Abstract
Objective
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains pandemic with considerable morbidity and mortality around the world. The aim of this study was to identify the predictors for clinical deterioration in patients with COVID-19 who did not show clinical deterioration upon hospital admission.
Methods
Two hundred fifty-seven patients with confirmed COVID-19 pneumonia admitted to Guangzhou Eighth People’s Hospital between 23 January and 21 March 2020 were retrospectively enrolled. Demographic data, symptoms, laboratory values, comorbidities and treatments were all collected. The study endpoint was clinical deterioration within 20 days from hospital admission. Univariate and multivariable logistic regression methods were used to explore the risk factors associated with clinical deterioration.
Results
A total of 49 (19%) patients showed clinical deterioration after admission. Compared with patients that did not experience clinical deterioration, clinically deteriorated patients had more dyspnea, cough and myalgia (65.3% versus 29.3%) symptoms and more had comorbidities (89.8% versus 36.1%). Clinical and laboratory characteristics at admission that were associated with clinical deterioration included senior age, diabetes, hypertension, myalgia, higher temperature, systolic blood pressure, C-reactive protein (CRP), procalcitonin, activated partial thromboplastin time, aspartate aminotransferase, alanine transaminase, direct bilirubin, plasma creatinine, lymphocytopenia, thrombocytopenia, decreased albumin and bicarbonate concentration. Medical history of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, calcium channel blockers and metformin were also risk factors.
Conclusion
The four best predictors for clinical deterioration were CRP, procalcitonin, age and albumin. A “best” multivariable prediction model, resulting from using a variable selection procedure, included senior age, presentation with myalgia, and higher level of CRP and serum creatinine (bias-corrected c-statistic = 0.909). Sensitivity and specificity corresponding to a cut point of CRP ≥18.45 mg/L for predicting clinical deterioration were 85% and 74%, respectively.
Keywords: COVID-19, predictor, clinical deterioration, myalgia, age, CRP, serum creatinine
Introduction
Coronavirus disease 2019 (COVID-19) is a newly recognized infectious disease that has spread rapidly throughout the world. So far, the number of COVID-19 infections and deaths has been increasing1–4. Outbreaks have led to shortage of medical resources and an impending economic crisis and recession.
Previous studies have been describing the epidemiological characteristics and the factors affecting the prognosis of patients with COVID-19, include age, hypertension, lymphopenia5–7, etc. However, the majority of these studies have focused on risk factors of mortality in COVID-19 patients, while ignoring risk factors leading to its clinical deterioration. Moreover, a lack of blood gas analysis results at admission is common. Acute Respiratory Distress Syndrome (ARDS) is one of the most important clinical manifestations of COVID-19, and thus several parameters derived from blood gas analysis at admission may be able to predict prognosis. In addition, the clinical manifestations of COVID-19 patients are diverse, family management of mildly infected patients has little effect, and some patients’ condition deteriorates suddenly, which increases the difficulty of epidemic control. Few studies have shown whether these symptoms provide prognostic information for patients with mild symptoms. Therefore, in order to give timely and appropriate treatment and allocate medical resources more rationally, it is particularly important to identify potentially severely deteriorating patients by combining clinical manifestations and laboratory tests.
This study retrospectively collected information on the non-severe-deterioration COVID-19 patients admitted from January to March in Guangzhou. It focuses on the risk factors leading to clinical deterioration in those patients, aiming to identify predictors of clinical deterioration in initially non-severe-deterioration patients upon admission, so as to help fight the epidemic.
Methods
Study population
We collected the data of 257 consecutive non-severe-deterioration patients with laboratory-confirmed SARS-CoV-2 infection admitted to Guangzhou Eighth People’s Hospital, a certified COVID-19 tertiary care hospital and designated center in Guangdong province, between 21 January and 23 March 2020. Patients were followed for 20 days after admission. Laboratory-confirmed cases were defined by a positive result on high-throughput sequencing or real-time reverse-transcriptase polymerase chain reaction (PCR) assay of nasal and/or pharyngeal swabs. This study was approved by the institutional review board at Guangzhou Eighth People’s Hospital. Written informed consent was waived by the Ethics Commission of the designated hospital for emerging infectious diseases.
Data collection
Date collected included patient demographic information, comorbidities, home medications, initial laboratory test, inpatient medications, treatments (including invasive mechanical ventilation and extracorporeal membrane oxygenation [ECMO]) and outcomes (including length of stay, discharge, clinical deterioration and mortality).
