Abstract
The neuropeptide corticotropin-releasing factor (CRF) plays a critical role in mediating anxiety-like responses to stressors, and dysfunction of the CRF system has been linked to the etiology of several psychiatric disorders. Extra-hypothalamic CRF can also modulate learning and memory formation, including amygdala-dependent learning. The basolateral nucleus of the amygdala (BLA) contains dense concentrations of CRF receptors, yet the distribution of these receptors on specific neuronal subtypes within the BLA has not been characterized. Here, we quantified the expression of CRF receptors on three nonoverlapping classes of GABAergic interneurons: those containing the calcium-binding protein parvalbumin (PV), and those expressing the neuropeptides somatostatin (SOM) or cholecystokinin (CCK). While the majority of PV+ neurons and roughly half of CCK1 neurons expressed CRF receptors, they were expressed to a much lesser extent on SOM1 inter-neurons. Knowledge of the distribution of CRF receptors within the BLA can provide insight into how manipulations of the CRF system modulate fear and anxiety-like behaviors.
1 | INTRODUCTION
The neuropeptide corticotropin-releasing factor (CRF) plays an important role in the behavioral, endocrine and autonomic responses to stress (Brown et al., 1982; Vale, Spiess, Rivier, & Rivier, 1981). Released by the paraventricular nucleus of the hypothalamus (PVN), it triggers a key component of the hypothalamic–pituitary–adrenal axis (Turnbull & Rivier, 1997). Inappropriate regulation of CRF levels can be associated with stress-related mood disorders (Holsboer, 1999; Nemeroff, 1988). For instance, chronic hyperactivation of the CRF system has been linked to anxiety and depression (Bale, 2005; Heinrichs & Koob, 2004). CRF is also produced by extra-hypothalamic structures including the amygdala, the bed nucleus of the stria terminalis, and locus coeruleus (Cummings, Elde, Ells, & Lindall, 1983; Curtis & Valentino, 1994). Amygdalar CRF has been implicated in mediating behavioral and emotional responses to stressful stimuli (Dunn & Berridge, 1990). Additionally, immobilization stress, neonatal stress, and predator stress all elevate extracellular CRF levels in the amygdala (Cook, 2004; Cratty, Ward, Johnson, Azzaro, & Birkle, 1995; Merlo Pich et al., 1995).
A considerable body of literature has identified the amygdala as a key component of neural circuitry in mediating fear conditioning (Fanselow & LeDoux 1999; Pape & Pare, 2010). Anatomical, electrophysiological, pharmacological, and transgenic studies have characterized the BLA as a crucial site of plasticity underlying the acquisition of conditioned fear (Pape & Pare, 2010; Schafe, Nader, Blair, & LeDoux, 2001). Related to fear conditioning is fear extinction, a new learning process that competes with and inhibits the original fear memory (Bouton, Westbrook, Corcoran, & Maren, 2006; Ji & Maren, 2007). Manipulation of the CRF system in the amygdala can affect both anxiety-like behaviors and fear learning. CRF receptor antagonists infused into the BLA disrupt contextual fear conditioning (Hubbard, Nakashima, Lee, & Takahashi, 2007) and impair memory formation of an inhibitory avoidance task (Roozendaal, Schelling, & McGaugh, 2008). Additionally, moderate increases in CRF enhance performance in spatial learning and visual discrimination tasks (Heinrichs et al., 1997), suggesting that in general CRF may enhance learning.
In contrast, we recently showed that intra-BLA infusions of CRF prior to fear extinction learning impair fear extinction consolidation, while application of a CRF receptor antagonist facilitates extinction (Abiri et al., 2014). These data are in line with work in human PTSD patients who show enhanced levels of CRF and deficits in their ability to extinguish learned fear (Peri, Ben-Shakhar, Orr, & Shalev, 2000; Risbrough & Stein, 2006). Similarly, increasing amygdalar CRF via deletion of GABAA a1 receptors specifically on CRF-containing neurons also impairs fear extinction (Gafford et al., 2012). Interestingly, when the gene which encodes NR1 is disrupted in CRF containing neurons, fear extinction is not affected, but mice show enhanced auditory fear conditioning and retention (Gafford, Jasnow, & Ressler, 2014). Thus manipulations of the amygdalar CRF system can have differential effects depending not only on the learning paradigm, but also on the manipulated cell types.
