Abstract
Pro-pregnancy hormone progesterone (P4) helps to maintain a quiescent status of uterine tissues during gestation. However, P4’s functional role in maintaining fetal membrane (amniochorion) integrity remains unclear. P4 functions through its membrane receptors (progesterone receptor membrane components (PGRMCs)) as fetal membrane cells lack nuclear receptors. This study screened the differential expression of PGRMCs in the fetal membranes and tested P4–PGRMC interactions under normal and oxidative stress (OS) conditions expected that can disrupt P4–PGRMC interactions impacting fetal membrane stability resulting in parturition. Human fetal membranes were collected from term and preterm deliveries (N = 5). Immunohistochemistry and western blot localized and determined differential expression of P4 receptors. Primary amnion epithelial, mesenchymal (AMCs), and chorion cell were treated with P4 alone or co-treated (P4 + OS induced by cigarette smoke extract (CSE)). Proximity ligation assay (PLA) documented P4–receptor binding, whereas P4 enzyme-linked immunosorbent assay documented culture supernatant levels. Immunohistology confirmed lack of nuclear progesterone receptors; however, confirmed expressions of PGRMC 1 and 2. Term labor (P = 0.01) and preterm rupture (P = 0.01) are associated with significant downregulation of PGRMC2. OS-induced differential downregulation of PGRMCs in both amnion and chorion cells (all P < 0.05) and downregulates P4 release (AMCs; P = 0.01). The PLA showed preferential receptor–ligand binding in amnion and chorion cells. Co-treatment of P4 + CSE did not reverse CSE-induced effects. In conclusion, P4–PGRMCs interaction maintains fetal membranes’ functional integrity throughout pregnancy. Increased OS reduces endogenous P4 production and cell type-dependent downregulation of PGRMCs. These changes can lead to fetal membrane-specific “functional progesterone withdrawal,” contributing to the dysfunctional fetal membrane status seen at term and preterm conditions.
Keywords: amnion membrane, progesterone, functional progesterone withdrawal, PGRMC2, PGRMC1, preterm birth, pPROM
Oxidative stress-induces cells type-dependent changes in progesterone receptor membrane component 1 and 2 expression, which could contribute to the disruption of human fetal membranes both at term and preterm labor.
Introduction
Preterm birth (PTB) is a major pregnancy complication [1]. The most common (~60%) phenotype of PTB occurs spontaneously, and ~40% of these cases are also complicated by preterm premature rupture of the membranes (pPROM), where fetal membrane (amniochorion) dysfunctions are one of the major contributing factors [2–6]. Significant knowledge exists on how the quiescent uterus and the cervix are transitioned to an active state of labor. This transition is an inflammatory state at term and preterm mediated by estrogen and progesterone (P4) and P4’s nuclear receptor-mediated functional changes [7–11]. The role of P4 on fetal side, specifically in the maintenance of fetal membrane homeostasis or transitioning them to a pro-labor inflammatory state or causing fetal membrane rupture to promote labor either at term or preterm, is unclear.
The fetal membranes are comprised of two epithelial layers, the amnion and chorion, which are separated by mesenchymal cells imbedded in the extracellular matrix. The fetal amniochorion layer is connected to the maternal decidua in utero forming the feto-maternal interface. The fetal membranes protect the fetus during gestation while undergoing constant remodeling of both cells and its extracellular matrix [12]. Multiple evidences suggest that fetal membranes undergo progressive senescence during gestation, which peaks at term in response to oxidative stress (OS) and creates an inflammatory environment [13, 14]. Fetal membrane senescence is mediated by p38 mitogen-activated kinase (MAPK), a responder of stress [15]. Premature activation of p38MAPK, senescence, and inflammation are seen in a major subset of PTB and pPROM [13]. P4 treatment failed to reduce OS (physiologically seen at term) and/or infection and infection-associated OS (pathologically seen at preterm) induced p38MAPK activation, senescence, and inflammation in fetal membranes in vitro [16]. This suggests that P4’s functional contribution to reduce inflammation in fetal membranes is muted when senescence is activated at term or preterm [16].
Mechanistically, P4’s actions are exerted via two known isoforms of nuclear progesterone receptors (PR), PR-A (96 kDa) and PR-B (116 kDa) [8, 9, 11] as well as a variety of membrane P4 receptors. Fetal membranes are not known to express PR-A and PR-B [9, 10, 17]. Therefore, ambiguity exists in P4’s functional role in maintaining fetal membrane integrity during pregnancy and if functional withdrawal of P4 can be a contributor to fetal membrane senescence and dysfunction. The P4 can perform signaling through nonnuclear PRs, including progesterone receptor membrane components 1 and 2 (PGRMC1 (gene ID: hsa10857) and PGRMC2 (gene ID: hsa10424)) and membrane PRs alpha and beta (mPα (gene ID: PAQR7) and mPβ (gene ID: PAQR8)) [18–25]. The PGRMC1 is a 195 amino acid protein located on the chromosome X, whereas PGRMC2, a 223 amino acid protein, is located on chromosome 4 [25, 26]. Both PGRMC 1 and 2 expressions have been reported in maternal and feto-maternal interface tissues [17, 22, 27, 28]. Feng et al. and Murtha et al. have reported PGRMC1-mediated P4 functional changes in normal and adverse pregnancy conditions [17, 27, 29, 30]. Concurrent studies on PGRMC1, PGRMC2, and other receptors will provide a theoretical model for P4 function and functional withdrawal in fetal membranes.
In vitro studies from our laboratory have shown that P4 helps to maintain an epithelial phenotype of amnion epithelial cells (AEC) and to promote transition of amnion mesenchymal stromal cells (AMC) to an epithelial state [31]. These cellular transitions and recycling help to constantly remodel fetal membranes during pregnancy. We hypothesize that P4, through its membrane PRs (mPRs), helps to maintain fetal membrane integrity, and receptor downregulation physiologically at term or pathologically at preterm causes fetal membrane dysfunction. Since membrane receptors are not that well characterized in fetal membranes, our objectives for this study were to screen for P4 receptors (nuclear and membrane) in fetal membranes from different clinical specimens at term and preterm parturition. Based on our primary screening, we tested the conditions that can contribute to differential expression of specific receptors.
Materials and methods
Institutional Review Board approval
Placentas for this study were collected from John Sealy Hospital (University of Texas Medical Branch (UTMB) at Galveston, TX, USA, according to the inclusion and exclusion criteria defined by our laboratory (see Section Inclusion Criteria for Term Categories and Exclusion Criteria for Term Categories). As discarded placentas were used after delivery for the study, subject recruitment or consenting was not done. The Institutional Review Board (IRB) at UTMB approved the study protocol, and placentas were collected according to the regulations of the IRB as an exempt protocol that allowed the use of the discarded placentas (UTMB 11-251). Under the same protocol, fetal membrane samples were also collected from cases of spontaneous PTB and pPROM. According to UTMB OB&GYN’s clinical practice standards, placenta collected from such cases are not subjected to histopathologic evaluation (e.g., chorioamnionitis or funisitis) are also considered as discarded samples. Studies using these samples are limited to determining receptor expression changes as clinical data collected are minimal. Correlative analyses between receptor expression and subject’s clinical status are also not performed as these samples are deidentified.
Inclusion criteria for term categories
Normal term birth were women with term labor (TL) and delivery (>390/7 weeks) and no pregnancy-related complications.