Study definitions
The diagnosis of severe clinical deterioration was made when one or more of the following conditions occurred: (1) shortness of breath, with respiratory rates (RR) ≥30 times/min; (2) arterial oxygen saturation (resting status) ≤93%; or (3) ratio of partial pressure of oxygen to fraction of inspiration O2 (PaO2/FiO2) ≤300 mmHg. Non-severe clinical deterioration was defined as not meeting any of the criteria for the diagnosis of severe clinical deterioration.
Acute kidney injury was defined by: (1) an increase in serum creatinine ≥0.3 mg/dL (27 μmol/L) within 48 h; (2) an increase to ≥1.5 times the presumed baseline value that is known or presumed to have occurred within the prior 7 days; or (3) a decrease in urine volume to <0.5 mL/kg/h over 6 h. Acute liver injury was defined by: (1) alanine transaminase (ALT) >3 times the upper limit of normal; (2) serum alkaline phosphatase (ALP) >2 times the upper limit of normal; or (3) total bilirubin >2 times the upper limit of normal. Acute heart failure (AHF) was defined by: (1) rapid onset or worsening of symptoms or signs of HF with elevated brain natriuretic peptide (BNP) or N-terminal pro brain natriuretic peptide (NT-proBNP) (if tested) during the study period.
Statistical analysis
Continuous variables are expressed as means ± SD. Time from illness onset to hospital admission are described as median (IQR). Categorical variables are expressed as frequencies (percentages). Continuous variables were compared using the Mann–Whitney U test and categorical variables compared using the chi-squared test or Fisher’s exact test where appropriate.
For analyses the outcome was clinical deterioration (yes/no) within 20 days from hospital admission. Univariate logistic regression was performed to estimate odds ratios and corresponding p values for predictors. For each predictor, we also report the c-statistic8, which is the same as area under the ROC curve (AUC)9 that treats the predictor variable as the decision variable for classifying patients as belonging to the deterioration or non-deterioration groups. The c-statistic estimates the probability that for a randomly selected pair of patients, one from the deterioration group and one from the non-deterioration group, the patient in the deterioration group will have a higher (lower) value of the decision variable, assuming that higher values are associated with higher (lower) likelihood of deterioration. We also constructed an ROC curve for the most significant single predictor to illustrate its ability to classify patients according to deteriorated status.
We used backward elimination variable selection to arrive at a final “best” multivariable logistic regression model for predicting severe clinical deterioration. For the backward elimination procedure, candidate variables consisted of those that showed a significant association (p < .05) in univariate logistic regression analysis. We present bias-corrected c-statistic and the odds ratios (with 95% confidence intervals) for the final model8.
We also performed a time-to-event analysis, where time was defined as the time from hospital admission until the date of clinical deterioration. In the non-deterioration group, all patients were followed up for 20 days whether discharged within 20 days or not. Times for patients discharged later than 20 days and without having experienced clinical deterioration in the first 20 days were censored at 20 days.
Kaplan–Meier estimates of the probability that a randomly selected patient did not experience clinical deterioration as a function of post-admission time were computed, and the resulting curves (probability versus time) were plotted to compare time-until-clinical-deterioration distributions for groups of patients defined by values of the predictors in the final backward-elimination model, with the time-until-event distributions compared by the log-rank test for each predictor.
Statistical analyses were done using SPSS software (version 13) unless otherwise indicated. A two-sided alpha of less than .05 was considered statistically significant. The bias-corrected c-statistic was computed using the R software package rms8.
Results
A total of 257 patients were enrolled after admission from Guangzhou. For analyses patients were classified into two groups, those that experienced clinical deterioration (“severe” patients) and those that did not experience clinical deterioration (“non-severe” patients). The mean age of 257 patients was 46 years old and 140 (54%) were male. Comorbidities were present in one third of patients, with hypertension being the most common comorbidity, followed by diabetes and coronary heart disease (Table 1). The most common symptom on admission was cough, followed by myalgia and sputum production (Table 1).
Table 1.
