Abstract
Sickle cell disease is the most common hemoglobinopathy and affects millions worldwide. The disease is associated with severe organ dysfunction, acute and chronic pain, and significantly decreased life expectancy. The large body of work demonstrating that hemolysis results in rapid consumption of the endogenous vasodilator nitric oxide, decreased nitric oxide production, and promotion of vaso-occlusion provides the basis for the hypothesis that nitric oxide bioavailability is reduced in sickle cell disease and that this deficit plays a role in sickle cell disease pain. Despite initial promising results, large clinical trials using strategies to increase nitric oxide bioavailability in sickle cell disease patients yielded no significant change in duration or frequency of acute pain crises. Further, recent investigations showed that sickle cell disease patients and mouse models have elevated baseline levels of blood nitrite, a reservoir for nitric oxide formation and a product of nitric oxide metabolism, regardless of pain phenotype. These conflicting results challenge the hypotheses that nitric oxide bioavailability is decreased and that it plays a significant role in the pathogenesis in sickle cell disease acute pain crises. Conversely, a large body of work demonstrates that nitric oxide, as a neurotransmitter, has a complex role in pain neurobiology, contributes to the development of central sensitization, and can mediate hyperalgesia in inflammatory and neuropathic pain. These results support an alternative hypothesis: one proposing that altered nitric oxide signaling may contribute to the development of neuropathic and/or inflammatory pain in sickle cell disease through its role as a neurotransmitter.
Keywords: Sickle cell disease, nitric oxide, nitrite, acute pain, chronic pain
Impact statement
For the past two decades, researchers studying the pathophysiology of sickle cell disease (SCD) have hypothesized that the bioavailability of the endogenous vasodilator nitric oxide (NO) is decreased in SCD and that this NO deficit could contribute to SCD-related organ injury as well as acute pain episodes. However, multiple clinical trials aimed at increasing NO-production or release using NO-potentiating agents or NO itself yielded no significant alteration or improvement on the course of acute pain crises in SCD patients. This work provides a review of the contradicting results surrounding that hypothesis and proposes an alternative framework of thought regarding NO’s role as a neurotransmitter possibly exacerbating SCD-pain.
Introduction
Sickle cell disease (SCD) is the most common monogenic hematologic disorder and affects over 100,000 Americans and millions worldwide.1,2 The disease is caused by a single point mutation in the gene encoding the β globin subunit, which results in the expression of a mutant sickle hemoglobin (HbS).3,4 Upon red blood cell deoxygenation and/or dehydration, HbS polymerizes, which leads to sickling of red blood cells, ongoing chronic hemolysis, and recurrent episodes of vaso-occlusion.3,5,6 These events result in chronic degenerative complications and as a result, SCD patients have a two- to three-decade reduction in life expectancy compared to the general population.7 Patients with SCD not only face premature mortality, but also have a poor overall quality of life due to acute and chronic pain and varying degrees of multi-system organ damage.2,8,9 In fact, SCD-related pain is a leading cause of morbidity, the main reason why SCD patients seek medical attention, and is associated with high health care costs. Data from 2018 show that acute pain episodes comprised a majority of the $2.98 billion total cost of care for SCD patients in the US.10,11 Therefore, SCD pain is associated with significant morbidity and high health care costs.
Unfortunately, the pathobiology and triggers of SCD acute pain crises are incompletely understood. Further, while acute pain crises are the hallmark of the disease, many SCD patients can develop chronic pain also by incompletely understood mechanisms. For the last two decades, many experimental and clinical studies have focused on the hypothesis that nitric oxide (NO) depletion and subsequent impaired vasodilation might play a role in the pathobiology of SCD complications including vaso-occlusion and acute pain. Indeed, among many functions, NO serves as a neurotransmitter and has been shown to be involved in the nociceptive process, in the development of central sensitization, and in mediating effects of analgesics including opioids.12 While it is known that NO plays a complex and dual (pro- and anti-nociceptive) role in modulating nociceptive processing, it remains unknown whether NO availability is depleted in SCD and whether altered NO availability contributes to SCD-pain. In this short review, we discuss the complex relationship between NO and pain phenotypes in the context of SCD acute and chronic pain and posit that additional investigations are needed to understand whether that is indeed a connection between NO availability and SCD pain.
Sickle cell disease, a painfully complex disorder
While the molecular etiology of SCD has been known since Linus Pauling and colleagues published their study in Science over 70 years ago,13 the mechanisms and triggers underlying SCD acute pain episodes, often referred to as vaso-occlusive crises (VOC), remain incompletely understood. A multiplicity of complex events is believed to take place and ultimately contribute to the development of vaso-occlusion and acute pain crises.14–20 Some of those events include the sickling of red blood cells in a background of existing chronic inflammation and endothelial damage, which can lead to the activation of neutrophils, mast cells, macrophages, and platelets, the release of inflammatory cytokines and cell adhesion molecules from leukocytes and endothelial cells, and an increase in platelet aggregation.3,21–23 This cascade of events thus illustrates the complexity of the underlying mechanisms of acute pain crises in SCD.
Given that few effective mechanism-based interventions are available, the current clinical approach to pain therapy for SCD acute pain crises is aimed at symptom control. High doses of opioid analgesics and fluids remain the mainstay of treatment during acute crises.24 One of the many challenges clinicians face when treating SCD-pain is the remarkable variability of pain phenotypes among SCD patients. Researchers have shown that 1% of SCD patients experience more than six acute pain crises per year, while 39% of patients report few or no episodes of severe pain yearly.25,26 Additionally, only 5% of SCD patients account for over 30% of all reported acute pain episodes requiring hospitalization.25,26 While the underlying reasons for such variability in SCD-pain phenotypes are incompletely understood, factors such as sickle cell genotype, genetic modifiers, age, and disease severity appear to play a role.25–27
There is mounting evidence demonstrating that some SCD patients will develop chronic pain, which can be punctuated with acute pain crises. In addition to recurrent episodes of pain crises, complications of SCD, such as skin ulcers and avascular necrosis can be associated with acute and chronic pain.28–30 This heterogeneity of pain phenotypes among SCD patients can certainly lead to therapeutic challenges and add complexity to the interpretation of results of clinical trials examining analgesic therapies. Typically, children with SCD are pain-free between acute pain crises and do not require chronic opioids.31 In contrast, as patients get older, some will persistently have some degree of pain between crises.31 In fact, publications reporting daily pain assessments of SCD patients show that more than 50% of patients experienced pain on more than half of the days evaluated.26 Additionally, patient reported outcome studies using validated cross-sectional questionnaires also document that some SCD patients have pain with neuropathic characteristics, such as hypersensitivity or allodynia.32,33 One study reported that almost 40% of the patients sampled used neuropathic pain descriptors to relate their pain experiences.32 In 2017, the American Pain Society Pain Taxonomy initiative developed an outline for the diagnosis of chronic SCD pain syndromes.34 The SCD experts in that working group proposed the existence of three common subtypes of chronic pain in SCD patients: chronic pain not resulting from SCD complications, chronic pain derived from a complication of SCD, and chronic pain with mixed presentation.34 In turn, recently, the American Society of Hematology issued a set of specific guidelines for the management of acute and chronic pain in SCD patients.28 Further, recognizing that SCD patients have such high pain burden, scientific societies as well as regulatory and funding agencies have dedicated great effort and resources to improve our understanding of the underlying mechanisms of SCD pain and to develop effective mechanism-based non-opioid pain therapies.