Exclusion criteria for term categories
Subjects with multiple gestations, placenta previa, fetal anomalies, and/or medical or surgeries (intervention for clinical conditions that are not linked to pregnancy) during pregnancy were excluded. Severe cases of preeclampsia or persistent symptoms (headache, vision changes, right upper quadrant (RUQ) pain) or abnormal laboratory findings (thrombocytopenia, repeated abnormal liver function tests, creatinine doubling or >1.2, or Hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome) or clinical findings (pulmonary edema or eclampsia) were excluded. Subjects who had any surgical procedure during pregnancy or who were treated for hypertension, preterm labor, or for suspected clinical chorioamnionitis (reports on foul smelling vaginal discharge, fetal tachycardia), positive Group B streptococcal (GBS) screening or diagnosis of bacterial vaginosis, behavioral issues (cigarette smoking, drug, or alcohol abuse), and delivered at term were excluded from the control groups.
Clinical fetal membrane samples
Fetal membranes were collected (amniochorion full thickness membranes) from the following categories of patients: term not in labor cesarean deliveries (TNIL; N = 5), TL vaginal deliveries (N = 5), pPROM (N = 6) (mean gestational age was 32 weeks) resulting in preterm delivery and spontaneous preterm labor, leading to imminent birth (PTB; N = 6) (mean gestational age was 34 weeks). The PTB samples were gestational matched as before or after 34 weeks (N = 6). A 4 × 4 cm piece of the fetal membrane section was cut out from the middle portion of the placenta (remote from weak and placental plate zones) and washed in warm saline solution (pH 7.4) within 15 min after delivery. Blood clots were subsequently removed by rinsing the fetal membranes back and forth in warm media. Tissue samples underwent an additional separation of chorion and decidua, accomplished by scraping off the decidual layer using a scalpel (stored at −80°C) until further use. Similarly, fetal membrane tissue from all categories was flash-frozen (stored at −80°C) until further experiments, whereas other biopsy punches (4–6 punches) were submerged in 0.5 mL 10% neutral buffered formalin for 24 h at 4°C for immunohistochemistry.
AEC in vitro culture
Human primary AECs were isolated and cultured as previously described by our laboratory (N = 5) [31–33]. Briefly, fetal membranes obtained from term-scheduled cesarean deliveries (TNIL) were received by our laboratory, and ~10 g of the amnion layer were separated from the chorion layer. The amnion was rinsed in saline and cut into small pieces of ~2 × 2 cm in size in a petri dish containing Hanks balanced salt solution (HBSS) (Mediatech Inc., Manassas, VA, USA). The amnion was then digested using 0.125% collagenase and 1.2% trypsin (Sigma-Aldrich, St. Louis, MO, USA) in HBSS for 35 min. The digestion step was repeated, and the tissue was filtered through a 70-μm cell strainer (Thermo Fisher Scientific, Waltham, MA, USA); trypsin was inactivated using complete Dulbecco modified Eagle medium:Nutrient Mixture F-12 media (DMEM/F12; Mediatech Inc.) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 10% penicillin/streptomycin (Mediatech Inc.), and 100 μg/mL epidermal growth factor (EGF; Sigma-Aldrich) following each digestion step. The filtrate was then centrifuged (3000 rpm, 10 min), and the pellet resuspended in complete DMEM/F12 media and cultured in T75 flasks at ~3–5 million cells/flask [34]. The flasks were incubated at 37°C, 5% CO2, and 95% air humidity until they reached 70–80% confluence and were ready to be passaged and treated.
AMC in vitro culture
Human primary AMCs (N = 5) were isolated from term placental tissue collected from repeat elective cesarean procedures. The AMCs were isolated as previously described [34–36], with slight modifications. Shiny amnion was identified and peeled from the chorion and rinsed three to four times in sterile HBSS (Cat# 21-021-CV, Corning) to remove blood debris. The tissue was then incubated with 0.05% trypsin/Ethylenediaminetetraacetic acid (EDTA) (Cat# 25-053-Cl, Corning) for 1 h at 37°C. The digested fetal membrane solution was neutralized using complete DMEM/F12 media (Cat# 10-092-CV, Corning), filtered using a 70-μm cell strainer, and centrifuged at 300 rpm for 10 min. The cell pellet was resuspended in complete DMEM/F12 media supplemented with 5% FBS (Cat# 35-010-CV, Corning), 100 U/mL penicillin G, and 100 mg/mL streptomycin (Cat# 30-001-Cl, Corning). The resuspended cells were subsequently seeded at a density of 2–3 million cells per T25 flask to yield cultures with 95–99% purity [37]. The mesenchymal nature of the primary cells culture was verified microscopically.
Chorion cell in vitro culture
Chorion cells were collected from term-scheduled cesarean deliveries. Separation of the chorion (N = 5) and decidual layers involved blunt and gentle scraping with a scalpel [34]. Tissues were first processed in a digestion buffer containing 2.4 U/mL dispase (Cat# D4693, Sigma) and incubated at 37°C for 8 min. Tissues were then allowed to rest for 5–10 min at room temperature in complete media (1:1 mixture of Ham F12/DMEM, supplemented with 10% FBS, 100 U/mL penicillin G, and 100 mg/mL streptomycin) (Cat# 30-001-Cl, Corning). The dispase incubation and the rest cycle were repeated once more. Subsequently, the tissue was incubated in a digestion buffer containing 0.75 mg/mL collagenase (Cat# C0130, Sigma) and 0.02% DNase I (Cat# DN25, Sigma) with rotation at 37°C for 3 h. Remaining tissue was incubated in a solution containing 0.25% trypsin (Cat# 85450c, Sigma) and 0.02% DNase I (Cat# DN25, Sigma) at 37°C for 5 min. The digested solution was then filtered through a 70-μm strainer for cell collection. After filtration, the cell solution was centrifuged at 3000 rpm for 10 min. Pelleted cells were resuspended in complete media and plated; the resulting culture contained a mixture of chorion mesenchymal and trophoblast cells. Cell viability was tested using the trypan blue exclusion method and microscopy.
Decidua cell in vitro culture
Decidua cells were collected from term-scheduled cesarean deliveries. The decidua was separated from the chorion by blunt dissection with forceps and a scalpel and minced by cross-cutting with scalpel blades [34]. Tissues were processed in a digestion buffer containing 0.125% trypsin (Cat# 85450c, Sigma), 0.2% collagenase (Cat# C0130, Sigma), and 0.02% DNase I (Cat# DN25, Sigma) and incubated at 37°C for 60–90 min. Samples were subsequently neutralized with complete media (1:1 mixture of Ham F12/DMEM, supplemented with 5% heat-inactivated FBS, 10 ng/mL EGF, 100 U/mL penicillin G, and 100 mg/mL streptomycin) (Cat# 30-001-CI, Corning). After filtration, the cell solution was centrifuged at 3000 rpm for 10 min. A cell separation gradient was prepared using an Optiprep column (Axis-Shield), with steps ranging from 4 to 40% of 4 mL each (4, 6, 8, 10, 20, 30, and 40%). Processed decidual cells were added to the top of the gradient and centrifuged (3000 rpm) at room temperature for 35 min. Cell densities of 1.027–1.038 g/mL represented the decidua layer. Harvested cells were washed with DMEM, centrifuged, resuspended in DMEM, and plated at densities of 80 000 (decidua) per well in six-well plates to yield cultures with 95–99% purity. Cell viability was tested using the trypan blue exclusion method. Decidua cells were used only as a comparison to measure P4 release.
