Abstract
Background
Standard care in severe SARS-CoV-2 pneumonia complicated by severe dyspnea and respiratory failure, consists of symptom reduction, ultimately supported by mechanical ventilation. Patients with severe SARS-CoV-2, a prominent feature of COVID-19, show several similar symptoms to Critical Asthma Syndrome (CAS) patients, such as pulmonary edema, mucus plugging of distal airways, decreased tissue oxygenation, (emergent) exhaustion due to severe dyspnea and respiratory failure. Prior application of elective phosphodiesterase (PDE)3-inhibitors milrinone and enoximone in patients with CAS yielded rapid symptomatic relief and reverted the need for mechanical ventilation, due to their bronchodilator and anti-inflammatory properties. Based on these observations, we hypothesized that enoximone may be beneficial in the treatment of patients with severe SARS-CoV-2 pneumonia and prominent CAS-features.
Methods
In this case report enoximone was administered to four consecutive patients (1 M; 3 F; 46–70 y) with emergent respiratory failure due to SARS-CoV-2 pneumonia. Clinical outcome was compared with three controls who received standard care only.
Results
After an intravenous bolus of enoximone 20 mg followed by 10 mg/h via perfusor, a rapid symptomatic relief was observed: two out of four patients recovered within a few hours, the other two (with comorbid COPD GOLD II/III) responded within 24–36 h. Compared to the controls, in the enoximone-treated patients respiratory failure and further COVID-19-related deterioration was reverted and mechanical ventilation was prevented, leading to reduced hospital/ICU time.
Discussion
Our preliminary observations suggest that early intervention with the selective PDE3-inhibitor enoximone may help to revert respiratory failure as well as avert mechanical ventilation, and reduces ICU/hospital time in patients with severe SARS-CoV-2 pneumonia. Our findings warrant further research on the therapeutic potential of PDE3-inhibition, alone or in combination with other anti-COVID-19 strategies.
Keywords: COVID-19, cytokine storm, enoximone, inflammation, mechanical, ventilation, PDE3-inhibitor, SARS-CoV-2 infection
Take home message
Phosphodiesterase3-inhibitor enoximone reverted respiratory failure and prevented mechanical ventilation in patients with severe SARS-CoV-2 pneumonia. This warrants further research on the potential of enoximone in COVID-19 treatment.
Introduction
While most patients with the corona virus disease 2019 (COVID-19), resulting from infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), experience only mild and self-limiting symptoms, a minority, even following initial recovery, develops pneumonia. Vulnerable patients as well as initially healthy individuals with SARS-CoV-2 pneumonia often end up in the ICU due to respiratory failure.1 Since effective treatment is still lacking, prolonged mechanical ventilation is often inevitable with usually a poor prognosis. Apart from a local inflammatory response pursuant to a cytokine storm,1 other pulmonary presentations of severe COVID-19 include massive pulmonary edema due to capillary leak syndrome (similar to ARDS) and thromboembolic events.1,2 These sequelae cause impairment in gas exchange with subsequent respiratory failure.1
Several pathophysiological similarities exist between patients with SARS-CoV-2 pneumonia and patients with critical asthma syndrome (CAS).3 Both (potentially fatal) conditions may present with respiratory failure based on ARDS-like phenomena, comprising pulmonary edema, capillary leakage, mucus plugging and coagulopathy, predominantly in the pulmonary vessels. In both conditions the same pro-inflammatory mediators and cytokines are involved, such as histamine, bradykinin, leukotrienes, thrombin, IL-1, IL-6, IL-8 and TNFα .4
Despite the growing number of targets and several potential (targeted) therapeutic modalities currently under development, so far there are no unambiguously effective treatment options for patients with severe SARS-CoV-2 pneumonia. Selective phosphodiesterase (PDE)3-inhibitors enoximone and milrinone previously showed clinical effectiveness in patients with CAS.5–7 PDE3-inhibitors relax airway smooth muscle,8,9 possess anti-inflammatory properties,9–11 improve mucosal barrier function and prevent endothelial leakage caused by vaso-active mediators (e.g. bradykinin, histamine and leukotrienes).10,12–14 In a study in children with enterovirus-A71 infection with neurogenic shock and pulmonary edema, milrinone effectively shortened the time of mechanical ventilation and improved survival, as compared with standard of care.15
Since the 1990s, enoximone has been included in the standard of care for hemodynamic management in the ICU setting, enhancing the myocardial function with oxygen-saving properties, and thus preventing organ failure. Based on these properties, as well as on enoximone’s previously reported beneficial effects in CAS and related pathophysiological conditions, we decided to administer enoximone to four consecutive patients with
SARS-CoV-2 with pulmonary edema due to capillary leakage and emergent respiratory failure. Subsequently, we monitored the effect on respiratory sequelae, the need for mechanical ventilation and duration of ICU/hospital stay and compared this to controls, receiving standard care only.
Patients and methods
The presented data are derived from observations from regular patient care and not from a randomized interventional study with an experimental product. During the first COVID-19 pandemic wave (March–July 2020), several COVID-19 patients were admitted to the COVID ward of a middle sized Dutch peripheral hospital. The patients received standard care: i.e., hydroxychloroquine, bronchodilators (e.g. salbutamol and ipratropium), nadroparine and supplemental oxygen. If needed patients were referred to ICU and mechanical ventilation was applied, mostly starting in prone position. During the first three days on mechanical ventilation, patients were administered ceftriaxone (1000 mg OD). Complications were treated according to existing standards.
On standard care, many patients showed a rapid disease progression, requiring mechanical ventilation, protracted hospitalization and an overall poor prognosis.1 Considering the complexity of the presentation of the COVID-19 patients with CAS-CS-features, hypoperfusion, pulmonary hypertension and the poor outcome to date, we strategized to focus on the distinct CAS-features in these deteriorating patients.
In view of its oxygen-sparing and positive haemodynamic effects as well as its known bronchodilator and anti-inflammatory properties,1 we started to treat COVID-19 patients referred to the ICU for (emergent) respiratory failure with enoximone, a PDE3-inhibitor regularly applied at the ICU. Key patient-related outcomes included reversion of respiratory failure, prevention of mechanical ventilation, and shorter ICU/hospital stay.
