Abstract
Objective
To evaluate whether early initiation of prophylactic anticoagulation compared with no anticoagulation was associated with decreased risk of death among patients admitted to hospital with coronavirus disease 2019 (covid-19) in the United States.
Design
Observational cohort study.
Setting
Nationwide cohort of patients receiving care in the Department of Veterans Affairs, a large integrated national healthcare system.
Participants
All 4297 patients admitted to hospital from 1 March to 31 July 2020 with laboratory confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and without a history of anticoagulation.
Main outcome measures
The main outcome was 30 day mortality. Secondary outcomes were inpatient mortality, initiating therapeutic anticoagulation (a proxy for clinical deterioration, including thromboembolic events), and bleeding that required transfusion.
Results
Of 4297 patients admitted to hospital with covid-19, 3627 (84.4%) received prophylactic anticoagulation within 24 hours of admission. More than 99% (n=3600) of treated patients received subcutaneous heparin or enoxaparin. 622 deaths occurred within 30 days of hospital admission, 513 among those who received prophylactic anticoagulation. Most deaths (510/622, 82%) occurred during hospital stay. Using inverse probability of treatment weighted analyses, the cumulative incidence of mortality at 30 days was 14.3% (95% confidence interval 13.1% to 15.5%) among those who received prophylactic anticoagulation and 18.7% (15.1% to 22.9%) among those who did not. Compared with patients who did not receive prophylactic anticoagulation, those who did had a 27% decreased risk for 30 day mortality (hazard ratio 0.73, 95% confidence interval 0.66 to 0.81). Similar associations were found for inpatient mortality and initiation of therapeutic anticoagulation. Receipt of prophylactic anticoagulation was not associated with increased risk of bleeding that required transfusion (hazard ratio 0.87, 0.71 to 1.05). Quantitative bias analysis showed that results were robust to unmeasured confounding (e-value lower 95% confidence interval 1.77 for 30 day mortality). Results persisted in several sensitivity analyses.
Conclusions
Early initiation of prophylactic anticoagulation compared with no anticoagulation among patients admitted to hospital with covid-19 was associated with a decreased risk of 30 day mortality and no increased risk of serious bleeding events. These findings provide strong real world evidence to support guidelines recommending the use of prophylactic anticoagulation as initial treatment for patients with covid-19 on hospital admission.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (covid-19), continues to spread worldwide. Deaths among people with covid-19 have been partially attributed to venous thromboembolism and arterial thromboses.1 2 In intensive care settings, prevalence of venous thromboembolism among patients with covid-19 has been reported at about 30%.3 In response, several expert organizations, including the American Society of Hematology,4 International Society on Thrombosis and Haemostasis,5 CHEST Guideline and Expert Panel,6 and others1 7 have recommended prophylactic anticoagulation for patients admitted with covid-19, who do not have a contraindication to this treatment, to reduce the risk of thromboembolism.
Heparin based anticoagulants are commonly used in hospital settings. Given evidence that shows these drugs might also possess anti-inflammatory properties,8 9 10 heparin-based treatments might be particularly effective in patients with covid-19.11 Randomized clinical trials evaluating the efficacy of prophylactic anticoagulation in patients with covid-19 are underway.12 Previous observational cohort studies have found evidence that use of anticoagulation in patients with covid-19 was associated with decreased risk of mortality13 14; however, these studies were limited in sample size or used relatively small healthcare systems. In this study we estimated the effect of early initiation of prophylactic anticoagulation compared with no anticoagulation on risk of 30 day mortality among patients admitted to hospital with covid-19 in the largest integrated healthcare system in the United States.
Methods
This study is reported according to the strengthening the reporting of observational studies in epidemiology (STROBE) and reporting of studies conducted using observational routinely collected health data (RECORD) guidelines (see supplementary appendix).
Study design and population
We conducted an observational cohort study using electronic health record data from the US Department of Veterans Affairs, which comprises more than 1200 points of care nationwide, including hospitals, medical centers, and community outpatient clinics. All care is recorded in a central data repository, with daily uploads into the Veterans Affairs Corporate Data Warehouse. Available data include demographics, outpatient and inpatient encounters, diagnoses, procedures, smoking and alcohol health behaviors, pharmacy dispensing records, vital signs, laboratory measures, and death information.
We included all patients admitted to hospital between 1 March and 31 July 2020 who had a laboratory confirmed positive SARS-CoV-2 test result on or within 14 days before hospital admission. We excluded patients who had no history of care (defined as at least one outpatient or inpatient encounter in the two years before 1 March 2020), received anticoagulation treatment in the 30 days before hospital admission (to mitigate the effect of prevalent use of anticoagulation), received a red blood cell transfusion within 24 hours of admission (as active bleeding or severe anemia could have been a contraindication for anticoagulation), or experienced any outcome within 24 hours of admission and therefore did not have equal chance to be classified as having received anticoagulation in this study.
Types and doses of anticoagulants
We extracted inpatient pharmacy records for warfarin, intravenous heparin, low molecular weight heparin (enoxaparin, fondaparinux, dalteparin), and direct oral anticoagulants (apixaban, rivaroxaban, dabigatran). Doses and routes considered prophylactic and therapeutic anticoagulation are listed in supplementary box 1 in the appendix.
Anticoagulation, outcomes, and follow-up
We compared prophylactic anticoagulation in the first 24 hours of hospital admission with no receipt of anticoagulation in the same time frame. The primary outcome was mortality within 30 days of hospital admission, which included in-hospital deaths (those during hospital admission) and those that occurred after discharge. Secondary outcomes were inpatient mortality and initiation of therapeutic anticoagulation. Algorithms to identify thromboembolic events during hospital admission of patients with covid-19 have yet to be validated; thus, we considered initiation of therapeutic levels of anticoagulation after the first 24 hours of admission to be a proxy for clinical deterioration, including thromboembolic events. For all outcomes, we followed patients from date of hospital admission until the earliest date of the outcome, a maximum of 30 days, or 30 August 2020.
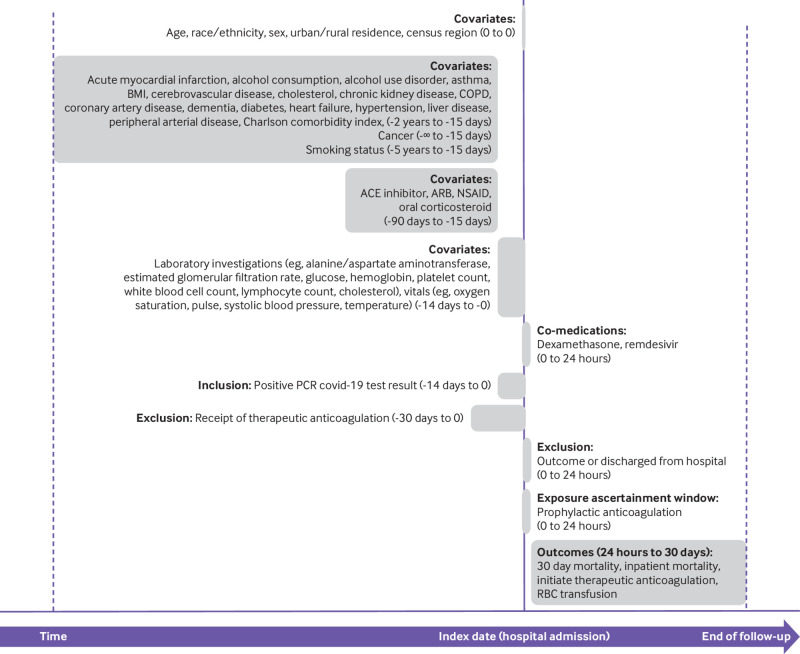
Some Veterans Affairs hospitals report observation periods and admissions separately, even when patients have not moved beds or changed providers. We combined these periods and considered a full hospital admission to begin at first presentation in a Veterans Affairs hospital and end when there was no subsequent hospital stay that began within 24 hours. Figure 1 shows the study design.
Fig 1.