Demographic data of patients on admission.
| Total (n = 257) | Non-deterioration (n = 208) | Deterioration (n = 49) | p Value | |
|---|---|---|---|---|
| Age, years | 46 ± 17 | 43 ± 16 | 60 ± 13) | <.001** |
| Sex | ||||
| Male | 140 (54) | 111 (43) | 29 (11) | .462 |
| Length of stay | 18 ± 10 | 17 ± 8 | 25 ± 16 | <.001** |
| Current smoker | 27 (10.5) | 21 (10.1) | 6 (12.2) | .659 |
| Signs and symptoms at admission | ||||
| Cough | 138 (63.7) | 106 (51) | 32 (65.3) | .07 |
| Sputum | 81 (31.5) | 61 (29.3) | 20 (40.8) | .119 |
| Myalgia | 93 (36.2) | 61 (29.3) | 32 (65.3) | <.001** |
| Headache | 34 (13.2) | 25 (12) | 9 (18.4) | .238 |
| Rhinorrhea | 17 (6.6) | 13 (6.3) | 4 (8.2) | .748 |
| Dyspnea | 43 (16.7) | 25 (12) | 18 (36.7) | <.001** |
| Comorbidity | ||||
| Hypertension | 53 (20.6) | 33 (15.9) | 20 (40.8) | <.001** |
| Diabetes | 15 (5.8) | 9 (4.3) | 6 (12.2) | .045* |
| Coronary heart disease | 16 (6.2) | 10 (4.8) | 6 (12.2) | .091 |
| Cerebrovascular disease | 8 (3.1) | 5 (2.4) | 3 (6.1) | .18 |
| Chronic obstructive lung disease | 2 (0.8) | 1 (0.5) | 1 (2) | .346 |
| Carcinoma | 6 (2.3) | 3 (1.4) | 3 (6.1) | .085 |
| Chronic kidney disease | 4 (1.6) | 2 (1) | 2 (4.1) | .165 |
| Hepatitis B | 12 (4.7) | 9 (4.3) | 3 (6.1) | .705 |
| Tuberculosis | 3 (1.2) | 3 (1.4) | 0 | 1 |
| Medical history | ||||
| ACEI/ARB | 26 (10.1) | 16 (7.7) | 10 (20.4) | .008* |
| CCB | 32 (12.5) | 21 (10.1) | 11 (22.4) | .018* |
| Diuretic | 3 (1.2) | 2 (1) | 1 (2) | .471 |
| β-blocker | 15 (5.8) | 11 (5.3) | 4 (8.2) | .496 |
| Metformin | 9 (3.5) | 5 (2.4) | 4 (8.2) | .07 |
| Vital signs at admission | ||||
| Respiratory rate | 19 ± 2 | 19 ± 2 | 20 ± 2 | <.001** |
| Fever (temperature ≥ 37.3 °C) | 156 (60.7) | 116 (55.8) | 40 (81.6) | <.001** |
| Pulse, beats per min | 86 ± 13 | 86 ± 13 | 87 ± 14 | .65 |
| Systolic blood pressure, mmHg | 126 ± 18 | 124 ± 17 | 135 ± 19 | .001** |
| Diastolic blood pressure, mmHg | 82 ± 11 | 82 ± 10 | 83 ± 14 | .29 |
Summary statistics are means ± SD or n (%). p Values were calculated by Mann–Whitney U test, χ² test, or Fisher’s exact test, as appropriate.
Abbreviations. ACEI, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin receptor blocker; CCB, Calcium channel blocker.
*p ≤ .05; **p ≤ .001.
The study endpoint of clinical deterioration occurred in 49 patients (19.1%), including 37 (75.5%) who were admitted to the intensive care unit, 1 (2.04%) who died and 11 who transferred to another hospital. The recovery rate in our hospital is about 95.3%. All patients in the deterioration group experienced clinical deterioration within 20 days and were deteriorated before discharge. None was censored in this group. In the non-deterioration group, 137 of the 208 patients were discharged within 20 days after admission, all these patients recovered without having experienced clinical deterioration at 20 days after admission. Thus, they were all censored at 20 days.
On admission most severe patients presented with abnormal respiratory rate, abnormal systolic blood pressure (SBP) and higher values of these laboratory results: leucocyte count, neutrophil count, levels of C-reactive protein (CRP), aspartate aminotransferase (AST), ALT, albumin, BNP, cardiac troponin I, D-dimer and procalcitonin (PCT). Moreover, these patients had significantly lower lymphocyte count and levels of bicarbonate concentration (HCO3) (all p < .05, Tables 1 and 2).
Table 2.
