Abstract
High unintended pregnancy rates are partially due to lack of effective nonhormonal contraceptives; development of safe, effective topical vaginal methods will address this need. Preclinical product safety and efficacy assessment requires in vivo testing in appropriate models. The sheep is a good model for the evaluation of vaginally delivered products due to its close similarities to humans. The study objective was to develop an ovine model for efficacy testing of female nonhormonal contraceptives that target human sperm. Fresh human semen was pooled from male volunteers. Nonpregnant female Merino sheep were treated with control or vaginal contraceptive product (IgG antibody with action against sperm or nonoxynol-9 [N9]). Pooled semen was added to the sheep vagina and mixed with product and vaginal secretions. Microscopic assessment of samples was performed immediately and progressive motility (PM) of sperm was compared between treatments. Cytokines CXCL8 and IL1B were assessed in vaginal fluid after instillation of human semen. No adverse reactions or elevations in proinflammatory cytokines occurred in response to human semen. N9 produced signs of acute cellular toxicity while there were no cellular changes after IgG treatment. N9 and IgG had dose-related effects with the highest dose achieving complete sperm immobilization (no sperm with PM). Surrogate post-coital testing of vaginally administered contraceptives that target human semen was developed in an ovine model established for vaginal product preclinical testing. This expanded model can aid the development of much needed nonhormonal topical vaginal contraceptives, providing opportunities for rapid iterative drug development prior to costly, time-intensive human testing.
Keywords: postcoital testing, topical nonhormonal contraceptive, sheep vaginal model, sperm inactivation, anti-sperm antibody
Summary Sentence Surrogate post-coital efficacy testing of topical vaginal nonhormonal contraceptives performed in a sheep vaginal model provides anatomical and structural similarities to humans and improvements over the current animal models for preclinical efficacy testing.
Introduction
Nearly half of all pregnancies in the USA are unintended, and despite numerous contraception options, only 60% of reproductive age women use contraception [1]. In total, 54% of women with unintended pregnancy did not use contraception and 41% did not use contraception consistently [2]. Many women do not use contraception due to concerns about health risks or side effects with systemic hormonal methods; the development of safe and effective female vaginal nonhormonal contraceptive agents has potential to address this unmet need [3]. Older topical vaginal contraceptives, such as nonoxynol-9 (N9), a nonionic surfactant, are not species-specific and work as spermicides. Unfortunately, they have also been shown to have clinical and subclinical toxic effects and therefore safer methods are needed. Alternative agents that can be used as vaginal contraceptives with specificity to human sperm, such as antibodies that inhibit sperm function, are undergoing development [4, 5].
Efficacy assessment of these emerging approaches requires use of conditions similar to that of intended use, including protocols that test product effects on human semen and that use systems that model key aspects of the in vivo human vaginal environment. Existing preclinical models, including the stump-tailed macaque and rabbit, have been utilized for in vivo semen testing, however, are not ideal for emerging vaginal nonhormonal contraceptive agents [5, 6]. The stump-tailed macaque model utilizes macaque sperm after mating, therefore does not allow for testing products with specific action against human sperm. New contraceptive agents delivered via human-sized devices cannot be accurately tested in stump-tailed macaques or rabbits, as neither has vaginal capacity sufficient for testing human doses or devices (e.g. drug-releasing intravaginal rings), and the differences in vaginal dimensions leaves uncertainty regarding appropriate dose scaling. Therefore, the availability of a cost-effective large animal model that can be used for assessing the safety and efficacy of promising agents could facilitate preclinical testing of novel contraceptive approaches prior to approval and use in humans.
The sheep vaginal tract has similarities in size and morphology to the human and has been shown to be a suitable model for testing safety and pharmacokinetics of vaginally administered drugs [7–9]. Human vagina measures 8–12 cm in length and the sheep vagina measures 9–13 cm in length, with slightly smaller diameter when compared to humans. The sheep vagina is composed of stratified squamous epithelium, similar, but slightly thinner than the human vaginal epithelium [7]. At the apex of the vagina in both humans and sheep, the single uterine cervix can be visualized, with an endocervical canal leading into the endometrial cavity. Sheep produce interleukin 1 beta (IL1B) and C-X-C motif chemokine ligand 8 (CXCL8) (also known as IL8), proinflammatory cytokines that have been shown to be important in human vaginal injury and susceptibility to vaginal infections [9]. The similarity in vaginal size of the sheep compared to the human, allowing for testing of drug-releasing devices, as well as to the availability of inflammatory markers, provide advantages for the use of sheep as a large animal model for development of vaginally administered drugs for prevention of infection and pregnancy.
Initially developed for infertility evaluation, the human postcoital test (PCT) has been used in early clinical studies to predict contraceptive efficacy of products undergoing development [10, 11]. The PCT involves the vaginal application of the product under investigation, condomless vaginal intercourse, and then evaluation of the postcoital vaginal secretions for sperm motility under ×40 objective by counting the number of progressively motile (PM) sperm and averaging over nine high powered fields (hpf). An average of <1 sperm with PM per hpf has been proposed as a cut-off for PCT contraceptive efficacy studies although variations exist between studies [10]. In this study, we have modified the standard PCT to test product effects on human sperm in an in vivo sheep model.