Primary cell culture experimental conditions
Primary cell cultures of AECs, AMCs, and chorion cells (trophoblasts and mesenchymal cells) were grown under (a) normal cell culture conditions (control), (b) cigarette smoke extract (CSE; 1:50 dilution of the concentrate), an OS inducer to mimic uterine OS conditions seen at TL, and (c) lipopolysaccharide (LPS; 100 ng/mL) to mimic an infectious inflammatory condition often seen in cases of PTB and pPROM. LPS from Escherichia coli 055:B5 that was reconstituted at a concentration of 1 mg/mL and then diluted to 100 ng/mL in media prior to use. This concentration is within the range of which is seen in the amniotic fluid of women with infection-associated pregnancy complications and used previously in our experiments [15]. The CSE was prepared by bubbling smoke drawn from a single-lit commercial cigarette (unfiltered Camel; R.J. Reynolds Tobacco Co., Winston Salem, NC, USA) through 25 mL of tissue culture medium (Ham F12/DMEM mixture with antimicrobial agents). In this study, we utilized CSE at a 1:50 ratio, which has been shown to induce endpoint phenotypes associated with labor such as senescence, Epithelial-mesenchymal transition (EMT), and inflammation [12, 15, 31]. Additional treatments were conducted where cells were treated either with progesterone (P4) 200 ng/mL, as documented in our prior manuscript [12], or co-treated P4 with CSE.
Immunohistochemistry
To screen for the presence of PRs, tissue sections were fixed in 4% paraformaldehyde (PFA) for 48 h and embedded in paraffin. Sections were cut at 5-μm thickness, adhered to a positively charged slide, and attached by keeping them at 57°C for 45 min. Slides were deparaffinized using xylene, rehydrated with 100% alcohol, 95% alcohol, and normal saline (pH 7.4), and stained. The following antihuman antibodies were used for immunohistochemistry: PR-A (Invitrogen MA5-14505), PR-B (Invitrogen MA5-14505), PGRMC1 (Invitrogen 13-856S), PGRMC2 (Invitrogen PA5-59465), mPα (Abcam 75508) and mPβ (Abcam 46535) at 1:800 dilution, and beta-actin 1:20 000 (Sigma-Aldrich, A5441). Development with 3, 3-diaminobenzidine (DAB) secondary substrate was optimized for each antibody (Ab) and used for the same time for each replicate. Standard hematoxylin and eosin staining was used as counter stain. Images from random sites from tissues from each group were taken at 10× and 40× magnification.
Protein extraction and immunoblot assay
AECs, AMCs, chorion cells, and human fetal membrane tissue were lysed with Radioimmunoprecipitation assay buffer (RIPA) lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% Triton X-100, and 1.0 mM EDTA pH 8.0, 0.1% sodium dodecyl sulfate (SDS)) supplemented with protease and phosphatase inhibitor cocktail and phenylmethylsulfonyl fluoride. After centrifugation at 10 000 rpm for 20 min, the supernatant was collected. Protein quantification was performed using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Western blot analyses were performed using 40 μg (tissue) and 22 μg (cells) of protein from each sample, applying standard protocols. The protein samples were separated using SDS–polyacrylamide gel electrophoresis on a gradient (4–15%) with Mini-PROTEAN TGX Precast Gels (Bio-Rad, Hercules, CA, USA) and transferred to the polyvinylidene fluoride membrane using the Bio-Rad Gel Transfer Device (Bio-Rad) at 120 V for about 45 min. Membranes were blocked in 5% nonfat milk in 1X Tris-buffered saline Tween 20 or in 5% bovine serum albumin buffer for a minimum of 1 h at room temperature and then probed (or reprobed) with primary Ab overnight at 4°C. The membrane was incubated with appropriate secondary Ab conjugated with horseradish peroxidase, and immunoreactive proteins were visualized using the chemiluminescence reagents ECL Western Blotting Detection System (Amersham Piscataway, NJ, USA). The stripping protocol followed the instructions of the Restore Western Blot Stripping Buffer (Thermo Fisher Scientific). No blots were used more than three times. The following antihuman antibodies were used for western blotting: PR-A (Invitrogen MA5-14505), PR-B (Invitrogen MA5-14505), PGRMC1 (Invitrogen 13-856S), PGRMC2 (Invitrogen PA5-59465), mPα (Abcam 75508) and mPβ (Abcam 46535) at 1:800 dilution, and beta-actin 1:20 000 (Sigma-Aldrich, A5441). The relative levels of the proteins in the specific bands were normalized densitometrically using associated beta-actin levels in the samples, applying the Bio-Rad Image Lab 6.0 software.
Proximity ligation assay
The proximity ligation assay (PLA) was used to determine binding between membrane P4 receptor (PGRMC1 or PGRMC2) and P4. For this, AECs, AMCs, and chorion cells were cultured on glass coverslips and treated with P4 (200 ng/mL) for 48 h. To validate the PLA kit and cell types, multiple positive (P4 (abcam, 1:300), PGRMC1 (1:100), PGRMC2 (2 μL/mL), cytokeratin-18 (CK-18; abcam, 1:800), and vimentin (abcam, 1:300)–immunocytochemistry protocol) and negative controls (positive and negative probe controls and true negative wells) were added to the experimental design. After 48 h with P4 treatment, cells were fixed with 4% PFA, permeabilized with 0.5% Triton X for 10 min, blocked for 1 h with PLA blocking agent, and probed with primary antibodies overnight at 4°C. Positive and negative probes, ligase, and amplification solution were added according to the manufacturer’s instructions (Duolink In Situ Detection Reagent Orange, Duolink In Situ PLA Probe Anti-Rabbit PLUS, Duolink In Situ PLA Probe Anti-Mouse MINUS). Slides were stained with 4′,6-diamidino-2-phenylindole (DAPI), and four random regions were imaged at 20× and 40× per biological replicate. The PLA complexes were quantitated using ImageJ partial analysis (0.0003-infinity) and normalized to the number of cells in the field of view.
Progesterone competitive enzyme-linked immunosorbent assay
Media from primary AECs, AMCs, chorion cells, and decidua cells were collected to measure the amount of P4 that was secreted in culture. A competitive enzyme-linked immunosorbent assay (ELISA; Invitrogen, EIAP4C21), one of four types of ELISA, was used by adding a competitive Ab after the addition of secondary Ab and performed following the manufacturer’s instructions. Standard curves were developed with recombinant protein samples of known quantities. Sample concentrations were determined by correlating the sample absorbance to the standard curve by linear regression analysis. To compare cell types grown under normal cell culture conditions in different sized flasks, we extrapolated and normalized P4 production for time (6 days) within a T75 flask surface area.
Statistical analysis
Statistical analysis for normally distributed data was performed using an analysis of variance with Tukey Multiple Comparisons Test (Figures 2,3, and5) and t-test (Figures 1,2, and5; Supplementary Figure 3). Statistical values were calculated using PRISM; P values of <0.054 were considered significant. Data are represented as mean ± SEM. All data were analyzed using Graph Pad Prism 6.
Figure 2.
