Abstract
Various metabolic and hormonal factors expressed in cumulus cells are positively correlated with the in vitro maturation (IVM) of oocytes. However, the role of hypoxia sensing both during maturation of cumulus–oocyte complexes (COCs) as well as during the resumption of meiosis remains uncertain. HIF1alpha plays major roles in cellular responses to hypoxia, and here we investigated its role during bovine COC maturation by assessing the expression of related genes in cumulus cells. COCs were divided into the following groups: immature (control), in vitro matured (IVM/control), or matured in the presence of a blocker of HIF1alpha activity (echinomycin, IVM/E). We found an inhibition of cumulus cell expansion in IVM/E, compared with the IVM/control. Transcript levels of several factors (n = 13) were assessed in cumulus cells. Decreased expression of HAS2, TNFAIP6, TMSB4, TMSB10, GATM, GLUT1, CX43, COX2, PTGES, and STAR was found in IVM/E (P < 0.05). Additionally, decreased protein levels were detected for STAR, HAS2, and PCNA (P < 0.05), while activated-Caspase 3 remained unaffected in IVM/E. Progesterone output decreased in IVM/E. The application of PX-478, another blocker of HIF1alpha expression, yielded identical results. Negative effects of HIF1alpha suppression were further observed in the significantly decreased oocyte maturation and blastocyst rates from COCs matured with echinomycin (P < 0.05) or PX-478 (P < 0.05). These results support the importance of HIF1alpha for COC maturation and subsequent embryo development. HIF1alpha is a multidirectional factor controlling intercellular communication within COCs, steroidogenic activity, and oocyte development rates, and exerting effects on blastocyst rates.
Keywords: HIF1alpha, bovine cumulus cells, in vitro maturation
Summary Sentence HIF1alpha is functionally involved in controlling in vitro embryo production output in cattle. Suppression of its expression and/or function in cumulus cells during in vitro maturation impacts intercellular communication, steroid production and, consequently, oocyte and blastocyst rates.
Introduction
In most mammalian species, in vivo maturation of the oocyte takes place in response to the preovulatory LH surge and involves its nuclear and cytoplasmic remodeling associated with the developmental transition from the primary to secondary oocyte (see reviewed in: [1]). Concomitantly, the secondary oocyte acquires its fertilization competence and becomes capable of promoting embryonic development [2]. The nuclear events are manifested by germinal-vesicle breakdown, i.e., resumption of meiotic division, resulting in the extrusion of the first polar body into the perivitelline space.
The oocyte is surrounded by layers of tightly packed follicular epithelial cells, constituting the cumulus–oocyte complex (COC), that are important in the acquisition of oocyte developmental competence [3–5]. They control nuclear and cytoplasmic maturation of the oocyte, keep the oocyte under meiotic arrest, and participate in the induction of meiotic resumption [3, 6–8]. Prior to ovulation in vivo, as well as during meiotic resumption in vitro, these compact cumulus granulosa cells disperse into a three-dimensional structure rich in extracellular matrix characterized by the synthesis of increased amounts of hyaluronic acid (HA) [9]. This process of cumulus expansion is associated with concomitant fundamental developmental changes of the oocyte, significantly affecting its maturation [10]. Besides being a structural molecule, HA is also an important signaling molecule and its production correlates positively with the resumption of oocyte meiotic competence [11, 12]. Several other molecules contribute to the formation of the matrix and/or are involved in the stabilization of HA, including the tumor necrosis factor alpha-induced protein 6 (TNFAIP6) [13]. Together with some other factors, both HA and TNFAIP6 act as indirect quality evaluation markers and indicators of oocyte maturation [14].
Due to the lack of vasculature within growing follicles, the communication between the oocyte and cumulus cells during oocyte maturation is guaranteed by gap junctions [15–17]. Functioning as the main intercellular link, gap junctions facilitate the transfer of small molecules including second messengers and ions [18]. The functionality of some gap junctions’ components, e.g., CX43, was proved indispensable for cumulus expansion and, thus, for oocyte maturation [19]. Cumulus cells excrete the steroid hormones, estrogens and progesterone (P4) [20, 21]. Besides being involved in controlling follicular growth, estradiol (E2) promotes the development of cumulus cells and the synthesis of HA as well as some of the gap junction proteins, including CX43 [22, 23]. Similarly, P4 secreted from cumulus cells is involved in the acquisition of oocyte meiotic competence and nuclear competence [24, 25]. This demonstrates that the oocyte and cumulus cells work as a functional entity and the presence of expanded cumulus cells is a conditio sine qua non for the oocyte to acquire fertilization competence. Following this line, arachidonic acid metabolites, like prostaglandins (PGs), are involved in the functional interplay between the cumulus and oocyte, due to their versatile mitogenic, differentiation, and immunomodulatory properties [21]. Other cumulus-derived factors used as specific markers of oocyte maturation include thymosin beta-4 and -10 (TMSB4 and TMSB10) [26, 27], small proteins (4–10 kDa) involved in several cellular processes like cell motility and differentiation or anti-inflammatory processes [28].
Oxygen (O2) is an essential component of the environment during oocyte maturation, both in vivo and in vitro. This also applies to the bovine species, in which varying O2 saturation conditions applied in vitro have been shown to affect oocyte maturation and developmental competence, as well as blastocyst rates and embryonic gene expression [29–32]. The actual O2 content of ovarian follicles is difficult to determine and the response to hypoxic conditions in vitro may depend on culture conditions, e.g., on energy substrate content [33], and may, thus, not fully reflect the situation in vivo. This can also lead to apparently contradictory conclusions, with reduced O2 tension being reported to exert either no, negative, or positive effects on in vitro maturation (IVM) of oocytes in different species [30, 31, 34–36], while supporting improved developmental competence of embryos, with higher blastocyst rates being observed. Thus, clearly, isolated COCs and developing blastocysts adapt to different in vitro experimental conditions.
The cellular effects of hypoxia are known to be mediated mainly through hypoxia-inducible factors (HIFs), with HIF1alpha (encoded by HIF1A gene) being the most known and thoroughly investigated. Responding to changing O2 concentrations, HIF1alpha acts as a transcription factor in heterodimer complexes with its constitutively expressed binding partner, HIF1beta [37–39]. Its expression in granulosa cells is FSH- and hCG-dependent [40]. Interestingly, the expression of both HIF1alpha and HIF1beta also appears to be dependent on the nuclear progesterone receptor (PGR), as shown in murine ovaries [41]. Thus, in PGR knockout mice an anovulatory phenotype was observed associated with reduced expression of HIF1alpha. Furthermore, blocking HIF1 complex activity by applying echinomycin, a specific inhibitor of HIF1alpha DNA binding capability, prevented ovulation and affected expression of PGR-dependent genes, establishing a causality between PGR and HIF1alpha functionality in regulating ovulation [41]. Moreover, HIF1alpha is a direct and potent regulator of STAR-mediated steroidogenesis in granulosa cells, as knocking down and/or blocking its functionality by echinomycin suppressed both basal and cAMP-induced STAR expression, under both normoxic (20% O2) and reduced oxygen tension conditions [42]. Most recently, the involvement of HIF1alpha in regulating aromatase (CYP19A1) expression in granulosa cells of cattle was shown [43].
Cumulatively, HIF1 complex is an important factor multidirectionally affecting cellular functions in virtually all body systems, including the reproductive organs (see reviewed in: [44]). Accordingly, here, considering the importance of O2 sensing in regulating ovarian function, we aimed to explore the involvement of HIF1alpha during IVM of COCs in a bovine model, by blocking HIF1alpha activity and/or expression. Our investigations were driven by the hypothesis that HIF1alpha mediates the expression of cumulus-derived markers of oocyte maturation and affects the development rates of embryos obtained from COCs in which HIF1alpha was blocked. Additionally, the steroidogenic output from cumulus cells, as well as apoptotic and proliferative effects were determined.
Materials and methods
Cumulus–oocyte complex isolation
Ovaries used in the present study were obtained at a local slaughterhouse complying with national animal welfare and meat quality guidelines. Only samples from healthy animals were used. After collection, ovaries were transported to the laboratory in a 0.9% NaCl solution with 0.06 g/ml penicillin and 0.1 g/ml streptomycin at 34–37 °C and processed within 3 h of collection. For COCs retrieval from follicles, ovaries were sliced using a sterile disposable scalpel blade and collected in wash medium (BO-WASH, IVF Bioscience, Cornwall, UK). Retrieved COCs were then filtered using a 100 μm cell strainer (Corning Falcon, Corning, NY, USA). Compact COCs were chosen based on their morphology, i.e., the presence of at least two layers of compact cumulus cells and evenly granulated ooplasm. Selected COCs were then washed four times with wash medium (BO-WASH, IVF Bioscience, Cornwall, UK) and used immediately in further experiments.