The objective of this study was to develop an experimental preclinical protocol for surrogate postcoital testing in an established safety and pharmacokinetics sheep model with the goal for rapid screening and optimization of emerging vaginal contraceptive products by assessing the efficacy of nonhormonal contraceptives that have action against sperm under in vivo conditions designed to simulate use in humans.
Methods
Human volunteers
Under a protocol approved by the University of Texas Medical Branch (UTMB) Institutional Review Board, male volunteers age 18–45 years old were screened for human immunodeficiency virus (HIV) and sexually transmitted diseases (STDs: Hepatitis B and C, syphilis, gonorrhea, and chlamydia) and with semen analysis for adequate volume (≥1.5 mL) and progressively motile sperm (≥32%) [12]. Eligible subjects were asked to return to the UTMB clinical research center to provide a fresh semen sample on predetermined days when sheep studies were planned. On the morning of the sheep studies, fresh human semen was collected from 3–5 previously screened male volunteers and then pooled for immediate use. The semen was evaluated for motility for each subject prior to pooling and for the pooled semen as described in the semen analysis section.
Sheep studies
Animal experiments were performed according to the National Institutes of Health (NIH) Guide for Care and Use of Experimental Animals and under a protocol approved by the UTMB Animal Care and Use Committee. Nonpregnant yearling female Merino sheep (n = 5) were housed indoors with 12:12 light/dark cycles and fed chow and water ad libitum. They were fasted overnight and then on each study day, were anesthetized with 5 mg/kg ketamine IM and 0.5–5% isoflurane by facemask and placed on a V-tilt table in supine position.
Sheep were then treated in a randomized blinded fashion with 1 mL vaginal human contraceptive antibody (HCA) IgG (333 or 33 ug/mL), N9 (Options Gynol II ES, Madison, NJ) contraceptive (3.0%, 1.5%, 0.06%, 0.03%), or control solution. Dulbecco’s phosphate-buffered saline (PBS), modified, without calcium chloride and magnesium chloride was used for dilution of IgG and N9 and the PBS control solution. The IgG antibody used was a monoclonal IgG antibody directed against CD52g, a ubiquitous antigen on the surface of human sperm, and was created by using the heavy and light chain of the published sequence of the H6-3C4 mAb (Supplemental File). The IgG dose range was selected based on our previous findings that effective sperm agglutination in whole semen is noted in vitro with 25 ug/mL IgG, and prior work in the topical microbicide field has indicated that a substantially greater dose must be delivered topically to achieve comparable protection as implied from in vitro potency measurements [13]. The antibody studies utilized PBS control because agglutination from the antibody could cause aggregates of sperm, which would not allow total sperm to be counted for calculation of %sperm with PM in those samples. Because N9 does not cause agglutination, a PBS control was not utilized for N9, allowing for a range of doses to be tested.
Simulated intercourse (SI) was performed for 15 strokes to mix product with the vaginal secretions. Pooled semen (1 mL) was added to the sheep vagina and SI was performed again for five strokes. After 2 min, a small Pederson vaginal speculum was used to expose the vaginal pool of secretions for collection and for examination of the vagina with a colposcope (Leisegang Model 3MTL-LED) for assessment of the vaginal mucosa. An early 2-min timepoint was selected for testing products that act within the vagina due to the rapid transport of sperm to the Fallopian tubes within 5 min of insemination [14].
Retrieved vaginal pool samples, which were a mix of product, human semen, and sheep vaginal secretions, were removed from the vagina using a syringe and immediately evaluated under light microscope as described in the semen analysis section. The vaginal cavity was flushed with PBS to remove study product and semen. After the procedures, sheep were recovered and returned to their housing area.
There was at least a week between study days for washout of semen and study drug for a total of nine study visits during the development of this model. The pooled semen and control group findings described in Table 1 are from Visits 1–9, with protocol optimization and testing of PBS control and other products (not reported herein) performed during visits 1–6, IgG treatments with PBS control administered during study visits 7–8, and dose-ranged N9 treatment administered during study Visit 9.
Table 1.
Characteristics of pooled semen used in this study before and after instillation into the control sheep
| Visita | PS at time of collection | PS in sheep | |||||
|---|---|---|---|---|---|---|---|
| PS Concentrationb | PM | Nonprogressive motility | Immotility | Male PIDsc | % PM in control sheepd | # Sheep | |
| (×106/mL) | % | % | % | ||||
| 1 | 92 | 68.9 | 8.9 | 22.2 | 3,4,6,8 | 19.9 | 3 |
| 2 | 53 | 66.3 | 10.0 | 23.7 | 2,4,6,7 | 25.0 | 3 |
| 3 | 57 | 86.9 | 6.3 | 6.9 | 2,4,6,7 | 15.3 | 3 |
| 4 | 91 | 76.8 | 6.0 | 17.2 | 6,9,10 | 9.1 | 5 |
| 5 | 103 | 74.1 | 7.3 | 18.5 | 2,4,6,10 | 40.1 | 5 |
| 6 | 173 | 62.8 | 8.9 | 28.4 | 2,4,6,10 | 32.0 | 5 |
| 7 | 106 | 62.2 | 14.4 | 23.4 | 2,4,6,9,10 | 42.9 | 5 |
| 8 | 100 | 63.5 | 6.8 | 29.7 | 2,3,4,6 | 35.0 | 5 |
| 9 | 52.2 | 56.3 | 12.2 | 31.4 | 3,4,6,9,10 | 26.7d | 5 |
aVisits 1–6: protocol optimization; visits 7–8: IgG study; visit 9: N9 study.