Laboratory markers at admission.
| Total (n = 257) | Non-deterioration (n = 208) | Deterioration (n = 49) | p Value | |
|---|---|---|---|---|
| Complete blood count (n = 257, 208 vs 49) | ||||
| White blood cell count × 109/L | 5.05 ± 1.89 | 4.85 ± 1.59 | 5.88 ± 2.67 | .016* |
| Lymphocyte count, × 109/L | 1.57 ± 2.38 | 1.70 ± 2.62 | 1.05 ± 0.51 | <.001** |
| Neutrophil count × 109/L | 3.33 ± 2.97 | 3.07 ± 3.00 | 3.07 ± 3.00 | <.001** |
| Red blood cell count × 1012/L | 4.53 ± 0.88 | 4.59 ± 0.92 | 4.29 ± 0.60 | .012* |
| Platelet count, × 109/L | 191.09 ± 60.21 | 194.90 ± 60.26 | 174.90 ± 57.80 | .016* |
| Inflammatory marker | ||||
| C-reactive protein, mg/L (n = 204, 161 vs 43) | 19.12 ± 22.27 | 13.58 ± 17.70 | 39.85 ± 25.41 | <.001** |
| Procalcitonin, ng/mL (n = 248, 201 vs 47) | 0.07 ± 0.10 | 0.06 ± 0.90 | 0.14 ± 0.12 | <.001** |
| Liver function test | ||||
| ALT, U/L (n = 257, 208 vs 49) | 25.08 ± 20.68 | 23.16 ± 16.34 | 33.23 ± 32.33 | .014* |
| AST, U/L (n = 257, 208 vs 49) | 24.65 ± 16.43 | 21.95 ± 11.42 | 36.11 ± 26.66 | <.001** |
| Albumin, g/L (n = 237, 197 vs 40) | 41.80 ± 21.93 | 43.00 ± 23.76 | 35.88 ± 5.53 | <.001** |
| Total bilirubin, μmol/L (n = 232, 193 vs 39) | 13.53 ± 46.71 | 13.96 ± 51.10 | 11.40 ± 7.84 | .729 |
| Direct bilirubin, μmol/L (n = 220, 183 vs 37) | 4.42 ± 2.89 | 4.21 ± 2.46 | 5.47 ± 4.35 | .078 |
| Renal function test | ||||
| Creatinine, μmol/L (n = 256, 207 vs 49) | 66.98 ± 28.28 | 63.87 ± 19.82 | 80.13 ± 48.44 | .095 |
| Cardiac markers | ||||
| CKMB, U/L (n = 245, 199 vs 46) | 11.83 ± 7.69 | 11.62 ± 5.94 | 12.76 ± 12.80 | .823 |
| Cardiac troponin I, ng/mL (n = 213, 175 vs 38) | 0.06 ± 0.54 | 0.05 ± 0.56 | 0.10 ± 0.47 | <.001** |
| Brain natriuretic peptide, pg/mL (n = 44, 21 vs 23) | 101.16 ± 170.64 | 35.57 ± 34.75 | 161.04 ± 219.00 | .002* |
| Coagulation test | ||||
| Prothrombin time, s (n = 256, 207 vs 49) | 13.54 ± 1.06 | 13.55 ± 0.98 | 13.52 ± 1.36 | .42 |
| APTT, s (n = 256, 207 vs 49) | 39.30 ± 4.49 | 38.94 ± 4.09 | 40.80 ± 5.69 | .010* |
| D-dimer, μg/L (n = 253, 204 vs 49) | 1880.28 ± 4617.27 | 1533.63 ± 3085.00 | 3323.47 ± 8308.95 | .001** |
| Arterial blood gas analysis | ||||
| Arterial blood PH (n = 240, 194 vs 46) | 7.39 ± 0.10 | 7.38 ± 0.10 | 7.40 ± 0.05 | .019* |
| PaO2, mmHg (n = 240, 194 vs 46) | 96.22 ± 32.21 | 100.6 ± 32.36 | 77.75 ± 24.25 | <.001** |
| SaO2, % (n = 240, 194 vs 46) | 95.61 ± 8.57 | 96.04 ± 8.54 | 93.78 ± 8.58 | <.001** |
| PaCO2, mmHg (n = 240, 194 vs 46) | 41.91 ± 19.37 | 42.18 ± 16.51 | 40.76 ± 28.67 | <.001** |
| HCO3, mmol/L (n = 238, 193 vs 45) | 24.50 ± 2.60 | 24.86 ± 2.13 | 22.95 ± 3.69 | .003* |
| Lactin, mmol/L (n = 231, 187 vs 44) | 1.83 ± 0.92 | 1.79 ± 0.86 | 2.00 ± 1.12 | .259 |
Summary statistics are means ± SD or n (%). p Values were calculated by Mann–Whitney U test, χ² test, or Fisher’s exact test, as appropriate.
Abbreviations. ALT, Alanine aminotransferase; AST, Aspartate transaminase; CKMB, Creatine phosphokinase-Mb; APTT, Activated partial thromboplastin time; PaO2, Partial pressure of oxygen; SaO2, Oxygen saturation; PaCO2, Partial pressure of carbon dioxide; HCO3, Bicarbonate concentration.