Study design. End of follow-up for all outcomes in patients admitted to hospital with coronavirus disease 2019 (covid-19) was earliest of date of outcome, a maximum of 30 days, or 30 August 2020. For the analysis of inpatient mortality and initiating therapeutic anticoagulation, patients were further censored at date of hospital discharge. BMI=body mass index; COPD=chronic obstructive pulmonary disease; ACE=angiotensin converting enzyme; ARB=angiotensin II receptor blocker; NSAID=non-steroidal anti-inflammatory drug; PCR=polymerase chain reaction; RBC=red blood cell
Covariates
Potential confounders in the relation between prophylactic anticoagulation and covid-19 mortality or thromboembolic events were identified by review of the existing literature and discussions with clinicians. We extracted information on age, race, ethnicity, sex, urban or rural residence, US census region, clinical comorbidities, Charlson comorbidity index, and substance use. Presence of clinical comorbidities was determined by one inpatient or two outpatient diagnoses using ICD-9 or ICD-10 (international classification of diseases, ninth or 10th revision) codes in the two years before hospital admission, except for cancer, which was considered present if diagnosed ever before hospital admission. Level of alcohol consumption was calculated using the most recent alcohol use disorder identification test-consumption (AUDIT-C)15 measure within two years before admission. Smoking status was determined by the most frequent response in the five years before hospital admission.16
We ascertained drug history pertinent to the 15 to 90 days before hospital admission. To account for acute health status at hospital admission, we captured vital signs and laboratory measures. Body mass index was calculated from height and weight measurements closest to hospital admission within five and two years, respectively, before admission. All other vital signs and all laboratory measures utilized the value closest to hospital admission within 14 days before admission. Figure 1 provides further details on covariate ascertainment windows. Supplementary box 2 in the appendix provides a list of potentially relevant covariates that could not be included owing to unavailability or high levels of missingness, including inflammatory markers D dimer and C reactive protein.
To account for potential effects of co-medications with other covid-19 treatments, we ascertained whether patients had received oral or intravenous dexamethasone17 at any dose, or intravenous remdesivir18 at any dose within the first 24 hours of hospital admission as well as treatments received after the first 24 hours.
Covariates with the largest proportion of missing data included alanine aminotransferase (13.5%), aspartate aminotransferase (15.2%), lymphocyte count (15.0%), and total cholesterol (14.1%): all other covariates had less than 10% of data missing.
Propensity score model
We used inverse probability of treatment weighting to estimate the average treatment effect. This contrast was chosen because we excluded patients with contraindications to prophylactic anticoagulation, and thus assume all included patients were eligible for anticoagulation. We first modeled the probability of receiving anticoagulation as a function of all measured covariates (apart from in-hospital treatments received after the first 24 hours so as to not use future information at baseline).19 Propensity scores (ie, the predicted probability of exposure) were estimated using a multivariable logistic regression model. Of 45 variables in the model, only four had missingness between 10% and 15%; all others had 10% or less, and most (30 of 45 variables) were complete. We included a missing category for covariates with missing data given the variables with most missingness do not drive the decision to administer prophylactic anticoagulation, but rather are markers for general health severity. Under the additional assumption that associations between fully observed covariates and receipt of prophylactic anticoagulation did not differ across missingness patterns, this approach produces unbiased estimates.20 21 Each patient was weighted by the inverse probability of receiving the exposure of interest, with the goal of balancing observable characteristics, including missingness patterns,22 between treatment groups. After inverse probability of treatment weighting, the distribution of propensity scores between the treatment groups overlapped nearly perfectly (see supplementary eFigure 1). We calculated absolute standardized mean differences between treatment groups and considered 0.2 or less as balanced,23 although most were 0.1 or less (see supplementary eFigure 2). Thus, the weighting produced treatment groups that were considered well balanced.
Statistical analysis
Covariates were summarized using descriptive statistics, stratified by treatment group. We calculated the inverse probability of treatment weighted sample sizes by multiplying weights by constant factor k, where k was the ratio of observed sample size to number in the pseudopopulation after weighting; in this study, k=4297/8576. This scaling was performed to force the sample size in the pseudopopulation after weighting to equal the sample size in the observed population. We generated inverse probability of treatment weighted Kaplan-Meier plots. Cox regression models with days since hospital admission as the timescale were used to estimate inverse probability of treatment weighted hazard ratios and 95% confidence intervals for the effect of early initiation of prophylactic doses of anticoagulation compared with no anticoagulation on 30 day mortality, inpatient mortality, and initiating therapeutic anticoagulation. Both secondary outcomes occur during hospital admission, wherein discharge from hospital was considered a competing event. If patients were censored at discharge, absolute risks derived from Kaplan-Meier analyses would be overestimated.24 25 We therefore displayed cumulative incidence rates by treatment group, treating discharge as a competing event (ie, no censoring at date of discharge). As our question was causal in nature, we nonetheless chose to display cause specific rather than subdistribution hazard ratios.26 These cause specific hazard ratios were interpreted as the effect of prophylactic anticoagulation compared with no anticoagulation on each of the outcomes irrespective of the effect on discharge. Proportional hazards were checked by examining the complementary log-log (or the log of negative log) of estimated survivor functions for groups that received or did not receive prophylactic anticoagulation versus the log of survival time. No evidence was found of proportional hazards violations. We used Microsoft SQL Server Management Studio v17.4 for data management and SAS version 9.4 (SAS Institute, Cary, NC) and Stata 16 MP for statistical analyses.
Sensitivity analyses
For each outcome we used quantitative bias analysis to calculate an e-value, which demonstrates the strength of association between an unmeasured confounder and exposure or outcome, conditional on measured covariates that would be necessary to fully explain observed effects.27 To assess for undue effects from outliers with very high or very low estimated propensity of treatment, we capped propensity score distributions at the 1st and 99th centiles and again at the 5th and 95th centiles. To account for potentially biased estimation of standard errors or influence from very high or very low weights, we performed sensitivity analyses using combinations of robust standard error estimation28 and stabilized weighting.29 We re-ran the primary analyses extending the exposure ascertainment window from 24 to 48 hours. Given the low frequency for use of direct oral anticoagulants in the cohort, we re-ran analyses excluding these drugs from the treated group.
Post hoc analyses
In post hoc analyses, we assessed the effect of prophylactic anticoagulation separately by the two most prescribed drugs in the cohort: subcutaneous heparin and enoxaparin. We stratified the primary model by whether patients were admitted to the intensive care unit (ICU) within the first 24 hours of hospital admission. To investigate safety associated with prophylactic anticoagulation, we fitted an additional model with bleeding that required red blood cell transfusion as the outcome.
Patient and public involvement
Patients were not consulted during the initial design of the study. However, in the course of peer review, we received thoughtful comments from a patient and carer and revised our manuscript accordingly. In addition, we engaged with the Veterans Affairs central office and chief medical officers during the entire course of this research.
Results
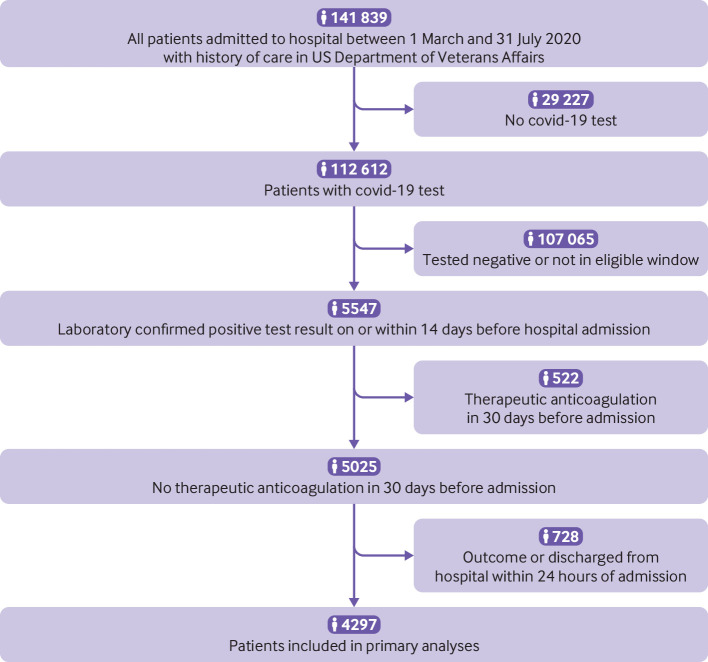
Overall, 4297 patients admitted to hospital with covid-19 between 1 March and 31 July 2020 were included in this analysis (fig 2). Median age in the cohort was 68 years (interquartile range 58-75 years); most patients were of non-Hispanic black (n=1940, 45.1%), non-Hispanic white (n=1603, 37.3%), and Hispanic (n=506, 11.8%) race or ethnicity. Most of the patients were men (n=4015, 93.4%), located in the south (n=2017, 46.9%), and resident in an urban area (n=3768, 87.7%) (table 1). More patients were admitted to hospital in July (n=1401, 32.6%) than any other month.
Fig 2.
Flow chart of patients admitted to hospital with coronavirus disease 2019 (covid-19)
Table 1.