bTotal number of sperm (×106) instilled in each sheep vagina is equal to sperm concentration (×106/mL) because 1 mL of sperm was utilized per sheep.
cPID of the human male subjects who provided semen sample at that visit.
dVisit 9, the dose ranging N9 study, did not have a PBS control, therefore the result shown reflects the %PM in the lowest dose N9 (0.03%) group. This low dose likely had an effect on %PM based on effects seen in epithelial cells.
PM, rogressive motility; PBS, phosphate-buffered saline; IgG, immunoglobulin G; PS, pooled sperm; PID, participant identification number.
To determine whether human semen had a proinflammatory effect on the sheep vagina, prior to use of fresh semen, frozen pooled semen was repeatedly introduced into the sheep vagina (n = 4) and mixed using SI as described above (on study day 0, 2, and 6). Vaginal secretions were obtained 48 h after instillation of semen (day 2 and 8) for quantification of cytokine C-X-C motif chemokine ligand 8 (CXCL8) and interleukin 1 beta (IL1B) levels using enzyme-linked immunosorbent assay as previously described with three technical replicates [9]. CXCL8 (also known as IL8) was obtained from EMD Millipore (capture MAB1044, detection AB1840) and IL1B was obtained from United States Biological (I7663-11 K, I7663-10E).
Semen analysis
Subjects and pooled semen
Semen from each volunteer was evaluated for volume, pH, and sperm motility. Semen (undiluted and diluted 1:20) was placed in a hemocytometer slide chamber and evaluated under ×20 objective of an inverted brightfield microscope (IX71, Olympus). A high sensitivity USB 3.0 CMOS camera, with 1280 × 1024 pixel resolution and a pixel size of 4.76 × 4.65 μm (DCC3240C Thorlabs, Inc), was used with ThorCam software (Thorlabs, Inc) to obtain digital videos for each of the 25 large squares (each surrounded by a thick or triple line border) of the central 1 mm2 grid of the hemocytometer counting chamber. Each of the 25 large squares contained 16 smaller 50 μm × 50 μm squares (each with a thin or single line border). At ×20 up to 6 large squares could be included within the field of view; a total of 6 videos were obtained in order to visualize each of the 25 large squares. Additionally, with the described microscope and camera set-up, each of the 25 large squares (with thick, triple-line borders) filled the field of view at ×40 magnification, therefore each square was equivalent to one high powered field.
Sperm with progressive motility (PM) had progressive, active movement, moving either linearly or in a large circle. Sperm with non-progressive motility (NPM) exhibited other types of movement, including maintaining a small circular pattern, minimal movement or flagellar beating without forward progression. Sperm with immotility (IM) demonstrated no movement [12]. Sperm were counted manually in each of the 25 large squares from the videos and the percent for each category (PM, NPM, and IM) was calculated for each subject as well as for the pooled semen. The lower reference limit is 32% for PM and 40% for total motility (PM + NPM). Sperm concentration was measured using the hemocytometer grids to provide number of sperm × 106/mL of semen; normal values are considered >15 × 106 sperm/mL of semen, or 39 × 106 spermatozoa per ejaculate [12].
Recovered semen/product/vaginal secretions mixture
Assessment of the recovered semen/product/vaginal secretions mixture included manual counting of PM from the digital videos for all samples. For the PBS control, the total number of sperm (PM, NPM, and IM) was counted manually. For the HCA contraceptive products, the total number of sperm could not be reliably counted due to agglutination, therefore the PBS control sheep was used for normalization of the samples during each study visit, so that the percent decrease in PM for each treatment could be calculated compared to the control. At the end of the study day (4–5 h after collection), the pooled semen was re-evaluated to determine if motility was adequate. In addition to human sperm, sheep vaginal epithelial cells were also visualized in the digital videos. Software methods were initially evaluated for counting, however, the presence of sheep vaginal cells affected the accuracy of the automated counts, therefore manual counts were used. Two manual counters (YZ, KV) evaluated the videos in a blinded fashion and had 66% agreement for all fields, 100% agreement for treatments with no motile sperm, and 90% agreement within ±1 sperm. Data presented was analyzed by the primary counter (YZ) because of his clinical lab experience.