In this observational set-up, we were only able to treat four COVID-19 patients with enoximone because the pandemic subsided over the summer (June 2020), and therefore we compared the outcomes with those of the three preceding COVID-19 cases admitted to the ICU who received standard of care only (Table 1). Inclusion criteria comprised adult patients (≥18 years), with SARS-CoV-2 infection confirmed by polymerase chain 86 reaction (PCR) and emergent respiratory failure related to SARS-CoV-2 pneumonia, requiring supplemental oxygen.
Table 1.
Clinical characteristics before treatment at ICU.
| Subject number | A* | B* | C* | 1# | 2# | 3# | 4# |
|---|---|---|---|---|---|---|---|
| AGE, GENDER | 71 (M) | 55 (F) | 63 (M) | 46 (M) | 54 (F) | 70 (F) | 55 (F) |
| COMORBIDITIES | obese | COPD Gold III (cachectic) | COPD Gold II (Centrilobular emphysema) | ||||
| SYMPTOMS | Feeling sick with fever | Fever (39-40°), headache, cough. | Fever, feeling sick. | Cough, fever, dyspnea, muscle pain, anosmia, loss of appetite. | Fever, dyspnea before admission. | Fever, cough, fatigue, shortness of breath. 08-12/05 admission for COPD (SARS-CoV-2 neg). 15-05 SARS-CoV-2 pos. 19-05 readmission for pneumonia. | Feeling sick, fever. 28/06/2020 tested SARS-CoV-2 negative. On 05/07/2020 tested again, this time SARS-CoV-2 positive. |
| DURATION OF FEVER IN DAYS | 14 | 7 | 7 | 5 | 6 | 12 | 11 |
| SORE THROAT | − | − | − | − | + | − | − |
| COUGH | + | + | − | − | + | + | − |
| SPUTUM PRODUCTION | + | − | − | − | − | − | − |
| FATIGUE | + | + | + | + | + | + | + |
| SHORTNESS OF BREATH | + | + | + | + | + | + | + |
| NAUSEA OR VOMITING | − | − | + | − | − | − | − |
= conventional treatment only (pts A-C);
= add on-enoximone (pts number 1–4).
Ethical considerations
In this emergency setting, enoximone was given (at much lower doses than marketed) within the scope of regular patient care and based on previous successful case reports…5,6,16 including our own experience in another indication (asthma) with a similar pathophysiology – all in line with good clinical practice (GCP). No prior ethical consent (MEC or IRB) was required. Administration was well-documented; all patients gave prior informed consent for the treatment; they also consented to possible publication of their data.
Dose and dosing regimen of enoximone
So far, enoximone (Perfan®, Carinopharm GmbH, Elze, Germany), has not been described in the literature in the context of COVID-19. The algorithm for a safe, well-tolerated and effective dose and dosing regimen was based on our previous experience in near fatal asthma and status asthmaticus in the emergency department and on pre-operative treatment of severe asthma patients.5,6,16 In our COVID-19 patients with severe pneumonia, we used an intravenous bolus of 20 mg (0.25 mg/kg) enoximone followed by 10 mg/h (via perfusor) (0.125 mg/kg/h (i.e., approximately 2.1 mcg/kg/min)) for approximately 24–48 h, which is approximately 10–12-fold lower than the commonly applied (marketed) dose for cardiovascular indications (i.e., doses up to 2400 mg i.v./d).
Statistical analysis
For statistical analysis regarding mechanical ventilation requirement, we used the Chi-Square tests, Fisher’s exact test, suitable for binary data in unpaired samples, i.e. the 2 × 2 table (SPSS 2.5). Mann-Whitney U test (one-tailed) was used for group comparison of a continuous endpoint, the number of days in the hospital and the number of days on mechanical ventilation (Table 3) (GraphPad Prism 5.0). Baseline laboratory values (Table 2) were tested with the same statistical power.
Results
Patient characteristics, essential laboratory data and specific ICU therapies
Following our decision to administer enoximone to patients with severe SARS-CoV-2 pneumonia to see if mechanical ventilation could be averted (Additional data: Rationale), we were able to treat the last four (consecutive) COVID-19 patients referred to the ICU during the first COVID-19 wave.
Upon ICU admission, all patients presented with progressive dyspnea, deteriorating dyspnea with an imminent need for mechanical ventilation, a high oxygen need (all had a non-rebreathing mask), indicative of severely impaired alveolar gas exchange, and an imminent respiratory failure. They also showed monosyllabic conversation, use of auxiliary respiratory muscles, and exhaustion due to increased respiratory difficulties caused by decreased pulmonary compliance, implying edema as a result of capillary leakage probably induced by (pro)inflammatory cytokines.
Controls received standard care (only) as described in the method section (Table 1). Baseline laboratory values including clinical chemistry and white blood cell differentials were comparable between the two groups (Table 2).
Table 2.
Laboratory parameters blood parameters counts before treatment at ICU.