Personal and clinical characteristics of 4297 patients admitted to hospital with coronavirus disease 2019 (covid-19) who received prophylactic anticoagulation within 24 hours of hospital admission, before and after weighting. Values are numbers (percentages) unless stated otherwise
| Characteristics | Unweighted | IPT weighted* | |||||
|---|---|---|---|---|---|---|---|
| No anticoagulation (n=670) | Prophylactic anticoagulation (n=3627) | SMD | No anticoagulation (n=2141) | Prophylactic anticoagulation (n=2156) | SMD | ||
| Personal characteristics | |||||||
| Median (interquartile range) age (years) | 69.0 (58.0-76.5) | 68.1 (58.2-74.8) | 0.04 | 69.4 (59.7-76.5) | 68.3 (58.2-75.0) | 0.04 | |
| Age groups (years): | |||||||
| 20-49 | 89 (13.3) | 446 (12.3) | 0.03 | 239 (11.2) | 265 (12.3) | 0.04 | |
| 50-59 | 105 (15.7) | 619 (17.1) | 0.04 | 313 (14.6) | 363 (16.8) | 0.06 | |
| 60-69 | 161 (24.0) | 951 (26.2) | 0.05 | 554 (25.9) | 558 (25.9) | 0.00 | |
| 70-79 | 188 (28.1) | 1056 (29.1) | 0.02 | 652 (30.5) | 626 (29.0) | 0.03 | |
| ≥80 | 127 (19.0) | 555 (15.3) | 0.10 | 383 (17.9) | 343 (15.9) | 0.05 | |
| Race or ethnicity: | |||||||
| White | 256 (38.2) | 1347 (37.1) | 0.02 | 832 (38.9) | 806 (37.4) | 0.03 | |
| Black | 291 (43.4) | 1649 (45.5) | 0.04 | 940 (43.9) | 974 (45.2) | 0.03 | |
| Hispanic | 74 (11.0) | 432 (11.9) | 0.03 | 238 (11.1) | 252 (11.7) | 0.02 | |
| Other | 22 (3.3) | 102 (2.8) | 0.03 | 76 (3.6) | 63 (2.9) | 0.04 | |
| Unknown | 27 (4.0) | 97 (2.7) | 0.08 | 55 (2.6) | 61 (2.8) | 0.02 | |
| Men | 620 (92.5) | 3395 (93.6) | 0.04 | 2019 (94.3) | 2014 (93.4) | 0.04 | |
| Urban residence | 587 (87.6) | 3181 (87.7) | 0.00 | 1915 (89.5) | 1893 (87.8) | 0.05 | |
| Census region: | |||||||
| Midwest | 79 (11.8) | 724 (20.0) | 0.22 | 343 (16.0) | 400 (18.6) | 0.07 | |
| Northeast | 139 (20.7) | 622 (17.1) | 0.09 | 432 (20.2) | 383 (17.8) | 0.06 | |
| South | 314 (46.9) | 1703 (47.0) | 0.00 | 998 (46.6) | 1012 (46.9) | 0.01 | |
| West | 138 (20.6) | 578 (15.9) | 0.12 | 367 (17.2) | 361 (16.7) | 0.01 | |
| Month of admission: | |||||||
| March | 116 (17.3) | 518 (14.3) | 0.08 | 323 (15.1) | 316 (14.7) | 0.01 | |
| April | 169 (25.2) | 868 (23.9) | 0.03 | 491 (22.9) | 522 (24.2) | 0.03 | |
| May | 70 (10.4) | 429 (11.8) | 0.04 | 277 (12.9) | 250 (11.6) | 0.04 | |
| June | 110 (16.4) | 616 (17.0) | 0.02 | 361 (16.9) | 364 (16.9) | 0.00 | |
| July | 205 (30.6) | 1196 (33.0) | 0.05 | 689 (32.2) | 704 (32.6) | 0.01 | |
| Clinical conditions | |||||||
| Acute myocardial infarction | 11 (1.6) | 66 (1.8) | 0.01 | 45 (2.1) | 39 (1.8) | 0.02 | |
| Asthma | 33 (4.9) | 176 (4.9) | 0.00 | 117 (5.5) | 105 (4.9) | 0.03 | |
| Cancer, any | 97 (14.5) | 494 (13.6) | 0.02 | 318 (14.9) | 298 (13.8) | 0.03 | |
| Cerebrovascular disease | 85 (12.7) | 369 (10.2) | 0.08 | 223 (10.4) | 230 (10.7) | 0.01 | |
| Chronic kidney disease | 136 (20.3) | 694 (19.1) | 0.03 | 436 (20.4) | 421 (19.5) | 0.02 | |
| COPD | 105 (15.7) | 544 (15.0) | 0.02 | 359 (16.8) | 328 (15.2) | 0.04 | |
| Coronary artery disease | 25 (3.7) | 90 (2.5) | 0.07 | 65 (3.0) | 59 (2.7) | 0.02 | |
| Dementia | 104 (15.5) | 378 (10.4) | 0.15 | 261 (12.2) | 244 (11.3) | 0.03 | |
| Diabetes | 269 (40.1) | 1573 (43.4) | 0.07 | 859 (40.1) | 924 (42.9) | 0.06 | |
| Heart failure | 77 (11.5) | 375 (10.3) | 0.04 | 265 (12.4) | 232 (10.8) | 0.05 | |
| Hypertension | 446 (66.6) | 2470 (68.1) | 0.03 | 1380 (64.5) | 1462 (67.8) | 0.07 | |
| Liver disease | 71 (10.6) | 322 (8.9) | 0.06 | 209 (9.8) | 199 (9.2) | 0.02 | |
| Peripheral arterial disease | 70 (10.4) | 387 (10.7) | 0.01 | 236 (11.0) | 229 (10.6) | 0.01 | |
| Charlson comorbidity index score: | |||||||
| 0 | 130 (19.4) | 765 (21.1) | 0.04 | 404 (18.9) | 450 (20.9) | 0.05 | |
| 1 | 110 (16.4) | 723 (19.9) | 0.09 | 388 (18.1) | 423 (19.6) | 0.04 | |
| 2 | 119 (17.8) | 657 (18.1) | 0.01 | 403 (18.8) | 384 (17.8) | 0.03 | |
| 3 | 74 (11.0) | 394 (10.9) | 0.01 | 252 (11.8) | 235 (10.9) | 0.03 | |
| 4 | 69 (10.3) | 324 (8.9) | 0.05 | 217 (10.2) | 194 (9.0) | 0.04 | |
| ≥5 | 168 (25.1) | 764 (21.1) | 0.10 | 476 (22.2) | 470 (21.8) | 0.01 | |
| Drug history | |||||||
| ACE inhibitor | 119 (17.8) | 807 (22.2) | 0.11 | 422 (19.7) | 463 (21.5) | 0.04 | |
| ARB | 78 (11.6) | 481 (13.3) | 0.05 | 261 (12.2) | 283 (13.1) | 0.03 | |
| NSAID | 144 (21.5) | 731 (20.2) | 0.03 | 408 (19.1) | 438 (20.3) | 0.03 | |
| Oral corticosteroid | 156 (23.3) | 875 (24.1) | 0.02 | 514 (24.0) | 516 (24.0) | 0.00 | |
| In-hospital treatments | |||||||
| Dexamethasone: | |||||||
| <24 hours | 74 (11.0) | 588 (16.2) | 0.15 | 309 (14.4) | 332 (15.4) | 0.03 | |
| >24 hours | 115 (17.2) | 892 (24.6) | 0.18 | 463 (21.6) | 508 (23.6) | 0.05 | |
| Remdesivir: | |||||||
| <24 hours | 35 (5.2) | 437 (12.0) | 0.24 | 204 (9.5) | 236 (10.9) | 0.05 | |
| >24 hours | 89 (13.3) | 791 (21.8) | 0.23 | 341 (15.9) | 447 (20.7) | 0.12 | |
| Substance use | |||||||
| Alcohol consumption status: | |||||||
| Abstinent | 51 (7.6) | 300 (8.3) | 0.02 | 178 (8.3) | 177 (8.2) | 0.00 | |
| Low risk | 360 (53.7) | 1831 (50.5) | 0.07 | 1059 (49.4) | 1098 (50.9) | 0.03 | |
| At risk | 148 (22.1) | 965 (26.6) | 0.11 | 574 (26.8) | 557 (25.8) | 0.02 | |
| Hazardous | 28 (4.2) | 161 (4.4) | 0.01 | 79 (3.7) | 94 (4.4) | 0.03 | |
| Alcohol use disorder | 3 (0.4) | 19 (0.5) | 0.01 | 11 (0.5) | 11 (0.5) | 0.00 | |