Statistics
Sperm with PM were counted in each of the 25 squares of the central grid for all samples and presented as mean (±standard deviation) number of sperm/hpf with PM. PM counts for each treatment were compared to the PBS control group in the IgG study (Visits 7,8) and compared with the lowest dose N9 for the N9 study (visit 9) and presented as a percent decrease in PM. Comparisons were made using analysis of variance with Tukey–Kramer post hoc analysis (Excel 2013 with Real Statistics add-in). A P value ≤ 0.05 was considered significant.
Results
Male volunteers
The age range of subjects was 24–41 years (average 27.9, median 24.5). Racial categories included 6 White (4 Hispanic, 2 Non-Hispanic), 2 Asian, 1 African American, and 1 mixed race. Two subjects were excluded; one for low volume and one for chlamydia infection. At each sheep study visit day, 4–5 subjects were asked to provide semen samples, however between 3 and 5 men brought samples for each study day. Table 1 shows the characteristics of the pooled semen for each study visit, demonstrating sperm concentrations and %PM within a normal range [11, 12].
Sheep vaginal response after placement of semen
No adverse reactions such as erythema, petechiae, or edema, were noted in the sheep vaginal mucosa on visual observation by colposcopy after placement of the human semen. Cytokines IL-8 and IL-1b were not elevated after instillation of human semen with SI (Figure 1). Past work has shown that a single application of N9 elevated both targets at 18 and 44 hours after treatment, when other markers of inflammation and irritation were reported [9, 15].
Figure 1.
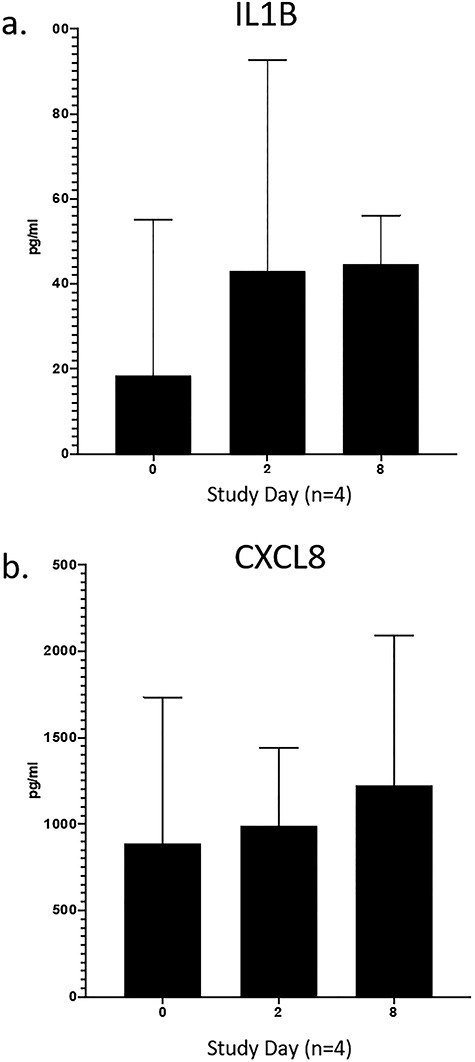
Proinflammatory cytokines IL-1b (a) and IL-8 (b) were not elevated 48 h after repeated dosing (day 0, 2, 6) of human sperm into the sheep vagina.
Semen analysis and handling
The semen motility was decreased after exposure to the vaginal environment in control sheep (Table 1—%PM in control sheep). This could be attributed in part to conditions in the vagina that affect motility (e.g. pH, mucus, and inflammatory markers) as well as storage conditions of the pooled semen prior to instillation into the vaginal cavity. For Visits 1–3, the freshly collected pooled semen (66.3–86.9% PM) was kept at room temperature (68-72F), and the PM fraction recovered from the control sheep vagina ranged from 15.3–25.0%. For visit 4, as a result of unanticipated delay in collection due to waiting for one subject who failed to bring in a semen sample, the entire study was extended by 1–1.5 h. The PM fraction decreased from 76.8% in the pooled semen to 9.1% in the recovered sample by the 4th sheep, with dosing ~ 6 h after collection of the semen. Completion of the first four collections illustrated the impact of the procedure on the data leading to a protocol change for storage of the pooled semen. For the latter collections, the samples were kept it in an incubator (37°C) during the initial semen analysis, and then the tube was wrapped with padded insulation during the sheep examinations, resulting in recovered secretion (semen/product/vaginal secretions) %PM in control sheep of 32.0–42.9%. Semen characteristics were maintained in the normal range (%PM > 32%) throughout the study day after these measures were put into place.
Surrogate post-coital testing
For HCA IgG treatments, normal sheep vaginal epithelial cells were noted. For the higher concentration of IgG (333 μg/mL), sperm were seen in aggregates. There were no sperm with PM visualized, which resulted in 100% decrease in sperm motility when compared with PBS treatment (P < 0.05) (Figures 2 and 3). For the lower IgG concentration (33 μg/mL), individual sperm and few isolated sperm aggregates were seen while the sperm counts showed an average of 3.3 ± 1.8 sperm/hpf with PM for a nonsignificant decrease in %PM compared with the PBS control (P = 0.08). In comparison, PBS-treated samples had normal vaginal epithelial cells and motile sperm with 4.1 ± 2.8 sperm/hpf with PM.