The BLA contains high densities of type I CRF (CRF1) receptors (Chen, Brunson, Muller, Cariaga, & Baram, 2000), while neurons in the CE express the CRF neuropeptide (Van Pett et al., 2000). Within the BLA, there is some evidence that the lateral nucleus (LA) contains a higher density of CRF receptors than the basal nucleus (BA) (Kuhne et al., 2012). The LA has traditionally been described as the main input station of the amygdala, with sensory inputs from the thalamus and cortex (LeDoux, Cicchetti, Xagoraris, & Romanski, 1990; Quirk, Repa, & LeDoux, 1995). BA neurons also change activity in response to fear conditioning, as they receive inputs from LA neurons (Herry et al., 2008; Smith & Pare, 1994). Extinction training alters the activity of both LA and BA neurons, depending on the responses initially acquired during fear conditioning (Amano, Duvarci, Popa, & Pare, 2011; Herry et al., 2008; Repa et al., 2001).
In vitro, acute CRF increases the excitability of rat BLA neurons by reducing the slow after-hyperpolarization (Rainnie, Fernhout, & Shinnick-Gallagher, 1992). It is unclear however, whether CRF affects the excitability of the principal excitatory neurons of the BLA or inhibitory interneurons or both.
Since neuronal activity in the BLA contributes to both fear learning and extinction, we here examine which types of neurons within the BLA express CRF receptors. The majority of neurons within the BLA are principal excitatory neurons which use glutamate as a neurotransmitter (McDonald, 1992). However, roughly 25% of the neurons of the BLA are inhibitory interneurons (McDonald & Augustine, 1993). These cells receive direct input from the auditory thalamus and cortex, as well as feedback from principal BLA excitatory cells (Smith, Pare, & Pare, 2000; Szinyei, Heinbockel, Montagne, & Pape, 2000). There is a growing realization that the activity and plasticity of inhibitory BLA neurons is essential for and modulates fear learning and extinction (Heldt, Mou, & Ressler, 2012; Li, Nair, & Quirk, 2009). Indeed, fear conditioning is associated with decreased mRNA levels of GABA-synthesizing enzymes and major GABAA receptor subunits, while fear extinction produces an upregulation of these GABAergic markers (Heldt & Ressler, 2007).
One of the major interneuron subclasses within the BLA expresses the calcium-binding protein parvalbumin (PV) (McDonald & Betette, 2001; Smith, Pare, & Pare, 1998). These neurons preferentially synapse on the soma of their target cells (McDonald & Betette, 2001; Muller, Mascagni, & McDonald, 2006). A second subclass of interneurons contains somatostatin (SOM), which preferentially contact distal dendrites (Muller et al., 2006). These two populations are non-overlapping (McDonald & Mascagni, 2002; Spampanato, Polepalli, & Sah, 2011), and recent data suggests that each has important and distinct roles during fear learning (Wolff et al., 2014).
A third class of interneurons within the BLA expresses cholecystokinin (CCK) with either calretinin or vasoactive intestinal peptide while a fourth class expresses CCK alone (Mascagni & McDonald, 2003). CCK neurons within the amygdala have been implicated in modulating both fear and anxiety. For example, the lesioning of neurokinin1 receptors on cells expressing CCK produces anxiety-like behaviors (Truitt, Johnson, Dietrich, Fitz, & Shekhar, 2009). Further, the CB1 cannabinoid receptors located exclusively on CCK-positive interneurons (Katona et al., 2001) modulate fear extinction (Marsicano et al., 2002). Chronic stimulation of CRF1 receptors in vivo results in an increase in CCK mRNA levels as well as higher anxiety-like behaviors (Sherrin et al., 2009). Thus, here we quantify the expression of CRF receptors on three classes of interneurons: those containing PV, SOM and CCK. Knowledge of the distribution of CRF receptors within the BLA can provide insight into how manipulations of the CRF system modulate fear and anxiety-like behaviors, as well as how different neuronal subpopulations contribute to fear learning and expression.
2 | MATERIALS AND METHODS
2.1 | Subjects
Adult male Sprague Dawley rats (Charles River Laboratories; 250–325 g) were housed individually with ad libitum access to food and water and maintained on a 12 hr light/dark cycle. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Columbia University’s Animal Care and Use Committee.