*p ≤ .05; **p ≤ .001.
Thirteen (5.1%) patients required invasive mechanical ventilation, including four patients that required ECMO. Two hundred eleven (82.1%) patients received antibiotics and 187 (72.8%) received antivirals. Acute liver injury was the most frequently observed complication, followed by acute kidney injury, acute heart failure and disseminated intravascular coagulation (DIC). Compared with non-severe patients, severe patients more often received mechanical ventilation and ECMO. The usage of antiviral treatment, intravenous immunoglobulin, glucocorticoids and antibiotics was higher among the patients who deteriorated (Table 3).
Table 3.
Treatment and outcome descriptive statistics.
| Total (n = 257) | Non-deterioration (n = 208) | Deterioration (n = 49) | |
|---|---|---|---|
| Time from illness onset to hospital admission, days | 3 (2–7) | 3 (1–6) | 5 (2.5–8.5) |
| Invasive mechanical ventilation | 13 (5.1) | 0 | 13 (27.1) |
| ECMO | 4 (1.6) | 0 | 4 (9.8) |
| Antiviral treatment | 187 (72.8) | 144 (69.2) | 43 (87.8) |
| Antibiotic | 211 (82.1) | 163 (78.4) | 48 (98) |
| Glucocorticoids | 62 (24.1) | 30 (14.4) | 32 (65.3) |
| Immunoglobulin therapy | 42 (16.3) | 12 (5.8) | 30 (61.2) |
| Acute kidney injury | 9 (3.5) | 2 (1) | 7 (14.3) |
| Acute liver injury | 22 (8.6) | 10 (4.8) | 12 (24.5) |
| Acute heart failure | 8 (3.1) | 1 (0.5) | 7 (14.3) |
| DIC | 1 (0.4) | 0 (0) | 1 (2) |
Summary statistics are median (IQR) or n (%).
Abbreviations. ECMO, Extracorporeal membrane oxygenation; DIC, Disseminated intravascular coagulation.
In univariable logistic regression, 21 clinical and laboratory characteristics at admission were associated with clinical deterioration, including advanced age, diabetes, hypertension, myalgia, higher temperature, SBP, CRP, procalcitonin, APTT, AST, ALT, direct bilirubin, plasma creatinine, lymphocytopenia, thrombocytopenia, decreased albumin and HCO3. Medical histories of ACEI/ARB, CCB or metformin were also risk factors (Table 4). Of these predictors, CRP had the highest c-statistic (0.833), and thus the highest discrimination ability, followed by procalcitonin (0.799), age (0.786) and albumin (0.7820). All other predictors had c-statistics ≤0.721.
Table 4.
Risk factors associated with in-hospital aggravation.
| c-Statistic | OR (95% CI) | p | |
|---|---|---|---|
| CRP (per 1 mg/L increase) | 0.833 | 1.053 (1.033–1.072) | <.001** |
| Procalcitonin (per 1 ng/L increase) | 0.799 | 1.008 (1.004–1.013) | <.001** |
| Age (per 10 years) | 0.786 | 2.043 (1.588–2.628) | <.001** |
| Albumin (per 5 g/L increase) | 0.782 | 0.291 (0.181–0.466) | <.001** |
| Lymphocyte count (per 1 × 109/L increase) | 0.721 | 0.202 (0.096–0.429) | <.001** |
| AST (per 10 U/L increase) | 0.692 | 1.513 (1.215–1.885) | <.001** |
| Myalgia | 0.687 | 4.820 (2.422–9.589) | <.001** |
| Temperature (per 1 °C increase) | 0.658 | 1.904 (1.310–2.769) | .001* |
| Systolic blood pressure (per 10 mmHg increase) | 0.653 | 1.393 (1.151–1.687) | .001* |
| HCO3 (per 1 mmol/L increase) | 0.630 | 0.787 (0.696–0.890) | <.001** |
| Platelet count (per 1 × 109/L increase) | 0.618 | 0.993 (0.987–0.999) | .021* |
| APTT (per 1 s increase) | 0.613 | 1.094 (1.017–1.176) | .015* |
| RBC (per 1 × 1012/L increase) | 0.626 | 0.407 (0.219–0.758) | .005* |
| Hypertension | 0.610 | 3.22 (1.586–6.537) | .001* |
| ALT (per 10 U/L increase) | 0.600 | 1.165 (1.008–1.346) | .039 * |
| Direct bilirubin (per 1 μmol/L increase) | 0.605 | 1.137 (1.021–1.267) | .019* |
| Creatinine (per 1 μmol/L increase) | 0.587 | 1.019 (1.008–1.030) | .001* |
| CCB | 0.561 | 2.544 (1.104–5.864) | .028* |
| ACEI/ARB | 0.550 | 2.595 (1.035–6.502) | .042* |
| Diabetes | 0.545 | 3.402 (1.145–10.103) | .027* |
| Metformin | 0.532 | 3.961 (1.020–15.368) | .047* |
Abbreviations. OR, Odds ratio; AST, Aspartate transaminase ; ALT, Alanine aminotransferase; ALB, Albumin; HCO3, Bicarbonate concentration; APTT, Activated partial thromboplastin time.