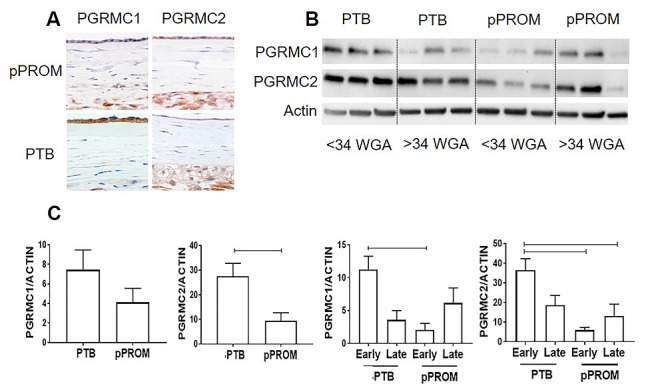
PGRMC expression during early and late preterm labor. (A) Immunohistochemistry localized PGRMC1 and PGRMC2 in fetal membrane tissues from preterm birth (PTB) and preterm premature rupture of membrane (pPROM) (×40); Scale bar = 30 μm. (B) Western blot analysis of PGRMC1 and PGRMC2 in full fetal membranes from early, <34 weeks gestational age (WGA), and late, >34 WGA; preterm birth deliveries were collected for analysis. (C) Densitometry showed PGRMC2 significantly decreased in pPROM membranes compared with PTB (P = 0.01), whereas the PGRMC1 level did not change. When divided into early and late phenotypes, early pPROM had significantly less PGRMC1 (P = 0.02) and PGRMC2 (P = 0.009) compared with early PTB. In addition, early PTB also expressed significantly higher PGRMC2 than late pPROM (P = 0.04) (N = 5; mean ± SEM). Scale bar is 50 μm.
Figure 3.
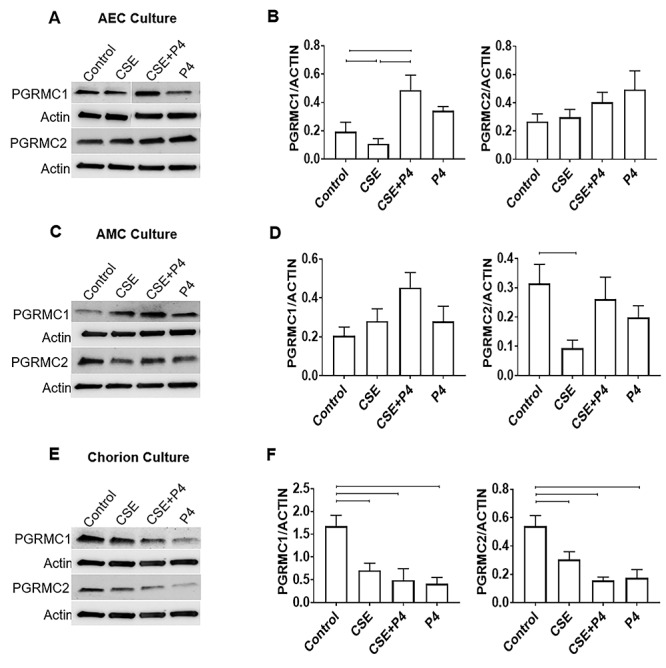
Effect of P4 on PGRMC expression in fetal membrane cells. Data represented in bar graphs are mean ± SEM. (A and B) Densitometry showed P4 co-treatment significantly increased PGRMC1 (P = 0.01) expression in AECs compared with CSE and control cells, whereas CSE alone significantly decreased PGRMC1 (P = 0.054) (N = 5). (C and D) Densitometry showed CSE treatment significantly decreased PGRMC2 expression (P = 0.01) in AMCs, whereas P4 co-treatment was able to increase both PGRMC1 and PGRMC2 compared with CSE alone (N = 5). (E and F) Densitometry showed CSE treatment significantly inhibited PGRMC1 (P = 0.006) and PGRMC2 (P = 0.03) expression, which was not recovered with co-treatment with P4 (PGRMC1: P = 0.007 and PGRMC2: P = 0.004). P4 alone also significantly reduced PGRMC1 (P = 0.007) and PGRMC2 (P = 0.004) expression (N = 5).
Figure 5.
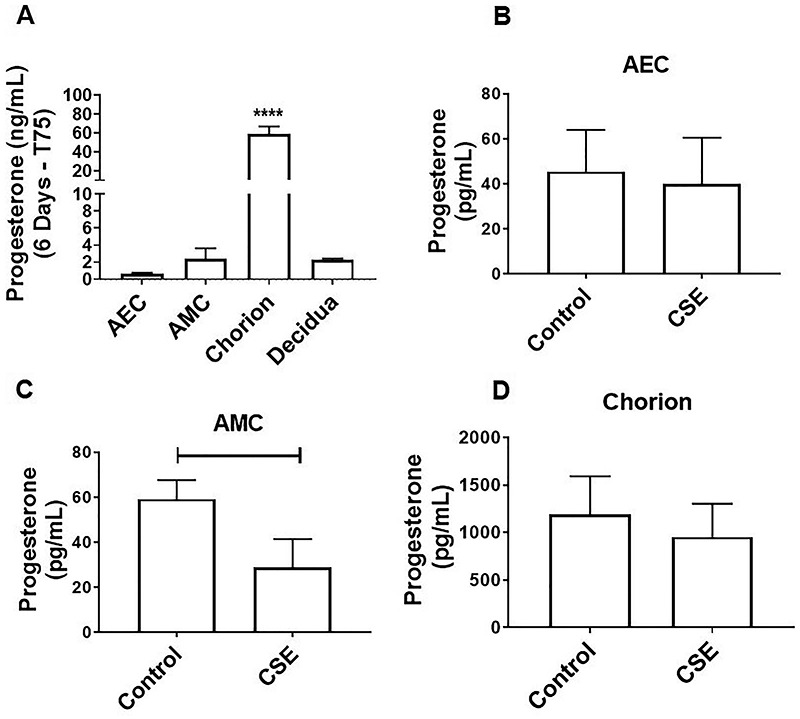
Oxidative stress inhibits local P4 release in AMCs. (A) Although all fetal membrane cells can produce progesterone, chorion cells produce higher levels compared with amnion epithelial and mesenchymal cells (AEC: 0.6 ± 0.1 ng/mL, AMC: 2.5 ± 1.2 ng/mL, chorion: 58.8 ± 8.1 ng/mL). In addition, full fetal membrane explants secrete significant levels of P4, which is likely produced by the chorion layer (P < 0.0001) (chorion: 58.8 ± 8.1 ng/mL, decidua: 2.3 ± 0.1 ng/mL) (N = 3; mean ± SEM). To compare cell types under normal cell culture conditions, we extrapolated and normalized P4 production for 6 days within a T75 flask. (B and D) CSE-induced oxidative stress did not affect P4 production in AECs (B), CSE decreased P4 production in AMCs (C) (P = 0.039; control: 59.2 ± 8.43 pg/mL, CSE: 28.95 ± 12.5 pg/mL) and chorion cells (D) (control: 1192 ± 401.6 pg/mL, CSE: 953.9 ± 350 pg/mL) (N = 3; mean ± SEM).
Figure 1.