In vitro embryo production and treatment with HIF1alpha blockers
In vitro embryo production (IVP) was performed following a standardized protocol and according to instructions from IVF Bioscience (Cornwall, UK). Thus, 30 COCs were pooled for each experimental group in every experiment. Experiments were repeated 3–10 times with ovaries from different animals in each experiment. The experimental design, presented schematically in Figure 1, included the following groups: immature COCs, IVM COCs, and IVM COCs in the presence of an HIF1alpha blocker. Two different classes of HIF1alpha blockers, with different modes of action, were used: echinomycin (a blocker of HIF1alpha DNA binding capability), was used throughout the study; and the PX-478 compound, which was used in some experiments. The latter decreases HIF1A mRNA expression and its translation, and inhibits deubiquitinization of HIF1alpha, cumulatively decreasing its cellular availability [45].
Figure 1.
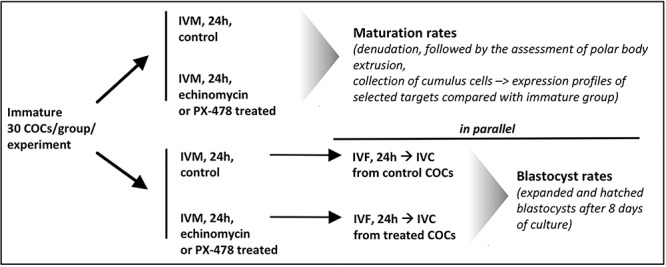
Experimental design of the study.
For the immature group, directly after slicing ovaries and selecting COCs, cumulus cells were separated from oocytes using 80 IU/ml hyaluronidase (GM501, Lensahn, DE) for 5 min at 37 °C. After denudation, cumulus cells were pooled by centrifugation (1000 g, 10 min), collected and stored at −80 °C until further analysis. The IVM protocol was applied for 24 h in 0.5 ml maturation medium (BO-IVM, IVF Bioscience, Cornwall, UK; medium containing gonadotropins with FSH and LH activities) at 38.5 °C, under a 5% CO2 and 20% O2 atmosphere, for both control and treatment groups. The concentrations of HIF1alpha blockers used were based on previous studies: 5 nM echinomycin [42, 43], and 25 and 50 μM PX-478 [45]. Following IVM, COCs were denuded using hyaluronidase as described above, and cumulus cells were stored at −80 °C. Then, oocytes were checked for the extrusion of the first polar body for the calculation of oocyte maturation rates. Additionally, the IVM medium (control and echinomycin-treated) was collected and stored at −80 °C for subsequent P4 content analysis by a competitive radioimmunoassay (RIA), as described previously [46]. Furthermore, in parallel, for each experiment, in vitro fertilization (IVF) was performed using all COCs from each IVM protocol (i.e., control vs. echinomycin- or PX-478-treated), and blastocyst rates were compared; only expanded and hatched blastocysts were counted at day 8 of in vitro culture (IVC) (Figure 1).
Briefly, the following IVF protocol was used. The density gradients used for sperm selection were created by the dilution of BoviPure with BoviDilute (both from Nidacon International AB, Gothenburg, SE) to concentrations of 80 and 40% (v/v). Equal volumes of both gradient layers were placed in one Eppendorf tube, with thawed cryopreserved sperm (0.25 ml) layered on top, and the preparation centrifuged at 300 g for 15 min. After removing the supernatant, the pellet was washed with Semen Preparation medium (IVF Bioscience, Cornwall, UK) and centrifuged twice at 350 g for 3 min. Then, 1 × 106 spermatozoa were added to each fertilization well with 30 COCs in 500 μl IVF medium (BO-IVF, IVF Bioscience, Cornwall, UK). Fertilization was performed at 38.5 °C under a 5% CO2 and 20% O2 atmosphere in a humidified incubator (Inkubator C16, Labotect, Göttingen, DE) for 19 h. For subsequent IVC of embryos, 0.5 ml IVC medium (BO-IVC, IVF Bioscience, Cornwall, UK), covered with 0.4 ml paraffin oil (BO-OIL, IVF Bioscience, Cornwall, UK), were placed in a humidified incubator (38.5 °C, 6–7% CO2, 6% O2) (Inkubator C16, Labotect) for 8 days. Blastocyst rates were assessed on day 8 after fertilization.
RNA isolation and semi-quantitative real time (TaqMan) PCR
The expression of selected target genes from different functional groups was determined in collected cumulus cells following IVM (Table 1). Those included: markers of cumulus expansion, indicators of meiotic resumption and oocyte developmental competence, steroidogenic markers, glucose transporter, as well as intercellular communication mediators and PG synthases. TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used for total RNA isolation following the manufacturer’s directions and as previously described [47]. For elimination of possible genomic DNA contamination, DNase-treatment was performed with RNase-free recombinant DNase I (Roche Diagnostics, Basel, CH), following the manufacturer’s instructions. The RT reaction was performed using reagents from Applied Biosystems by Thermo Fisher (Waltham, USA), utilizing random hexamers as primers, according to the manufacturer’s instructions. cDNA corresponding to 100 ng DNase-treated total RNA was used for each target gene reaction in semi-quantitative real time (TaqMan) PCR performed as described previously [47] in an automated fluorometer ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Thermo Fisher, Waltham, USA). Custom-made primers and the FAM/TAMRA-labeled probes were obtained from Microsynth (Balgach, CH), while commercially available systems were obtained from Thermo Fisher. Detailed information about TaqMan systems is provided in Table 1. All samples were analyzed in duplicate, negative controls were performed either by replacing cDNA with autoclaved water or with RNA samples that were not reverse transcribed (RT-minus control). The comparative Ct method (ΔΔCt method), following the instructions of the ABI PRISM 7500 fluorometer and as used previously [47–49], was applied for relative quantification of target genes expression relative to the expression of two reference genes (GAPDH and SDHA). Immature COCs were used as calibrators in each experiment for determining relative gene expression.
Table 1.
