santelli A, Abumoawad A, Eirin A, Ferguson CM, Woollard JR, Lerman A, Textor SC, Puranik AS, Lerman LO. Loss of renal peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension 2018; 72:1180–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otani K, Yokoya M, Kodama T, Hori K, Matsumoto K, Okada M, Yamawaki H. Plasma exosomes regulate systemic blood pressure in rats. Biochem Biophys Res Commun 2018; 503:776–83 [DOI] [PubMed] [Google Scholar]
- 66.Zhang G, Lin X, Shao Y, Su C, Tao J, Liu X. Berberine reduces endothelial injury and arterial stiffness in spontaneously hypertensive rats. Clin Exp Hypertens 2020; 42:257–65 [DOI] [PubMed] [Google Scholar]
- 67.Good ME, Musante L, La Salvia S, Howell NL, Carey RM, Le TH, Isakson BE, Erdbrügger U. Circulating extracellular vesicles in normotension restrain vasodilation in resistance arteries. Hypertension 2020; 75:218–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown IAM, Diederich L, Good ME, DeLalio LJ, Murphy SA, Cortese-Krott MM, Hall JL, Le TH, Isakson BE. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler Thromb Vasc Biol 2018; 38:1969–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC. Vascular smooth muscle contraction in hypertension. Cardiovasc Res 2018; 114:529–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenblum WI. Endothelium-dependent responses in the microcirculation observed in vivo. Acta Physiol 2018; 224:e13111. [DOI] [PubMed] [Google Scholar]
- 71.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol 2009; 157:527–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr., Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 1998; 95:9220–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sansone R, Baaken M, Horn P, Schuler D, Westenfeld R, Amabile N, Kelm M, Heiss C. Endothelial microparticles and vascular parameters in subjects with and without arterial hypertension and coronary artery disease. Data Brief 2018; 19:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feng B, Chen Y, Luo Y, Chen M, Li X, Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis 2010; 208:264–9 [DOI] [PubMed] [Google Scholar]
- 75.Lovren F, Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin Chem 2013; 59:1166–74 [DOI] [PubMed] [Google Scholar]
- 76.Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis 2001; 158:277–87 [DOI] [PubMed] [Google Scholar]
- 77.Taguchi K, Narimatsu H, Matsumoto T, Kobayashi T. ERK-containing microparticles from a diabetic mouse induce endothelial dysfunction. J Endocrinol 2019; 241:221–33 [DOI] [PubMed] [Google Scholar]
- 78.Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 2008; 112:2512–9 [DOI] [PubMed] [Google Scholar]
- 79.Zou X, Wang J, Chen C, Tan X, Huang Y, Jose PA, Yang J, Zeng C. Secreted monocyte miR-27a, via mesenteric arterial mas receptor-eNOS pathway, causes hypertension. Am J Hypertens 2020; 33:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roman MJ, Saba PS, Pini R, Spitzer M, Pickering TG, Rosen S, Alderman MH, Devereux RB. Parallel cardiac and vascular adaptation in hypertension. Circulation 1992; 86:1909–18 [DOI] [PubMed] [Google Scholar]
- 81.Aalkjaer C, Heagerty AM, Petersen KK, Swales JD, Mulvany MJ. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation–contraction coupling in isolated resistance vessels from essential hypertensives. Circ Res 1987; 61:181–6 [DOI] [PubMed] [Google Scholar]
- 82.Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, Kawecki C, Guillot A, Martiny L, Debelle L, Maurice P. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res 2016; 110:298–308 [DOI] [PubMed] [Google Scholar]
- 83.Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol 2012; 110:49–55 [DOI] [PubMed] [Google Scholar]
- 84.Ren XS, Tong Y, Qiu Y, Ye C, Wu N, Xiong XQ, Wang JJ, Han Y, Zhou YB, Zhang F, Sun HJ, Gao XY, Chen Q, Li YH, Kang YM, Zhu GQ. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. J Extracell Vesicles 2019; 9:1698795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Furmanik M, Chatrou M, van Gorp RH, Akbulut A, Willems B, Schmidt HH, van Eys G, Bochaton-Piallat ML, Proudfoot D, Biessen EA, Hedin U, Matic L, Mees B, Shanahan CM, Reutelingsperger C, Schurgers LJ. Reactive oxygen-forming Nox5 links vascular smooth muscle cell phenotypic switching and extracellular vesicle-mediated vascular calcification. Circ Res 2020; 127:911–27 [DOI] [PubMed] [Google Scholar]