Figure 2.
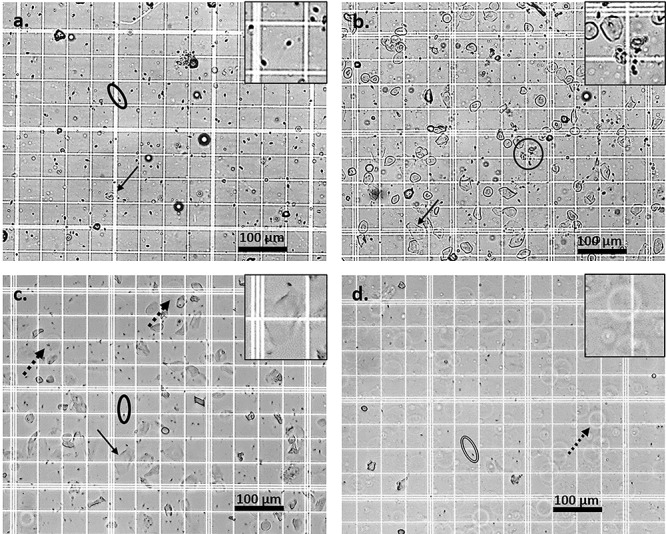
Microscopy findings after treatment with IgG and N9 (still images were taken from videos that are available online). Normal superficial vaginal epithelial cells (black arrow) and progressively motile sperm (surrounded by oval; PM detectable in video) were visible in the PBS control group (a, inset: sperm enlarged ×2). Aggregated non-progressively motile sperm (circle encompassing aggregate; lack of PM notable in video) were seen after treatment with the highest IgG dose of 333 ug/mL (b, inset: sperm aggregate enlarged ×2). After the lowest dose N9 treatment (0.03%), progressively motile sperm (surrounded by oval; PM detectable in video) and a mix of normal (black arrow) and abnormal (black dashed arrow) epithelial cells were seen (c, inset: normal epithelial cell enlarged ×2). After N9 (1.5%) treatment, immotile sperm (surrounded by oval with double line), lack of motility notable in video) and abnormal faint, enlarged, spherical, vaginal epithelial cells with brighter halos (black open arrow) were visualized, consistent with cellular swelling, an early sign of toxicity (d, inset: abnormal epithelial cell enlarged ×2). Images represent 1:20 dilution; scale bar 100 μm. Unmodified videos were utilized for analysis of motility of sperm (see online video clips), however to enhance visualization of still image findings, the following modifications were made to the still images only: increase sharpness 50%, brightness 20% and contrast 40%.
Figure 3.
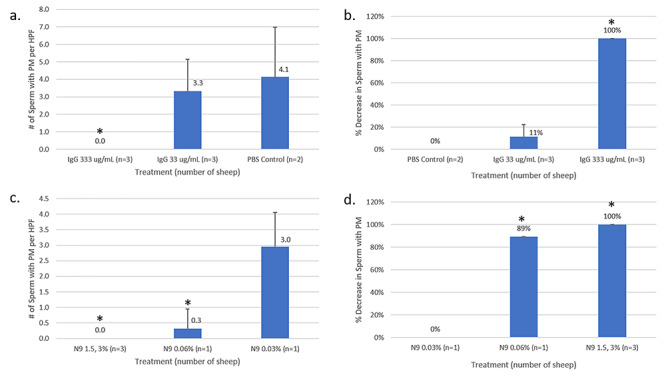
Number of sperm/hpf with PM and decrease in sperm with PM after treatment with IgG and N9. There was a dose response to IgG and no visible sperm with progressive motility (PM) in any counted field (a), which corresponded to 100% reduction in sperm with PM in the higher dose (333 μg IgG) group (b). No significant decrease in motility was seen in the lower dose (33 μg IgG) group (P = 0.08). N9 treatment (3%, 1.5%) resulted in no visible sperm with PM in any counted field (c), which corresponded to 100% decrease in PM sperm when compared with the lowest dose of N9 (0.03%). Diluted N9 (0.06%) produced an average of 0.32 sperm/hpf, resulting in an 89% reduction when compared to the lowest dose of N9 (0.03%) (d). *P < 0.05. Error bars represent standard deviation.
For all N9 treatments, abnormal sheep vaginal epithelial cells were visualized by microscopy (Figure 2); the cells were faint, transparent, enlarged, and spherical with halos, consistent with cell swelling, a sign of acute cell injury [13]. For the 3% and 1.5% N9 treatments, abnormal epithelial cells and only immotile sperm were seen; for 0.06% and 0.03% groups, progressively motile sperm as well as a mixture of normal and abnormal epithelial cells were seen. The abnormal epithelial cell findings at this short timepoint are consistent with the action of N9, which lyses cell membranes; findings of epithelial cell injury and sloughing have been reported at later timepoints (24–48 h) as a sign of toxicity [13]. N9 treatment at 3% and 1.5% resulted in no visible sperm with PM (100% decrease), and the 0.06% N9 solution had 0.3 ± 0.6 sperm/hpf with PM, which was decreased by 89% when compared with 0.03% N9 (P < 0.05). The lowest dose N9 group (0.03%) had 3.0 ± 1.1 sperm/hpf with PM (Figure 3).