| Gene | Name | Accession number | Taqman system | Product length (bp) |
|---|---|---|---|---|
| HIF1alpha (HIF1A) | Hypoxia-inducible factor 1 alpha | NM_174339.3 | Commercially available: Applied Biosystems, prod. no. Bt03259341_m1 | 109 |
| Markers of cumulus expansion (HA metabolism) | ||||
| HAS | Hyaluronan synthase 2 | NM_174079.2 | Commercially available: Applied Biosystems, prod. no. Bt03212695_g1. | 119 |
| TNFAIP6 | Tumor necrosis factor alpha—stimulated gene-6 protein | NM_001007813.2 | Commercially available: Applied Biosystems, prod. no. Bt03210224_m1 | 124 |
| Indicators of meiotic resumption | ||||
| TMSB4 | Thymosin beta-4 | XM_005222037.4 | Forward 5′-GCG CCT CTG CAA CCA TGT-3′ Reverse 5′-CGA AGG CAG TGG ATT TCT CTC T-3′ TaqMan probe 5′-CCC GAT ATG GCT GAG ATT GAG AAG TTC G-3′ |
107 |
| TMSB10 | Thymosin beta-10 | AF294616.1 | Forward 5′-GGA TTC TCC ACC GCA TCA TCT-3′ Reverse 5′-GGG TTC ACA GTG CAG CTT GTC-′ TaqMan probe 5′-CCC TAG CCG TGA TGT GGA CCA AGA CC-3′ |
96 |
| Steroidogenic markers | ||||
| STAR | Steroidogenic acute regulatory protein | NM_174189 | Forward 5′-AA GTC CCT CAA GGA CCA AAC TC-3′ Reverse 5′-TG CGA GAG GAC CTG GTT GAT-3′ TaqMan probe 5′-ACC TCA AGG GAT GGC TGC CGA AGA-3′ |
90 |
| HSD3B | 3beta-hydroxysteroid dehydrogenase (3betaHSD) | NM_174343 | Forward 5′-CAC ACC GCC TCT GTC ATT GA-3′ Reverse 5′-GTA CGC TGG CCT GGA CAC A-3′ TaqMan probe 5′-TGC TGT CCC GCG AGA CCA TCA-3′ |
78 |
| Indicators of oocyte developmental competence | ||||
| GATM | Glycine amidinotransferase | NM_001045878.1 | Commercially available: Applied Biosystems, prod. no. Bt03237895_m1 | 110 |
| NPR2 | Natriuretic peptide. Receptor 2 | NM_174126.2 | Commercially available: Applied Biosystems, prod. no. Bt03212860_g1. | 96 |
| Glucose transporter | ||||
| SLC2A1 (GLUT1) | Solute carrier family 2 member 1 (glucose transporter 1) | NM_174602.2 | Commercially available: Applied Biosystems, prod. no. Bt03215311_g1 | 102 |
| Mediator of intercellular communication | ||||
| GJA1 (CX43) | Gap junction alpha-1 protein (connexin 43) | NM_174068.2 | Commercially available: Applied Biosystems, prod. no. Bt03244351_m1 | 67 |
| Prostaglandin synthases | ||||
| PTGS2 (COX2) | Prostaglandin 2 synthase (Cyclooxygenase-2) | NM_174445 | Forward 5′-GCA CAA ATC TGA TGT TTG CAT TC-3′ Reverse 5′-GGT CCT CGT TCA AAA TCT GTC T-3′ TaqMan probe 5′-TTG CCC AGC ACT TCA CCC ATC AAT T-3′ |
76 |
| PTGES | Prostaglandin E2 synthase | NM_174443 | Forward 5′-CAA GTG AGG CTG CGG AAG A-3′ Reverse 5′-AGG CAG CGT TCC ACA TCT G-3′ TaqMan probe 5′-TTT GCC AAC CCC GAG GAC GCT C-3′ |
101 |
| AKR1B1 (20alphaHSD/PGFS) | 20alpha-hydroxysteroid dehydrogenase (prostaglandin F2alpha synthase) | NM_001012519 | Forward 5′-ACC TGG ACC TCT ACC TCA TCC A-3′ Reverse 5′-TCC TCA TCC AAT GGG AAG AAG T-3′ TaqMan probe 5′-CCC ACA GGC TTC AAG CCT GGG A-3′ |
73 |
| Reference genes | ||||
| GAPDH | Glyceraldehyde-3- phosphate dehydrogenase | NM_001034034 | Forward 5′-GCG ATA CTC ACT CTT CTA CCT TCG A-3′ Reverse 5′-TCG TAC CAG GAA ATG AGC TTG AC-3′ TaqMan probe 5′-CTG GCA TTG CCC TCA ACG ACC ACT-3′ |
82 |
| SDHA | Succinate dehydrogenase complex flavoprotein subunit alpha | NM_174178 | Forward 5′-ATG GAA GGT CTC TGC GCT AT-3′ Reverse 5′-ATG GAC CCG TTC TTC TAT GG-3′ TaqMan probe 5′-ACA GAG CGA TCA CAC CGC GG-3′ |
119 |
Protein preparation and Western blot
Depending on the availability of the protein material and commercial antibodies, Western Blot analyses included selected target proteins: proliferating-cell-nuclear-antigen (PCNA), activated caspase-3 (activated-CASP3), steroidogenic acute regulatory protein (STAR) and hyaluronan synthase 2 (HAS2), and was determined in immature cumulus and compared with in vitro matured cumulus with/without echinomycin. Following denudation of oocytes, cumulus cells were collected by centrifugation (1000 g, 10 min), the supernatant was discarded and the cell pellet resuspended in 10 μl NET-2 lysis buffer (50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 0.05% NP-40). Proteins were then sonicated (Vibra-Cell 75186, Weilburg, USA) at 75 W for 15 s and solubilized in sample buffer (25 mM Tris-Cl, pH 6.8, 1% SDS, 5% b-mercaptoethanol, 10% glycerol, 0.01% bromophenol blue). Protein concentration was measured by Bradford assay in a Smart Spec Plus spectrophotometer (Bio-Rad Laboratories, Munich, DE) and the Western blot assay was performed as previously described [49]. Proteins (20–30 μg) were loaded onto 12 or 15% polyacrylamide gel (Bio-Rad), transferred into methanol-activated polyvinylidene difluoride (PVDF) membranes (Bio-Rad) and probed with primary antibodies: anti-HIF1alpha (HIF1A, rabbit polyclonal, Novus Biologicals Europe, Abington, UK, catalog number NB100-134, diluted 1:1000), anti-STAR (polyclonal rabbit, a gift from Dr DM Stocco, Texas Tech University Health Sciences Center, Lubbock, TX, USA [50], diluted 1:5000), anti-PCNA (proliferation marker; mouse monoclonal, ABCAM, Cambridge, UK, catalog number: AB29, diluted 1:1000), anti-HAS2 (HA synthase; polyclonal rabbit, catalog number: LS-C411405/113976, LSBio, Seattle, Washington, USA, diluted 1:1000), and anti-activated-CASP3 (rabbit monoclonal, catalog number: 559565, BD Biosciences, Allschwill, CH, diluted 1:200). For loading controls and semi-quantitation of target protein expression, PVDF membranes were reblotted with mouse monoclonal antibody against B-Actin (sc-81178) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), diluted 1:1000. The following secondary antibodies were used: donkey anti-rabbit HRP-labeled IgG, catalog number: 31460 from Pierce Biotechnology, Rockford, IL, USA, and HRP conjugated anti-mouse IgG, catalog number: W402B from Promega, Dübendorf, CH, both used at dilution 1:30 000. Signals were detected with SuperSignalWest chemiluminescent substrate (Pierce Biotechnology, Rockford, USA) according to the manufacturer’s protocol and visualized with a ChemiDoc XRS + System and Image Lab Software, in the presence of the Precision PlusProtein Standard (molecular weight marker) ranging from 10 to 250 kDa (all from Bio-Rad). A fine-point phosphorescent ink marking pen Glow Writer (MIDSCI, St. Louis, Valley Park, USA) was used to annotate the visible protein ladder in the ChemiDoc XRS + System. Semi-quantification of protein expression was performed using ImageJ Software (US National Institutes of Health, Bethesda, MD, USA). Representative Western blots are shown (Figure 5).
Figure 5.
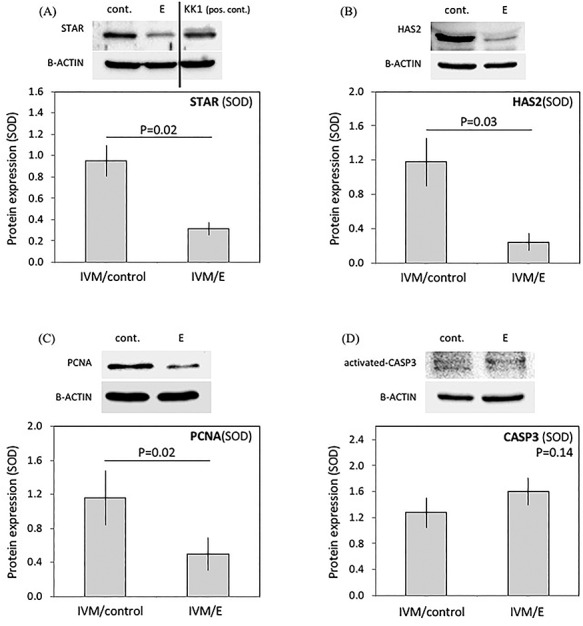
Effects of functional HIF1alpha suppression on protein expression of STAR, HAS2, PCNA, and activated-CASP-3 in cumulus cells. Cumulus cells were collected from COCs matured in vitro in the absence (IVM/control) or presence of echinomycin (5 nM) (IVM/E). Cells were denuded, collected, and homogenized; 25–30 μg of total protein were used for Western blot analysis. Representative Western blots are shown. Student t-test, unpaired, two-tailed, was applied; the average standardized optical density is shown (mean ± SEM) for: (A) STAR 30 kDa, (B) HAS2, 64 kDa, (C) PCNA 29 kDa, and (D) activated-CASP3, 17kDa. B-ACTIN was used as loading control (45 kDa). For STAR expression, mouse granulosa KK1 cells were used [42]. Solid lines placed over bars indicate a statistically significant difference between groups.