- 86.Ryu JH, Jeon EY, Kim SJ. Indoxyl sulfate-induced extracellular vesicles released from endothelial cells stimulate vascular smooth muscle cell proliferation by inducing transforming growth Factor-Beta production. J Vasc Res 2019; 56:129–38 [DOI] [PubMed] [Google Scholar]
- 87.Wang D, Gao B, Yue J, Liu F, Liu Y, Fu W, Si Y. Exosomes from mesenchymal stem cells expressing miR-125b inhibit neointimal hyperplasia via myosin IE. J Cell Mol Med 2019; 23:1528–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niu C, Wang X, Zhao M, Cai T, Liu P, Li J, Willard B, Zu L, Zhou E, Li Y, Pan B, Yang F, Zheng L. Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. J Am Heart Assoc 2016; 5:e004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vajen T, Benedikter BJ, Heinzmann ACA, Vasina EM, Henskens Y, Parsons M, Maguire PB, Stassen FR, Heemskerk JWM, Schurgers LJ, Koenen RR. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J Extracell Vesicles 2017; 6:1322454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tong Y, Ye C, Ren XS, Qiu Y, Zang YH, Xiong XQ, Wang JJ, Chen Q, Li YH, Kang YM, Zhu GQ. Exosome-Mediated transfer of ACE (Angiotensin-Converting enzyme) from adventitial fibroblasts of spontaneously hypertensive rats promotes vascular smooth muscle cell migration. Hypertension 2018; 72:881–8 [DOI] [PubMed] [Google Scholar]
- 91.Zeng C, Villar VA, Eisner GM, Williams SM, Felder RA, Jose PA. G protein-coupled receptor kinase 4: role in blood pressure regulation. Hypertension 2008; 51:1449–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol 2012; 2:2393–442 [DOI] [PubMed] [Google Scholar]
- 93.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004; 101:13368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou H, Kajiyama H, Tsuji T, Hu X, Leelahavanichkul A, Vento S, Frank R, Kopp JB, Trachtman H, Star RA, Yuen PS. Urinary exosomal Wilms' tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol 2013; 305:F553–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalani A, Mohan A, Godbole MM, Bhatia E, Gupta A, Sharma RK, Tiwari S. Wilm's tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One 2013; 8:e60177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol 2014; 25:1401–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karpman D, Ståhl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol 2017; 13:545–62 [DOI] [PubMed] [Google Scholar]
- 98.Mallegol J, van Niel G, Heyman M. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol Dis 2005; 35:11–6 [DOI] [PubMed] [Google Scholar]
- 99.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 2009; 20:363–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rojas-Vega L, Jiménez-Vega AR, Bazúa-Valenti S, Arroyo-Garza I, Jiménez JV, Gómez-Ocádiz R, Carrillo-Pérez DL, Moreno E, Morales-Buenrostro LE, Alberú J, Gamba G. Increased phosphorylation of the renal Na+-Cl- cotransporter in male kidney transplant recipient patients with hypertension: a prospective cohort. Am J Physiol Renal Physiol 2015; 309:F836–42 [DOI] [PubMed] [Google Scholar]
- 101.Mayan H, Attar-Herzberg D, Shaharabany M, Holtzman EJ, Farfel Z. Increased urinary Na-Cl cotransporter protein in familial hyperkalaemia and hypertension. Nephrol Dial Transplant 2008; 23:492–6 [DOI] [PubMed] [Google Scholar]
- 102.Tutakhel OAZ, Moes AD, Valdez-Flores MA, Kortenoeven MLA, Vrie MVD, Jeleń S, Fenton RA, Zietse R, Hoenderop JGJ, Hoorn EJ, Hilbrands L, Bindels RJM. NaCl cotransporter abundance in urinary vesicles is increased by calcineurin inhibitors and predicts thiazide sensitivity. PLoS One 2017; 12:e0176220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zachar R, Jensen BL, Svenningsen P. Dietary Na(+) intake in healthy humans changes the urine extracellular vesicle prostasin abundance while the vesicle excretion rate, NCC, and ENaC are not altered. Am J Physiol Renal Physiol 2019; 317:F1612–22 [DOI] [PubMed] [Google Scholar]