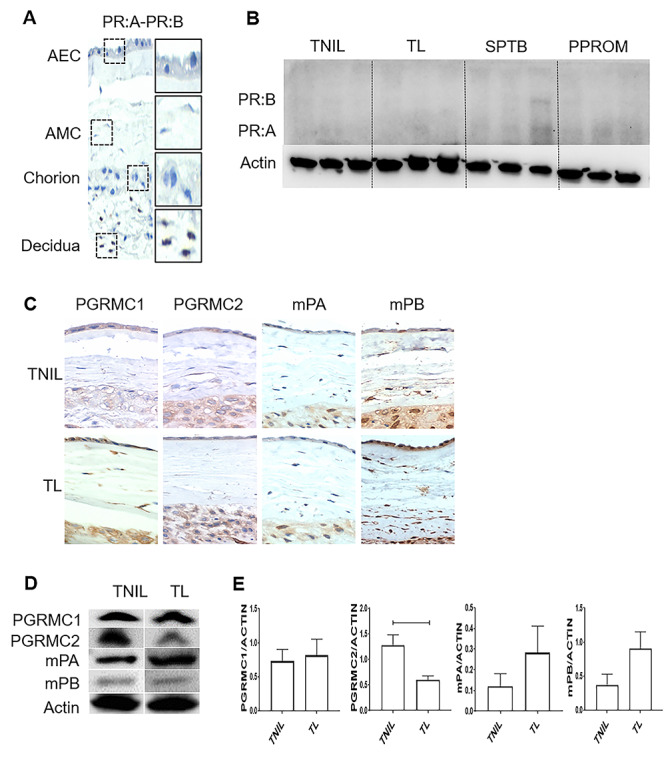
Differential expression of P4 membrane receptors in human fetal membranes at term. (A) Fetal membrane cells, including amnion epithelial cells (AECs), amnion mesenchymal cells (AMCs), and chorion cells, were negative for nuclear progesterone receptors A and B (PR:A and PR:B) (×40). Immunohistochemistry localized PRA and PRB in the maternal decidua, confirming the specificity of staining. (B) Western blot analysis of nuclear P4 receptors (PR:A–PR:B) in term labor (TL), term not in labor (TNIL), and preterm birth fetal membranes (amnion–chorion). (C) All four P4 membrane receptors, progesterone receptor membrane component (PGRMC) 1, PGRMC2, mPα, and mPβ, in fetal membrane cells were localized by immunohistochemistry at term, regardless of the labor status (×40) (N = 3); Scale bar = 50 μm. (D) Western blot analysis of membrane P4 receptors at TL and TNIL in fetal membranes (amnion–chorion). (E) Densitometry analysis of TL showed significantly lower expression of PGRMC2 compared with TNIL sample (P = 0.01), whereas PGRMC1, mPα, and mPβ expression did not change in TL compared with TNIL fetal membranes (N = 5; mean ± SEM). Full gels for (C) can be found in Supplementary Figure 2. PGRMC1 and PGRMC2 data also seen in Richardson et al. [12].
Results
Analysis of nuclear and mPRs in fetal membranes from term deliveries
Conflicting data exist in the literature regarding the expression of P4 nuclear PRs in the fetal membranes, where most recent studies show their absence [9, 10, 22, 28, 38]. To confirm the absence of nuclear PRs, immunohistochemistry was conducted on TNIL fetal membranes. All fetal membrane tissues, including AEC, AMC, and chorion cells, were negative for nuclear PR-A:PR-B, whereas the maternal decidua showed nuclear PRs stained brown (Figure 1A; Supplementary Figure 1) (N = 3). Western blot data on term and preterm fetal membrane tissue confirmed the prior reports and validated the specificity of the antibodies used (Figure 1B) (N = 3). However, one PTB tissue did show PR:A–PR:B likely due to immune cell infiltration. Immunohistochemistry was performed on TNIL and TL fetal membranes to determine the expression of the four mPRs. Regardless of the labor status, fetal membrane cells expressed PGRMC1, PGRMC2, mPα, and mPβ, as seen by brown stains predominantly in the cell membranes, but also in the cytoplasm and the perinuclear regions of AECs, AMCs, and chorion cells (Figure 1C; Supplementary Figure 1) (N = 3). Thus, we confirm the prior reports that fetal membranes do not express nuclear PRs [10, 28]; however, they express all four types of mPRs, regardless of the labor status.
Differential expression of mPRs in human fetal membranes at term
After confirming the lack of nuclear PR expression in fetal membranes and verifying the expression of all mPRs, we determined their differential expressions at labor using western blot analysis. Protein expression of full thickness fetal membranes from TNIL and TL groups was analyzed for PGRMC1, PGRMC2, mPα, and mPβ (Figure 1D; Supplementary Figure 2) (N = 5). Densitometry analysis of intact fetal membranes showed that PGRMC2 expression, and not any other mPRs, was affected by the onset of labor. The PGRMC2 expression was significantly lower in TL compared with TNIL samples (P = 0.01). The PGRMC1, mPα, and mPβ expression did not significantly change, regardless of the labor status (Figure 1E). Based on these data, we further focused our studies on both PGRMCs. In previous studies, PGRMC1 expression was different in fetal membrane cells [17, 27, 28], although its expression did not change in intact fetal membranes between the conditions in our early histology and western analysis. Due to functional similarities between PGRMC 1 and 2 and because the observed differences were likely caused by specific cell type difference, we included PGRMC1 in all our further analyses and compared it with PGRMC2.
Characterization of PGRMCs in human fetal membranes from preterm deliveries
Full fetal membranes from pPROM (N = 6) and PTB (N = 6) were screened for PGRMCs using immunohistochemistry, (N = 3) was <34 weeks of gestational age (WGA) and (N = 3) >34 WGA. Immunohistochemistry localized PGRMC1 and PGRMC2 in fetal membrane tissues from PTB and pPROM deliveries predominantly in the cell membrane, cytoplasm, and perinuclear regions of AECs, AMCs, and chorion layers (Figure 2A). Differential expression of PGRMC1 and PGRMC2 was assessed by western blot analysis (Figure 2B). Overall, PGRMC2 was significantly decreased in fetal membranes from pPROM subjects compared with PTB (P = 0.01), whereas the PGRMC1 level did not change (Figure 2C). In a stratified analysis based on early vs. late (before and after 34 WGA), both early <34 WGA and late >34 WGA pPROM membrane tissue expressed significantly less PGRMC1 (P = 0.02) and PGRMC2 (P = 0.009) compared with early PTB (Figure 2C). The data on PGRMC1 supported the prior reports by Feng et al. [17]. Our results confirmed that full fetal membranes from pPROM and PTB differentially expressed PGRMC1 and PGRMC2, and pPROM are associated with significant downregulation of PGRMC2 (Figure 2C).
Infectious and noninfectious OS induction and P4 effect on PGRMC expression in fetal membrane cells
After documenting significant PGRMC2 downregulation at term and pPROM fetal membrane tissue, we tested the hypothesis that OS at term and infectious or noninfectious OS in preterm may mediate a reduction of PGRMCs, potentially contributing to a withdrawal of P4’s function in fetal membranes. The OS conditions were recreated using CSE (1:50), a well-known OS inducer whose effect on fetal membrane cells in vitro mimics uterine conditions at TL [13, 14, 32]. To demonstrate the effect of OS on PGRMC 1 and 2 expressions, we used primary cells from fetal membranes (N = 5). Western blot analysis revealed differential expressions of PGRMC1 and PGRMC2 in AECs, AMC, and chorion cells (trophoblasts and mesenchymal cells) under OS. In AECs, CSE induced a significant reduction in PGRMC1, but not in PGRMC2, compared with the controls (CSE; P = 0.054) (Figure 3A and B). In AMCs, PGRMC1 was not affected by CSE (Figure 3C and D); however, AMCs treated with CSE showed a significant reduction in PGRMC2 expression (P = 0.01). In chorion cells, CSE significantly decreased both PGRMC1 (P = 0.006) and PGRMC2 (P = 0.03) compared with the control (Figure 3E and F). The above results confirmed our proposed hypothesis that OS exerts cell-specific and stimulant-dependent differential expression of PGRMCs. In summary, OS at term or preterm (as mimicked by CSE treatment in our study) downregulates PGRMC1 in both AECs and chorion cells, whereas it downregulates PGRMC2 in both AMCs and chorion cells.