The NO deficiency hypothesis in SCD
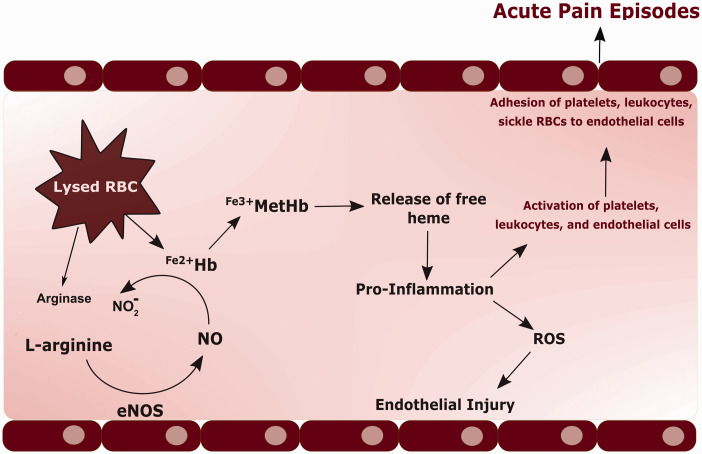
During the past 20 years, a number of preclinical 35–37 and clinical studies 35,38–44 have centered on the hypothesis that decreased NO availability and signaling play a central role in the pathobiology of SCD complications including vaso-occlusion and pain. Several molecular events could certainly increase NO scavenging and decrease NO production, thereby leading to decreased NO bioavailability (Figure 1) in SCD. Sickling and hemolysis of red blood cells caused by HbS polymerization result in the release of cell free hemoglobin that rapidly and potently scavenges NO from plasma,14,45 thereby increasing NO consumption. Hemolysis also results in the release of arginase, an enzyme that degrades the substrate for NO synthesis, L-arginine, effectively decreasing NO production by NO synthase (NOS) enzymes. In fact, plasma arginine levels have been shown to be reduced in SCD patients compared to controls.46 Further, arginine deficiency resulting from increased arginase activity has been linked to decoupling of NOS enzymes and the production of superoxide that then reacts with NO to form peroxynitrite, which further reduces NO bioavailability.46,47 Vascular inflammation and superoxide production in SCD potentially result in a deficiency of the essential NOS cofactor, tetrahydrobiopterin, which has been implicated in the uncoupling of NOS enzymes, rendering them unable to produce NO.48–50 Moreover, SCD patients have been shown to have elevations of asymmetric dimethylarginine (ADMA), the arginine metabolite and endogenous NOS inhibitor, implying increased eNOS inhibition and decreased NO production.51 Therefore, the “NO deficiency hypothesis” postulates that various events triggered by hemolysis result in decreased NO bioavailability in SCD. In turn, decreased NO bioavailability would lead to vasoconstriction, endothelial injury, reactive oxygen species generation, platelet activation, leukocyte adhesion, vaso-occlusion, and downstream ischemia, possibly contributing to the development of acute pain crises.
Figure 1.
The NO-deficiency hypothesis. Hemolysis of red blood cells (RBCs) in sickle cell patients results in the release of free ferrous hemoglobin (Fe2+Hb) that then rapidly reacts with nitric oxide (NO) to form nitrite (NO2) and methemoglobin (Fe3+MetHb). This results in the release of free heme, which promotes inflammation, oxidative stress with reactive oxygen species (ROS) production, and endothelial injury. The release of free heme additionally activates platelets, leukocytes, and endothelial cells, leading to their adhesion to the endothelium. Hemolysis also results in the release of arginase, an enzyme that degrades the substrate for NO synthesis, L-arginine, effectively decreasing NO production by NO synthase (NOS) enzymes. This complex cascade of events is believed to contribute to vaso-occlusion, downstream ischemia, and possibly acute pain crises.
These findings and concepts have formed the basis for several clinical trials of strategies aimed at increasing NO bioavailability in SCD patients to treat a number of SCD complications including acute pain crises and other vascular complications such as chronic kidney disease, stroke, and pulmonary hypertension.40,41,52 While the theory behind this hypothesis has been reviewed in great detail,53,54 investigations into the extent that reduced NO bioavailability contributes to the pathophysiology of SCD acute pain episodes have produced conflicting results.35,38,55,56
The challenges of measuring nitric oxide levels in biological matrices—The proof is in the measurement
Studies of NO availability and its signaling are fraught with challenges related to its measurement given NO’s very short half-life (100 ms half-life average) and the complexity of its metabolism.57,58 Nitric oxide rapidly reacts with hemoglobin, is metabolized into nitrite and nitrate,59,60 and can react with lipids, proteins, and thiols to form peroxynitrite, N-nitrosamines (RNNOs), and S-nitrosothiols (RSNOs).61,62 Several of these NO-generated compounds actually have physiological functions and can regenerate NO.62,63 Therefore, the challenges associated with the measurement of NO are not to be underestimated and need to be considered when interpreting studies of its bioavailability.
The NO metabolites, nitrite, and nitrate, are commonly measured in combination and referred to as NOx, which is a surrogate measure of NO levels. However, NOx measurements tend to reflect mostly nitrate levels, which are well known to vary greatly with human diets. The other NO metabolite, nitrite, serves as a reservoir pool for NO production and as a signaling molecule itself.64,65 A great deal of work has shown that nitrite can re-generate NO by NOS-independent pathways, especially during hypoxic and ischemic conditions, and as such, nitrite can mediate vasodilation and cytoprotection in the setting of ischemia/reperfusion.66–72 Therefore, specific measurement of nitrite has been regarded as an indirect measurement of NO availability.
One caveat when measuring nitrite levels is the fact that nitrite is commonly found in collection vials, laboratory glassware, and reagents, generating concern for sample contamination during collection, processing, and nitrite measurement.73 Protocols for blood collection to avoid sample nitrite contamination have been developed and used to examine nitrite levels in blood compartments and biological matrices.73,74 With such methods, the measurement of nitrite is an acceptable surrogate measure of NO. In addition to NO metabolites, such as nitrite and nitrate, secondary messengers such as cyclic guanosine monophosphate (cGMP) have been cited as potential indicators of downstream NO signaling and NO levels.
Altered NO availability in SCD: What is the evidence?