- 104.Esteva-Font C, Wang X, Ars E, Guillén-Gómez E, Sans L, González Saavedra I, Torres F, Torra R, Masilamani S, Ballarín JA. Fernández-Llama P. Are sodium transporters in urinary exosomes reliable markers of tubular sodium reabsorption in hypertensive patients? Nephron Physiol 2010; 114:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu CC, Katerelos M, Choy SW, Crossthwaite A, Walker SP, Pell G, Lee M, Cook N, Mount PF, Paizis K, Power DA. Pre-eclampsia is associated with altered expression of the renal sodium transporters NKCC2, NCC and ENaC in urinary extracellular vesicles. PLoS One 2018; 13:e0204514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oliveira RA, Diniz LF, Teotônio LO, Lima CG, Mota RM, Martins A, Sanches TR, Seguro AC, Andrade L, Silva GB, Jr., Libório AB, Daher EF. Renal tubular dysfunction in patients with American cutaneous leishmaniasis. Kidney Int 2011; 80:1099–106 [DOI] [PubMed] [Google Scholar]
- 107.Andersen H, Friis UG, Hansen PB, Svenningsen P, Henriksen JE, Jensen BL. Diabetic nephropathy is associated with increased urine excretion of proteases plasmin, prostasin and urokinase and activation of amiloride-sensitive current in collecting duct cells. Nephrol Dial Transplant 2015; 30:781–9 [DOI] [PubMed] [Google Scholar]
- 108.Qi Y, Wang X, Rose KL, MacDonald WH, Zhang B, Schey KL, Luther JM. Activation of the endogenous Renin-Angiotensin-Aldosterone system or aldosterone administration increases urinary exosomal sodium channel excretion. J Am Soc Nephrol 2016; 27:646–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA. Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem 2013; 46:1131–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salih M, Fenton RA, Zietse R, Hoorn EJ. Urinary extracellular vesicles as markers to assess kidney sodium transport. Curr Opin Nephrol Hypertens 2016; 25:67–72 [DOI] [PubMed] [Google Scholar]
- 111.Gracia T, Wang X, Su Y, Norgett EE, Williams TL, Moreno P, Micklem G, Karet Frankl FE. Urinary exosomes contain MicroRNAs capable of paracrine modulation of tubular transporters in kidney. Sci Rep 2017; 7:40601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tonneijck L, Muskiet MHA, Blijdorp CJ, Smits MM, Twisk JW, Kramer MHH, Danser AHJ, Diamant M, Joles JA, Hoorn EJ, van Raalte DH. Renal tubular effects of prolonged therapy with the GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes mellitus. Am J Physiol Renal Physiol 2019; 316:F231–f40 [DOI] [PubMed] [Google Scholar]
- 113.Almeida LF, Tofteng SS, Madsen K, Jensen BL. Role of the renin-angiotensin system in kidney development and programming of adult blood pressure. Clin Sci 2020; 134:641–56 [DOI] [PubMed] [Google Scholar]
- 114.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59:251–87 [DOI] [PubMed] [Google Scholar]
- 115.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol 2016; 310:H137–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 2015; 116:960–75 [DOI] [PubMed] [Google Scholar]
- 117.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 2015; 131:2120–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via ang II receptor type I/NADPH oxidase/rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol 2011; 31:1898–907 [DOI] [PubMed] [Google Scholar]
- 119.Moriya H, Kobayashi S, Ohtake T, Tutumi D, Mochida Y, Ishioka K, Oka M, Maesato K, Hidaka S, Nomura S. Aliskiren, a direct renin inhibitor, improves vascular endothelial function in patients on hemodialysis independent of antihypertensive effect – a pilot study. Kidney Blood Press Res 2013; 37:190–8 [DOI] [PubMed] [Google Scholar]
- 120.de la Cuesta F, Baldan-Martin M, Moreno-Luna R, Alvarez-Llamas G, Gonzalez-Calero L, Mourino-Alvarez L, Sastre-Oliva T, López JA, Vázquez J, Ruiz-Hurtado G, Segura J, Vivanco F, Ruilope LM, Barderas MG. Kalirin and CHD7: novel endothelial dysfunction indicators in circulating extracellular vesicles from hypertensive patients with albuminuria. Oncotarget 2017; 8:15553–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perez-Hernandez J, Olivares D, Forner MJ, Ortega A, Solaz E, Martinez F, Chaves FJ, Redon J, Cortes R. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J Transl Med 2018; 16:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle K, Chalmers J, Schmittgen TD, Phelps MA. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles 2017; 6:1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, Kim J, Kanai M, Bi L, Brown K, Di Paolo G, Homma S, Sims PA, Topkara VK. Vunjak-Novakovic G. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng 2018; 2:293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol 2020; 17:685–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med 2012; 10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]