| Missing | 80 (11.9) | 351 (9.7) | 0.07 | 241 (11.3) | 219 (10.1) | 0.04 | |
| Smoking status: | |||||||
| Never | 17 (2.5) | 63 (1.7) | 0.06 | 42 (2.0) | 41 (1.9) | 0.00 | |
| Former | 258 (38.5) | 1431 (39.5) | 0.02 | 753 (35.1) | 842 (39.1) | 0.08 | |
| Current | 225 (33.6) | 1355 (37.4) | 0.08 | 905 (42.3) | 793 (36.8) | 0.11 | |
| Missing | 170 (25.4) | 778 (21.5) | 0.09 | 442 (20.6) | 479 (22.2) | 0.04 | |
| Vital signs | |||||||
| Body mass index: | |||||||
| <26 | 206 (30.7) | 938 (25.9) | 0.11 | 630 (29.4) | 576 (26.7) | 0.06 | |
| 26-32 | 258 (38.5) | 1436 (39.6) | 0.02 | 940 (43.9) | 854 (39.6) | 0.09 | |
| ≥33 | 169 (25.2) | 1113 (30.7) | 0.12 | 494 (23.1) | 637 (29.5) | 0.15 | |
| Missing | 37 (5.5) | 140 (3.9) | 0.08 | 78 (3.6) | 89 (4.1) | 0.03 | |
| Oxygen saturation (%): | |||||||
| <93 | 72 (10.7) | 582 (16.0) | 0.16 | 315 (14.7) | 329 (15.2) | 0.02 | |
| 93-96 | 182 (27.2) | 1147 (31.6) | 0.10 | 669 (31.3) | 666 (30.9) | 0.01 | |
| ≥96 | 396 (59.1) | 1775 (48.9) | 0.21 | 1077 (50.3) | 1091 (50.6) | 0.01 | |
| Missing | 20 (3.0) | 123 (3.4) | 0.02 | 80 (3.7) | 71 (3.3) | 0.02 | |
| Pulse (beats/min): | |||||||
| <90 | 438 (65.4) | 2200 (60.7) | 0.10 | 1327 (62.0) | 1327 (61.6) | 0.01 | |
| ≥90 | 232 (34.6) | 1427 (39.3) | 0.10 | 814 (38.0) | 828 (38.4) | 0.01 | |
| Systolic blood pressure (mm Hg): | |||||||
| <140 | 446 (66.6) | 2360 (65.1) | 0.03 | 1347 (62.9) | 1405 (65.2) | 0.05 | |
| ≥140 | 224 (33.4) | 1267 (34.9) | 0.03 | 794 (37.1) | 751 (34.8) | 0.05 | |
| Temperature (°C): | |||||||
| ≤37 | 356 (53.1) | 1701 (46.9) | 0.12 | 1045 (48.8) | 1033 (47.9) | 0.02 | |
| 37-37.9 | 244 (36.4) | 1292 (35.6) | 0.02 | 737 (34.4) | 771 (35.8) | 0.03 | |
| ≥38 | 70 (10.4) | 634 (17.5) | 0.20 | 359 (16.8) | 352 (16.3) | 0.01 | |
IPT=inverse probability of treatment; SMD=absolute value of the standardized mean difference; COPD=chronic obstructive pulmonary disease; ACE=angiotensin converting enzyme; ARB=angiotensin II receptor blocker; NSAID=non-steroidal anti-inflammatory drug.
Counts after IPT weighting were calculated by multiplying weights by constant factor k, where k was the ratio of observed sample size to number in the pseudopopulation after weighting; in this study, k=4297/8576.
In this cohort, 3627 (84.4%) patients received prophylactic anticoagulation within 24 hours of hospital admission. Among those who received prophylactic anticoagulation, the most common drugs were heparin based: either subcutaneous heparin (n=1094, 30.2%) or enoxaparin (n=2506, 69.1%).
At hospital presentation, the group of patients who received prophylactic anticoagulation, compared with the group of patients who did not, had a higher proportion with an oxygen saturation level less than 93% (table 1; 16.0% v 10.7%), heart rate at 90 beats/min or higher (39.3% v 34.6%), and temperature of 38°C or higher (17.5% v 10.4%). In contrast, the burden of prevalent comorbid disease (Charlson comorbidity index score ≥5) was lower among those who received prophylactic anticoagulation compared with those who did not (21.1% v 25.1%). Treatment for covid-19 using other drugs within the first 24 hours of hospital admission were more common among those who received prophylactic anticoagulation compared with those who did not (16.2% v 11.0% for dexamethasone; 12.0% v 5.2% for remdesivir). After inverse probability of treatment weighting, however, differences were minimized between the two treatment groups (all standardized mean differences ≤0.2, with most ≤0.1; table 1 and table 2 and supplementary eFigure 2).
Table 2.
Laboratory findings in 4297 patients admitted to hospital with coronavirus disease 2019 (covid-19) who received prophylactic anticoagulation within 24 hours of hospital admission, before and after weighting
| Laboratory findings | Unweighted | IPT weighted* | |||||
|---|---|---|---|---|---|---|---|
| No anticoagulation (n=670) | Prophylactic anticoagulation (n=3627) | SMD | No anticoagulation (n=2141) | Prophylactic anticoagulation (n=2156) | SMD | ||
| Alanine aminotransferase (U/L): | |||||||
| ≤30 | 297 (44.3) | 1722 (47.5) | 0.06 | 1081 (50.5) | 1014 (47.0) | 0.07 | |
| >30 | 241 (36.0) | 1456 (40.1) | 0.09 | 751 (35.1) | 847 (39.3) | 0.09 | |
| Missing | 132 (19.7) | 449 (12.4) | 0.20 | 310 (14.5) | 295 (13.7) | 0.02 | |
| Aspartate aminotransferase (U/L): | |||||||
| ≤30 | 225 (33.6) | 1171 (32.3) | 0.03 | 717 (33.5) | 701 (32.5) | 0.02 | |
| >30 | 301 (44.9) | 1949 (53.7) | 0.18 | 1056 (49.3) | 1124 (52.1) | 0.06 | |
| Missing | 144 (21.5) | 507 (14.0) | 0.20 | 367 (17.2) | 330 (15.3) | 0.05 | |
| eGFR (mL/min/1.73 m2): | |||||||
| ≥60 | 354 (52.8) | 2034 (56.1) | 0.07 | 1164 (54.4) | 1196 (55.5) | 0.02 | |
| 30-59 | 159 (23.7) | 957 (26.4) | 0.06 | 555 (25.9) | 559 (25.9) | 0.00 | |
| <30 | 82 (12.2) | 418 (11.5) | 0.02 | 274 (12.8) | 252 (11.7) | 0.03 | |
| Missing | 75 (11.2) | 218 (6.0) | 0.19 | 147 (6.9) | 149 (6.9) | 0.00 | |
| Glucose (mmol/L): | |||||||
| ≤9.44 | 486 (72.5) | 2766 (76.3) | 0.09 | 1636 (76.4) | 1628 (75.5) | 0.02 | |
| >9.44 | 135 (20.1) | 759 (20.9) | 0.02 | 426 (19.9) | 449 (20.8) | 0.02 | |
| Missing | 49 (7.3) | 102 (2.8) | 0.21 | 79 (3.7) | 78 (3.6) | 0.00 | |
| Hemoglobin (g/L): | |||||||
| ≤140 | 387 (57.8) | 2151 (59.3) | 0.03 | 1248 (58.3) | 1276 (59.2) | 0.02 | |