Discussion
The ovine model can be used to assess the effectiveness of vaginally administered contraceptives targeting human sperm in preclinical testing, similar to the post-coital test that is frequently used in early phase human clinical trials. The sheep vaginal model provides important advantages compared to other animal models, especially the opportunity to observe the effect of the intervention in an environment with anatomical shape and size, temperature and mixing conditions similar to that found in humans [7]. This study also emphasizes the value of an animal model system where vaginal contraceptives that are specific to human semen, such as human anti-sperm antibody, can be tested using human sperm. Importantly, our study showed that human semen did not elicit an inflammatory response in the sheep and could be safely used under appropriate biosafety conditions. The cytokines measured and previously validated in sheep mimic the inflammatory response seen in humans, adding an additional level of safety testing to the model.
In standard PCT testing in humans, results are typically reported as average number of sperm with PM per hpf. In infertility studies, there is a wide range of normal counts associated with future fertility, while in contraceptive studies the cut-off values used for acceptable baseline normal counts and for determining contraceptive efficacy after contraceptive use are also variable among different study designs [10]. Treatment with the higher doses of IgG (333 ug/mL) and N9 (3%, 1.5%) achieved the proposed cut-off of <1 sperm with PM per hpf in this study. However, because a consensus has not been established for cut-off values for a satisfactory contraceptive PCT, we also looked at decrease in PM between treatments and the control group. For example, Amaral et al. [11] reported the average baseline control cycle group as having 17.94 sperm with PM per hpf, which decreased to 0.07 per hpf after treatment with N9. Conversion of the human data to % decrease in PM would equate to a 99.6% decrease in PM, which is comparable to the 100% decrease noted in this study after application of 3.0% and 1.5% N9.
N9, a surfactant that non-specifically lyses the sperm cell membrane, can increase the risk of acquisition of STDs and HIV, therefore is not recommended for use in high risk patients. In this study N9 treatment induced vaginal epithelial cell swelling, an early sign of vaginal toxicity [16]; later signs of vaginal toxicity including loss of the superficial epithelial cell layer and thinning of the vaginal epithelium have been previously reported in sheep and women [15, 17]. Emerging nonhormonal topical contraceptives aim to target sperm motility in safer and more specific ways, such as binding to an antigen that is only present on human sperm, therefore mating studies with nonhuman sperm are not adequate for testing these compounds. The remaining limitations of the macaque and rabbit models include a much smaller vaginal tract that affects SI, sample distribution, and dosing, making them less similar to women than the sheep model. We evaluated the impact of a novel anti-human sperm antibody product on PM in this model. The higher dose of IgG was 100% effective at decreasing %PM when compared with controls confirming the potential of using HCA as a vaginally administered contraception.
Pooling the semen samples from 3–5 male volunteers provided high percentages (56.3–86.9%, median 66.3%) of PM while providing the volume necessary for multiple tests at 1 mL semen per sheep. There were variations between study days, even when the samples from the same subjects were pooled. After placement in the vagina, without regard to heat loss in the pooled semen, the %PM was 15.3–25%, however with optimization of the protocol to include insulation and temperature regulation of the pooled semen prior to placement in the vagina, the %PM in PBS controls was 32.0–42.9%. While the pooled semen %PM was slightly lower for the visit 9 N9 study (56.3%) compared with the median for all visits (66.3%), it remained within the WHO criteria for a normal sample [12]. The %PM in the lowest dose N9 (0.03%) group was 26.7%, which was lower than the PBS controls in the IgG study (Visits 7, 8; 35.0–42.9% PM); this was more likely attributed to the spermicidal effect rather than the initial pooled semen %PM, as some vaginal epithelial cells in the lowest dose group had abnormalities that were noted after N9 at all doses. While the number of subjects did not adversely affect the motility of the pooled sample, however it did affect the volume of the sample impacting the number of sheep that can be tested in a given day. For these studies, we found that requesting equal or more subjects than sheep for that study day was helpful to ensure adequate sample volume.
The primary limitations associated with the use of pooled human semen were that the semen had undergone liquefaction prior to instillation into the sheep vagina and that the timing of recovered vaginal secretion collection did not allow for measure of sperm capacitation. In this study, we used pooled semen to reduce variability, therefore it was not feasible to use semen that had not yet liquefied due to timing and logistical factors involved with sample collection, privacy of the study volunteers, and separation between clinical and animal research space. In the human vagina, the semen initially coagulates into a thick seminal clot, which protects the sperm from the vaginal environment and could also protect it from a vaginal contraceptive product [18]. However, this protection would be temporary because as the seminal clot liquefies, the sperm can come into contact with the contraceptive. We chose an early timepoint for collection of the recovered vaginal secretion mixture (i.e., semen/product/vaginal secretions) because of rapid transport of sperm to the Fallopian tubes [14]. Capacitation, a process that can take several hours, appears to occur within the cervical mucus and upper tract, after sperm transit from the vagina to the cervix [18]. The primary aim of this study was to provide a surrogate postcoital test to detect motility of sperm in the lower genital tract, therefore it did not allow for simultaneous assessment of motility and sperm capacitation.