| Standard care | Add-on enoximone | p value MW (one tailed) | ||||||
|---|---|---|---|---|---|---|---|---|
| BLOOD PARAMETERS COUNTS (ref. values) |
A* | B* | C* | 1# | 2# | 3# | 4# | |
| Haemoglobin (6.8–9.3 mmol/L) | 6.9 | 6.8 | 8.1 | 9.0 | 6.5 | 7.5 | 6.2 | 0.4 |
| C-reactive protein (0–10 mg/L) | 116 | 199 | 243 | 6 | 234 | 191 | 173 | 0.3 |
| Albumin (30–50g/L) | 17 | 19 | 19 | 27 | 22 | 14 | 25 | 0.2 |
| Bilirubin (µmol/L) | 13 | 8 | 18 | – | 5 | 8 | 6 | 0.1 |
| D-dimer (0–0.5mg/L) | 2.1 | 0.21 | 2.8 | 0.54 | 0.33 | 1.3 | 0.47 | 0.3 |
| Lactate (mmol/L) | 1.5 | 1.1 | 1.2 | 2.9 | 0.8 | 1.3 | 0.7 | 0.3 |
| White blood cell, (4.0–10.0 × 109/L) | 23.7 | 8.5 | 13.8 | 5.3 | 9.7 | 11.0 | 4.4 | 0.1 |
| Neutrophils, (2.0–6.0 × 109/L) | 9.0 | 7.6 | 12.5 | 2.8 | 7.9 | 9.7 | 3.6 | 0.2 |
| Lymphocytes (1.0–3.5 × 109/L) | 12 | 0.7 | 0.7 | 1.5 | 1.4 | 0.8 | 0.8 | 0.3 |
| Monocytes (1.0–3.5 × 109/L) | 0.86 | 0.13 | 1.00 | 0.51 | 0.32 | 0.43 | 0.12 | 0.2 |
| Eosinophils (1.0–3.5 × 109/L) | 0.25 | 0.02 | 0.01 | 0.13 | 0.01 | 0.08 | 0.00 | 0.4 |
| Platelet count (1.3–3.6 × 109/L) | 5.3 | 2.0 | 3.1 | 5.1 | 3.2 | 3.8 | 1.5 | 0.5 |
= conventional treatment only (pts A-C);.
= add on-enoximone (pts number 1-4).
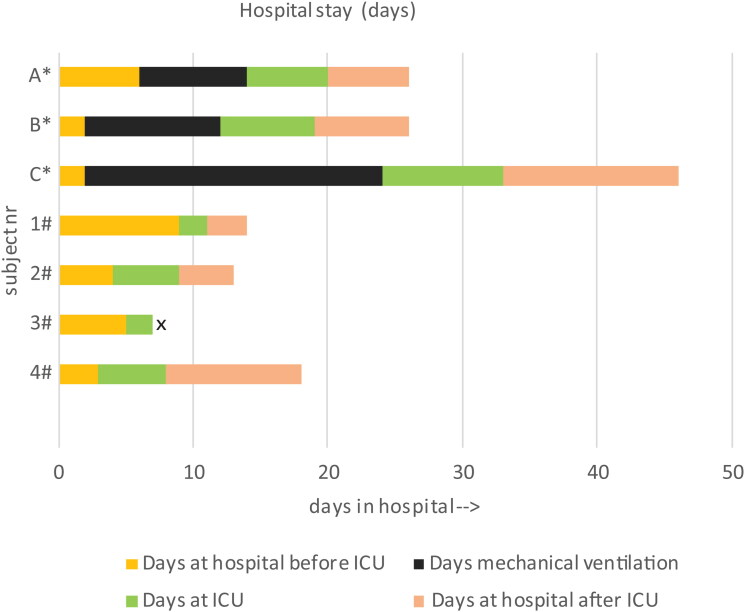
Similar to our previous experiences in CAS, enoximone achieved a rapid symptomatic relief in two out of four patients, with full recovery within a few hours (patients 1 and 4), while the other two patients (patients 2 and 3, with comorbid obesity or COPD GOLD III) required a longer time to respond (24–36 h) due to sputum retention. Compared to controls, the enoximone-treated patients had no need for mechanical ventilation (Chi-Square Tests, Fisher’s exact test p = 0.029), had a shorter stay in ICU (2–5 days for enoximone-treated patients versus 12–31 days for controls), and an overall shorter stay in hospital (13–15 days for enoximone-treated patients versus 22–46 days for controls) as well as a shorter recovery time (Figure 1; Table 3) (Mann-Whitney U test p = 0.05). Blood gas analysis (Supplementary Table 1) showed a minimal improvement in three out of four patients between before and 1 h after enoximone. The apparent alkalosis is mainly due to hyperventilation (in turn due to patient anxiety). pO2 levels are all at the right side of the sigmoid curve of saturation, reflecting maximal oxygen saturation despite severe dyspnea (Supplementary Table 1).
Figure 1.
Hospital stay days.
Patient 3# passed away, according to her own wish
*conventional treatment only (pts A-C); # add on-enoximone (pts number 1-4)
x patient 3# passed away on 26-05-2020, according to her own wishes
Table 3.
Duration of hospital stay, chest radiography, mechanical ventilation characteristics and intervention.
| Standard care | Add-on enoximone | p value MW (one tailed) | ||||||
|---|---|---|---|---|---|---|---|---|
| Subject number | A* | B* | C* | 1# | 2# | 3# | 4# | |
| Age (years) | 71 | 55 | 63 | 46 | 54 | 70 (COPD Gold III) | 55 (COPD) | |
| Gender | Male | Female | Male | Male | Female | Female | Female | |
| Days from onset to (date) HA (Hospital Admission)/to (date) ICU admission [i] | 14 28-03 HA 04-04 ICU |
7 05-04 HA 07-04 ICU |
7 06-04 HA 08-04 ICU |
5 08-05 HA 17-05 ICU |
7 20-05 HA 24-05 ICU |
5 19-05 HA (readmission; 08-12/05 prev. admission for COPD (SARS-CoV-2 neg)) 24-05 ICU |
11 05-07 HA 08-07 ICU |
|
| Days at hospital before ICU [ii] | 7 | 2 | 2 | 9 | 4 | 5 | 3 | ns |
| Days at ICU [iii] | 12 | 13 | 31 | 2 | 5 | 2 | 5 | p0.05 |
| Days mechanical ventilation [x] | 8 | 10 | 22 | 0 | 0 | 0 | 0 | p0.05 |
| Days at hospital after ICU [iv] | 6 | 7 | 13 | 3 | 4 | – | 7 | ns |
| Date of discharge hosp. | 22-04-2020 | 27-04-2020 | 22-05-2020 | 22-05-2020 | 02-06-2020 | ---** | 20-07-2020 | |
| Total days in hosp. [=ii + iii + iv] | 25 | 22 | 46 | 14 | 13 | ---** | 15 | p≤0.05 |
| Total duration of disease (onset-discharge) [=i + ii + iii + iv) |
39 | 29 | 53 (to rehab facility) |
19 | 20 | ---** | 26 (to transition ward) |
p0.05 |
| Chest radiography findings | CAT-scan: No embolus. Bilateral spotty consolida-tions. Viral pneumonia | CAT-scan: No embolus, bi-lateral spotty infiltrations, some emphysema | CAT-scan: Embolus in left lower lobe and small one right mediobasal. Infiltrations predominantly in upper lobes. Spotty infiltrations compatible with COVD-19 | Cat-scan: No embolus. Bi-lateral ground glass, notably lower lobes, right lower lobe consolidated | CAT-scan: Disseminated infiltrations and spotty consolidations | Chest X-Ray: Diffuse bilateral infiltrations | CAT-scan: Embolus right lower lobe + small embolus more peripherally. Centrilobular emphysema. Infiltrations and fibrosis consistent with COVID-19 in both upper lobes. | |