Statistical analysis
Statistical analysis was performed by either one-way analysis of variance (ANOVA) or unpaired, two-tailed Student t-test. In the case of ANOVA yielding P ≤ 0.05, the analysis was followed by a Tukey–Kramer multiple comparisons test for pairwise comparisons. For all tests, P ≤ 0.05 was considered to be statistically significant. Results are presented as mean and SEM. The statistics software program GraphPad 3.06 (GraphPad Software, San Diego, CA, USA) was used for all analyses.
Results
HIF1alpha activity during IVM is needed for cumulus expansion and expression of selected target genes in bovine cumulus cells
Following IVM, cumulus expansion was observed in the control group of COCs, as expected, but it was greatly inhibited in the echinomycin treated group (Figure 2). At the gene expression level, transcripts of all targets were clearly detectable, and when compared with immature COCs, significantly elevated levels of the following genes were induced during IVM: HAS2, TMSB4 and -10, CX43, PTGES (P < 0.001 each), TNAIP6 and COX2 (P < 0.01 each), GATM, GLUT1, and STAR (P < 0.05 each) (Figure 3). Echinomycin treatment prevented these effects for all these genes (Figure 3), and apart from HAS2 and GATM, the expression levels were similar to those of immature COCs (P > 0.05). HAS2 and GATM appeared to be strongly HIF1alpha dependent, as functional suppression of HIF1alpha yielded lower transcript levels than in immature cumulus cells (P < 0.05 and P < 0.01, respectively; Figure 3). IVM was associated with decreased expression of NPR2, AKR1B1 (20alphaHSD/PGFS), as well as of HSD3B (P < 0.05), regardless of the presence of echinomycin (Figure 3), with no statistically significant differences between both IVM groups, i.e., either treated or not with echinomycin (P > 0.05).
Figure 2.
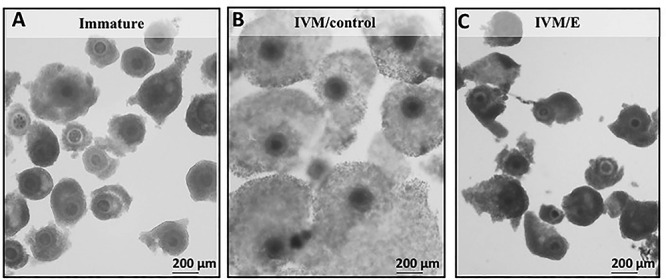
Representative micrographs showing the morphology of COCs after echinomycin treatment. (A) immature COCs, (B) COCs following control IVM (IVM/control), (C) COCs treated with echinomycin (5 nM) during IVM (IVM/E).
Figure 3.
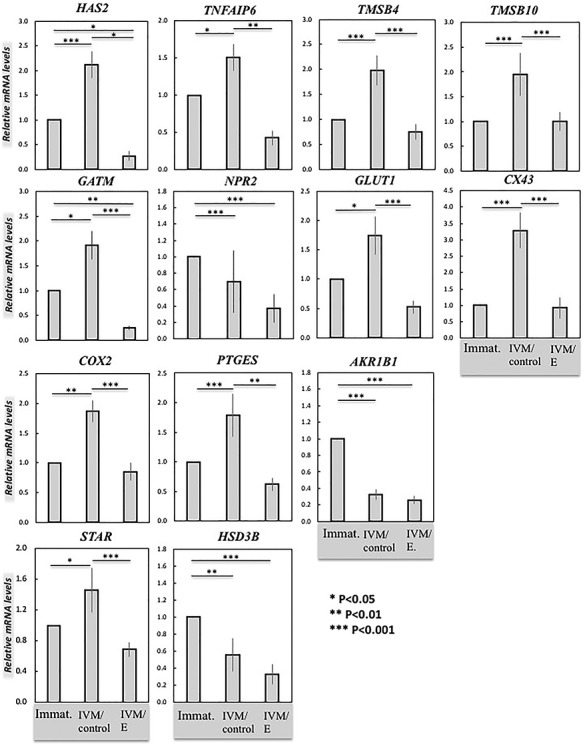
Effects of functional HIF1alpha suppression on target gene expression in cumulus cells derived from COCs as evaluated by semi-quantitative (TaqMan) real time PCR. Three experimental groups were involved: immature COCs (Immat.), control in vitro matured COCs (IVM/control), and COCs treated with echinomycin (5 nM) during IVM (IVM/E). The effect of functional HIF1alpha suppression on gene expression of target genes in cumulus cells was tested by one-way ANOVA, revealing: HAS2 (P < 0.0001), TNFAIP6 (P < 0.0001), TMSB4 (P = 0.0001), TMSB10 (P = 0.005), GATM (P < 0.0001), NPR2 (P = 0.036), GLUT1 (P = 0.0008), CX43 (P = 0.0001), COX2 (P = 0.001), PTGES (P < 0.0001), AKR1B1 (PGFS/20αHSD) (P < 0.0001), STAR (P = 0.0001), and HSD3B (P < 0.0001). Tukey–Kramer multiple comparisons post-test was applied to test the effect of treatment on gene expression (solid lines). Bars with asterisks differ at: *P < 0.05, **P < 0.01, ***P < 0.001, as indicated on the figure. Results are presented as mean ± SEM.
The expression of target genes was also evaluated in the cumulus cells of COCs exposed to PX-478. The levels of HIF1A transcript, which were increased during IVM (P < 0.05), decreased significantly following exposure to PX-478 (P < 0.01) compared with IVM/control, with both dosages of the blocker (25 and 50 μM) being equally effective. Similar effects were observed at the protein level. Thus, while HIF1A increased (P < 0.001) in IVM/control group, its levels were suppressed (P < 0.01) in PX-478-treated cumulus cells, but not in those cumulus cells derived from echinomycin-treated COCs (Figure 4). The expression of expansion and maturation-related target genes was significantly reduced (P < 0.05) in cells from treated COCs, compared with their levels in IVM/control cumulus cells (Figure 4), with similar expression patterns to those observed with echinomycin treatment. The expression of NPR2 was significantly lower than that observed in IVM/control cells in response to 50 μM PX-478 treatment (P < 0.05). Details of statistical analyses are presented on Figure 4.
Figure 4.
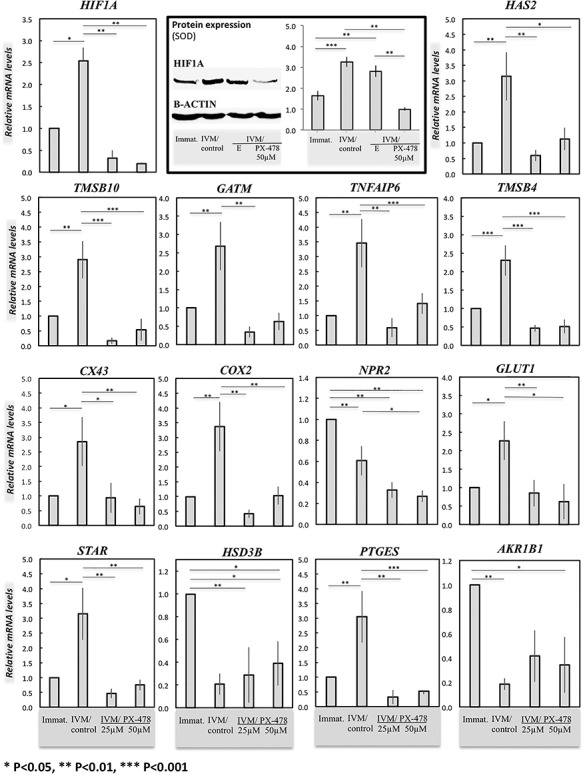
Effects of the suppression of HIF1alpha expression by PX-478 on target gene expression in cumulus cells derived from COCs as evaluated by semi-quantitative (TaqMan) real time PCR. The following experimental groups were involved: immature COCs (Immat.), control in vitro matured COCs (IVM/control), and COCs treated with two dosages of PX-478 (25 and 50 μM) during IVM (IVM/25 μM PX-478 and IVM/50 μM PX-478). The one-way ANOVA was applied to test the effect of HIF1alpha suppression on gene expression in treated cells, revealing: HIF1A (P = 0.0016), HAS2 (P = 0.018), TNFAIP6 (P = 0.0006), TMSB4 (P < 0.0001), TMSB10 (P = 0.0002), GATM (P = 0.002), NPR2 (P < 0.0001), GLUT1 (P = 0.004), CX43 (P = 0.06), COX2 (P = 0.001), PTGES (P = 0.005), AKR1B1 (P = 0.004), STAR (P = 0.002), and HSD3B (P = 0.002). Additionally, the HIF1A protein levels were determined by Western blot analysis in all experimental groups, i.e., immature, as well as in control and PX-478 (50 μM) and echinomycin (5 nM) treated IVM groups. The one-way ANOVA was applied revealing P < 0.0001. Tukey–Kramer multiple comparisons post-test was applied to test the effect of treatment on gene and/or protein expression (solid lines). Bars with asterisks differ at: *P < 0.05, **P < 0.01, ***P < 0.001, as indicated on the figure. Results are presented as mean ± SEM.