Limitations of the study inherent in the use of sheep include collection of the sample from the vaginal pool of secretions rather than within the endocervical canal mucus, however this is also a limitation in the other established animal models (i.e., stump-tailed macaque, rabbit [5, 6]. Vaginal secretion volumes in humans and sheep have been reported to be 300–500 μL, therefore not likely to significantly dilute the 2 mL of vaginal product and semen added during these studies [19, 20]. Because sheep, like most animals, have estrus rather than a menstrual cycle, it is challenging to clinically determine the stage of estrus. Estrus (ovulation) lasts a single day, and therefore timing of coincident studies would be impractical [21]. Sheep experience seasonal anestrus, which is induced by an increasing number of daylight hours as summer approaches, however our indoor provides unchanging equal light:dark cycles and we have observed ovulation by direct visualization of ovaries, hormonal measures, and ultrasound findings during the summer months and throughout the year [21].
Another limitation of the study is the number of sheep that can be examined within a single day. As previously described, the sperm in the pooled sample can lose motility over time, which limited the length of the experiment to 4–5 h after semen collection in our studies [22]. We were able to mitigate this problem with use of protocol changes to minimize heat loss of the pooled sperm, however the %PM still decreased over time. Systematic testing of insulating conditions may be carried out in future studies to further optimize these parameters. Additionally, enacting measures to decrease the time for each sheep evaluation may also prevent loss of motility by decreasing the total time of the sheep studies for each study day. A final limitation in the use of sheep, common to all animal vaginal models used in the field, is that vaginal pH at baseline ranges from 5.5–8.5, which is higher than that of women [7]. However after intercourse in women, vaginal pH is 7.2, comparable to pH of 8.0 after instillation of human semen into sheep in this study [23]. As pH is a common limitation regardless of animal model, the strengths of the sheep model, including advantages of scale and structure similar to human and no indication of inflammation due to human sperm, outweigh the pH and other limitations.
In summary, use of the sheep vaginal model as a surrogate post-coital test for preclinical efficacy testing of topical contraceptives allows for a reproducible, quantitative method that is most representative of product use in humans based on anatomical shape and size, temperature, mixing conditions, and the use of human sperm. The addition of efficacy testing in this well-established safety model can aid in the development of much needed nonhormonal topical vaginal contraceptives, providing opportunity for rapid iterative optimization of drugs during development using a preclinical model prior to initiating regulated, costly, and more time-intensive human clinical testing.
Conflict of Interest
There are no conflicts of interest with respect to the work reported herein.
Author Contributions
T.M., S.L, and K.V. conceived of the presented idea. J.S., P.V., and G.V. prepared the imaging equipment. Y.Z., J.S., R.P. and K.V. carried out the experiment. Y.Z., S.B., R.P. and K.V. performed data analysis. K.V. wrote the manuscript with support from Y.Z. G.V., S.L., T.M., R.P. and M.M. All authors discussed the results and contributed to the final manuscript.
Supplementary Material
Acknowledgements
We would like to thank Mr. Justin Vincent for his editorial assistance in preparing the videos for upload.
Footnotes
† Grant support: This research was funded by National Institutes of Health NICHD R43HD097941 and conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1 TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Yong Zhu, Department of Ophthalmology and Visual Sciences, University of Texas Medical Branch, Galveston, TX, USA.
Jamal Saada, Department of Ophthalmology and Visual Sciences, University of Texas Medical Branch, Galveston, TX, USA.
Shrestha Bhawana, University of North Carolina Eshelman School of Pharmacy, Chapel Hill, NC, USA.
Sam Lai, University of North Carolina Eshelman School of Pharmacy, Chapel Hill, NC, USA.
Paula Villarreal, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX, USA.
Richard Pyles, Department of Pediatrics, University of Texas Medical Branch, Galveston, TX, USA.
Massoud Motamedi, Department of Ophthalmology and Visual Sciences, University of Texas Medical Branch, Galveston, TX, USA.
Gracie Vargas, Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX, USA.
Tom Moench, Mucommune, LLC, Carrboro, NC, USA.
Kathleen L Vincent, Department of Obstetrics and Gynecology, University of Texas Medical Branch, Galveston, TX, USA.
References
- 1. CDC fact sheet on unintended pregnancy. https://www.cdc.gov/reproductivehealth/contraception/unintendedpregnancy/index.htm. Accessed 27 January 2020.