| >140 | 231 (34.5) | 1353 (37.3) | 0.06 | 812 (37.9) | 792 (36.7) | 0.02 | |
| Missing | 52 (7.8) | 123 (3.4) | 0.19 | 81 (3.8) | 88 (4.1) | 0.02 | |
| Platelet count (×109/L): | |||||||
| ≤230 | 421 (62.8) | 2433 (67.1) | 0.09 | 1472 (68.8) | 1430 (66.3) | 0.05 | |
| >230 | 195 (29.1) | 1097 (30.2) | 0.02 | 594 (27.7) | 647 (30.0) | 0.05 | |
| Missing | 54 (8.1) | 97 (2.7) | 0.24 | 75 (3.5) | 78 (3.6) | 0.01 | |
| White blood cell count (×109/L): | |||||||
| ≤6 | 299 (44.6) | 1747 (48.2) | 0.07 | 1038 (48.5) | 1025 (47.6) | 0.02 | |
| >6 | 319 (47.6) | 1786 (49.2) | 0.03 | 1031 (48.2) | 1054 (48.9) | 0.02 | |
| Missing | 52 (7.8) | 94 (2.6) | 0.24 | 72 (3.4) | 76 (3.5) | 0.01 | |
| Lymphocyte count (×109/L): | |||||||
| ≤0.6 | 100 (14.9) | 600 (16.5) | 0.04 | 353 (16.5) | 352 (16.3) | 0.01 | |
| >0.6 | 431 (64.3) | 2523 (69.6) | 0.11 | 1428 (66.7) | 1479 (68.6) | 0.04 | |
| Missing | 139 (20.7) | 504 (13.9) | 0.18 | 359 (16.8) | 326 (15.1) | 0.05 | |
| Total cholesterol (mmol/L): | |||||||
| ≤3.37 | 152 (22.7) | 678 (18.7) | 0.10 | 398 (18.6) | 414 (19.2) | 0.02 | |
| >3.37 | 415 (61.9) | 2444 (67.4) | 0.11 | 1410 (65.9) | 1435 (66.6) | 0.01 | |
| Missing | 103 (15.4) | 505 (13.9) | 0.04 | 333 (15.6) | 307 (14.2) | 0.04 | |
| HDL cholesterol (mmol/L): | |||||||
| <0.98 | 180 (26.9) | 917 (25.3) | 0.04 | 551 (25.7) | 548 (25.4) | 0.01 | |
| 0.98-1.28 | 202 (30.1) | 1213 (33.4) | 0.07 | 719 (33.6) | 710 (33.0) | 0.01 | |
| ≥1.29 | 181 (27.0) | 988 (27.2) | 0.01 | 535 (25.0) | 587 (27.2) | 0.05 | |
| Missing | 107 (16.0) | 509 (14.0) | 0.05 | 337 (15.7) | 311 (14.4) | 0.04 | |
| Triglycerides (mmol/L): | |||||||
| <1.55 | 58 (8.7) | 310 (8.5) | 0.00 | 175 (8.2) | 183 (8.5) | 0.01 | |
| 1.55-2.32 | 133 (19.9) | 628 (17.3) | 0.07 | 362 (16.9) | 385 (17.9) | 0.02 | |
| ≥2.33 | 370 (55.2) | 2165 (59.7) | 0.09 | 1259 (58.8) | 1268 (58.8) | 0.00 | |
| Missing | 109 (16.3) | 524 (14.4) | 0.05 | 344 (16.1) | 319 (14.8) | 0.03 | |
IPT=inverse probability of treatment; SMD=absolute value of the standardized mean difference; eGFR=estimated glomerular filtration rate; HDL=high density lipoprotein.
Counts after IPT weighting were calculated by multiplying weights by constant factor k, where k was the ratio of observed sample size to number in the pseudopopulation after weighting; in this study, k=4297/8576.
Absolute and relative risks
Overall, 622 deaths (622/4297, 14.5%) occurred within 30 days of hospital admission, 513 among those who received prophylactic anticoagulation (table 3). Most deaths (510/622, 82%) occurred during hospital admission. In inverse probability of treatment weighted analyses, the cumulative incidence of mortality at 30 days was 14.3% (95% confidence interval 13.1% to 15.5%) for patients receiving prophylactic anticoagulation and 18.7% (15.1% to 22.9%) for those receiving no anticoagulation (table 3). Receiving prophylactic anticoagulation was associated with a 27% decreased risk of death over the first 30 days (hazard ratio 0.73, 95% confidence interval 0.66 to 0.81; table 3 and fig 3) compared with receiving no anticoagulation. Similar associations were found for inpatient mortality (0.69, 0.61 to 0.77) and initiating therapeutic anticoagulation (0.81, 0.73 to 0.90).
Table 3.
Absolute and relative risks associated with prophylactic anticoagulation in the first 24 hours of hospital admission with coronavirus disease 2019
| Outcomes | No of patients | No of events | Unweighted | IPT weighted | ||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Cumulative incidence (95% CI) | Hazard ratio (95% CI) | ||||
| 30 day mortality: | ||||||
| Prophylactic anticoagulation | 3627 | 513 | 0.85 (0.69 to 1.05) | 14.3 (13.1 to 15.5) | 0.73 (0.66 to 0.81) | |
| No anticoagulation | 670 | 109 | Ref | 18.7 (15.1 to 22.9) | Ref | |
| Inpatient mortality: | ||||||
| Prophylactic anticoagulation | 3627 | 418 | 0.82 (0.66 to 1.03) | 11.7 (10.7 to 12.8) | 0.69 (0.61 to 0.77) | |
| No anticoagulation | 670 | 92 | Ref | 16.4 (13.0 to 20.5) | Ref | |
| Initiating therapeutic anticoagulation: | ||||||
| Prophylactic anticoagulation | 3627 | 573 | 1.14 (0.91 to 1.42) | 15.6 (14.4 to 16.8) | 0.81 (0.73 to 0.90) | |
| No anticoagulation | 670 | 92 | Ref | 18.8 (15.2 to 23.1) | Ref | |
IPT=inverse probability of treatment.
Fig 3.
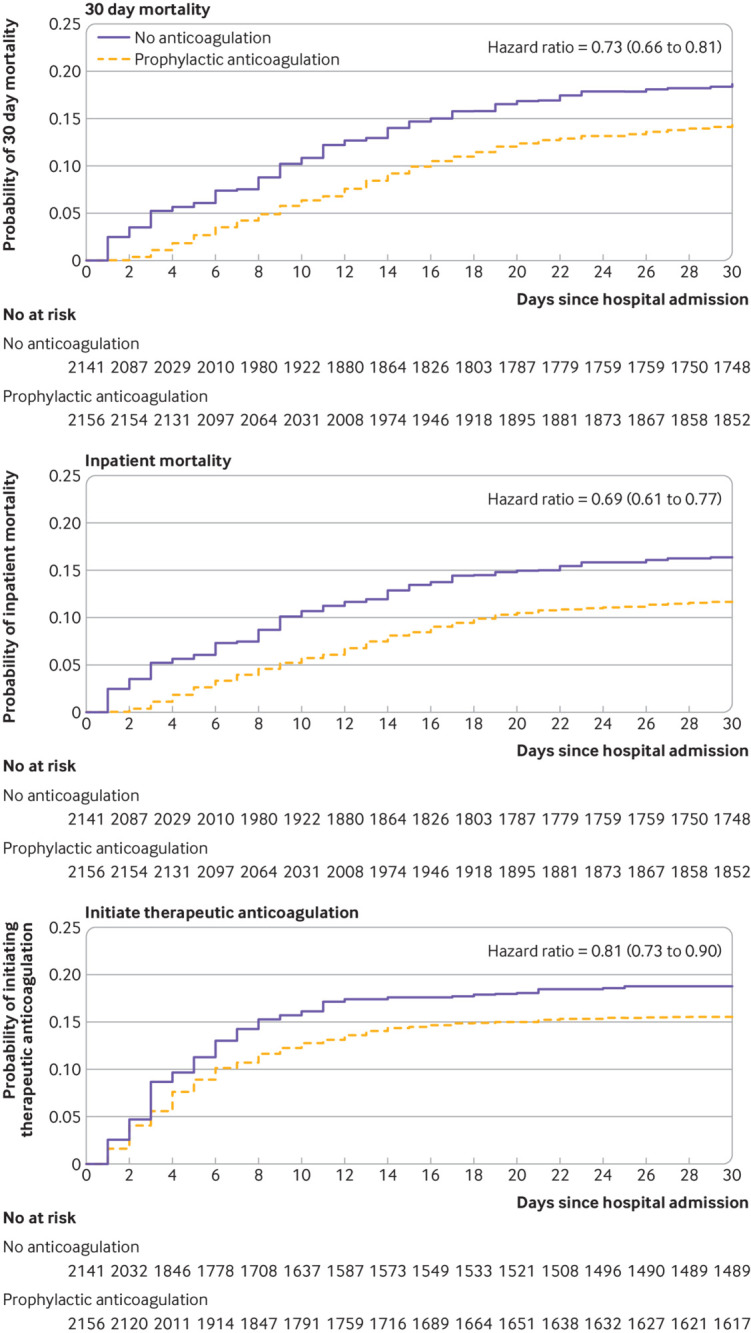
Inverse probability treatment weighted Kaplan-Meier plots. Numbers at risk were calculated by multiplying weights by constant factor k, where k was the ratio of observed sample size to number in the pseudopopulation after inverse probability treatment weighting; in this study, k=4297/8576
Sensitivity analyses
Quantitative bias analysis showed that an unmeasured confounder would need to be strongly associated with receiving prophylactic anticoagulation and each outcome to explain the observed associations: lower 95% confidence limit for e-value was 1.77 for 30 day mortality, 1.92 for inpatient mortality, and 1.46 for initiating therapeutic anticoagulation (supplementary eFigure 3). Results were robust to capping propensity scores (supplementary eTable 1), using stabilized weighting and robust variance estimation (supplementary eTable 2), extending the window for anticoagulation from 24 to 48 hours (supplementary eTable 3), and excluding direct oral anticoagulants from the exposure definition (supplementary eTable 4).