Apart from the characterization of a cascade of events that could lead to increased consumption and decreased production of NO, studies conclusively demonstrating decreased NO availability in SCD are lacking. In support of decreased NO bioavailability, one study showed that SCD patients with higher plasma heme concentrations, and therefore increased NO consumption, had lower forearm blood flow measurements, suggesting that increased NO scavenging by heme leads to decreased NO bioavailability and less vasodilation in SCD.14 Researchers also investigated lactate dehydrogenase (LDH) as a biomarker of intravascular hemolysis and NO consumption.75,76 In one study, researchers showed that patients with high LDH had more hemolysis, higher plasma NO consumption, and diminished vasodilatory responses to nitroprusside, a NO donor.76 Further, those patients were found to be at increased risk of developing pulmonary hypertension, cutaneous leg ulcers, and priapism, and of having early mortality.76 While the study was not powered to establish a relationship between high LDH and incidence of strokes or other disease complications, the authors concluded that increased hemolysis was associated with decreased NO availability and vascular complications in SCD patients.76 Together, these studies provided indirect evidence that NO availability might be decreased in SCD.
Researchers have shown that SCD patients display diminished vasodilatory responses to NO donors and these finding led to the hypothesis of a “nitric oxide resistance state” in SCD patients.77 However, studies investigating the vasodilatory responses of SCD patients to NO-dependent stimuli have produced conflicting results.42–44 One study showed that forearm blood flow was higher in SCD patients than in healthy subjects in response to the eNOS dependent vasodilator acetylcholine,43 thus suggesting an increased synthesis or release of NO in response to pharmacologic stimuli in these patients.42,43 Interestingly, in that same study there was no significant difference in forearm blood flow changes upon administration of NG-Methyl-L-arginine (L-NMMA), a nonspecific NOS inhibitor, comparing SCD patients and healthy subjects, suggesting that both groups had similar baseline NO levels.43 Moreover, administration of the NO donor, sodium nitroprusside, produced similar vasodilatory responses in SCD patients and healthy subjects, indicating normal NO-mediated vasodilation in SCD patients.43 Together, these findings suggest normal basal production of NO and upregulated acetylcholine-dependent NO production, which is not consistent with impaired vessel diameter adjustments in SCD. These results are in agreement with other blood flow studies demonstrating increased vasodilatory response to NO donors or pharmacologic stimuli in SCD patients or mice compared to controls.42,78 Together, these results dispute the hypothesis of decreased NO bioavailability and/or nitric oxide resistance in SCD.
Also contradicting the decreased NO availability hypothesis, there are multiple animal and human studies suggesting that nitrite and NO bioavailability are actually elevated, rather than reduced in SCD during steady state (baseline) conditions. For example, despite methodological limitations mentioned previously, researchers measuring plasma NOx (nitrite and nitrate combined) concentrations in SCD patients and controls showed that these NO metabolites were elevated in SCD patients at steady state.79 This was corroborated by a later study showing an eight-fold increase of plasma nitrite levels in SCD patients compared to control subjects during baseline conditions.55 In addition to elevations of plasma nitrite, expression of endothelial NOS was shown to be increased in cremaster muscle and kidneys of transgenic SCD mice.80,81 These increases in endothelial NOS expression were coupled with lower mean arterial pressure 80 and higher urinary nitrite measurements,81 which were altered with NOS substrates and reversed with NOS inhibitors.80,81 Others have examined the secondary messenger in the NO signaling pathway, cGMP, at baseline and showed that vascular cGMP is elevated in aortic rings of SCD mice.37 The results of these studies were further supported by our recent study showing elevated nitrite and cGMP levels in blood compartments of sickle cell patients and mice when compared with respective controls.35 Together, these findings further contradict the hypothesis that NO availability is decreased in SCD.
For proponents of the decreased NO availability hypothesis, the reported elevations of plasma NO metabolites and secondary messengers are regarded as paradoxical in the setting of endothelial dysfunction and hemolysis, which is characteristic of SCD22. While a molecular mechanism explaining such elevations has not been identified, a possible alternative explanation may consider that the elevation of nitrite levels and eNOS expression in SCD results from a compensatory response to chronic inflammation and vaso-constriction that is seen in SCD. We have recently shown that the accumulation of nitrite in blood of SCD patients and mice results in part from slowed nitrite metabolism caused by decreased hemoglobin concentrations observed in SCD patients and mice.35 Although it is possible for cGMP and nitrite levels to be upregulated by NO-independent mechanisms, 82,83 the fact that nitrite, cGMP, and NOS expression levels are not decreased at steady state in SCD suggests that NO bioavailability is not decreased and might actually be increased in SCD patients.
NO’s role (or lack thereof) in SCD acute pain
The extent to which reduced NO bioavailability contributes to the pathophysiology of SCD acute pain episodes has been examined in translational and clinical studies.35,38,55,56 Studies demonstrating reductions of NOx during acute pain episodes supported the notion that reduced NO bioavailability played a role in exacerbating pain episodes. One study measuring plasma NOx in SCD patients at steady state and during acute pain crises showed a significant reduction in NO metabolites during acute pain episodes.84 These results are corroborated by multiple other studies finding decreased NOx during acute pain episodes compared to steady state.85–87
When we investigated the relationship between pain burden and blood compartment nitrite levels in SCD subjects, we found that subjects with low (defined as ≤ 2 pain related hospitalizations per year) and high pain burden (defined as ≥ 3 pain related hospitalizations per year) have similarly elevated nitrite levels.35 Those findings thus suggest a lack of correlation between steady state nitrite levels, NO bioavailability, and yearly number of pain crises in SCD patients.35 Another study measured serial serum NOx levels and pain scores of SCD patients receiving emergency department analgesic intervention for VOC and showed no significant correlation between changes in NOx levels and changes in pain levels during the overall treatment period. 38 Taken together, these findings suggest that nitrite/nitrate levels are unrelated to the frequency, severity, or duration of pain episodes in SCD patients or animal models.38
While in one study, researchers proposed that increased hemolysis would promote vasoconstriction and increase the frequency of acute pain crises,76 the degree of hemolysis based on LDH levels does not correlate with the frequency of acute pain crises in a larger cohort of SCD patients.88 In fact, a sub-group of those patients with chronically increased LDH exhibited fewer acute pain crises than patients with lower LDH values, suggesting that more hemolysis would actually be associated with fewer acute pain episodes.88 Additionally, as pointed out by others,89 the observed levels of plasma hemoglobin in SCD are 10-fold lower than those levels in paroxysmal nocturnal hemoglobinuria, another hemolytic disorder, which is not associated with acute pain episodes.89 Combined, these results further challenge the hypothesis that an increase in NO scavenging by cell-free hemoglobin causes increased frequency of pain crises in SCD.