HIF1alpha regulates expression of functional protein markers in cumulus cells during IVM
The expression of representative markers of steroidogenesis (STAR), hyaluronan synthase (HAS2), proliferation marker (PCNA), and proapoptotic activated-CASP3 was assessed by Western blot analysis of cumulus cells matured in the presence and absence of echinomycin (Figure 5). Although clearly detectable and abundantly expressed in normally matured cumulus cells, STAR, HAS2, and PCNA expression was significantly suppressed in treated cumulus cells (P < 0.05; Figure 5). For the STAR expression assessment, the specificity of the antibody to recognize bovine protein was verified by including a positive control with proteins from murine immortalized granulosa KK1 cells cultured as previously described [42]. The expression of activated-CASP3 was generally low in cumulus cells from both groups and was not significantly altered in treated cumulus cells (P = 0.14; Figure 5).
Functional suppression of HIF1alpha during in vitro maturation reduces steroidogenic output from cumulus cells and alters IVM/IVP outcome from treated COCs
The biological effects of HIF1alpha suppression by echinomycin or PX-478 were assessed by evaluating oocyte maturation rates (first polar body extrusion) and blastocyst rates (expanded and hatched blastocysts) obtained from treated COCs. The oocyte maturation rates were decreased in echinomycin-treated COCs, compared with their untreated controls (77 vs. 44%; P < 0.001, Figure 6A), as were the blastocyst rates, calculated based on matured COCs (IVP output; 45 vs. 28% P < 0.001, Figure 6A). In addition, P4 output was significantly lower in treated COCs (Figure 6B; P < 0.05).
Figure 6.
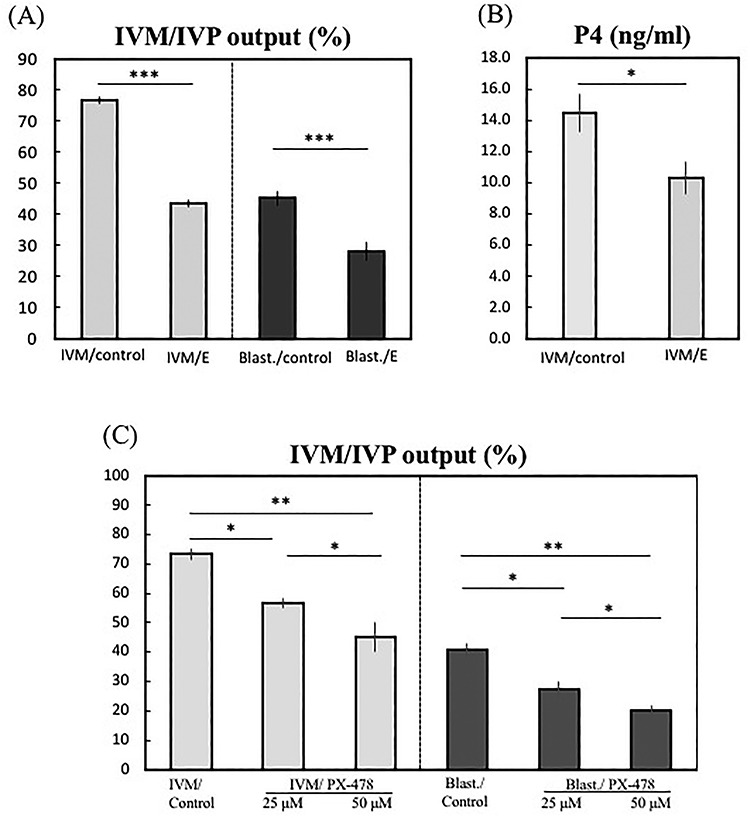
Effects of functional inhibition of HIF1alpha or suppression of its expression, on oocyte maturation rates (IVM output) and steroidogenic output (progesterone, P4), and on blastocyst rates (IVP output) from bovine COCs treated with HIF1alpha blockers. COCs were treated with either echinomycin (5 nM) or PX-478 (25 and 50 μM) and the IVM output was determined compared with untreated COCs (IVM/control) (A and C). In parallel experiments, control COCs and those treated with blockers were further used for IVF, and IVP output was determined by comparing numbers of expanded and hatched blastocysts at day 8 of culture (A and C). The steroidogenic output was determined by RIA in media from control and echinomycin-treated COCs (B). Student t-test, unpaired, two-tailed, was applied in (A) and (B), and one-way ANOVA was applied in (C), separately for IVM and IVP output (mean ± SEM). Following ANOVA (P < 0.002 and 0.001 for IVM/IVP output, respectively), Tukey–Kramer multiple comparisons post-test was applied to test the effect of the treatment on gene expression (solid lines). Bars with asterisks differ with: *P < 0.05, **P < 0.01, and ***P < 0.001.
Similar functional effects were observed following treatment with PX-478 (Figure 6C). Thus, the oocyte maturation rates decreased significantly in COCs treated with 25 μM PX-478 and 50 μM PX-478 (73 vs. 57 or 45%, respectively; P < 0.05 and P < 0.01). Blastocyst yields were also significantly lower from COCs exposed to PX-478 during IVM: 43 vs. 34% (P < 0.01) after 25 μM PX-478 treatment, and 43 vs. 24% (P < 0.01) after 50 μM PX-478 treatment.
Discussion
HIF1alpha is one of the main factors modulating cellular response to hypoxia. As shown in vitro, it regulates functions of steroidogenic cells under reduced O2 tension as well as under normoxic conditions [42, 51]. This, at least in part, appears to be due to its responsiveness to different stimuli, constituting the O2-independent regulation of HIF1alpha availability [52]. Consequently, although apparently deviating from physiological conditions, cells in vitro appear to cope with varying O2 levels, still maintaining their HIF1alpha responsiveness. Here, using a standardized IVP protocol, we addressed the hypothesis that HIF1alpha could be involved in regulating cumulus cell function, mirrored in alterations of expression of genes regarded as markers of oocyte maturation. The ultimate goal was to provide a functional insight into the underlying molecular and biological effects of HIF1 complexes and their importance for successful IVM in cattle. The functional consequences of interference with biological functions of HIF1alpha during IVM were further assessed by determining oocyte maturation rates (IVM output). Taking it further, in parallel experiments, oocytes from control COCs and those treated with HIF1alpha inhibitors were fertilized, allowing the determination of their functionality in terms of blastocyst output.
By applying a specific blocker of HIF1alpha transcriptional activity, echinomycin [53], we were able to show an inhibition of cumulus expansion (Figure 2). We then investigated the expression of HAS2 and TNFAIP6, selected as markers of cumulus expansion and maturation [14], and found their transcript levels were decreased (Figure 3). Concomitantly, the expression of HAS2 protein was significantly diminished in cumulus cells from echinomycin-treated COCs (Figure 5). The decreased expansion of cumulus was also associated with reduced proliferation of cumulus cells, reflected in strongly lowered expression of PCNA protein (Figure 5). These alterations in cell-cycle progression were, however, not related to the pro-apoptotic activities, as indicated by unaltered, generally low, levels of activated-CASP3 protein expression (Figure 5). Thus, despite the cellular level disturbances caused by HIF1alpha withdrawal, there was no increased apoptosis observed. However, blocking of HIF1alpha also affected other mechanisms considered key for oocyte maturation. For example, the decreased expression of CX43 suggested negative effects of HIF1alpha blocking on gap junction functionality. Interestingly, CX43 expression is increased in cumulus cells from COCs with a high developmental competence, when compared to those with a lower developmental competence, in vitro [54]. Also, here, CX43 appeared to serve as an indicator of oocyte quality. Furthermore, GLUT1 downregulation in cumulus cells from echinomycin-treated COCs suggests a negative effect of the treatment on glucose transport and therefore on the maintenance of cellular homeostasis and metabolic capacity and function of COC elements, i.e., cumulus cells and the oocyte [55]. Based on these observations, echinomycin-mediated inhibition of HIF1alpha activity seemed to affect the communication between cumulus cells, as well as between the corona radiata cells and the oocyte, possibly affecting its metabolic balance.