Post hoc analyses
The effect of prophylactic anticoagulation compared with no anticoagulation on 30 day mortality was similar when stratified by whether patients received subcutaneous heparin (hazard ratio 0.73, 95% confidence interval 0.64 to 0.84) or enoxaparin (0.78, 0.68 to 0.89; supplementary eTable 5). Some evidence suggested that the effect of prophylactic anticoagulation compared with no anticoagulation on 30 day mortality differed among patients who were admitted to the ICU within the first 24 hours of hospital admission (admitted to ICU: 0.91, 0.76 to 1.09; not admitted to ICU: 0.68, 0.60 to 0.77; P=0.009). Bleeding events that required transfusion were relatively rare (n=198, 4.6%). Prophylactic anticoagulation compared with no anticoagulation was not associated with an increased risk of bleeding events that required transfusions (0.87, 0.71 to 1.05).
Discussion
In a nationwide cohort of 4297 patients admitted to hospital with covid-19 in the largest integrated healthcare system in the United States, initiation of predominantly heparin based prophylactic anticoagulation compared with no anticoagulation within the first 24 hours of admission was associated with a relative risk reduction of 30 day mortality as high as 34% and an absolute risk reduction of 4.4% in the context of an absolute risk of 18.7% among patients who did not receive anticoagulation. These results persisted in sensitivity analyses. The evidence of benefit was strongest among patients not admitted to the ICU within the first 24 hours of admission. Additionally, severe bleeding as measured by requirement for blood transfusions was a relatively rare event and not associated with receipt of prophylactic anticoagulation. We observed similar protective effects for secondary outcomes, including inpatient mortality and initiation of therapeutic anticoagulation—a proxy for clinical deterioration that included thromboembolic events.
Comparison with other evidence
Results from previous studies investigating the role of anticoagulation among patients with covid-19 have varied.13 14 30 31 32 33 34 35 36 37 Variations in reported associations probably derive from different definitions of anticoagulation, for both drug type and dose. Additionally, different patient populations (eg, disease specific cohorts), comparator groups, and inclusion and exclusion criteria were used. One of the largest observational studies to date reported that both prophylactic and therapeutic anticoagulation were associated with a reduction in inpatient mortality by up to 55% compared with no anticoagulation across five hospitals in New York City.13 However, the study allowed patients to switch treatment groups during follow-up without comprehensively accounting for time updated confounding by indication that could have affected the results. Recently, interim results from three aggregated randomized trials encompassing 1000 inpatients with covid-19 reported study discontinuation because therapeutic doses of anticoagulation were associated with a reduction in the rate of ventilation or organ supportive interventions compared with prophylactic doses of anticoagulation.38 The interim results did not report the effect of anticoagulation on mortality, venous thromboembolism, or bleeding. Although consistent with the aggregated trial results suggesting therapeutic benefit for anticoagulation, our findings on more than 4000 inpatients extend this aggregated study suggesting benefit from prophylactic doses of anticoagulation compared with no anticoagulation, primarily in patients not requiring ICU level care. Further, we report associations with mortality and treatment intensification as well as safety data on serious bleeding events.
Our study was designed to emulate a hypothetical clinical trial, in which we used inverse probability treatment weighting to balance the distribution of covariates between treatment groups at hospital admission (analogous to randomization), defined eligibility through inclusion and exclusion criteria (eg, removed prevalent users and those with contraindications), defined time zero as the initiation of prophylactic anticoagulation, and assigned patients into treatment groups based on dose specific use of anticoagulation within 24 hours of admission. We conducted an intention-to-treat analysis because the data required to account for time updated exposures and confounders (eg, marginal structural modeling) may not be available for acute hospital admissions. For example, many hospital systems report all diagnoses that occur during a given hospital admission at discharge. Therefore, we could not determine if initiation of prophylactic anticoagulation later in hospital admission confers benefit. Multiple clinical trials are in progress to determine dosing and timing for anticoagulation during the clinical course of covid-19.12 Until clinical trial data are available, our results provide strong evidence for the use of prophylactic anticoagulation as initial treatment for patients with covid-19 on hospital admission.
Thromboembolic events in the context of covid-19 are strongly associated with mortality.30 36 39 40 41 The cause of elevated thrombosis risk remains unclear, although proposed mechanisms have included systemic inflammation, endothelialitis, and activation of the complement system.42 43 44 Increases in a variety of inflammatory pathways, including bradykinin, interleukin 6, C reactive protein, and growth differentiation factor 15, have been described in covid-19.10 11 45 46 47 48 49 50 51 52 53 54 Further, heparin has been shown to block the SARS-CoV-2 viral spike protein from binding in experimental studies.55 56 57 We postulate that the combination of the known antithrombotic and potential anti-inflammatory effects of heparin,8 9 in addition to attenuation of viral infectivity might, at least in part, explain the observed benefit associated with prophylactic anticoagulation.
Strengths and limitations of this study
Although this study has many strengths, including the availability of detailed, longitudinal, electronic health record data on a nationwide cohort of patients admitted to hospital with covid-19, the use of rigorous pharmacoepidemiological methodology, and findings that were robust to sensitivity analyses, we recognize possible limitations. First, owing to the observational nature of the study, a degree of uncertainty persists that can only be addressed through randomized trials, which have the benefit of blinding and prospective, standardized measurement of patient characteristics, cotreatments, and outcomes. Nonetheless, we took several steps to mitigate potential confounding. We comprehensively accounted for chronic and acute health conditions at hospital admission in addition to other potential covid-19 treatments to achieve balance of these potential confounders between treatment groups. Further, we demonstrated that our results were robust to unmeasured confounding using quantitative bias analysis, which showed that an unmeasured confounder would need to be strongly associated with receipt of prophylactic anticoagulation and each of the outcomes considered to explain the observed effects. Second, information on cause of death was not available at the time of analysis. However, based on single center reports,58 we suspect that venous and arterial thrombosis are contributing causes of death. Third, a validated algorithm to directly identify thromboembolic events as an outcome was not available. We acknowledge that the initiation of therapeutic anticoagulation might occur as a result of many reported complications of covid-19, including venous thromboembolism, arterial thromboembolism, cardiac arrhythmia, and disseminated intravascular coagulation.59 60 61 62 63 64 65 66 We surmised that an intensification of anticoagulation indicated an adverse change in clinical condition, including these events. Fourth, this study was conducted on veterans currently receiving care in the Veterans Affairs healthcare system, who are older and have a higher prevalence of chronic health conditions and risk behaviors than the general US population.67 68 69 However, previous research has established that after adjusting for age, sex, race, ethnicity, region, and residence type, all of which were accounted for in this study, total disease burden between veterans and non-veterans does not differ.69 In separate analyses, we developed a predictive index based on Veterans Affairs data70 and have since shown that the risk of covid-19 mortality associated with age, sex, and comorbid disease diagnoses that we observed in Veterans Affairs data was consistent across other academic and national healthcare samples in the US.71 Our key finding in the current analysis has also been shown in smaller, non-veteran healthcare systems13 14; thus, effects reported in this study are probably generalizable to the wider US population. Fifth, although individuals in Veterans Affairs care represent a diversity of backgrounds, women represented a small proportion of the sample population and thus results might not be generalizable to women. Sixth, some risk factors for venous thromboembolism were not included in this dataset because they were unavailable or had high levels of missingness—for example, immobility, paresis, and biomarkers, including D dimer, C reactive protein, and fibrinogen.72 Finally, we excluded patients with no history of care in the two years before study start; this only accounted for 2% of eligible patients, however, and therefore probably did not have an impact on our observed results.