Increasing NO bioavailability failed to ameliorate acute pain in SCD patients
For years, hydroxyurea had been the only FDA-approved disease-modifying treatment for SCD and the mechanism of its salutary effects has been predominantly attributed to its effect on fetal hemoglobin synthesis. However, researchers have shown that hydroxyurea directly generates NO in erythroid cells 90 and that the hydroxyurea-associated induction of fetal hemoglobin is mediated by NO-dependent activation of soluble guanylate cyclase.91 Additionally, others have shown that in models of tumor necrosis factor-α-induced acute vaso-occlusion in SCD mice, hydroxyurea improves leukocyte rolling and decreases leukocyte adhesion and red blood cell/leukocyte interactions, which were coupled with beneficial effects on vaso-occlusive mechanisms and survival.92 That same group also showed that acutely, hydroxyurea ameliorates the rapid inflammatory response observed in models of acute hemolysis.93 Importantly, in those two studies, these acute beneficial effects of hydroxyurea were not associated with increases in fetal hemoglobin and were mediated by NO donation and increased NO/cGMP signaling.92,93 Together these reports suggest that in addition to increasing fetal hemoglobin, hydroxyurea might have acute salutary effects, which result from increases in NO/cGMP signaling.
These findings together with the notion that NO availability could be decreased in SCD formed the basis for clinical trials of therapeutic strategies aiming at increasing NO delivery/production (NO donors, arginine salt, increasing NOS activity) or NO signaling (phosphodiesterase inhibitors) in SCD patients. Unfortunately, the trials using strategies to increase NO availability conducted to date have failed to show benefits on the frequency and course of acute pain episodes, thus calling into question the decreased NO availability hypothesis and its role in modulating acute pain in SCD patients.39–41,52 A multicenter clinical trial of sildenafil, a phosphodiesterase inhibitor, which inhibits cGMP degradation and increases NO signaling, was discontinued prematurely due to increased pain crises frequency among patients on sildenafil.39,52 Furthermore, a multi-institutional controlled trial of inhaled NO revealed no therapeutic benefit to adults with acute pain crises as length of hospital stay, visual analog pain scores, cumulative opioid use, and rate of acute chest syndrome remained unchanged compared with placebo.40 Notably, oral arginine, a substrate for NOS, yielded a significant decrease in total analgesic use but no change in total length of hospital stay in pediatric SCD patients.41 Together, these results contradict the idea that increasing NO availability benefits SCD patients during acute pain crises.
In SCD mice, an attempt at chronic nitrite supplementation was associated with elevations in plasma nitrite, increases in cGMP,35 and a paradoxical decrease in muscle nitrite.56 These decreases in muscle nitrite levels were associated with an improvement in grip force, suggesting an improvement in muscle function and in muscle hyperalgesia. In contrast, elevations in plasma nitrite and cGMP levels with nitrite supplementation yielded no changes in sensory fiber sensitization measured by thermal and electrical stimulation.35 Given these results from preclinical and human trials, one must consider that further increases in NO availability might not be beneficial and, in some instances, may be detrimental. One must also consider the possibility that the effect of given treatments on frequency and hospital course of acute pain crises may not inform or predict the effects of those treatments on other complications of SCD. Nevertheless, while strategies to increase NO donation/nitrite supplementation may not be suitable approaches for SCD, it is worth noting that other phosphodiesterase inhibitors (phosphodiesterase-9) and cGMP amplifying agents are currently under various stages of investigation for the treatment of vascular SCD complications.94–96 Whether these agents will have a role in acute pain crises or chronic pain in SCD is yet unknown.
The role of NO signaling in pain—A balancing act
The role of NO signaling in the neurobiology of pain is complex in that it can have both, anti-and pro-nociceptive effects. For example, researchers have shown that activation of NO/cGMP signaling pathways by peripheral administration of NO donors yields analgesia in models of peripheral inflammatory pain and that NOS and cGMP inhibitors block these analgesic effects.97,98 Also supporting the anti-nociceptive effects of NO signaling are a number of preclinical investigations indicating that opioid-induced peripheral analgesia in inflammatory pain is dependent on the activation of NO/cGMP signaling in peripheral sensory nerves.99 Further, reports suggest NO may transiently have anti-nociceptive properties within the spinal cord, depending on the type of neurons activated.100 For example, intrathecal administration of L-arginine increased mechanical tail withdrawal thresholds in rats.101 Together these studies support the notion that activation of NO/cGMP signaling can be beneficial in inflammatory pain by preventing and/or mitigating excitability of peripheral sensory neurons.
Conversely, a large body of literature implicates NO and its signaling in pro-nociceptive effects.102,103 Animal studies have shown that inhibition of NO or cGMP production can reduce inflammatory and neuropathic pain.102,104,105 For example, two studies showed that inhibition of NO synthesis by the non-specific NOS inhibitor L-NAME reduced thermal and mechanical hyperalgesia induced by inflammation.106,107 Other studies have reported a reduction in inflammatory and neuropathic pain markers and associated nocifensive behaviors upon selective neuronal NOS (nNOS) and inducible NOS (iNOS) inhibition.108–111 Additionally, administration of NO and cGMP activators have been shown in multiple studies to increase hypersensitivity of sensory neurons in pain models.112–120 One proposed mechanism of its pro-nociceptive effects is that NO accentuates the dysregulated spreading potentiation of nociceptive signals in the spinal cord, which leads to neuropathic pain in hindpaw ischemia models.112 This effect was effectively blocked by spinal application of L-NAME and is absent in neuronal NOS knockout mice.112
Another study investigating inflammatory pain in mice deficient in NOS isoforms showed significant reductions in thermal hyperalgesia in nNOS, eNOS, and iNOS knock out mice.104 Interestingly, nNOS deficient mice had little thermal hyperalgesia and displayed no mechanical allodynia with inflammatory pain.104 We have additionally shown that genetic deficiency of the three NOS isoforms nNOS, iNOS, and endothelial NOS (eNOS) differentially alters baseline nocifensive behavior.121 Neuronal NOS-deficient animals had evidence of increased tolerance to electrical stimulation of myelinated (Aβ and Aδ) and non-myelinated (C) sensory nerve fibers, suggesting that inhibition of nNOS and subsequent decreases in neuronal NO attenuates sensory fiber hyperalgesia. Inducible NOS-deficient animals also had increased tolerance only to C-fiber stimulation, 121 with another study further implicating iNOS in inflammatory pain processing.122 However, investigations of eNOS deficiency have produced conflicting results. Our study found that eNOS-deficient animals had decreased tolerance to Aβ and Aδ sensory fiber stimulation, 121 while other studies found no evidence to support that eNOS is involved in pain processing.110 Together, these investigations indicate that alterations in NO production can significantly alter the phenotype of inflammatory and neuropathic pain models. While it is clear that NO may exert pro- and anti-nociceptive roles (Table 1), it remains unclear what exactly determines whether NO and cGMP signaling will serve nociceptive or anti-nociceptive functions in neuropathic or inflammatory pain.
Table 1.