Negative effects of functional suppression of HIF1alpha were also observed with regard to the members of the PGs system, i.e., COX2 (PTGS2) and PTGES. An increased production of PGs has been associated with ovulation in several species [56–58] and the COX2-derived PGE2 has been shown to be essential for ovulation, e.g., in mice [59]. Furthermore, besides being involved in ovulation, PGE2 signaling through its EP2 (PTGER2) receptor is involved in the limitation of excessive integrin-mediated extracellular matrix assembly in the cumulus, making it resistant to sperm hyaluronidase and, thus, preventing sperm penetration [60]. With this, PGE2 contributes also to the successful fertilization of the oocyte. Based on our findings, both COX2 and PTGES appear to be targets for the cumulus-expressed HIF1alpha in bovine COCs. Interestingly, this does not seem to be the case for AKR1B1 (PGFS/20alphaHSD). Previously known as AKR1B5 [61, 62], the recently renamed bos taurus AKR1B1 is an aldose reductase, which not only has the capability to convert PGH2 into PGF2α in a species-specific manner, but also metabolizes P4 to 20alpha-hydroxyprogesterone [61]. The latter is a weaker agonist of PGR [63]. The expression of AKR1B1 was decreased in matured cumulus cells and was not affected by the HIF1alpha activity. By decreasing its levels during IVM, AKR1B1 may thus contribute to the maintenance of progesterone availability in the cumulus. Indeed, steroid production is an important feature of follicular granulosa cells, with their synthesis via STAR modulated by HIF1alpha [42, 64], as confirmed in the present study. Although, the expression of STAR mRNA and protein was significantly increased during IVM, as expected, it was strongly suppressed in the IVM/E group of COCs. In our previous findings, neither the expression of HSD3B protein, nor that of the P450scc (CYP11A1; an enzyme catalyzing the first metabolic step in steroid synthesis, the conversion of cholesterol to pregnenolone), seemed to be regulated by O2 sensing in granulosa cells [42]. Accordingly, as shown here, the expression of HSD3B was not affected by the suppression of HIF1α. With this, STAR appears to be the HIF1alpha -dependent, rate limiting candidate gene controlling the steroidogenic output from cumulus cells during IVM. This is further supported by the significantly suppressed P4 output measured in medium from treated COCs.
Possible functional consequences of HIF1alpha suppression on oocyte development were reflected in the suppressed expression of TMSB4 and TMSB10, used as markers of meiotic resumption [28]. In fact, as shown previously and confirmed here, paralleled by upregulated levels of COX2, HAS2, as well as of PGR, the expression of TMSB4 and TMSB10 was increased in a time-dependent manner in bovine cumulus cells during IVM [28]. It was associated with oocyte maturation, but not with their competence for fertilization and early development in vitro [28], as the levels were similar in cumulus from oocytes that did and did not develop to the blastocyst stage. GATM, a mitochondrial enzyme involved in arginine metabolism, was previously associated with blastocyst fate [65]. It was highly expressed in cumulus from COCs with higher developmental competence towards blastocyst, and was thus proposed as a biomarker for high-potential COCs in the cow [65]. In our study, GATM was revealed to be a highly HIF1alpha sensitive gene; diminishing HIF1alpha functionality by echinomycin not only prevented its upregulation during IVM, but similar to HAS2, suppressed its expression levels to below those seen in immature cumulus cells. Contrasting with thymosin’s and GATM, the expression of cumulus-derived NPR2 decreased during IVM. Its function is to maintain the oocyte under meiotic arrest, thereby preventing precocious meiotic resumption of oocytes in tertiary follicles [66, 67]. Interestingly, the expression of NPR2 in cumulus appears to be regulated reciprocally by the oocyte, as its expression decreases when the oocyte is removed, and it can be restored when the same cumulus cells are incubated with denuded oocytes [68]. Its decreased expression was thus expected in our experiments during meiotic resumption. However, although its expression was apparently decreased, there was no statistically significant difference between echinomycin-treated vs. non-treated COCs. Nevertheless, its expression was significantly suppressed when HIF1alpha expression was diminished by PX-478 (discussed below).
Having observed the effects of echinomycin on cumulus gene expression during IVM, and despite knowing its function in blocking transcriptional activity of HIF1alpha, it could however still be considered that echinomycin could interfere with the functionality of other transcriptional factors, thereby inhibiting the expression of their target genes. At this point, it is important to mention that in previous studies similar effects to those obtained with echinomycin could be achieved upon expression of STAR, CYP19A1 (aromatase), or VEGF, by applying RNA silencing [42, 43]. Here, in order to confirm our findings we decided to employ PX-478, a new generation blocker with alternative modes of action to echinomycin in targeting and suppressing HIF1alpha expression [45, 69]. Accordingly, contrasting with the functional inhibition of HIF1alpha by echinomycin not affecting the protein levels of HIF1A, we were able to confirm the inhibitory effects of PX-478 on HIF1A mRNA and protein expression in bovine cumulus cells and observed that its effects in treated COCs were identical and confirmatory with those observed with echinomycin.
An interesting observation was made with regard to the expression of NPR2, which was strongly suppressed in treated cumulus, below the levels observed in two other experimental groups (i.e. immature and IVM/control). This allowed the conclusion that NPR2 is also a HIF1alpha responsive gene. With this, as for the other markers of meiotic resumption, HIF1alpha seems to actively contribute to the NPR2-mediated control of meiotic arrest in cattle. Functional studies in support of this conclusion would be of interest.
Finally, the most important functional finding from our study was that disturbances in the expression of cumulus markers of COC maturation were followed by significantly decreased oocyte maturation rates in response to both HIF1alpha blockers, corresponding with the suppressed thymosin’s expression. Furthermore, as indicated by decreased GATM expression, in parallel experiments reduced numbers of expanded and hatched blastocyst were observed from treated COCs. Consequently, by showing lower blastocyst rates obtained from treated oocytes, the treatment appeared to affect developmental competence of oocytes. Our findings demonstrate that HIF1alpha is functionally involved in controlling IVP output in cattle, while proposing the underlying mechanism and several target genes. Whether these effects are directly or indirectly mediated through HIF1alpha needs to be further determined for each gene. These mechanisms appear to be diverse, involving several HIF1alpha-mediated pathways, like HA-, TNFAIP6-, and PGE2-dependent mechanisms controlling cumulus matrix assembly and cumulus cell proliferation. Other mechanisms involve functional and metabolic effects characterized by lowered steroidogenic output and suppression of GLUT1-mediated energy supply. They also affect cell-to-cell communication and, finally, factors controlling oocyte meiotic and developmental competence.
Besides highlighting possible pathways and biological mechanisms involved in hypoxia sensing in COCs, our findings emphasize the importance of HIF1alpha in successful IVP, providing a basis for further investigations into the underlying mechanisms of hypoxia-mediated effects during oocyte maturation and, possibly, leading to further optimization of IVP protocols.
Conflict of interests
The authors declare that they have no conflict of interests. All authors read and approved the final version of the manuscript.
Authors’ contribution
AT was involved in developing the concept of the present study, experimental design, generating data, analysis and interpretation of data and writing of the manuscript. MTP was involved in drafting and revising the manuscript, knowledge transfer. GS was involved in the laboratory part of the project, knowledge transfer. UB was involved in developing the concept of the study, experimental design, analysis and interpretation of data, and revising the manuscript. MPK designed and supervised the project, was involved in analysis and interpretation of the data and writing and revising of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The technical expertise and contributions of Ricardo Fernandez Rubia from the Institute of Veterinary Anatomy, Vetsuisse Faculty, University of Zurich (UZH) and Sabine Feller from the Clinic for Obstetrics, Gynecology and Andrology of Large and Small Animals, Justus-Liebig-University, Giessen, Germany, are greatly appreciated. Authors are thankful to Dr. Sharon Mortimer (Oozoa Biomedical, Inc., Vancouver, Canada) for careful editing of the manuscript. Part of the laboratory work was performed using the logistics of the Center for Clinical Studies, Vetsuisse Faculty, University of Zurich.