Conclusions and implications
We studied a nationwide cohort of patients admitted to hospital with covid-19 and found that initiation of prophylactic, heparin based anticoagulation compared with no anticoagulation within 24 hours of admission was associated with a lower risk of 30 day mortality, in-hospital mortality, and initiation of therapeutic anticoagulation probably indicative of clinical deterioration, including thromboembolic events. This benefit seemed to be greater among patients not admitted to the ICU within 24 hours of hospital admission. Early initiation of prophylactic anticoagulation was not associated with an increased risk of bleeding that required transfusion. Our results provide strong real world evidence to support guidelines recommending the use of prophylactic anticoagulation as initial treatment for patients with covid-19 on hospital admission.
What is already known on this topic
Deaths among patients with coronavirus disease 2019 (covid-19) are partially attributed to venous thromboembolism and arterial thromboses
Anticoagulants prevent thrombosis formation, possess antiviral and potentially anti-inflammatory properties, and might be particularly effective in patients with covid-19
Evaluations of the efficacy of prophylactic anticoagulation in patients with covid-19 in randomized clinical trials are underway but not yet reported; previous observational studies have been limited in sample size or used relatively small healthcare systems
What this study adds
This study found that initiation of prophylactic anticoagulation compared with no anticoagulation within 24 hours of hospital admission was associated with a relative risk reduction of 30 day mortality as high as 34% and an absolute risk reduction of 4.4% among patients admitted to hospital with covid-19
In a post hoc safety analysis, receipt of prophylactic anticoagulation was not associated with increased risk of bleeding that required transfusion
These findings provide strong real world evidence to support guidelines recommending the use of prophylactic anticoagulation as initial treatment for patients with covid-19 on hospital admission
Acknowledgments
The Veterans Affairs Medications, Safety, and Effectiveness Collaboratory Workgroup vetted the study design and statistical approach before study approval.
Web extra.
Extra material supplied by authors
Supplementary information: additional boxes, figures, and tables, and STROBE and RECORD statements
Contributors: CTR and JAB contributed equally to this work. ACJ and MSF are joint principal investigators. CTR, JAB, ACJ, and MSF conceived the study. CTR, CA, FK, MS, YH, MFL, and JPT curated the data. CTR performed the formal analysis. CTR, DA, ACJ, and MSF acquired funding. CTR, JAB, LT, WFG, IJD, JPT, SJWE, DA, ACJ, and MSF designed the methodology. CTR, DA, ACJ, and MSF managed and coordinated the project. CTR, DA, ACJ, and MSF procured resources to carry out the study. CTR, CA, FK, MS, and JPT developed programming. CTR, JAB, DA, ACJ, and MSF provided oversight and leadership of the project. CTR prepared data visualizations. CTR and JAB wrote the first draft of the manuscript. All the authors wrote (reviewed and edited) the manuscript. CTR and MSF are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding This work was supported by the National Institute on Alcohol Abuse and Alcoholism (U01-AA026224, U24-AA020794, U01-AA020790, U10-AA013566) and by the Department of Veterans Affairs Health Services Research and Development (C19 20-405) and Office of Research and Development (MVP000). Research was also sponsored by the Laboratory Directed Research and Development Program (LOIS:10074) of Oak Ridge National Laboratory, managed by UT-Battelle, LLC for the US Department of Energy under contract DE-AC05-00OR22725. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication. The views and opinions expressed in this manuscript are those of the authors and do not necessarily represent those of the Department of Veterans Affairs or the United States government.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: This work was supported by the National Institute on Alcohol Abuse and Alcoholism (U01-AA026224, U24-AA020794, U01-AA020790, U10-AA013566) and by the Department of Veterans Affairs Health Services Research and Development (C19 20-405) and Office of Research and Development (MVP000). Research was also sponsored by the Laboratory Directed Research and Development Program (LOIS:10074) of Oak Ridge National Laboratory, managed by UT-Battelle, LLC for the US Department of Energy under contract DE-AC05-00OR22725. JAB reports consulting with Amgen, Bayer, JanOne, and Janssen. He serves on the data safety monitoring committee for Novartis. PMH is supported by grants from National Heart, Lung, and Blood Institute, VA Health Services Research & Development, and University of Colorado School of Medicine. He has a research agreement with Bristol-Myers Squibb through the University of Colorado. He serves as the deputy editor for Circulation: Cardiovascular Quality and Outcomes. IJD reports grants from UK National Health Service National Institute for Health Research and has received unrestricted research grants and holds shares in GlaxoSmithKline, outside of the submitted work. All other authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the institutional review boards of Yale University and VA Connecticut Healthcare System (ref #0013). It has been granted a waiver of informed consent and is compliant with the Health Insurance Portability and Accountability Act.
Data sharing: Owing to US Department of Veterans Affairs (VA) regulations and our ethics agreements, the analytic data sets used for this study are not permitted to leave the VA firewall without a data use agreement. This limitation is consistent with other studies based on VA data. However, VA data are made freely available to researchers with an approved VA study protocol. For more information, please visit https://www.virec.research.va.gov or contact the VA Information Resource Center at VIReC@va.gov.
The manuscript’s guarantors (CTR and MSF) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: This study was conducted under a waiver of consent, which explicitly prohibits direct patient contact. However, we have reported these findings to the Veterans Affairs central office and chief medical officers who have taken steps to implement our findings in Veterans Affairs clinical care. Study results will be shared with the public through press release, social media, and conference presentations.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bikdeli B, Madhavan MV, Jimenez D, et al. Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:2950-73. 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wise J. Covid-19 and thrombosis: what do we know about the risks and treatment? BMJ 2020;369:m2058. 10.1136/bmj.m2058 [DOI] [PubMed] [Google Scholar]
- 3. Zhang R, Ni L, Di X, et al. Prevalence of venous thromboembolic events in novel coronavirus disease-2019 patients: Systematic review and meta-analysis. J Vasc Surg Venous Lymphat Disord 2020;S2213-333X(20)30654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreuziger LB, Lee A, Garcia D, et al. COVID-19 and VTE/Anticoagulation: Frequently Asked Questions (version 5.1). American Society of Hematology, https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation (2020, accessed January 24, 2021).
- 5. Spyropoulos AC, Levy JH, Ageno W, et al. Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1859-65. 10.1111/jth.14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest 2020;158:1143-63. 10.1016/j.chest.2020.05.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis 2020;50:72-81. 10.1007/s11239-020-02138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nasiripour S, Gholami K, Mousavi S, et al. Comparison of the Effects of Enoxaparin and Heparin on Inflammatory Biomarkers in Patients with ST-segment Elevated Myocardial Infarction: A prospective Open Label Pilot Clinical Trial. Iran J Pharm Res 2014;13:583-90. [PMC free article] [PubMed] [Google Scholar]
- 9. Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-Inflammatory Effects of Heparin and Its Derivatives: A Systematic Review. Adv Pharmacol Sci 2015;2015:507151. 10.1155/2015/507151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Li L, Shi Y, Yu S, Ma X. Different signaling pathways involved in the anti-inflammatory effects of unfractionated heparin on lipopolysaccharide-stimulated human endothelial cells. J Inflamm (Lond) 2020;17:5. 10.1186/s12950-020-0238-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife 2020;9:e59177. 10.7554/eLife.59177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Search on ClinicalTrials.gov, https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=anticoagulation (accessed November 24, 2020).