| Intervention 1st day of ICU admission | Chloroquine (continued from COVID ward) | Chloroquine (continued from COVID ward) | Chloroquine (continued from COVID ward) | Enoximone | Enoximone | Enoximone | Enoximone Remdesivir on 10-07 (for 5 days) | |
| vv-ECMO (days) | – | – | – | – | – | – | – | |
| Specific characteristics of mechanical ventilation | PCV followed by assist ventilation | PCV followed by assist ventilation | PCV followed by assist ventilation | Optiflow™ started at 45% oxygen and 60 L flow | Optiflow™ started at 100% oxygen and 50 L flow | Optiflow™ started at 100% oxygen and 60 L flow |
Optiflow™ started at 55% oxygen and 60 L flow | |
| Plateau pressure (cm H2O) | 26 | 24 | 26 | − | − | − | − | |
| PEEP (cm H2O) | 14 | 10 | 10 | − | − | − | − | |
| Compliance (ml/cmH2O) | 34 | 30.1 | 28.6 | − | − | − | − | |
| Prone position | + | + | + | − | + (without help) | − | − | |
| Inhaled pulmonary vasodilators | − | − | + | − | − | − | + | |
| Extracorporeal membrane oxygenation | − | − | − | − | − | − | − | |
| Echocardiogram completed | − | − | − | − | − | − | − | |
| Echocardiogram showing new left ventricular dysfunction | − | − | − | − | − | − | − | |
| Neuromuscular blockade | Yes | No | Yes | No | No | No | No | |
| Vasopressors | − | + | + | − | − | − | + | |
| Renal replacement therapy | − | − | − | − | − | − | − | |
Abbreviations: – vv-ECMO : veno-venous extracorporeal membrane oxygenation – date HA : Hospital Admission - PCV: Pressure Controlled Ventilation.
= conventional treatment only (pts A-C); #= add on-enoximone (pts number 1-4).
Patient 3# passed away on 26-05-2020, according to her own wishes. Used medication: formoterol 12-24 mcg/d, ipratropium 250 mcg up to 6 x dd, salbutamol 100 mcg up to 4 x dd, ceftriaxone 2000 mg 1 x dd, morphine up to 6 x dd 5 mg subcutaneously.
The pretreatment oxygen demand and the key respiratory characteristics upon ICU admission are shown in Table 4. Following enoximone treatment, the oxygenation substantially improved (Table 4). Patient-reported outcome confirmed the observed improvements; all patients were very relieved about their perceived sudden recovery and not needing mechanical ventilation. At the doses used, enoximone was well-tolerated in all patients and no clinically relevant adverse events were observed. In all patients the beneficial effect persisted even after discontinuation of enoximone. Given the nature of this report, which is not based on a formal study protocol, there are no data available on the after-COVID-19-disease wear-off following hospital discharge (e.g. blood gas analysis, imaging, lung function testing/diffusion capacity test, etc.).
Tabel 4.
Respiratory characteristics before Enoximone (admission ICU) – after 24 h – after 48 h.
| Standard care | PATIENT | DATE | RESP. SUPPORT | FiO2 | RESP. RATE |
TIDAL VOLUME |
MINUTE VOLUME | PEAK/PEEP PRESSURE | RESP. VALUES | TIME |
|---|---|---|---|---|---|---|---|---|---|---|
| A* | 04-04-2020 | PCV | 80% | 22 | 475 | 5,1 | 22/14 | - | 18.24 h | |
| 05-04-2020 | PCV | 40% | 20 | 413 | 4,9 | 31/09 | - | 18.24 h | ||
| 06-04-2020 | Pr Supp | 40% | 21 | 484 | 6,1 | 14/10 | - | 18.24 h | ||
| B* | 07-04-2020 | PCV | 55% | 20 | 422 | 6,2 | 23/14 | - | 18.18 h | |
| 08-04-2020 | Pr Supp | 40% | 21 | 492 | 10,0 | 16/08 | - | 18.18 h | ||
| 09-04-2020 | Pr Supp | 55% | 15 | 433 | 7,6 | 15/10 | - | 18.18 h | ||
| C* | 08-04-2020 | PCV | 55% | 20 | 458 | 6,6 | 23/14 | - | 12.35 h | |
| 09-04-2020 | PCV | 50% | 24 | 435 | 6,4 | 25/10 | - | 12.35 h | ||
| 10-04-2020 | PCV | 41% | 24 | 430 | 6,0 | 25/11 | - | 12.35 h | ||
| Add-on enoximone | 1# | 17-05-2020 | Optiflow™ | 45% | 16 | – | – | – | 45%/60 L | 16.24 h |
| 18-05-2020 | none | – | 15 | – | – | – | 6 L O2 (nasal probe) |
16.24 h | ||
| 19-05-2020 | none | – | 16 | – | – | – | 2 L O2 (nasal probe) |
16.24 h | ||
| 2# | 24-05-2020 | Optiflow™ | 65% | – | – | – | – | 65%/50 L | 11.26 h | |
| 25-05-2020 | Optiflow™ | 60% | – | – | – | – | 60%/60 L | 11.26 h | ||
| 26-05-2020 | none | – | – | – | – | – | 6 L O2 (nasal probe) |
11.26 h | ||
| 3# | 24-05-2020 | Optiflow™ | 100% | 24 | – | – | – | 100%/60 L | 22.27 h | |
| 25-05-2020 | Optiflow™ | 55% | 12 | – | – | – | 55%/50 L | 22.27 h | ||
| 26-05-2020 | Optiflow™ | 60% | 13 | – | – | – | 60%/60 L | 22.27 h | ||
| 4# | 08-07-2020 | Optiflow™ | 55% | – | – | – | – | 55%/60 L | 12.51 h | |
| 09-07-2020 | Optiflow™ | 50% | – | – | – | – | 50%/55 L | 12.51 h | ||
| 10-07-2020 | Optiflow™ | 50% | – | – | – | – | 50%/50 L | 12.51 h |
= conventional treatment only (pts A-C);
= add on-enoximone (pts number 1-4).