Dual effect of nitric oxide (NO) and cyclic guanosine-3′,5′-monophosphate (cGMP) signaling in nociception.
| Anti-nociceptive effects of nitric oxide signaling | Pro-nociceptive Effects of nitric oxide signaling |
|---|---|
| Increased NO/cGMP signaling yields anti-nociceptive responses to prostaglandin-E2 induced hyperalgesia119 | NO contributes to development and maintenance of central sensitization115 |
| Peripheral opioid analgesia is dependent on NO/cGMP signaling96 | NO/cGMP signaling mediates glutamate induced hyperalgesia110 |
| L-arginine increases mechanical thresholds in rats98 | Neuronal NOS knockout mice show 50% reduction in thermal hyperalgesia and abolished mechanical hyperalgesia in models of inflammatory pain101 |
| NOS inhibition attenuates nerve-injury induced mechanical hyperalgesia102 and reduce thermal and mechanical hyperalgesia in inflammatory pain models97,103,104,112,127 |
NOS: nitric oxide synthase.
Beyond acute pain: Implications for chronic pain syndromes in SCD
There are multiple lines of evidence supporting the presence of inflammatory and neuropathic components in SCD pain. Preclinical studies in SCD mice suggest that in SCD there can be dysregulation of sensory neuronal signaling within the peripheral and central nervous systems.123–127 SCD mice display evidence of peripheral neuropathy manifested by thin epidermal skin layers and decreased innervation.128 There are also extensive reports of hypersensitivity to mechanical and thermal stimuli in SCD mouse models, further supporting the existence of abnormalities of the peripheral nervous system and sensitization of sensory nerve fibers.123,127,128 We have shown that SCD mice have sensitization of both myelinated (Aβ and Aδ sensory fibers) and non-myelinated (C) sensory fibers thus suggesting a neuropathic component to the nociception phenotype in the model.127 Furthermore, SCD mice exhibit increased expression of calcitonin gene related peptide (CGRP) and substance P in skin layers, and exhibit increased expression of TLR-4 and inflammatory cytokines such as IL-6 in spinal cords, all of which are upregulated in neuropathic and inflammatory pain conditions.128 Together these preclinical studies suggest that central sensitization and neuropathic processes could contribute to the development of chronic pain in SCD.33,34
Given the known role of NO/cGMP signaling in nociception, in the context of SCD, one must consider the possibility that elevations of NO metabolites and cGMP could contribute to inflammatory and neuropathic pain syndromes in SCD patients.35,55,56,79 As discussed here, SCD patients (and mice) have elevated blood nitrite and cGMP levels in plasma and red cells at baseline, and while those elevated levels do not correlate with the frequency of acute pain episodes, they are associated with sensory fiber sensitization. Given these findings and the evidence suggesting that altered NO bioavailability can contribute to neuropathic and inflammatory pain syndromes, a hypothesis that increased NO availability could contribute to the development of chronic pain syndromes in SCD is reasonable and worthy of testing. Further, research to determine whether there are alterations of NO and cGMP signaling at the level of sensory neurons and central nervous system and to determine the triggers and mechanisms underlying acute pain crises and chronic pain syndromes in SCD patients are certainly needed. In this regard, we propose a shift in the framework of thought in order to understand the direct pathophysiological consequences of accumulated nitrite and cGMP signaling in SCD to determine whether there is indeed a painful connection.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Paulette Price (NIH).
AUTHORS’ CONTRIBUTIONS: All authors participated in the writing and review of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Intramural Program from the National Institutes of Health Clinical Center, NIH (Grant numbers 1ZIACL090052-01, 1ZIACL090053-01, and 1ZIACL090054-01) supported this work.
ORCID iD: Zenaide MN Quezado https://orcid.org/0000-0001-9793-4368
References
- 1.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med 2017; 376:1561–73 [DOI] [PubMed] [Google Scholar]
- 2.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010; 38:S512–21 [DOI] [PubMed] [Google Scholar]
- 3.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997; 337:762–9 [DOI] [PubMed] [Google Scholar]
- 4.Ingram VM. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature 1956; 178:792–4 [DOI] [PubMed] [Google Scholar]
- 5.Coletta M, Hofrichter J, Ferrone FA, Eaton WA. Kinetics of sickle haemoglobin polymerization in single red cells. Nature 1982; 300:194–7 [DOI] [PubMed] [Google Scholar]
- 6.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010; 376:2018–31 [DOI] [PubMed] [Google Scholar]
- 7.Lubeck D, Agodoa I, Bhakta N, Danese M, Pappu K, Howard R, Gleeson M, Halperin M, Lanzkron S. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open 2019; 2:e1915374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, Smith-Whitley K, Wang W. Health-related quality of life in children with sickle cell disease: a report from the comprehensive sickle cell centers clinical trial consortium. Pediatr Blood Cancer 2010; 55:485–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizio AA, Bhor M, Lin X, McCausland KL, White MK, Paulose J, Nandal S, Halloway RI, Bronté-Hall L. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res 2020; 29:1533–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999-2007. Am J Prev Med 2010; 38:S536–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. Am J Hematol 2009; 84:323–7 [DOI] [PubMed] [Google Scholar]
- 12.Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011; 25:243–54 [DOI] [PubMed] [Google Scholar]
- 13.Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–8 [DOI] [PubMed] [Google Scholar]
- 14.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 2002; 8:1383–9 [DOI] [PubMed] [Google Scholar]
- 15.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res 1987; 61:866–79 [DOI] [PubMed] [Google Scholar]
- 16.Canalli AA, Franco-Penteado CF, Saad STO, Conran N, Costa FF. Increased adhesive properties of neutrophils in sickle cell disease may be reversed by pharmacological nitric oxide donation. Haematologica 2008; 93:605–9 [DOI] [PubMed] [Google Scholar]
- 17.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr., Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 1995; 96:60–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenpeck KL, Gauthier TW, Albertine KH, Lefer AM. Role of P-selectin in microvascular leukocyte-endothelial interaction in splanchnic ischemia-reperfusion. Am J Physiol 1994; 267:H622–H30 [DOI] [PubMed] [Google Scholar]
- 19.Gauthier TW, Davenpeck KL, Lefer AM. Nitric oxide attenuates leukocyte-endothelial interaction via P-selectin in splanchnic ischemia-reperfusion. Am J Physiol 1994; 267:G562–68 [DOI] [PubMed] [Google Scholar]