To further evaluate the functional role of PGRMCs in P4 signaling, fetal membrane cells were co-treated with CSE + P4. In AECs, CSE + P4 co-treatment significantly upregulated PGRMC1 expression compared with CSE alone (P = 0.01) (Figure 3A and B). Although not significant, CSE + P4 co-treatment in AMCs increased both PGRMC1 and PGRMC2 compared with CSE alone (Figure 3C and D). This trend is interesting and suggests that under stress conditions, with weakened fetal membranes, P4 promotes its own receptor expression, likely to promote fetal membrane integrity. In chorion cells, co-treatment with P4 or P4 alone significantly downregulated PGRMC expression (PGRMC1: P = 0.007 and PGRMC2: P = 0.004) (Figure 3E and F). In summary, OS-induced changes are likely restorable on the amnion side, whereas P4 does not perform a similar function on the chorion side.
In addition, we also tested the ability of LPSs to mimic inflammatory conditions often associated with preterm. We have already reported that distinct stimuli produce OS responses that are different in fetal membrane cells. Conversely, LPS did not change the expression of PGRMC1 or PGRMC2 in AEC, AMC, or chorion cells (Supplementary Figure 3) compared with cell controls.
Confirming P4 binding to both PGRMC1 and PGRMC2 in all fetal membrane cell types
The functional properties of P4 rely on its binding to these receptors. To prove that P4 can bind to PGRMC1 and PGRMC2, a PLA was carried out in AECs, AMCs, and chorion cells after 48-h treatment with P4 (N = 3). Additional immunocytochemistry was conducted to validate the cell type (CK-18; red, vimentin-green, DAPI-blue (AECs-co-express, AMCs-dominate vimentin, low CK-18, chorion trophoblast-CK-18+, chorion mesenchymal cells-vimentin+)) of interest and the PLA primary antibodies (P4 (red) and PGRMC1 and PGRMC2 (green)). As an experimental control, internal kit controls for plus and minus PLA probes (which bind to the receptor and P4) were added to detect any nonspecific binding. The P4 bound directly to PGRMC1 and PGRMC2 in AECs (as seen by red dots), although it bound more to PGRMC2 (Figure 4A). The P4 bound directly to both PGRMC1 and PGRMC2, similar as in AMCs (Figure 4B). Chorion cells exhibited the most P4 receptor interactions compared with other fetal membrane cell types and showed equal binding to both receptors (Figure 4C).
Figure 4.
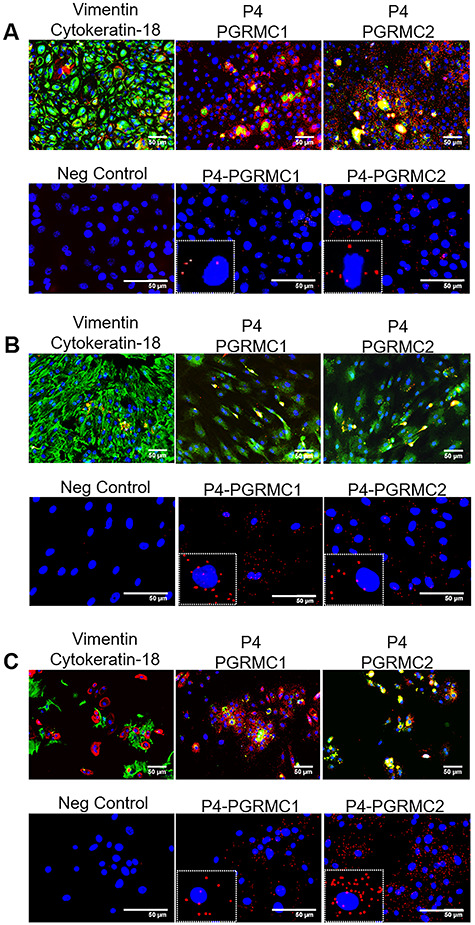
Proximity ligation assay shows P4 binding to both PGRMCs in all fetal membrane cell types. Top left panel: intermediate filament markers vimentin (mesenchymal marker; green) and cytokeratin-18 (epithelial marker; red) confirm primary cell type. Middle and left panel: immunocytochemistry controls validating P4 (red), PGRMC1 (green), and PGRMC2 (green) antibodies. Bottom left panel: positive and negative probes only without primary antibodies to act as a negative (Neg) control. (A) P4 bound directly to PGRMC1 and PGRMC2 in AECs (as seen by red dots); however, P4 binding was more significant to PGRMC2 (N = 3). (B) P4 bound directly to both PGRMC1 and PGRMC2, as in AMCs (N = 3). (C) Chorion cells exhibited the most P4 receptor interactions of all cells types, but did not significantly prefer PGRMC2 to PGRMC1. Scale bar is 50 μm (N = 3).
Inhibition of P4 release with OS
To determine whether fetal membrane cells can synthesize and secrete their own P4, we collected cell supernatant from AEC, AMC, and chorion cells after 6 days in culture (Figure 5A). All fetal membrane cells produced P4; however, chorion cells produced higher levels compared with AECs and AMCs (AEC: 0.6 ± 0.1 ng/mL, AMC: 2.5 ± 1.2 ng/mL, chorion: 58.8 ± 8.1 ng/mL). In addition, full fetal membrane explants secreted significant levels of P4, most likely produced by the underlying intact chorion layer (P = 0.0001) (chorion: 58.8 ± 8.1 ng/mL, decidua: 2.3 ± 0.1 ng/mL). After subjecting primary cells to OS conditions, CSE did not affect P4 production in AECs (Figure 5B), whereas CSE significantly decreased P4 release in AMCs (Figure 5C) (P = 0.039; control: 59.2 ± 8.43 pg/mL, CSE: 28.95 ± 12.5 pg/mL) and chorion cells (Figure 5D) (control: 1192 ± 401.6 pg/mL, CSE: 953.9 ± 350 pg/mL). This data shows that in fetal membranes, OS can induce local inhibition of P4 release at the cellular level.
Discussion
The function of P4 has hardly been studied in fetal membranes, partly because this tissue lacks nuclear receptors [10, 28] and hence is often not considered to have any specific role. Recent reports on PGRMC expression in fetal membranes have ignited renewed interest in studying the role of P4 in fetal membrane functions [7, 17, 18, 27, 28]. In this report, we tested the hypothesis that PGRMC expression in fetal membranes is cell type dependent, and its differential expression in response OS-associated in utero conditions, seen during parturition, causes their downregulation, contributing to fetal membrane dysfunction. The results presented here demonstrate the OS-induced cell type-dependent changes in PGRMCs, which could contribute to the disruption of human fetal membranes both at TL and in pPROM. Our study reports the following: (1) fetal membrane cells (AECs, AMCs, and chorion cells) are sources of P4; (2) fetal membrane cells express all four P4 membrane receptors and lack classic nuclear receptors; (3) PGRMC 1 and 2 are differentially expressed, whereas mPα and mPβ are constitutive; (4) PLA suggests that both PGRMC1 and PGRMC2 are very proximal to P4 receptors and likely to bind and have a functional role as reported in our prior reports; (5) changes in PGRMC 1 and 2 expressions are cell- and stimulant-dependent; (6) OS causes differential downregulation of PGRMCs in cells by reducing PGRMC1 in AECs, PGRMC2 in AMCs, and both of them in chorion cells; (7) OS-mediated effects in vitro are similar to those observed in fetal membranes from TL and pPROM conditions; and (8) OS decreases localized P4 production in AMCs and chorion cells. In summary, the cell- and receptor type-dependent P4 function is to maintain fetal membrane homeostasis. The OS at term or OS at pPROM, potentially due to infection or sterile inflammation, downregulates PGRMCs, which can interrupt the normal functions of the fetal membrane.