Cases
Three preceding COVID-19 patients receiving standard care at the ICU served as ‘historical’ controls. All controls received standard of care, including Optiflow™ and subsequent intubation if no/insufficient effect.
Control (standard of care only) patients (March–May 2020)
Patient A* – a 71 year-old male, admitted to the COVID ward on 28/03/2020; on 04/04/2020 was transferred to the ICU (with non-rebreathing mask) due to respiratory exhaustion – he was intubated, ventilated and directly put in prone position. In total 12 days ICU, with 8 days of mechanical ventilation and two periods in prone position. Returned to the COVID ward on 16/04/2020 and discharged from hospital on 22/04/2020.
Patient B* – a 55 year-old female, admitted to the COVID ward on 05/04/2020; on 07/04/2020 transferred to ICU (with oxygen nasal probe 6 L/min) for intubation and mechanical ventilation. From 09/04/2020 to 12/04/2020 and from 13/04 to 15/04 in prone position. Extubated on 17/04/2020, followed by two more days of OptiflowTM. Patient returned to the COVID ward on 20/04/2020 and was discharged from hospital on 27/04/2020. A total of 13 days ICU, 10 days of which mechanically ventilated, with two periods in prone position and two days on Optiflow TM.
Patient C* – a 63 year-old male, admitted to the COVID ward on 04/04/2020; he was transferred to the ICU (with non-rebreathing mask) with emergent exhaustion on 08/04/2020, subsequently intubated, ventilated and immediately placed in prone position. In total 31 days ICU: 22 days mechanically ventilated, with three periods of in total 10 days in prone position. His disease course was complicated by a transient renal insufficiency (without needing dialysis), atrial fibrillation and multiple pulmonary embolisms. On 22/05/2020 patient was discharged from the hospital and transferred to a rehabilitation facility.
Enoximone (on top of standard of care)-treated patients (May–July 2020)
Patient 1# – a 46 year-old male with COVID-19 related symptoms since 03/05/2020; admitted to hospital on 08/05/2020. Transferred to ICU with severe dyspnea, emergent exhaustion and need for mechanical ventilation on 17/05/2020. Upon arrival at the ICU, the patient was connected to the OptiflowTM. Enoximone was started as mentioned above. Upon ICU entry, patient was monosyllabic; 10 min after initiation of enoximone treatment, he was breathing calmly and was able to call his wife. Enoximone dosing was reduced to 0 within 24 h and on the next day (19/05/2020) the patient was able to return to the COVID ward and was discharged from hospital on 22/05/2020.
Patient 2# – a 54 year-old female with COVID-19 related symptoms since 13/05/2020; admitted to hospital on 20/05/2020. Transferred to ICU (with non-rebreathing mask) on 24/05/2020 due to increasing dyspnea, impeding exhaustion and need for mechanical ventilation. Upon arrival at the ICU, OptiflowTM was started with insufficient effect. Enoximone was started as above-mentioned. Due to sputum retention, it took approximately 24 h for the dyspnea to subside. On 25/05/2020 patient could be mobilized (out of bed); that same day the enoximone was reduced to 5 mg/h and discontinued on 27/05/2020. The patient returned to the COVID ward on 29/05/2020 and was discharged from hospital on 02/06/2020.
Patient 3# – a 70 year-old cachectic female with COPD GOLD III and SARS-CoV-2 PCR positive since 14/05/2020. On 24/05/2020, patient was transferred to ICU with severe dyspnea and emergent respiratory failure. Upon arrival at the ICU (with non-rebreathing mask) OptiflowTM was tried, with little effect. Enoximone was started as above-mentioned. Enoximone dosing was reduced to 0 within approximately 36 h (on 26/05/2020); the patient resumed her usual breathing pattern (COPD taken into account) and the OptiflowTM could be weaned to 0. Patient was then able to tell us her life story, and that she had actually given up on life. Her only reason to get "better" was to be able to hear whether her husband survived his SARS-CoV-2 infection. When the message of his recovery arrived, despite the significant improvement in her own condition, she decided she was done and asked to discontinue the treatment. Patient received palliative medication and passed away peacefully, according to her wish.
Patient 4# – a 55 year-old female with respiratory symptoms since 24/06/2020; on 28/06/2020 she was tested negative for SARS-CoV-2. This early test was done because of her prior diagnosis with COPD GOLD II. Her condition deteriorated and she was referred to hospital on 05/07/2020; this time she tested SARS-CoV-2-positive. On 08/07/2020 she was transferred to the ICU (with non-rebreathing mask) because of severe dyspnea and exhaustion. Enoximone was started as above-mentioned and followed by i.v. perfusor (NB: 5 mg/h during 24 h). Within 10 minutes patient was able to breath calmly and to communicate normally with the nursing staff. On 13/07/2020 she was back on the COVID ward; on 20/07/2020 she could be discharged.
In summary, following successful reversal of the COVID-19 related symptoms and signs in all four patients, none of the enoximone-treated patients required mechanical ventilation and three of them could be safely transferred to the COVID ward for further recovery and subsequent discharge. One patient (no 3) with comorbid severe COPD, following initial recovery, chose to discontinue further treatment and asked for palliative sedation based on personal circumstances. The enoximone-treated patients could be discharged from the hospital within 5–12 days after start of enoximone treatment, while in the three preceding controls this took 18–44 days following start of mechanical ventilation (Figure 1).