- 2. Guttmacher fact sheet on contraceptive use. https://www.guttmacher.org/fact-sheet/contraceptive-use-united-states?gclid=EAIaIQobChMI2b-2iO2l5wIVhp6zCh0DegCLEAAYASAAEgJANPD_BwE. Accessed 24 January 2020
- 3. Darroch JE, Sedgh BH. Contraceptive Technologies: Responding to Women’s Needs. New York: Guttmacher Institute; 2011. [Google Scholar]
- 4. Vickram AS, Dhama K, Chakraborty S, Abdul Samad H, Latheef SK, Sharun K, Khurana SK, Archana K, Tiwari R, Bhatt P, Vyshali K, Chaicumpa W. Role of antisperm antibodies in infertility, pregnancy, and potential for contraceptive and antifertility vaccine designs: Research progress and pioneering vision. Vaccine 2019; 7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castle PE, Whaley KJ, Hoen TE, Moench TR, Cone RA. Contraceptive effect of sperm-agglutinating monoclonal antibodies in rabbits. Biol Reprod 1997; 56:153–159. [DOI] [PubMed] [Google Scholar]
- 6. Zatuchni B, Hahn DW, Zaneveld LJD. Postcoital, vaginal, spermicidal potency of formulations: The macaca arctoides (stumptailed macaque) as animal model. Fertil Steril 1981; 35:683–690. [DOI] [PubMed] [Google Scholar]
- 7. Vincent KL, Bourne N, Bell BA, Vargas G, Tan A, Cowan D, Stanberry LR, Rosenthal SL, Motamedi M. High resolution imaging of epithelial injury in the sheep cervicovaginal tract: A promising model for testing safety of candidate microbicides. Sex Transm Dis 2009; 36:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moss JA, Baum M, Malone AM, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Willis RA, Vincent KL, Motamedi M, Smith TJ. Tenofovir and tenofovir disoproxil fumarate pharmacokinetics from intravaginal rings. AIDS 2012; 26:707–710 PMID: 22210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Milligan GN, Vargas G, Vincent KL, Zhu Y, Bourne N, Motamedi M. Evaluation of immunological markers of ovine vaginal irritation: Implications for preclinical assessment of non-vaccine HIV preventive agents. J Reprod Immunol 2017 Nov; 124:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mauck CK, Vincent KL. The postcoital test in the development of new vaginal contraceptives. Biol Reprod 2020; 103:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amaral E, Perdigaõ A, Souza MH, Mauck C, Waller D, Zaneveld L, Faúndes A. Postcoital testing after the use of a bio-adhesive acid buffering gel (ACIDFORM) and a 2% nonoxynol-9 product. Contraception 2004; 70:492–497. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization (2010). WHO laboratory manual for the examination and processing of human semen, 5th ed World Health Organization; https://apps.who.int/iris/handle/10665/44261. Accessed 3 Frebruary 2020. [Google Scholar]
- 13. Anderson DJ, Politch JA, Cone RA, Zeitlin L, Lai SK, Santangelo PJ, Moench TR, Whaley KJ. Engineering monoclonal antibody-based contraception and multipurpose prevention technologies. Biol Reprod 2020; 103:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Settlage DSF, Motoshima M, Tredway DR. Sperm transport from the external cervical os to the fallopian tubes in women: A time and quantitation study. Fertil Steril 1973; 24:655–661. [DOI] [PubMed] [Google Scholar]
- 15. Vincent KL, Vargas G, Wei J, Bourne N, Motamedi M. Monitoring vaginal epithelial thickness changes noninvasively in sheep using optical coherence tomography. Am J Obstet Gynecol 2013; 208:282.e1–282.e2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller MA, Zachary JF. Chapter 1 Mechanisms and Morphology of Cellular Injury, Adaptation, and Death In: Pathologic Basis of Veterinary Disease. Mosby: Sixth Edition; 2017. [Google Scholar]
- 17. Vincent KL, Stanberry LR, Moench TR, Breitkopf CR, Loza ML, Wei J, Grady J, Paull J, Motamedi M, Rosenthal SL. Optical coherence tomography compared with colposcopy for assessment of vaginal epithelial damage: A randomized controlled trial. Obstet Gynecol 2011; 118:1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brannigan R, Lipshultz L. Glob libr women's med, (ISSN: 1756–2228) 2008; DOI 10.3843/GLOWM.1031 [DOI] [Google Scholar]
- 19. Bélec L, Meillet D, Lévy M, Georges A, Tévi-Bénissan C, Pillot J. Dilution assessment of cervicovaginal secretions obtained by vaginal washing for immunological assays. Clin Diagn Lab Immunol 1995; 2:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Churchman SA, Moss JA, Baum MM. Accurate measurement of female genital tract fluid dilution in cervicovaginal lavage samples. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1017-1018:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schatten H, Constantinescu GM. Comparative Reproductive Biology. Ames, Iowa: Blackwell Publishing; 2007. [Google Scholar]
- 22. Valsa J, Skandhan KP, Sumangala B, Jaya V. Time bound changes (in 24 h) in human sperm motility and level of calcium and magnesium in seminal plasma. Alexandria Journal of Medicine 2016; 52:235–241. [Google Scholar]
- 23. Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radiotelemetry of vaginal pH during human coitus. J Reprod Fertil 1973; 33:69–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