Our prior report has shown that the functional contributions of P4 strengthen the fetal membrane during pregnancy by promoting cellular transitions. This property is mediated through specific membrane receptors in each cell compartment, which we characterized in this study. Specifically, P4, through PGRMC1, maintains the epithelial state in AECs, and via PGRMC2, it promotes the transition of AMCs to become AECs [12]. P4 promotes this transition (mesenchymal - to - epithelial transition [MET]) [37, 39–41] through the activation of protooncogene c-MYC. This phenomenon has also been reported in other systems [19] as well as in ovine amnion cells [42].
In normal fetal membranes, the AEC:AMC ratio is ~10:1, and this ratio is maintained by the recycling of cells. The chorion of the fetal membranes produces large amount of P4 using substrates such as pregnenolone, pregnenolone sulfate, and 20 alpha-dihydroprogesterone than cholesterol as substrate [43] in utero. In addition, under term conditions, OS decreases P4 production, which could contribute to fetal membrane weakening. A steady supply of P4, either self-generated in addition to P4 supplied by the placenta, and the expression of specific receptors in each cell type are essential for chorion’s function as a barrier at the decidual interface. This is likely to minimize inflammation or to maintain immune homeostasis at the chorio-decidual interface constantly induced by resident decidual immune cells or by immunocompetent decidual cells. OS can cause functional P4 withdrawal by two mechanisms, primarily by reducing P4 production [44], as well as by downregulating its receptors. As reported already, OS at term induces fetal membrane senescence [34, 43], the accumulation of AMCs in the stroma, and inflammation, which are classic signs of a dysfunctional fetal membrane status. Decrease in endogenous P4 and reduction in PGRMCs are likely to aid this process at term, weakening the fetal membranes.
Mechanistically, downregulation of PGRMC2 causes accumulation of AMCs in the fetal membrane matrix region due to the lack of MET. This condition predisposes fetal membranes to weaken, as AMCs respond to OS much more rigorously, causing localized inflammation. The functional contributions of these receptors in chorion cells have not been tested in our laboratory; however, Feng et al. have shown that chorionic cells undergo senescence when PGRMC1s are downregulated [27–29]. This also occurs in response to OS. Thus, P4 plays a specific role in different cell layers of the fetal membranes, and P4 functional withdrawal is distinct than what is reported in myometrium. This difference could be due to the lack of classical nuclear PRs in the fetal membranes, whereas myometrial transitions (quiescence to activation) are primarily reported through P4’s action via nuclear receptors. One of the limitations of this study is that this is a descriptive study and not mechanistic or comprehensive. The role of other P4 receptors along with glucocorticoid receptor (GR) should be evaluated to fully ascertain functional role of P4 receptors and P4 in fetal membranes.
Clinically, these findings are relevant, specifically in adverse pregnancy outcomes such as pPROM, a condition associated with OS [13, 44]. The P4 and PGRMC downregulations are features of OS and identifying a woman with high risk status may benefit strategies that will induce P4 and PGRMCs. However, P4 or 17-OHP supplementation has not been successful in pPROM, as P4 alone is insufficient to produce the desired functional effect without an adequate expression of PGRMCs. Minimizing OS and OS-induced damages are better strategies than P4 supplementation, as P4 alone is not sufficient to increase receptor expressions throughout the fetal membrane cells or to produce P4–PGRMC-mediated beneficial effects. TL fetal membrane data support this hypothesis, as transition from not in labor to labor is associated with increased OS and, likely, OS-induced P4 and PGRMC downregulations. The OS-induced P4 effect is not an isolated event by P4; OS causes a transforming growth factor beta increase in fetal membrane cells and promotes EMT [33]. This phenomenon is well reported in nonlabor to labor transitions [33, 45, 46]. Therefore, P4 withdrawal can provide an additive effect to ensure fetal membrane dysfunction, required at term delivery.
Our data on PGRMC expressions are supportive of most recent reports on the expression of fetal membrane PGRMCs. We were able to accomplish this study using well-characterized primary cell types isolated from fetal membranes. However, our study has a few limitations. Here our study focuses on P4, instead of P4 interaction with other pregnancy-related hormones such as estrogen, androgen, Human chorionic gonadotropin (HCG), and relaxin. Although PLA is not an evidence for P4–PGRMC binding and signaling, multiple reports have shown PGRMC-mediated signaling in various systems, including pregnancy [7, 18, 19, 25–28, 47]. Gene silencing and over-expression of PGRMCs have shown the impact of P4 on fetal membrane and other cell types, further supporting the concept that such receptor–ligand interactions are functionally relevant. This is not a mechanistic study, but mostly a descriptive work based on expression profiles of P4 and its membrane receptors. However, our prior mechanistic data are supportive of the data presented here.
Supplementary Material
Grant Support: L.R. was a fellow in the Environmental Toxicology Training Program (T32ES007254), which is supported by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) of the United States and administered through the University of Texas Medical Branch in Galveston, TX, USA.
This study is partly supported by the National Institute of Child Health and Human Development (NICHD) grant (grant number 1R03HD098469-01) to R.M.
Contributor Information
Violetta Lozovyy, Division of Maternal-Fetal Medicine & Perinatal Research, Department of Obstetrics & Gynecology, The University of Texas Medical Branch at Galveston, Galveston, TX, USA.
Lauren Richardson, Division of Maternal-Fetal Medicine & Perinatal Research, Department of Obstetrics & Gynecology, The University of Texas Medical Branch at Galveston, Galveston, TX, USA.
George Saade, Division of Maternal-Fetal Medicine & Perinatal Research, Department of Obstetrics & Gynecology, The University of Texas Medical Branch at Galveston, Galveston, TX, USA.
Ramkumar Menon, Division of Maternal-Fetal Medicine & Perinatal Research, Department of Obstetrics & Gynecology, The University of Texas Medical Branch at Galveston, Galveston, TX, USA.
Data Availability
Data will be made available upon request.