- 20.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 2013; 122:3892–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 2017; 127:750–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med 1980; 302:992–5 [DOI] [PubMed] [Google Scholar]
- 23.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest 2000; 106:411–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE, Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014; 312:1033–48 [DOI] [PubMed] [Google Scholar]
- 25.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. N Engl J Med 1991; 325:11–6 [DOI] [PubMed] [Google Scholar]
- 26.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med 2008; 148:94–101 [DOI] [PubMed] [Google Scholar]
- 27.McClish DK, Smith WR, Dahman BA, Levenson JL, Roberts JD, Penberthy LT, Aisiku IP, Roseff SD, Bovbjerg VE. Pain site frequency and location in sickle cell disease: the PiSCES project. Pain 2009; 145:246–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandow AM, Carroll CP, Creary S, Edwards-Elliott R, Glassberg J, Hurley RW, Kutlar A, Seisa M, Stinson J, Strouse JJ, Yusuf F, Zempsky W, Lang E. American society of hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv 2020; 4:2656–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aguilar CM, Neumayr LD, Eggleston BE, Earles AN, Robertson SM, Jergesen HE, Stulberg BN, Vichinsky EP. Clinical evaluation of avascular necrosis in patients with sickle cell disease: children's hospital Oakland hip evaluation scale – a modification of the Harris hip score. Arch Phys Med Rehabil 2005; 86:1369–75 [DOI] [PubMed] [Google Scholar]
- 30.Mahadeo KM, Oyeku S, Taragin B, Rajpathak SN, Moody K, Santizo R, Driscoll MC. Increased prevalence of osteonecrosis of the femoral head in children and adolescents with sickle-cell disease. Am J Hematol 2011; 86:806–8 [DOI] [PubMed] [Google Scholar]
- 31.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood 2012; 120:3647–56 [DOI] [PubMed] [Google Scholar]
- 32.Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014; 61:512–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkie DJ, Molokie R, Boyd-Seal D, Suarez ML, Kim YO, Zong S, Wittert H, Zhao Z, Saunthararajah Y, Wang ZJ. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc 2010; 102:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT diagnostic criteria for chronic sickle cell disease pain. J Pain 2017; 18:490–8 [DOI] [PubMed] [Google Scholar]
- 35.Almeida LEF, Kamimura S, de Souza Batista CM, Spornick N, Nettleton MY, Walek E, Smith ML, Finkel JC, Darbari DS, Wakim P, Quezado ZMN. Sickle cell disease subjects and mouse models have elevated nitrite and cGMP levels in blood compartments. Nitric Oxide 2020; 94:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet S-F, Wang X, Kevil CG. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 2005; 115:1232–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nath KA, Shah V, Haggard JJ, Croatt AJ, Smith LA, Hebbel RP, Katusic ZS. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol 2000; 279:R1949–R55 [DOI] [PubMed] [Google Scholar]
- 38.Lopez BL, Davis-Moon L, Ballas SK, Ma X-L. Sequential nitric oxide measurements during the emergency department treatment of acute vasoocclusive sickle cell crisis. Am J Hematol 2000; 64:15–9 [DOI] [PubMed] [Google Scholar]
- 39.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Goldsmith JC, Woolson R, Gordeuk VR, Gibbs S, Little JA, Kato GJ, Schraufnagel DE, Krishnamurti L, Girgis RE, Morris CR, Berman-Rosenzweig E, Badesch DB, Waclawiw MA, Gladwin M. Safety and efficacy of sildenafil therapy for Doppler-Defined pulmonary hypertension in patients with sickle cell disease: preliminary results of the Walk-PHaSST clinical trial. Blood 2009; 114:571 [Google Scholar]
- 40.Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, Hagar RW, Howard T, Nuss R, Okam MM, Tremonti CK, Berman B, Villella A, Krishnamurti L, Lanzkron S, Castro O, Gordeuk VR, Coles WA, Peters-Lawrence M, Nichols J, Hall MK, Hildesheim M, Blackwelder WC, Baldassarre J, Casella JF. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 2011; 305:893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris CR, Kuypers FA, Lavrisha L, Ansari M, Sweeters N, Stewart M, Gildengorin G, Neumayr L, Vichinsky EP. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 2013; 98:1375–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eberhardt RT, McMahon L, Duffy SJ, Steinberg MH, Perrine SP, Loscalzo J, Coffman JD, Vita JA. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. Am J Hematol 2003; 74:104–11 [DOI] [PubMed] [Google Scholar]
- 43.Belhassen L, Pelle G, Sediame S, Bachir D, Carville C, Bucherer C, Lacombe C, Galacteros F, Adnot S. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress–mediated vasodilation. Blood 2001; 97:1584–89 [DOI] [PubMed] [Google Scholar]
- 44.Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter CD, Schenke WH, Csako G, Waclawiw MA, Panza JA, Cannon RO. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation 2003; 107:271–78 [DOI] [PubMed] [Google Scholar]
- 45.Ponka P. Cell biology of heme. Am J Med Sci 1999; 318:241–56 [DOI] [PubMed] [Google Scholar]
- 46.Schnog J-J, Jager E, Dijs F, Duits A, Moshage H, Muskiet F, Muskiet F. Evidence for a metabolic shift of arginine metabolism in sickle cell disease. Ann Hematol 2004; 83:371–5 [DOI] [PubMed] [Google Scholar]
- 47.Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, Van-Assche T, Cunnington C, Tousoulis D, Pillai R, Ratnatunga C, Stefanadis C, Channon KM. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J 2009; 30:1142–50 [DOI] [PubMed] [Google Scholar]
- 48.Li L, Chen W, Rezvan A, Jo H, Harrison DG. Tetrahydrobiopterin deficiency and nitric oxide synthase uncoupling contribute to atherosclerosis induced by disturbed flow. Arterioscler Thromb Vasc Biol 2011; 31:1547–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood KC, Hebbel RP, Lefer DJ, Granger DN. Critical role of endothelial cell-derived nitric oxide synthase in sickle cell disease-induced microvascular dysfunction. Free Radic Biol Med 2006; 40:1443–53 [DOI] [PubMed] [Google Scholar]
- 50.Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci 2009; 30:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato GJ, Wang Z, Machado RF, Blackwelder WC, Taylor JGt, Hazen SL. Endogenous nitric oxide synthase inhibitors in sickle cell disease: abnormal levels and correlations with pulmonary hypertension, desaturation, haemolysis, organ dysfunction and death. Br J Haematol 2009; 145:506–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, Gibbs JS, Little JA, Schraufnagel DE, Krishnamurti L, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Onyekwere O, Castro OL, Sachdev V, Waclawiw MA, Woolson R, Goldsmith JC, Gladwin MT. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood 2011; 118:855–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. Am J Physiol Lung Cell Mol Physiol 2015; 308:L314–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaer DJ, Buehler PW. Cell-free hemoglobin and its scavenger proteins: new disease models leading the way to targeted therapies. Cold Spring Harb Perspect Med 2013; 3:a013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elias DBD, Rocha LBdS, Cavalcante MB, Pedrosa AM, Justino ICB, Gonçalves RP. Correlation of low levels of nitrite and high levels of fetal hemoglobin in patients with sickle cell disease at baseline. Rev Bras Hematol Hemoter 2012; 34:265–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Almeida LEF, Kamimura S, van der Meulen JH, Nagaraju K, Quezado M, Wakim P, Quezado ZMN. The role of nitrite in muscle function, susceptibility to contraction injury, and fatigability in sickle cell mice. Nitric Oxide 2018; 80:70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem 1998; 273:18709–13 [DOI] [PubMed] [Google Scholar]