Footnotes
† Grant Support: This project was partially supported by the Ministry of Higher Education of the Republic of Turkey through the Scholarship Program YLSY and from the Vetsuisse Faculty of Zurich via Institute of Veterinary Anatomy and Clinic of Reproductive Medicine.
Contributor Information
Aslihan Turhan, Vetsuisse Faculty, Institute of Veterinary Anatomy, University of Zurich (UZH), Zurich, Switzerland; Department of Farm Animals, Clinic of Reproductive Medicine, Vetsuisse Faculty University of Zurich, Zurich, Switzerland.
Miguel Tavares Pereira, Vetsuisse Faculty, Institute of Veterinary Anatomy, University of Zurich (UZH), Zurich, Switzerland.
Gerhard Schuler, Clinic for Obstetrics, Gynecology and Andrology of Large and Small Animals, Justus-Liebig-University, Giessen, Germany.
Ulrich Bleul, Department of Farm Animals, Clinic of Reproductive Medicine, Vetsuisse Faculty University of Zurich, Zurich, Switzerland.
Mariusz P Kowalewski, Vetsuisse Faculty, Institute of Veterinary Anatomy, University of Zurich (UZH), Zurich, Switzerland.
References
- 1. Mehlmann LM Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 2005; 130:791–799. [DOI] [PubMed] [Google Scholar]
- 2. Eppig JJ Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 1996; 8:485–489. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Jiang S, Wozniak PJ, Yang X, Godke RA. Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol Reprod Dev 1995; 40:338–344. [DOI] [PubMed] [Google Scholar]
- 4. Tanghe S, Van Soom A, Mehrzad J, Maes D, Duchateau L, de Kruif A. Cumulus contributions during bovine fertilization in vitro. Theriogenology 2003; 60:135–149. [DOI] [PubMed] [Google Scholar]
- 5. Ortiz-Escribano N, Smits K, Piepers S, Van den Abbeel E, Woelders H, Van Soom A. Role of cumulus cells during vitrification and fertilization of mature bovine oocytes: Effects on survival, fertilization, and blastocyst development. Theriogenology 2016; 86:635–641. [DOI] [PubMed] [Google Scholar]
- 6. Fukui Y, Sakuma Y. Maturation of bovine oocytes cultured in vitro: relation to ovarian activity, follicular size and the presence or absence of cumulus cells. Biol Reprod 1980; 22:669–673. [DOI] [PubMed] [Google Scholar]
- 7. Chian RC, Niwa K, Sirard MA. Effects of cumulus cells on male pronuclear formation and subsequent early development of bovine oocytes in vitro. Theriogenology 1994; 41:1499–1508. [DOI] [PubMed] [Google Scholar]
- 8. Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev 2002; 61:414–424. [DOI] [PubMed] [Google Scholar]
- 9. Chen L, Mao SJT, Mclean LR, Powers RW, Larsen WJ. Proteins of the inter-alpha-trypsin inhibitor family stabilize the cumulus extracellular-matrix through their direct binding with hyaluronic-acid. J Biol Chem 1994; 269:28282–28287. [PubMed] [Google Scholar]
- 10. Fulop C, Kamath RV, Li YF, Otto JM, Salustri A, Olsen BR, Glant TT, Hascall VC. Coding sequence, exon-intron structure and chromosomal localization of murine TNF-stimulated gene 6 that is specifically expressed by expanding cumulus cell-oocyte complexes. Gene 1997; 202:95–102. [DOI] [PubMed] [Google Scholar]
- 11. Fulop C, Salustri A, Hascall VC. Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus-oocyte complex. Arch Biochem Biophys 1997; 337:261–266. [DOI] [PubMed] [Google Scholar]
- 12. Salustri A, Yanagishita M, Underhill CB, Laurent TC, Hascall VC. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol 1992; 151:541–551. [DOI] [PubMed] [Google Scholar]
- 13. Lee TH, Wisniewski HG, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (Tsg-6) is a member of the family of hyaluronate binding-proteins, closely related to the adhesion receptor Cd44. J Cell Biol 1992; 116:545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Assidi M, Dufort I, Ali A, Hamel M, Algriany O, Dielemann S, Sirard M-A. Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol Reprod 2008; 79:209–222. [DOI] [PubMed] [Google Scholar]
- 15. Feng GX, Shi DS, Yang SF, Wang XL. Co-culture embedded in cumulus clumps promotes maturation of denuded oocytes and reconstructs gap junctions between oocytes and cumulus cells. Zygote 2013; 21:231–237. [DOI] [PubMed] [Google Scholar]
- 16. Furger C, Cronier L, Poirot C, Pouchelet M. Human granulosa cells in culture exhibit functional cyclic AMP-regulated gap junctions. Mol Hum Reprod 1996; 2:541–548. [DOI] [PubMed] [Google Scholar]
- 17. Santiquet NW, Develle Y, Laroche A, Robert C, Richard FJ. Regulation of gap-junctional communication between cumulus cells during in vitro maturation in swine, a gap-FRAP study. Biol Reprod 2012; 87. [DOI] [PubMed] [Google Scholar]
- 18. Buccione R, Schroeder AC, Eppig JJ. Interactions between somatic-cells and germ-cells throughout mammalian oogenesis. Biol Reprod 1990; 43:543–547. [DOI] [PubMed] [Google Scholar]
- 19. Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol 2000; 226:167–179. [DOI] [PubMed] [Google Scholar]
- 20. McNatty KP, Smith DM, Makris A, Osathanondh R, Ryan KJ. Steroidogenesis by the human oocyte-cumulus cell complex in vitro. Steroids 1980; 35:643–651. [DOI] [PubMed] [Google Scholar]
- 21. Nuttinck F, Reinaud P, Tricoire H, Vigneron C, Peynot N, Mialot JP, Mermillod P, Charpigny G. Cyclooxygenase-2 is expressed by cumulus cells during oocyte maturation in cattle. Mol Reprod Dev 2002; 61:93–101. [DOI] [PubMed] [Google Scholar]
- 22. Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Mol Endocrinol 2010; 24:2303–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schams D, Berisha B. Steroids as local regulators of ovarian activity in domestic animals. Domest Anim Endocrinol 2002; 23:53–65. [DOI] [PubMed] [Google Scholar]
- 24. Salhab M, Tosca L, Cabau C, Papillier P, Perreau C, Dupont J, Mermillod P, Uzbekova S. Kinetics of gene expression and signaling in bovine cumulus cells throughout IVM in different mediums in relation to oocyte developmental competence, cumulus apoptosis and progesterone secretion. Theriogenology 2011; 75:90–104. [DOI] [PubMed] [Google Scholar]
- 25. Aparicio IM, Garcia-Herreros M, O'Shea LC, Hensey C, Lonergan P, Fair T. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol Reprod 2011; 84:910–921. [DOI] [PubMed] [Google Scholar]
- 26. Chen C, Li M, Yang H, Chai H, Fisher W, Yao Q. Roles of thymosins in cancers and other organ systems. World J Surg 2005; 29:264–270. [DOI] [PubMed] [Google Scholar]
- 27. Huff T, Muller CS, Otto AM, Netzker R. Hannappel E. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol 2001; 33:205–220. [DOI] [PubMed] [Google Scholar]
- 28. Salhab M, Papillier P, Perreau C, Guyader-Joly C, Dupont J, Mermillod P, Uzbekova S. Thymosins beta-4 and beta-10 are expressed in bovine ovarian follicles and upregulated in cumulus cells during meiotic maturation. Reprod Fertil Dev 2010; 22:1206–1221. [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto S, Minami N, Takakura R, Yamada M, Imai H, Kashima N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocyte complexes. Mol Reprod Dev 2000; 57:353–360. [DOI] [PubMed] [Google Scholar]
- 30. Pinyopummintr T, Bavister BD. Optimum gas atmosphere for in vitro maturation and in vitro fertilization of bovine oocytes. Theriogenology 1995; 44:471–477. [DOI] [PubMed] [Google Scholar]
- 31. Oyamada T, Fukui Y. Oxygen tension and medium supplements for in vitro maturation of bovine oocytes cultured individually in a chemically defined medium. J Reprod Dev 2004; 50:107–117. [DOI] [PubMed] [Google Scholar]
- 32. Fujitani Y, Kasai K, Ohtani S, Nishimura K, Yamada M, Utsumi K. Effect of oxygen concentration and free radicals on in vitro development of in vitro-produced bovine embryos. J Anim Sci 1997; 75:483–489. [DOI] [PubMed] [Google Scholar]