*p ≤ .05; **p ≤ .001.
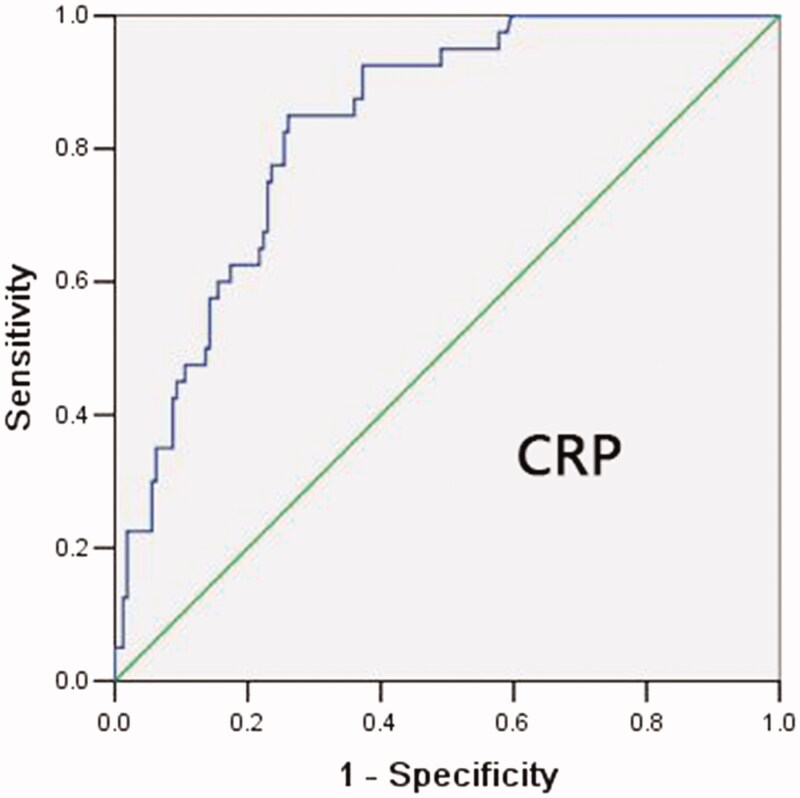
The ROC curve is shown in Figure 1. Each point on the ROC curve provides sensitivity and 1 − specificity levels resulting from classifying patients as experiencing severe versus non-severe deterioration using a particular cutoff value for CRP. For instance, a value of 18.45 mg/L for CRP corresponds to the ROC curve point of (1 − specificity = 0.26, sensitivity = 0.85).
Figure 1.
ROC curve for CRP as a predictor of severe COVID-19 during first 20 days after admission. Each point on the ROC curve provides sensitivity and 1 − specificity levels resulting from classifying patients as experiencing severe versus non-severe deterioration using a particular cutoff value for CRP. For instance, a value of 18.45 mg/L for CRP corresponds to the ROC curve point of (1 − specificity = 0.26, sensitivity = 0.85).