- 58.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A 2001; 98:355–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piknova B, Schechter AN. Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods Mol Biol 2011; 704:39–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta 1999; 1411:273–89 [DOI] [PubMed] [Google Scholar]
- 61.Toledo JC, Jr., Augusto O. Connecting the chemical and biological properties of nitric oxide. Chem Res Toxicol 2012; 25:975–89 [DOI] [PubMed] [Google Scholar]
- 62.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 2009; 15:391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiss C, Strauer BE, Feelisch M, Kelm M. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest 2002; 109:1241–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, 3rd, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr., Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol 2005; 1:308–14 [DOI] [PubMed] [Google Scholar]
- 65.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 2008; 7:156–67 [DOI] [PubMed] [Google Scholar]
- 66.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Jr., Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol 2009; 5:865–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z, Naughton D, Winyard PG, Benjamin N, Blake DR, Symons MCR. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun 1998; 249:767–72 [DOI] [PubMed] [Google Scholar]
- 68.Basu S, Azarova NA, Font MD, King SB, Hogg N, Gladwin MT, Shiva S, Kim-Shapiro DB. Nitrite reductase activity of cytochrome c. J Biol Chem 2008; 283:32590–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim-Shapiro DB, Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide 2014; 38:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia–reperfusion damage. Proc Natl Acad Sci U S A 2004; 101:13683–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikula I, Durocher S, Martasek P, Mutus B, Slama-Schwok A. Isoform-specific differences in the nitrite reductase activity of nitric oxide synthases under hypoxia. Biochem J 2009; 418:673–82 [DOI] [PubMed] [Google Scholar]
- 72.Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, Webb AJ, Caulfield MJ, Hobbs AJ, Ahluwalia A. Enhanced vasodilator activity of nitrite in hypertension. Hypertension 2013; 61:1091–102 [DOI] [PubMed] [Google Scholar]
- 73.Almeida LEF, Kamimura S, Nettleton MY, de Souza Batista CM, Walek E, Khaibullina A, Wang L, Quezado ZMN. Blood collection vials and clinically used intravenous fluids contain significant amounts of nitrite. Free Radic Biol Med 2017; 108:533–41 [DOI] [PubMed] [Google Scholar]
- 74.Almeida LE, Kamimura S, Kenyon N, Khaibullina A, Wang L, de Souza Batista CM, Quezado ZM. Validation of a method to directly and specifically measure nitrite in biological matrices. Nitric Oxide 2015; 45:54–64 [DOI] [PubMed] [Google Scholar]
- 75.Neely CL, Wajima T, Kraus AP, Diggs LW, Barreras L. Lactic acid dehydrogenase activity and plasma hemoglobin elevations in sickle cell disease. Am J Clin Pathol 1969; 52:167–9 [DOI] [PubMed] [Google Scholar]
- 76.Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr., Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 2006; 107:2279–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med 2008; 44:1506–28 [DOI] [PubMed] [Google Scholar]
- 78.Waltz X, Pichon A, Lemonne N, Mougenel D, Lalanne-Mistrih M-L, Lamarre Y, Tarer V, Tressières B, Etienne-Julan M, Hardy-Dessources M-D, Hue O, Connes P. Normal muscle oxygen consumption and fatigability in sickle cell patients despite reduced microvascular oxygenation and hemorheological abnormalities. PLoS One 2012; 7:e52471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rees DC, Cervi P, Grimwade D, O'Driscoll A, Hamilton M, Parker NE, Porter JB. The metabolites of nitric oxide in sickle-cell disease. Br J Haematol 1995; 91:834–7 [DOI] [PubMed] [Google Scholar]
- 80.Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. Am J Physiol Heart Circ Physiol 2000; 278:H1799–806 [DOI] [PubMed] [Google Scholar]
- 81.Bank N, Aynedjian HS, Qiu JH, Osei SY, Ahima RS, Fabry ME, Nagel RL. Renal nitric oxide synthases in transgenic sickle cell mice. Kidney Int 1996; 50:184–9 [DOI] [PubMed] [Google Scholar]
- 82.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol 1987; 252:H721–32 [DOI] [PubMed] [Google Scholar]
- 83.Thomas G, Ramwell P. Induction of vascular relaxation by hydroperoxides. Biochem Biophys Res Commun 1986; 139:102–8 [DOI] [PubMed] [Google Scholar]
- 84.Antwi-Boasiako C, Campbell AD. Low nitric oxide level is implicated in sickle cell disease and its complications in Ghana. VHRM 2018; 14:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morris CR, Kuypers FA, Larkin S, Vichinsky EP, Styles LA. Patterns of arginine and nitric oxide in patients with sickle cell disease with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol 2000; 22:515–20 [DOI] [PubMed] [Google Scholar]
- 86.Lopez BL, Kreshak AA, Morris CR, Davis-Moon L, Ballas SK, Ma XL. L-arginine levels are diminished in adult acute vaso-occlusive sickle cell crisis in the emergency department. Br J Haematol 2003; 120:532–4 [DOI] [PubMed] [Google Scholar]
- 87.Stuart MJ, Setty BN. Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood 1999; 94:1555–60 [PubMed] [Google Scholar]
- 88.Taylor JGt Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS One 2008; 3:e2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 2010; 116:687–92 [DOI] [PubMed] [Google Scholar]
- 90.Lou TF, Singh M, Mackie A, Li W, Pace BS. Hydroxyurea generates nitric oxide in human erythroid cells: mechanisms for gamma-globin gene activation. Exp Biol Med 2009; 234:1374–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cokic VP, Smith RD, Beleslin-Cokic BB, Njoroge JM, Miller JL, Gladwin MT, Schechter AN. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest 2003; 111:231–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Almeida CB, Scheiermann C, Jang JE, Prophete C, Costa FF, Conran N, Frenette PS. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood 2012; 120:2879–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Almeida CB, Souza LE, Leonardo FC, Costa FT, Werneck CC, Covas DT, Costa FF, Conran N. Acute hemolytic vascular inflammatory processes are prevented by nitric oxide replacement or a single dose of hydroxyurea. Blood 2015; 126:711–20 [DOI] [PubMed] [Google Scholar]