Discussion
This report includes four cases with severe SARS-CoV-2 pneumonia in whom early intervention (i.e., before intubation) with the selective PDE3-inhibitor enoximone, yielded symptomatic relief, helped to revert respiratory failure and thus prevented mechanical ventilation, while, compared to controls, accelerating recovery and shortening the overall ICU/hospital stay. In the context of the clinical (observational) setting, it should be noted that blood gas samples may not have been obtained at the most representative timepoints and may thus not be fully informative. Blood gas analysis (Supplementary Table 1) and oxygen saturation did not reflect patients’ clinical status accurately; despite an apparent improvement in pO2 approximately 1 h post-treatment they were still severely dyspnoeic. The ability to speak (nodding yes/shaking no, monosyllabic speech, short sentences or complete sentences) seems to be a more adequate marker of the physical status and respiratory function of very severe COVID-19 patients.
An obvious limitation of this report is the small sample size, however, the reported outcomes are clinically relevant and the presented cases cover a fair spectrum of the COVID-19 population, ranging from relatively young to older age, from prior good health to comorbid (severe) COPD, and from normal body weight to obesity. The overrepresentation of women in our case report does not correspond to the global men/women ratio previously reported for COVID-19.
Our preliminary observations are promising and warrant larger (controlled) studies with enoximone in COVID-19. Baseline laboratory values in Table 2 were tested with statistical power with the same strength as tested for Table 3. This statistical test that show significant differences earlier (1 tail instead of 2 tails) if there is a difference. The result of no difference in baseline laboratory values illustrates the beneficial effect of enoximone on preventing mechanical ventialtion.
While the initial (marketed) indication for enoximone is heart failure at daily i.v. doses up to 2400 mg, administered at the ICU with close monitoring,17 much lower oral doses (150 mg daily, 6 months) have been safely given to patients (n = 1854) with advanced heart failure in an ambulatory setting and were found to be similar to placebo in regard to adverse events.18 In another study, low-dose oral enoximone was safely administered to young children (aged 0.5–191 months) with congenital heart failure in an ambulatory setting upon discharge from ICU (0.5 mg/kg3 × daily).19 In addition, patients with advanced COPD have been previously reported to benefit from low-dose enoximone (unpublished observation; see also Table 1 – patients 3 and 4).
In the presented cases, we applied even lower doses of enoximone based on our experience with oral enoximone in asthma patients (>80 patients and approximately 300 patient years) 16 in an ambulatory setting. These patients received a maximum oral daily dose of 25 mg enoximone in a customized fashion (i.e., once daily or less) without noticeable side effects.16 At the currently applied dosing regimen, enoximone was well-tolerated on top of other medications and, as expected given no reported interactions or contraindications, no clinically relevant adverse events were noted.
Based on previous observations,5 supported by preclinical and translational data,10,12–14,20,21 PDE3-inhibitors (including enoximone) have been shown to possess anti-inflammatory activities, modulator and broncho-protective properties, which, in ARDS models and critical respiratory conditions in humans, helped to prevent the sequelae of the associated cytokine storm.22 This may have accounted for the clinical efficacy observed in our COVID-19 patients.
The key pro-inflammatory mediators and cytokines involved in respiratory viral infections, including histamine, bradykinin, leukotrienes, members of the IL-1 family, IL-8, TNFα and thrombin, directly affect microvascular permeability and cause capillary leakage due to actin skeleton disruption, resulting in pulmonary edema.10,12–14,20,21 Apart from decreasing the production of pro-inflammatory cytokines,13 PDE3-inhibitors prevent microvascular leakage via direct modulation and normalization of the cytoskeleton by increasing intracellular cAMP.10,14,21 PDE3-inhibition has also been associated with improving the cilia function of the epithelial cells within the respiratory tract 23 and restoring mucociliary clearance function. Actin skeleton disruption reduces PDE3A–Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) interaction. PDE3 is located within the microdomain of the cAMP-dependent CFTR, and PDE3A clusters with CFTR, which indicates, that PDE3-inhibition can directly influence CFTR via one of its active mechanisms: the resorption of interstitial fluid and the reduction of pulmonary oedema.20,24,25 TNF-alpha induces disruption of the F-actine cytoskeleton, resulting I) dysfunction of the tight junctions and cellular barrier, and II) impaired organization of microdomains in the cell and thus an impaired function of CFTR. Similar impairment occurs for the Na and Cl channels and aquaporins that normally play a key role in keeping the airways (relatively) dry.20 SARS-CoV-2 may interfere with GPCR signaling pathways to dysregulate ion and fluid transport within the lungs.33 Furthermore, PDE3-inhibitors prevent mast cell degranulation 26 and subsequent release of pro-inflammatory mediators (e.g. histamine, bradykinin, and LTs), which have been reported to contribute to SARS-CoV-2-induced inflammation.4 A major consideration is that several of the pro-inflammatory mediators released by effector cells in COVID-19 act on airway smooth muscle cells and induce bronchoconstriction,4,27,28 which can be relieved by (combined) PDE3-inhibitors producing (potent and sustained) bronchodilation.5,7–10,21
Recently, based on their broad anti-inflammatory activity, systemic corticosteroids have been advocated in severe COVID-19 patients requiring supplemental oxygen (i.e., with a similar indication as our COVID-19 cases) in line with the preliminary findings in the RECOVERY study.29 However, high-dosed (systemic) corticosteroids may also induce deleterious effects and impose health risks, especially in vulnerable patients.29 Therefore, it is worthwhile to explore safer treatment options with comparable anti-inflammatory effects.11
PDE inhibitors might have potential in the treatment of COVID-19,30 mainly through anti-inflammatory effects resulting from PDE3 and/or PDE4 inhibition by reducing cytokines including TNF-a levels.11,31,32 Additionally, PDE3 inhibitors are potent bronchodilators through relaxation of the airway smooth muscle cells and reduces pulmonary edema.5–7,15 Presently, several selective PDE inhibitors (Apremilast, Ensifentrine, Pentoxifyline, Dipyridamole and Ibudilast) targeting PDE3, PDE4, PDE5 and PDE10 are under investigation in COVID19 clinical trials {clinical trial.gov}.