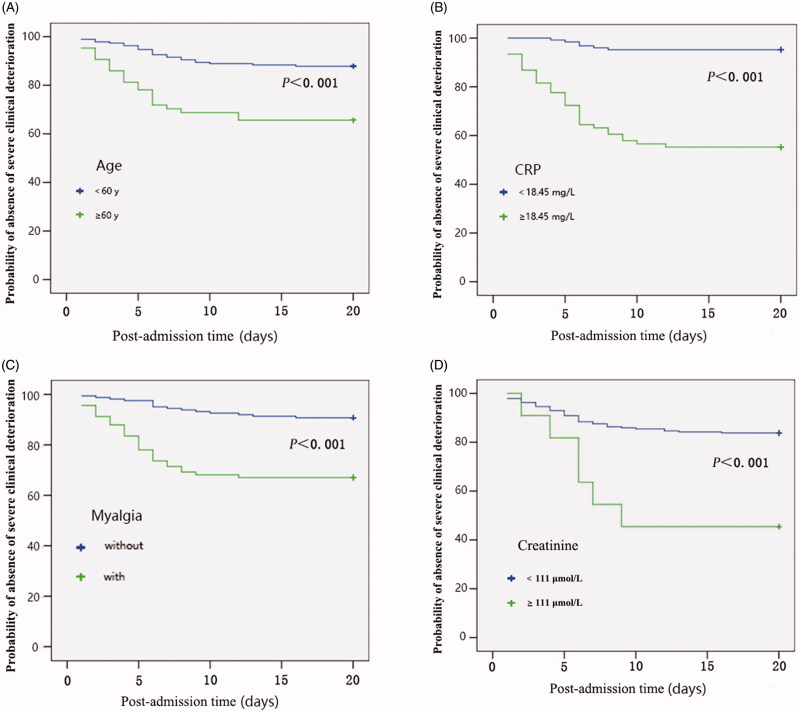
The final model using backward elimination included four predictors: senior age (OR 2.197; 95% CI: 1.367–3.531; p = .001), myalgia onset (OR 3.386; 95% CI: 1.069–10.724; p = .038), higher level of CRP (OR 1.037; 95% CI: 1.008–1.066; p = .011) and creatinine (OR 1.032; 95% CI: 1.005–1.060; p = .021) (Table 5). The bias-corrected c-statistic was 0.909. Noting that myalgia was marginally significant, we also fit the model without it, resulting in c = 0.892. The small difference between the adjusted c values with and without myalgia implies that myalgia may provide limited additional information. Kaplan–Meier analysis for the four variables in the final model shows the time-to-even distributions for myalgia and dichotomized versions of age (≥60/<60), CRP (≥18.45/<18.45 mg/L) and creatinine (≥111/<111 μmol/L), with higher values of age, CRP, creatinine and presence of myalgia associated with markedly higher clinical deterioration (Figure 2).
Figure 2.
(A–D) Kaplan–Meier plot of clinical deteriorated probability. Kaplan–Meier curves of post-admission time until severe clinical deterioration in patients with COVID-19. Time until severe clinical deterioration was significantly less in patients with senior age, with myalgia, creatinine ≥111 μmol/L and CRP ≥18.45 mg/L (all p < .001 by log-rank test).
Table 5.
Independent risk factors associated with in-hospital aggravation.
| Adjusted c-statistic | OR (95% CI) | p | |
|---|---|---|---|
| CRP (per 1 mg/L increase) | 0.909 | 1.037 (1.008–1.066) | .011 |
| Age (per 10 years) | 2.197 (1.367–3.531) | .001 | |
| Creatinine (per 1 μmol/L increase) | 1.032 (1.005–1.060) | .021 | |
| Myalgia | 3.386 (1.069–10.724) | .038 |
Abbreviations. OR, Odds ratio; CRP, C-reactive protein.
Discussion
This retrospective cohort study identified 21 statistically significant predictors of clinical deterioration among initially non-severe patients with COVID-19 in Guangzhou. The four most useful single predictors were CRP (c = 0.833), procalcitonin (c = 0.799), age (c = 0.786) and albumin (c = 0.782). All other predictors had c-statistics less than 0.721. A “best” multivariable prediction model, resulting from using backward elimination, included CRP, age, creatinine and myalgia, for which the bias-corrected c-statistic was 0.909. All four variables were positively associated with clinical deterioration. We noted that the contribution to the model from myalgia, although statistically significant, was small in terms of the increase in classification ability resulting from including it.
The findings that elderly COVID-19 patients face a significantly increased risk of developing severe illness are consistent with previous studies6,10. The possible mechanisms may be as follows. First, comorbidity and complications such as diabetes or hypertension are more common in elderly patients (see Supplement Table), which may negatively affect prognosis6,11. Second, elderly patients are at increased risk of secondary bacterial infections. Patients with virus infection are susceptible to bacterial co-infection and have high risk for secondary bacterial pneumonia for several weeks because of host immunosuppression12. Wu et al.13 reported that patients who had developed ARDS had significantly higher neutrophil counts than those without ARDS, perhaps leading to the activation of neutrophils to execute an immune response against the infections. We found that older patients with COVID-19 had higher PCT (see Supplement Table). Therefore, timely administration of antibiotics to prevent infection might reduce complications and mortality for COVID-19 patients with weakened immune functions.
Moreover, it is known that cellular and humoral immune functions become altered with age. Bronchoalveolar lavage fluids in healthy elderly subjects showed an increase in the proportion of neutrophils, an increased in the proportion of CD4+/CD8+ lymphocytes and an increase in the ability of alveolar macrophages to release superoxide anion in response to stimuli14. Oxidant-mediated injury to the lung matrix ultimately impairs gas exchange throughout the alveolar membrane14.