Authors’ Contributions
V.L. and L.R. conducted experiments. V.L. and L.R. performed data analysis and drafted the manuscript. G.S. helped with overall experimental concept. R.M. conceived the project, designed experiments, provided funding and helped with data analysis and interpretation, and prepared the manuscript.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, Saade GR, Menon R. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol 2015; 213:359.e1–359.e16. [DOI] [PubMed] [Google Scholar]
- 3. Mogami H, Kishore AH, Word RA. Collagen type 1 accelerates healing of ruptured fetal membranes. Sci Rep 2018; 8:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caughey AB, Robinson JN, Norwitz ER. Contemporary diagnosis and management of preterm premature rupture of membranes. Rev Obstet Gynecol 2008; 1:11–22. [PMC free article] [PubMed] [Google Scholar]
- 5. Richardson LS, Vargas G, Brown T, Ochoa L, Sheller-Miller S, Saade GR, Taylor RN, Menon R. Discovery and characterization of human amniochorionic membrane microfractures. Am J Pathol 2017; 187:2821–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menon R, Richardson LS. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin Perinatol 2017; 41:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ackerman WET, Summerfield TL, Mesiano S, Schatz F, Lockwood CJ, Kniss DA. Agonist-dependent downregulation of progesterone receptors in human cervical stromal fibroblasts. Reprod Sci 2016; 23:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun 2016; 7:11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merlino A, Welsh T, Erdonmez T, Madsen G, Zakar T, Smith R, Mercer B, Mesiano S. Nuclear progesterone receptor expression in the human fetal membranes and decidua at term before and after labor. Reprod Sci 2009; 16:357–363. [DOI] [PubMed] [Google Scholar]
- 10. Kumar D, Springel E, Moore RM, Mercer BM, Philipson E, Mansour JM, Mesiano S, Schatz F, Lockwood CJ, Moore JJ. Progesterone inhibits in vitro fetal membrane weakening. Am J Obstet Gynecol 2015; 213:520.e1–520.e9. [DOI] [PubMed] [Google Scholar]
- 11. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002; 87:2924–2930. [DOI] [PubMed] [Google Scholar]
- 12. Richardson LS, Taylor RN, Menon R. Reversible EMT and MET mediate amnion remodeling during pregnancy and labor. Sci Signal 2020; 13:eaay1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol 2014; 184:1740–1751. [DOI] [PubMed] [Google Scholar]
- 14. Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, Papaconstantinou J, Taylor RN. Senescence of primary amniotic cells via oxidative DNA damage. PLoS One 2013; 8:e83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixon CL, Richardson L, Sheller-Miller S, Saade G, Menon R. A distinct mechanism of senescence activation in amnion epithelial cells by infection, inflammation, and oxidative stress. Am J Reprod Immunol 2017; 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayad MT, Taylor BD, Menon R. Regulation of p38 mitogen-activated kinase-mediated fetal membrane senescence by statins. Am J Reprod Immunol 2018; 80:e12999. [DOI] [PubMed] [Google Scholar]
- 17. Feng L, Antczak BC, Lan L, Grotegut CA, Thompson JL, Allen TK, Murtha AP. Progesterone receptor membrane component 1 (PGRMC1) expression in fetal membranes among women with preterm premature rupture of the membranes (PPROM). Placenta 2014; 35:331–333. [DOI] [PubMed] [Google Scholar]
- 18. Cahill MA The evolutionary appearance of signaling motifs in PGRMC1. Biosci Trends 2017; 11:179–192. [DOI] [PubMed] [Google Scholar]
- 19. Zhu X, Ji M, Han Y, Guo Y, Zhu W, Gao F, Yang X, Zhang C. PGRMC1-dependent autophagy by hyperoside induces apoptosis and sensitizes ovarian cancer cells to cisplatin treatment. Int J Oncol 2017; 50:835–846. [DOI] [PubMed] [Google Scholar]
- 20. Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology 2009; 150:3186–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang R, Sheehan PM, Brennecke SP. Changes in myometrial expression of progesterone receptor membrane components 1 and 2 are associated with human parturition at term. Reprod Fertil Dev 2016; 28:618–627. [DOI] [PubMed] [Google Scholar]
- 22. Wu W, Shi SQ, Huang HJ, Balducci J, Garfield RE. Changes in PGRMC1, a potential progesterone receptor, in human myometrium during pregnancy and labour at term and preterm. Mol Hum Reprod 2011; 17:233–242. [DOI] [PubMed] [Google Scholar]
- 23. Peluso JJ Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin Reprod Med 2007; 25:198–207. [DOI] [PubMed] [Google Scholar]
- 24. Peluso JJ Progesterone receptor membrane component 1 and its role in ovarian follicle growth. Front Neurosci 2013; 7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cahill MA Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol 2007; 105:16–36. [DOI] [PubMed] [Google Scholar]
- 26. Wendler A, Wehling M. PGRMC2, a yet uncharacterized protein with potential as tumor suppressor, migration inhibitor, and regulator of cytochrome P450 enzyme activity. Steroids 2013; 78:555–558. [DOI] [PubMed] [Google Scholar]
- 27. Feng L, Allen TK, Marinello WP, Murtha AP. Roles of progesterone receptor membrane component 1 in oxidative stress-induced aging in chorion cells. Reprod Sci 2018; 26:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meng Y, Murtha AP, Feng L. Progesterone, inflammatory cytokine (TNF-alpha), and oxidative stress (H2O2) regulate progesterone receptor membrane component 1 expression in fetal membrane cells. Reprod Sci 2016; 23:1168–1178. [DOI] [PubMed] [Google Scholar]
- 29. Allen TK, Feng L, Nazzal M, Grotegut CA, Buhimschi IA, Murtha AP. The effect of progestins on tumor necrosis factor alpha-induced matrix metalloproteinase-9 activity and gene expression in human primary amnion and chorion cells in vitro. Anesth Analg 2015; 120:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murtha AP, Feng L, Yonish B, Leppert PC, Schomberg DW. Progesterone protects fetal chorion and maternal decidua cells from calcium-induced death. Am J Obstet Gynecol 2007; 196:257.e1–257.e5. [DOI] [PubMed] [Google Scholar]
- 31. Richardson L, Menon R. Proliferative, migratory, and transition properties reveal metastate of human amnion cells. Am J Pathol 2018; 188:2004–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sheller S, Papaconstantinou J, Urrabaz-Garza R, Richardson L, Saade G, Salomon C, Menon R. Amnion-epithelial-cell-derived exosomes demonstrate physiologic state of cell under oxidative stress. PLoS One 2016; 11:e0157614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richardson L, Dixon CL, Aguilera-Aguirre L, Menon R. Oxidative stress-induced TGF-beta/TAB1-mediated p38MAPK activation in human amnion epithelial cells. Biol Reprod 2018; 99:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin J, Richardson L, Sheller-Miller S, Zhong N, Menon R. Oxidative stress induces p38MAPK-dependent senescence in the feto-maternal interface cells. Placenta 2018; 67:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sato BL, Collier ES, Vermudez SA, Junker AD, Kendal-Wright CE. Human amnion mesenchymal cells are pro-inflammatory when activated by the Toll-like receptor 2/6 ligand, macrophage-activating lipoprotein-2. Placenta 2016; 44:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richardson L, Jeong S, Kim S, Han A, Menon R. Amnion membrane organ-on-chip: an innovative approach to study cellular interactions. FASEB J 2019; 33:8945–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Canciello A, Russo V, Berardinelli P, Bernabò N, Muttini A, Mattioli M, Barboni B. Progesterone prevents epithelial-mesenchymal transition of ovine amniotic epithelial cells and enhances their immunomodulatory properties. Sci Rep 2017; 7:3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oh SY, Kim CJ, Park I, Romero R, Sohn Y-K, Moon KC, Yoon BH. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am J Obstet Gynecol 2005; 193:1156–1160. [DOI] [PubMed] [Google Scholar]
- 39. van der Horst PH, Wang Y, Vandenput I, Kühne LC, Ewing PC, van Ijcken WFJ, van der Zee M, Amant F, Burger CW, Blok LJ. Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS One 2012; 7:e30840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chaffer C Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs 2007; 7–19. [DOI] [PubMed] [Google Scholar]
- 41. Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res 2006; 66:11271–11278. [DOI] [PubMed] [Google Scholar]
- 42. Stinson LF, Ireland DJ, Kemp MW, Payne MS, Stock SJ, Newnham JP, Keelan JA. Effects of cytokine-suppressive anti-inflammatory drugs on inflammatory activation in ex vivo human and ovine fetal membranes. Reproduction 2014; 147:313–320. [DOI] [PubMed] [Google Scholar]
- 43. Menon R, Richardson LS, Lappas M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta 2018; 79:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Menon R Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol 2014; 5:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mogami H, Hari Kishore A, Akgul Y, Word RA. Healing of preterm ruptured fetal membranes. Sci Rep 2017; 7:13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Janzen C, Sen S, Lei MY, Gagliardi de Assumpcao M, Challis J, Chaudhuri G. The role of epithelial to mesenchymal transition in human amniotic membrane rupture. J Clin Endocrinol Metab 2017; 102:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peluso J Progesterone receptor membrane component 1 and its role in ovarian follicle growth. Front Neurosci 2013; 7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.