- 33. Clark AR, Stokes YM. Follicle structure influences the availability of oxygen to the oocyte in antral follicles. Comput Math Methods Med 2011; 2011:287186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology 2000; 54:741–756. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi M Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev 2012; 58:1–9. [DOI] [PubMed] [Google Scholar]
- 36. Adam AA, Takahashi Y, Katagiri S, Nagano M. Effects of oxygen tension in the gas atmosphere during in vitro maturation, in vitro fertilization and in vitro culture on the efficiency of in vitro production of mouse embryos. Jpn J Vet Res 2004; 52:77–84. [PubMed] [Google Scholar]
- 37. McNamee EN, Johnson DK, Homann D, Clambey ET. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol Res 2013; 55:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity 2014; 41:518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA 2009; 106:17910–17915. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Henriquez S, Kohen P, Munoz A, Godoy A, Orge F, Strauss JF 3rd, Devoto L. In-vitro study of gonadotrophin signaling pathways in human granulosa cells in relation to progesterone receptor expression. Reprod Biomed Online 2017; 35:363–371. [DOI] [PubMed] [Google Scholar]
- 41. Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 2009; 150:3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kowalewski MP, Gram A, Boos A. The role of hypoxia and HIF1alpha in the regulation of STAR-mediated steroidogenesis in granulosa cells. Mol Cell Endocrinol 2015; 401:35–44. [DOI] [PubMed] [Google Scholar]
- 43. Baddela VS, Sharma A, Michaelis M, Vanselow J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci Rep 2020; 10:3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ziello JE, Jovin IS, Huang Y. Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 2007; 80:51–60. [PMC free article] [PubMed] [Google Scholar]
- 45. Koh MY, Spivak-Kroizman T, Venturini S, Welsh S, Williams RR, Kirkpatrick DL, Powis G. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol Cancer Ther 2008; 7:90–100. [DOI] [PubMed] [Google Scholar]
- 46. Hoffmann B, Kyrein H, Ender M. An efficient procedure for the determination of progesterone by radioimmunoassay applied to bovine peripheral plasma. Horm Res Paediatr 1973; 4:302–310. [DOI] [PubMed] [Google Scholar]
- 47. Kowalewski MP, Schuler G, Taubert A, Engel E, Hoffmann B. Expression of cyclooxygenase 1 and 2 in the canine corpus luteum during diestrus. Theriogenology 2006; 66:1423–1430. [DOI] [PubMed] [Google Scholar]
- 48. Gram A, Buchler U, Boos A, Hoffmann B, Kowalewski MP. Biosynthesis and degradation of canine placental prostaglandins: prepartum changes in expression and function of prostaglandin F2alpha-synthase (PGFS, AKR1C3) and 15-hydroxyprostaglandin dehydrogenase (HPGD). Biol Reprod 2013; 89:2. [DOI] [PubMed] [Google Scholar]
- 49. Kowalewski MP, Dyson MT, Manna PR, Stocco DM. Involvement of peroxisome proliferator-activated receptor gamma in gonadal steroidogenesis and steroidogenic acute regulatory protein expression. Reprod Fertil Dev 2009; 21:909–922. [DOI] [PubMed] [Google Scholar]
- 50. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 1994; 269:28314–28322. [PubMed] [Google Scholar]
- 51. Nishimura R, Okuda K. Multiple roles of hypoxia in ovarian function: roles of hypoxia-inducible factor-related and -unrelated signals during the luteal phase. Reprod Fertil Dev 2016; 28:1479–1486. [DOI] [PubMed] [Google Scholar]
- 52. Chun YS, Kim MS, Park JW. Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci 2002; 17:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res 2005; 65:9047–9055. [DOI] [PubMed] [Google Scholar]
- 54. Read CC, Willhelm G, Dyce PW. Connexin 43 coupling in bovine cumulus cells, during the follicular growth phase, and its relationship to in vitro embryo outcomes. Mol Reprod Dev 2018; 85:579–589. [DOI] [PubMed] [Google Scholar]
- 55. Herrick JR, Brad AM, Krisher RL. Chemical manipulation of glucose metabolism in porcine oocytes: effects on nuclear and cytoplasmic maturation in vitro. Reproduction 2006; 131:289–298. [DOI] [PubMed] [Google Scholar]
- 56. Sirois J, Dore M. The late induction of prostaglandin G/H synthase-2 in equine preovulatory follicles supports its role as a determinant of the ovulatory process. Endocrinology 1997; 138:4427–4434. [DOI] [PubMed] [Google Scholar]
- 57. Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod 2001; 7:731–739. [DOI] [PubMed] [Google Scholar]
- 58. Kowalewski MP, Kautz E, Hogger E, Hoffmann B, Boos A. Interplacental uterine expression of genes involved in prostaglandin synthesis during canine pregnancy and at induced prepartum luteolysis/abortion. Reprod Biol Endocrinol 2014; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matsumoto H, Ma W, Smalley W, Trzaskos J, Breyer RM, Dey SK. Diversification of cyclooxygenase-2-derived prostaglandins in ovulation and implantation. Biol Reprod 2001; 64:1557–1565. [DOI] [PubMed] [Google Scholar]
- 60. Tamba S, Yodoi R, Segi-Nishida E, Ichikawa A, Narumiya S, Sugimoto Y. Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. Proc Natl Acad Sci USA 2008; 105:14539–14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Madore E, Harvey N, Parent J, Chapdelaine P, Arosh JA, Fortier MA. An aldose reductase with 20 alpha-hydroxysteroid dehydrogenase activity is most likely the enzyme responsible for the production of prostaglandin F2 alpha in the bovine endometrium. J Biol Chem 2003; 278:11205–11212. [DOI] [PubMed] [Google Scholar]
- 62. Schuler G, Teichmann U, Kowalewski MP, Hoffmann B, Madore E, Fortier MA, Klisch K. Expression of cyclooxygenase-II (COX-II) and 20alpha-hydroxysteroid dehydrogenase (20alpha-HSD)/prostaglandin F-synthase (PGFS) in bovine placentomes: implications for the initiation of parturition in cattle. Placenta 2006; 27:1022–1029. [DOI] [PubMed] [Google Scholar]
- 63. Veomett MJ, Daniel JC Jr. Termination of pregnancy after accelerated lactation in the rat. IV. Relationship to 20alpha-hydroxysteroid dehydrogenase activity and plasma progesterone concentration. J Reprod Fertil 1975; 44:529–536. [DOI] [PubMed] [Google Scholar]
- 64. Fadhillah YS, Nishimura R, Yamamoto Y, Kimura K, Okuda K. Hypoxia-inducible factor 1 mediates hypoxia-enhanced synthesis of progesterone during luteinization of granulosa cells. J Reprod Dev 2017; 63:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bunel A, Jorssen EP, Merckx E, Leroy JL, Bols PE, Sirard MA. Individual bovine in vitro embryo production and cumulus cell transcriptomic analysis to distinguish cumulus-oocyte complexes with high or low developmental potential. Theriogenology 2015; 83:228–237. [DOI] [PubMed] [Google Scholar]
- 66. Kiyosu C, Tsuji T, Yamada K, Kajita S, Kunieda T. NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary. Reproduction 2012; 144:187–193. [DOI] [PubMed] [Google Scholar]
- 67. Zhang W, Yang Y, Liu W, Chen Q, Wang H, Wang X, Zhang Y, Zhang M, Xia G. Brain natriuretic peptide and C-type natriuretic peptide maintain porcine oocyte meiotic arrest. J Cell Physiol 2015; 230:71–81. [DOI] [PubMed] [Google Scholar]
- 68. Xi G, An L, Jia Z, Tan K, Zhang J, Wang Z, Zhang C, Miao K, Wu Z, Tian J. Natriuretic peptide receptor 2 (NPR2) localized in bovine oocyte underlies a unique mechanism for C-type natriuretic peptide (CNP)-induced meiotic arrest. Theriogenology 2018; 106:198–209. [DOI] [PubMed] [Google Scholar]
- 69. Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther 2004; 3:233–244. [PubMed] [Google Scholar]