- 13. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J Am Coll Cardiol 2020;76:1815-26. 10.1016/j.jacc.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094-9. 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiellin DA, Reid MC, O’Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med 2000;160:1977-89. 10.1001/archinte.160.13.1977 [DOI] [PubMed] [Google Scholar]
- 16. McGinnis KA, Justice AC, Tate JP, et al. VACS Project Group Using DNA methylation to validate an electronic medical record phenotype for smoking. Addict Biol 2019;24:1056-65. 10.1111/adb.12670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horby P, Lim WS, Emberson JR, et al. RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med 2020; published online 17 July. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beigel JH, Tomashek KM, Dodd LE, et al. ACTT-1 Study Group Members Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813-26. . 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149-56. 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blake HA, Leyrat C, Mansfield KE, et al. Propensity scores using missingness pattern information: a practical guide. Stat Med 2020;39:1641-57. 10.1002/sim.8503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blake HA, Leyrat C, Mansfield KE, Tomlinson LA, Carpenter J, Williamson EJ. Estimating treatment effects with partially observed covariates using outcome regression with missing indicators. Biom J 2020;62:428-43. 10.1002/bimj.201900041 [DOI] [PubMed] [Google Scholar]
- 22. D’Agostino RB, Jr, Rubin DB. Estimating and using propensity scores with partially missing data. J Am Stat Assoc 2000;95:749 10.1080/01621459.2000.10474263 [DOI] [Google Scholar]
- 23. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med 1993;12:737-51. 10.1002/sim.4780120803 [DOI] [PubMed] [Google Scholar]
- 25. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695-706. [DOI] [PubMed] [Google Scholar]
- 26. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244-56. 10.1093/aje/kwp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med 2017;167:268-74. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 28. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016;35:5642-55. 10.1002/sim.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656-64. 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paolisso P, Bergamaschi L, D’Angelo EC, et al. Preliminary Experience With Low Molecular Weight Heparin Strategy in COVID-19 Patients. Front Pharmacol 2020;11:1124. 10.3389/fphar.2020.01124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paranjpe I, Fuster V, Lala A, et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J Am Coll Cardiol 2020;76:122-4. 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salah HM, Naser JA, Calcaterra G, Bassareo PP, Mehta JL. The Effect of Anticoagulation Use on Mortality in COVID-19 Infection. Am J Cardiol 2020;134:155-7. 10.1016/j.amjcard.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wijaya I, Andhika R, Huang I. The Use of Therapeutic-Dose Anticoagulation and Its Effect on Mortality in Patients With COVID-19: A Systematic Review. Clin Appl Thromb Hemost 2020;26:1076029620960797. 10.1177/1076029620960797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rossi R, Coppi F, Talarico M, Boriani G. Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID-19 era. Eur J Intern Med 2020;77:158-60. 10.1016/j.ejim.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis 2020;50:298-301. 10.1007/s11239-020-02162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferguson J, Volk S, Vondracek T, Flanigan J, Chernaik A. Empiric Therapeutic Anticoagulation and Mortality in Critically Ill Patients With Respiratory Failure From SARS-CoV-2: A Retrospective Cohort Study. J Clin Pharmacol 2020;60:1411-5. 10.1002/jcph.1749 [DOI] [PubMed] [Google Scholar]
- 37. Billett HH, Reyes-Gil M, Szymanski J, et al. Anticoagulation in COVID-19: Effect of Enoxaparin, Heparin, and Apixaban on Mortality. Thromb Haemost 2020;120:1691-9. . 10.1055/s-0040-1720978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Heart, Lung, and Blood Institute (NHLBI), https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients (accessed January 24, 2021).
- 39. Zhang L, Feng X, Zhang D, et al. Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020;142:114-28. 10.1161/CIRCULATIONAHA.120.046702 [DOI] [PubMed] [Google Scholar]
- 40. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA 2020;324:799-801. 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daughety MM, Morgan A, Frost E, et al. COVID-19 associated coagulopathy: Thrombosis, hemorrhage and mortality rates with an escalated-dose thromboprophylaxis strategy. Thromb Res 2020;196:483-5. 10.1016/j.thromres.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carvelli J, Demaria O, Vély F, et al. Explore COVID-19 IPH group. Explore COVID-19 Marseille Immunopole group Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020;588:146-50. 10.1038/s41586-020-2600-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020;383:120-8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meini S, Giani T, Tascini C. Intussusceptive angiogenesis in Covid-19: hypothesis on the significance and focus on the possible role of FGF2. Mol Biol Rep 2020;47:8301-4. 10.1007/s11033-020-05831-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Masi P, Hékimian G, Lejeune M, et al. Systemic Inflammatory Response Syndrome Is a Major Contributor to COVID-19-Associated Coagulopathy: Insights From a Prospective, Single-Center Cohort Study. Circulation 2020;142:611-4. 10.1161/CIRCULATIONAHA.120.048925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607-8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu PP, Blet A, Smyth D, Li H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation 2020;142:68-78. 10.1161/CIRCULATIONAHA.120.047549 [DOI] [PubMed] [Google Scholar]
- 48. Simpson JM, Newburger JW. Multisystem Inflammatory Syndrome in Children in Association With COVID-19. Circulation 2020;142:437-40. . 10.1161/CIRCULATIONAHA.120.048726 [DOI] [PubMed] [Google Scholar]
- 49. Nicolai L, Leunig A, Brambs S, et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation 2020;142:1176-89. 10.1161/CIRCULATIONAHA.120.048488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Most ZM, Hendren N, Drazner MH, et al. The Striking Similarities of Multisystem Inflammatory Syndrome in Children and a Myocarditis-like Syndrome in Adults: Overlapping Manifestations of COVID-19. Circulation 2021;143:4-6. 10.1161/CIRCULATIONAHA.120.050166 [DOI] [PubMed] [Google Scholar]
- 51. Chau VQ, Giustino G, Mahmood K, et al. Cardiogenic Shock and Hyperinflammatory Syndrome in Young Males With COVID-19. Circ Heart Fail 2020;13:e007485. 10.1161/CIRCHEARTFAILURE.120.007485 [DOI] [PubMed] [Google Scholar]
- 52. Myhre PL, Prebensen C, Strand H, et al. Growth Differentiation Factor 15 Provides Prognostic Information Superior to Established Cardiovascular and Inflammatory Biomarkers in Unselected Patients Hospitalized With COVID-19. Circulation 2020;142:2128-37. . 10.1161/CIRCULATIONAHA.120.050360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li X, Pan X, Li Y, et al. Cardiac injury associated with severe disease or ICU admission and death in hospitalized patients with COVID-19: a meta-analysis and systematic review. Crit Care 2020;24:468. 10.1186/s13054-020-03183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Allenbach Y, Saadoun D, Maalouf G, et al. DIMICOVID Development of a multivariate prediction model of intensive care unit transfer or death: A French prospective cohort study of hospitalized COVID-19 patients. PLoS One 2020;15:e0240711. 10.1371/journal.pone.0240711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tree JA, Turnbull JE, Buttigieg KR, et al. Unfractionated heparin inhibits live wild‐type SARS‐CoV‐2 cell infectivity at therapeutically relevant concentrations. Br J Pharmacol 2021;178:626-35. 10.1111/bph.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tandon R, Sharp JS, Zhang F, et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J Virol 2021;95:e01987-20. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clausen TM, Sandoval DR, Spliid CB, et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020;183:1043-1057.e15. 10.1016/j.cell.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Poissy J, Goutay J, Caplan M, et al. Lille ICU Haemostasis COVID-19 Group Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 2020;142:184-6. 10.1161/CIRCULATIONAHA.120.047430 [DOI] [PubMed] [Google Scholar]
- 59. Singh B, Kaur P, Ajdir N, Gupta S, Maroules M. Covid-19 Presenting as Acute Limb Ischemia. Cureus 2020;12:e9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Naudin I, Long A, Michel C, et al. Acute aortoiliac occlusion in a patient with novel coronavirus disease-2019. J Vasc Surg 2021;73:18-21. 10.1016/j.jvs.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bozzani A, Arici V, Tavazzi G, et al. Acute arterial and deep venous thromboembolism in COVID-19 patients: Risk factors and personalized therapy. Surgery 2020;168:987-92. 10.1016/j.surg.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136:489-500. 10.1182/blood.2020006520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Helms J, Tacquard C, Severac F, et al. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089-98. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peltzer B, Manocha KK, Ying X, et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol 2020;31:3077-85. 10.1111/jce.14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lala A, Johnson KW, Januzzi JL, et al. Mount Sinai COVID Informatics Center Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol 2020;76:533-46. 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gawałko M, Kapłon-Cieślicka A, Hohl M, Dobrev D, Linz D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc 2020;30:100631. 10.1016/j.ijcha.2020.100631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U.S. Military, veteran, and civilian men. Am J Prev Med 2012;43:483-9. 10.1016/j.amepre.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 68. Lehavot K, Hoerster KD, Nelson KM, Jakupcak M, Simpson TL. Health indicators for military, veteran, and civilian women. Am J Prev Med 2012;42:473-80. 10.1016/j.amepre.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 69. RAND Health Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. RAND Corporation, 2015. [PMC free article] [PubMed] [Google Scholar]
- 70. King JT, Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: The Veterans Health Administration COVID-19 (VACO) Index. PLoS One 2020;15:e0241825. 10.1371/journal.pone.0241825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. King JT, Jr, Yoon JS, Bredl ZM, et al. Accuracy of the Veterans Health Administration COVID-19 (VACO) Index for predicting short-term mortality among 1,307 Yale New Haven Hospital inpatients and 427,224 Medicare patients. medRxiv. Epub ahead of print January 4, 2021. 10.1101/2021.01.01.20249069. [DOI] [PMC free article] [PubMed]
- 72. Darzi AJ, Karam SG, Charide R, et al. Prognostic factors for VTE and bleeding in hospitalized medical patients: a systematic review and meta-analysis. Blood 2020;135:1788-810. 10.1182/blood.2019003603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional boxes, figures, and tables, and STROBE and RECORD statements