Considerations
In the beginning of the SARS-CoV2 pandemic, no effective treatment options were available due to the lack of our knowledge on the new Coronavirus and its sequelae. With increasing insight into the complex pathophysiology of COVID-19, treatment has been subject to changes over the past few months, e.g. successively off-label hydroxychloroquine and remdesivir and, more recently, off-label systemic corticosteroids, but none of them has proven (fully) adequate.
The advantage of using already registered drugs (repurposing) is the existing knowledge of their mode of action and safety profile. This may obviously help shorten the trajectory of registration for new indications. In clinical trial.gov and published papers,30 several studies with existing (classes of) off-label drugs for COVID-19 have been initiated/proposed, with different strategies and targets, including virus replication, inflammatory sequelae, pulmonary capillary leakage and/or thrombo-embolic events. Targeting the complex SARS-CoV-2 cascade at different levels seems rational. Hence systemic application of PDE3-inhibitors in COVID-19 seems plausible, given the wide scope of targets for this drug class within the complex pathophysiology of this syndrome.5,23,30 Obviously this requires further research.
In conclusion, our preliminary data based on small patient numbers suggest that the progressive course of the SARS-CoV-2 infection in COVID-19 patients can be modulated by early intervention with enoximone, making this drug a valid candidate for further research. Respiratory failure could be averted, no mechanical ventilation was needed and overall ICU/hospital time was significantly shorter.
Supplementary Material
Acknowledgements
The authors wish to thank Maarten van Lieshout, MD, Dianne Hoelen, MD, Cynthia Blanker MD for their contribution to clinical performance and acquiring the data for this study.
Glossary
Abbreviations
- ARDS
Acute Respiratory Distress Syndrome
- cAMP
cyclic Adenosine Monophosphate
- CAS
Critical Asthma Syndrome
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- cGMP
cyclic Guanosine Monophosphate
- ICU
intensive care unit
- PDE3
phosphodiesterase 3
Disclosure statement
J.B., P.B. and B.B. are scientific/clinical advisors and shareholders in BMR BV. A.K.J. is scientific advisor of BMR, relatives of A.K.J. are shareholders of BMR BV. BMR has a patent pending for respiratory indications of enoximone.
Z.D. has acted as Executive and Scientific Medical Director at a phase I/II pharmacological unit (QPS-NL) performing clinical trials with several pharmaceutical companies until mid 2020. Furthermore, Z.D. received honoraria, consultancy and speaker fees from Acucort, Astrazeneca, ALK, Boehringer Ingelheim, GlaxoSmithKline, HAL Allergy, Merck Sharp & Dohme and Sanofi-Genzyme; all outside this report. Z.D. also acts as an advisor for BMR BV.
All authors declared no relationship with Carinopharm GmbH, Elze, Germany, the licensee of Perfan® (iv enoximone).
Author contributions
J.B., P.B., B.B., J.T., Z.D. and A.K. contributed to, wrote and edited the manuscript; J.B., P.B., B.B., J.T., Z.D. and A.K., were involved in analyzing the data and reviewing and shaping the manuscript. All authors approved the final version before submission.
Prof. Zuzana Diamant, MD PhD and Alex KleinJan, PhD contributed equally.
References
- 1.Chen N, Zhou M, Dong X, et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermejo JF, Munoz-Fernandez MA.. Severe acute respiratory syndrome, a pathological immune response to the new coronavirus-implications for understanding of pathogenesis, therapy, design of vaccines, and epidemiology. Viral Immunol. 2004;17(4):535–544. doi: 10.1089/vim.2004.17.535. [DOI] [PubMed] [Google Scholar]
- 3.Kenyon N, Zeki AA, Albertson TE, Louie S.. Definition of critical asthma syndromes. Clin Rev Allergy Immunol. 2015;48(1):1–6. doi: 10.1007/s12016-013-8395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kritas SK, Ronconi G, Caraffa A, Gallenga CE, Ross R, Conti P.. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents. 2020; 34(1): 9–14. doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 5.Beute J. Emergency treatment of status asthmaticus with enoximone. Br J Anaesth. 2014;112(6):1105–1108. doi: 10.1093/bja/aeu048. [DOI] [PubMed] [Google Scholar]
- 6.Schulz O, Wiesner O, Welte T, et al. . Enoximone in status asthmaticus. ERJ Open Res. 2020;6(1):00367–2019. doi: 10.1183/23120541.00367-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobhy A, Eldin DMK, Zaki HV.. The use of milrinone versus conventional treatment for the management of life-threatening bronchial asthma. TOATJ. 2019;13(1):12–17. doi: 10.2174/2589645801913010012. [DOI] [Google Scholar]
- 8.Leeman M, Lejeune P, Melot C, Naeije R.. Reduction in pulmonary hypertension and in airway resistances by enoximone (MDL 17,043) in decompensated COPD. Chest. 1987;91(5):662–666. doi: 10.1378/chest.91.5.662. [DOI] [PubMed] [Google Scholar]
- 9.Franciosi LG, Diamant Z, Banner KH, et al. . Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med. 2013;1(9):714–727. doi: 10.1016/S2213-2600(13)70187-5. [DOI] [PubMed] [Google Scholar]
- 10.Beute J, Lukkes M, Koekoek EP, et al. . A pathophysiological role of PDE3 in allergic airway inflammation. JCI Insight. 2018;3(2)doi: 10.1172/jci.insight.94888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santarpino G, Caroleo S, Onorati F, et al. . Inflammatory response to cardiopulmonary bypass with enoximone or steroids in patients undergoing myocardial revascularization: a preliminary report study. CP. 2009;47(02):78–88. doi: 10.5414/CPP47078. [DOI] [PubMed] [Google Scholar]