- 94.Charnigo RJ, Beidler D, Rybin D, Pittman DD, Tan B, Howard J, Michelson AD, Frelinger A, III, Clarke N. PF-04447943, a phosphodiesterase 9A inhibitor, in stable sickle cell disease patients: a phase Ib randomized, Placebo-Controlled study. Clin Transl Sci 2019; 12:180–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zimmer DP, Shea CM, Tobin JV, Tchernychev B, Germano P, Sykes K, Banijamali AR, Jacobson S, Bernier SG, Sarno R, Carvalho A, Chien YT, Graul R, Buys ES, Jones JE, Wakefield JD, Price GM, Chickering JG, Milne GT, Currie MG, Masferrer JL. Olinciguat, an oral sGC stimulator, exhibits diverse pharmacology across preclinical models of cardiovascular, metabolic, renal, and inflammatory disease. Front Pharmacol 2020; 11:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conran N, Torres L. cGMP modulation therapeutics for sickle cell disease. Exp Biol Med (Maywood) 2019; 244:132–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferreira SH, Lorenzetti BB, Faccioli LH. Blockade of hyperalgesia and neurogenic oedema by topical application of nitroglycerin. Eur J Pharmacol 1992; 217:207–09 [DOI] [PubMed] [Google Scholar]
- 98.Duarte IDG, Lorenzetti BB, Ferreira SH. Peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Eur J Pharmacol 1990; 186:289–93 [DOI] [PubMed] [Google Scholar]
- 99.Gomes FIF, Cunha FQ, Cunha TM. Peripheral nitric oxide signaling directly blocks inflammatory pain. Biochem Pharmacol 2020; 176:113862. [DOI] [PubMed] [Google Scholar]
- 100.Luo ZD, Cizkova D. The role of nitric oxide in nociception. Curr Rev Pain 2000; 4:459–66 [DOI] [PubMed] [Google Scholar]
- 101.Zhuo M, Meller ST, Gebhart GF. Endogenous nitric oxide is required for tonic cholinergic inhibition of spinal mechanical transmission. Pain 1993; 54:71–8 [DOI] [PubMed] [Google Scholar]
- 102.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain 1993; 52:127–36 [DOI] [PubMed] [Google Scholar]
- 103.Schmidtko A, Tegeder I, Geisslinger G. No no, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci 2009; 32:339–46 [DOI] [PubMed] [Google Scholar]
- 104.Boettger MK, Uceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, Sommer C. Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur J Pain 2007; 11:810–8 [DOI] [PubMed] [Google Scholar]
- 105.Guan Y, Yaster M, Raja SN, Tao Y-X. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain 2007; 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Semos ML, Headley PM. The role of nitric oxide in spinal nociceptive reflexes in rats with neurogenic and non-neurogenic peripheral inflammation. Neuropharmacology 1994; 33:1487–97 [DOI] [PubMed] [Google Scholar]
- 107.Osborne MG, Coderre TJ. Effects of intrathecal administration of nitric oxide synthase inhibitors on carrageenan-induced thermal hyperalgesia. Br J Pharmacol 1999; 126:1840–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain 1993; 54:291–300 [DOI] [PubMed] [Google Scholar]
- 109.Sekiguchi F, Mita Y, Kamanaka Y, Kawao N, Matsuya H, Taga C, Kawabata A. The potent inducible nitric oxide synthase inhibitor ONO-1714 inhibits neuronal NOS and exerts antinociception in rats. Neurosci Lett 2004; 365:111–15 [DOI] [PubMed] [Google Scholar]
- 110.Tao F, Tao YX, Zhao C, Doré S, Liaw WJ, Raja SN, Johns RA. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience 2004; 128:421–30 [DOI] [PubMed] [Google Scholar]
- 111.LaBuda CJ, Koblish M, Tuthill P, Dolle RE, Little PJ. Antinociceptive activity of the selective iNOS inhibitor AR-C102222 in rodent models of inflammatory, neuropathic and post-operative pain. Eur J Pain 2006; 10:505–05 [DOI] [PubMed] [Google Scholar]
- 112.Onishi T, Watanabe T, Sasaki M, Kamiya Y, Horie M, Tsukano H, Hishida R, Kohno T, Takebayashi H, Baba H, Shibuki K. Acute spatial spread of NO-mediated potentiation during hindpaw ischaemia in mice. J Physiol 2019; 597:3441–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferreira J, Santos ARS, Calixto JB. The role of systemic, spinal and supraspinal l-arginine–nitric oxide–cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology 1999; 38:835–42 [DOI] [PubMed] [Google Scholar]
- 114.Song X-J, Wang Z-B, Gan Q, Walters ET. cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglia compression. J Neurophysiol 2006; 95:479–92 [DOI] [PubMed] [Google Scholar]
- 115.Meller ST, Dykstra C, Gebhart GF. Production of endogenous nitric oxide and activation of soluble guanylate cyclase are required for N-methyl-D-aspartate-produced facilitation of the nociceptive tail-flick reflex. Eur J Pharmacol 1992; 214:93–96 [DOI] [PubMed] [Google Scholar]
- 116.Kitto KF, Haley JE, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett 1992; 148:1–5 [DOI] [PubMed] [Google Scholar]
- 117.Machelska H, Przewłocki R, Radomski MW, Przewłocka B. Differential effects of intrathecally and intracerebroventricularly administered nitric oxide donors on noxious mechanical and thermal stimulation. Pol J Pharmacol 1998; 50:407–15 [PubMed] [Google Scholar]
- 118.Lin Q, Palec˘Ek J, Palec˘Ková V, Peng YB, Wu J, Cui M, Willis WD, Nitric oxide mediates the Central sensitization of primate spinothalamic tract neurons. J Neurophysiol 1999; 81:1075–85 [DOI] [PubMed] [Google Scholar]
- 119.Garry MG, Abraham E, Hargreaves KM, Aanonsen LM. Intrathecal injection of cell-permeable analogs of cyclic 3′, 5′-guanosine monophosphate produces hyperalgesia in mice. Eur J Pharmacol 1994; 260:129–31 [DOI] [PubMed] [Google Scholar]
- 120.Naik AK, Tandan SK, Kumar D, Dudhgaonkar SP. Nitric oxide and its modulators in chronic constriction injury-induced neuropathic pain in rats. Eur J Pharmacol 2006; 530:59–69 [DOI] [PubMed] [Google Scholar]
- 121.Finkel J, Guptill V, Khaibullina A, Spornick N, Vasconcelos O, Liewehr DJ, Steinberg SM, Quezado ZM. The three isoforms of nitric oxide synthase distinctively affect mouse nocifensive behavior. Nitric Oxide 2012; 26:81–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guhring H, Gorig M, Ates M, Coste O, Zeilhofer HU, Pahl A, Rehse K, Brune K. Suppressed injury-induced rise in spinal prostaglandin E2 production and reduced early thermal hyperalgesia in iNOS-deficient mice. J Neurosci 2000; 20:6714–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood 2011; 118:3376–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garrison SR, Kramer AA, Gerges NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Mol Pain 2012; 8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain 2015; 156:722–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Uhelski ML, Gupta K, Simone DA. Sensitization of C-fiber nociceptors in mice with sickle cell disease is decreased by local inhibition of anandamide hydrolysis. Pain 2017; 158:1711–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kenyon N, Wang L, Spornick N, Khaibullina A, Almeida LE, Cheng Y, Wang J, Guptill V, Finkel JC, Quezado ZMN. Sickle cell disease in mice is associated with sensitization of sensory nerve fibers. Exp Biol Med 2015; 240:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood 2010; 116:456–65 [DOI] [PMC free article] [PubMed] [Google Scholar]