In our study, a rise in CRP increases the probability of deterioration in our study. The expression level of CRP is usually low, but it increases rapidly and significantly during the acute inflammatory response15,16. Patients infected with 2019-nCoV also had high amounts of proinflammatory cytokines in serum, possibly leading to activated T-helper-1 (Th1) cell responses. Moreover, patients requiring ICU admission had higher concentrations of pro-inflammatory cytokines than those who did not require ICU admission, suggesting that the cytokine storm was associated with disease severity1. Another study indicated that the cytokine and chemokine signaling networks are altered. The induction of proinflammatory cytokines after septic stimuli is not adequately controlled by anti-inflammatory mechanisms in elderly patients17. The imbalance between pro- and anti-inflammatory cytokines in the elderly may explain the worse prognosis.
Interestingly, we found the incidence of myalgia is higher than reported in previous studies (11.5–22%)6,18,19, for which a possible explanation is that patients’ condition in our study is better, which gave them more chance to express their symptoms. Some studies have shown the levels of lactate dehydrogenase, creatine kinase and myoglobin increasing in severe patients6,19,20, which suggests muscle injury may be related to prognosis. Our study is the first to identified myalgia as a predictor of clinical deterioration, which has been similarly observed for the Ebola virus disease21. Therefore, we should pay attention to the onset of myalgia in non-severe COVID-19 patients to avoid clinical deterioration.
Previous studies have reported that a higher level of serum creatinine is associated with poor prognosis in COVID-19 patients22,23, which is consistent with our research. Some studies have shown that deceased patients had higher levels of D-dimer compared with recovered patients, indicating a marked tendency to thrombosis, as the changes of other parameters indicating a bleeding tendency, such as severely low platelets or fibrinogen levels, were absent19,24,25. Our study did not show that high D-dimer levels can predict prognosis independently. The discrepancy may be explained by the relatively mild condition for patients on admission in our study.
This study has some limitations. First, as a retrospective study, data on some other parameters that may also predict the prognosis of COVID-19 were missing in some patients. For example, brain natriuretic peptide was not tested in all patients, but it might be an important predictor. Second, information on the dynamic changes in laboratory variables was lacking, and the data collected for each patient on admission may come from different disease stages. Third, this research was conducted in single center; data from larger populations and multiple centers are warranted to further confirm the results. Despite these limitations, the presented clinical data provide a useful tool for predicting the clinical deterioration of COVID-19 patients upon admission.
Conclusion
The four most useful single predictors for clinical deterioration in patients with COVID-19 were CRP, procalcitonin, age and albumin. A “best” multivariable prediction model, resulting from using a variable selection procedure, included CRP, age, serum creatinine and myalgia.
Supplementary Material
Acknowledgements
We thank all the doctors, nurses, and civilians working together to fight against SARS-CoV-2.
Transparency
Declaration of funding
The authors were supported by The Fundamental Research Funds for the Central Universities [19ykpy10].
Declaration of financial/other relationships
No potential conflict of interest was reported by the author(s).
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules CI, Marston HD, Fauci AS.. Coronavirus infections – more than just the common cold. JAMA. 2020;323(8):707. [DOI] [PubMed] [Google Scholar]
- 3.Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Mao B, Liang S, et al. ; Shanghai Clinical Treatment Experts Group for COVID-19. Association between ages and clinical characteristics and outcomes of COVID-19. Eur Respir J. 2020;55(5):2001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Zhou H, Zhou Y, et al. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect. 2020;81(1):e95–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrell FE Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Dordrecht (The Netherlands): Springer, 2015. [Google Scholar]
- 9.Hanley JA, McNeil BJ.. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Ning R, Tao Y, et al. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: a retrospective study. J Am Geriatr Soc. 2020;68(6):E19–E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36(7):e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heo JY, Song JY, Noh JY, et al. Effects of influenza immunization on pneumonia in the elderly. Hum Vaccin Immunother. 2018;14(3):744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma G, Goodwin J.. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan C, Huang Y, Shi F, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92(7):856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opal SM, Girard TD, Ely EW.. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(7):S504–S512. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han H, Xie L, Liu R, et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan. J Med Virol. 2020;92(7):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartley M-A, Young A, Tran A-M, et al. Predicting Ebola severity: a clinical prioritization score for Ebola virus disease. PLoS Negl Trop Dis. 2017;11(2):e0005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.