- 12.Svensjo E, Andersson KE, Bouskela E, Cyrino FZ, Lindgren S.. Effects of two vasodilatory phosphodiesterase inhibitors on bradykinin-induced permeability increase in the hamster cheek pouch. Agents Actions. 1993;39(1-2):35–41. doi: 10.1007/BF01975712. [DOI] [PubMed] [Google Scholar]
- 13.Wright LC, Seybold J, Robichaud A, Adcock IM, Barnes PJ.. Phosphodiesterase expression in human epithelial cells. Am J Physiol. 1998;275(4):L694–700. doi: 10.1152/ajplung.1998.275.4.L694. [DOI] [PubMed] [Google Scholar]
- 14.Surapisitchat J, Beavo JA.. Regulation of endothelial barrier function by cyclic nucleotides: the role of phosphodiesterases. Handb Exp Pharmacol. 2011;(204):193–210. doi: 10.1007/978-3-642-17969-3_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi CY, Khanh TH, Thoa Le PK, et al. . Milrinone therapy for enterovirus 71-induced pulmonary edema and/or neurogenic shock in children: a randomized controlled trial. Crit Care Med. 2013; 41(7):1754–1760. doi: 10.1097/CCM.0b013e31828a2a85. [DOI] [PubMed] [Google Scholar]
- 16.Beute J. (Oral) Enoximone in asthma. ERJ Open Res. 2020;6(4):00319–2020. doi: 10.1183/23120541.00319-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kereiakes D, Chatterjee K, Parmley WW, et al. . Intravenous and oral MDL 17043 (A new inotrope-vasodilator agent) in congestive heart failure: Hemodynamic and clinical evaluation in 38 patients. J Am Coll Cardiol. 1984;4(5):884–889. doi: 10.1016/S0735-1097(84)80047-9. [DOI] [PubMed] [Google Scholar]
- 18.Metra M, Eichhorn E, Abraham WT, et al. . Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur Heart J. 2009;30(24):3015–3026. doi: 10.1093/eurheartj/ehp338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furck AK, Bentley S, Bartsota M, Rigby ML, Slavik Z.. Oral Enoximone as an Alternative to Protracted Intravenous Medication in Severe Pediatric Myocardial Failure. Pediatr Cardiol. 2016; 37(7):1297–1301. doi: 10.1007/s00246-016-1433-4. [DOI] [PubMed] [Google Scholar]
- 20.Londino JD, Lazrak A, Collawn JF, Bebok Z, Harrod KS, Matalon S.. Influenza virus infection alters ion channel function of airway and alveolar cells: mechanisms and physiological sequelae. Am J Physiol Lung Cell Mol Physiol. 2017;313(5):L845–L58. doi: 10.1152/ajplung.00244.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Yu C, Yang F, Paganini-Hill A, Fisher MJ.. Phosphodiesterase inhibitor modulation of brain microvascular endothelial cell barrier properties. J Neurol Sci. 2012;320(1-2):45–51. doi: 10.1016/j.jns.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosutova P, Mikolka P, Balentova S, Adamkov M, Calkovska A, Mokra D.. Effects of PDE3 inhibitor olprinone on the respiratory parameters, inflammation, and apoptosis in an experimental model of acute respiratory distress syndrome. Int J Mol Sci. 2020; 21(9).doi: 10.3390/ijms21093382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cervin A, Lindgren S.. The effect of selective phosphodiesterase inhibitors on mucociliary activity in the upper and lower airways in vitro. Auris Nasus Larynx. 1998;25(3):269–276. doi: 10.1016/S0385-8146(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 24.Penmatsa H, Zhang W, Yarlagadda S, et al. . Compartmentalized cyclic adenosine 3',5'-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Mol Biol Cell. 2010;21(6):1097–1110. doi: 10.1091/mbc.e09-08-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Veilleux A, Zhang L, et al. . Dynamic activation of cystic fibrosis transmembrane conductance regulator by type 3 and type 4D phosphodiesterase inhibitors. J Pharmacol Exp Ther. 2005;314(2):846–854. doi: 10.1124/jpet.105.083519. [DOI] [PubMed] [Google Scholar]
- 26.Beute J, Ganesh K, Nastiti H, et al. . PDE3 inhibition reduces epithelial mast cell numbers in allergic airway inflammation and attenuates degranulation of basophils and mast cells. Front Pharmacol. 2020;11(470).doi: 10.3389/fphar.2020.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamant Z, Timmers MC, van der Veen H, Booms P, Sont JK, Sterk PJ.. Effect of an inhaled neutral endopeptidase inhibitor, thiorphan, on airway responsiveness to leukotriene D4 in normal and asthmatic subjects. Eur Respir J. 1994;7(3):459–466. doi: 10.1183/09031936.94.07030459. [DOI] [PubMed] [Google Scholar]
- 28.Ding S, Zhang J, Yin S, et al. . Inflammatory cytokines tumour necrosis factor-α and interleukin-8 enhance airway smooth muscle contraction by increasing L-type Ca2+ channel expression. Clin Exp Pharmacol Physiol. 2019; 46(1):56–64. doi: 10.1111/1440-1681.13030. [DOI] [PubMed] [Google Scholar]
- 29.Horby P, Lim WS, et al. . Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020.doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giorgi M, Cardarelli S, Ragusa F, et al. . Phosphodiesterase inhibitors: Could they be beneficial for the treatment of COVID-19? Int J Mol Sci. 2020; 21(15): 5338.doi: 10.3390/ijms21155338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalamaga M, Karampela I, Mantzoros CS.. Commentary: Phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Covid-19 in Metabolism. 2020;109(154282). doi: 10.1016/j.metabol.2020.154282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santaniello A, Vigone B, Beretta L.. Letter to the editor: Immunomodulation by phosphodiesterase-4 inhibitor in COVID-19 patients. Metabolism - Metabolism. 2020;110:154300. VolOpen AccessPublished:June 20, doi: 10.1016/j.metabol.2020.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hameid RA, Cormet-Boyaka E, Kuebler WM, Uddin M, Berdiev BK.. SARS-CoV-2 may hijack GPCR signaling pathways to dysregulate lung ion and fluid transport. Am J Physiol Lung Cell Mol Physiol. 2021. doi: 10.1152/ajplung.00499.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.