2.2 | Immunohistochemistry
Animals were given an overdose of sodium pentobarbital (100 mg/kg), and then perfused transcardially with 0.9% saline and 4% paraformaldehyde in 0.1M phosphate buffer (PB). Tissue was postfixed for 4 h then transferred to a 20% sucrose solution. Tissue was sliced into 80 lm coronal sections using a Vibratome. Every other slice was collected, to obviate correction for double counting. 10 slices per animal were collected, covering the entire rostro-caudal length of the BLA. To examine the distribution of CRF1 receptors throughout the amygdala, sections were treated with 1.8% H2O2 for 5 min to reduce endogenous peroxidase activity, and then washed in PBT (0.1M PB with 1% Triton). Sections were blocked in 1% bovine serum albumin BSA (in PBT) for 1 hour and incubated at 4 °C for 48 hr in goat anti-CRF-RI/II polyclonal antibody (1:5,000; Santa Cruz, sc-1757). Slices were washed in PBT and incubated with biotinylated donkey antigoat secondary antibody (1:200; Jackson ImmunoResearch Labs) for 1 hr at room temperature. Slices were processed with avidin–biotin horseradish peroxidase complex (Vectastain ABC Kit, Vector Labs). Horseradish peroxidase was visualized with 3,3′diaminobenzidine in a 3M sodium acetate buffer containing 0.05% H2O2. Sections were washed, mounted on slides, coverslipped, and examined using light microscopy. For double labeling experiments, tissue was washed in 0.1M PB 3 times, then in PBT 3 times for 5 min each wash. Slices were blocked in 1% BSA in PBT for 1 hr and incubated for 48 hr at 4 °C in primary antibodies in BSA. Primaries used were a combination of goat anti-CRF-RI/II polyclonal antibody (1:2,000; Santa Cruz, sc-1757) and one of the following: mouse anti-GAD67 (1:500; Millipore MAB5406), rabbit antisomatostatin polyclonal antibody (1:400; Santa Cruz, sc-13099), mouse anti-PV monoclonal antibody (1:1,000, Millipore MAB1572), or rabbit anti-CCK-8 polyclonal antibody (1:1,000; Immunostar 20078). Slices were then washed in PBT and incubated in secondary antibodies for 1 hr. Secondary antibodies used were donkey Alexa Fluor-488, Alexa-555 or Alexa-594 (1:200; Life Technologies). Slices were washed in PBS, mounted on slides and coverslipped.
2.3 | Microscopy
Double-labeling was assessed by confocal microscopy using a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss, Thornwood, NY) with LSM 510-META scanning confocal attachments. Stacked images were collected as 1 mm multitract optical sections. LSM 510 software (Carl Zeiss) was used to visualize doubly-labeled fluorescent slices at 2503 and to capture images. Manual counting of doubly-labeled cells was performed on images of dorsal and ventral sections of the BLA in each hemisphere per animal.
2.4 | Specificity of antibodies
We used a goat polyclonal anti-CRF RI/II antibody (Santa Cruz, sc-1757). This antibody was raised against a peptide corresponding to amino acid sequence 396–415 mapping at the C-terminus of the CRF1 receptor of human origin. There is a three-amino acid difference between the rat CRF1 and CRF2 receptor at the region that corresponds to this sequence (Chen et al., 2000). However, western blot analysis has revealed a positive band corresponding to the predicted molecular weight of the CRF1 receptor in mice (77–80 kDa) (Chen et al., 2000; Radulovic, Sydow, & Spiess, 1998). The specificity of the antibody has also been tested by preadsorbing the antiserum overnight with purified CRF1 blocking peptide (Chen et al., 2000; Korosi et al., 2007), suggesting that this antibody preferentially binds to the CRF1 receptor. Further, this antibody does not crossreact with the CRF2 receptor as it produces no labeling in sections of mouse heart, which is known to express the CRF2 but not CRF1 receptor (Chen et al., 2000). Convincingly, this specific antibody has been tested in CRF1 knockout animals and a complete lack of immunoreactivity was observed (Waselus, Nazzaro, Valentino, & Van Bockstaele, 2009). We performed additional controls to demonstrate the specificity of the antibody. Immunoreactivity was absent from tissue in which the primary antisera was omitted from the incubation solution. Preadsorbing the antiserum with the CRF1 blocking peptide (sc-1757P, Santa Cruz Biotechnology) also led to an elimination of neuronal staining, consistent with previous studies using this antibody (Rajbhandari, Baldo, & Bakshi, 2015; Korosi et al., 2007). Antibodies directed against GAD67, PV, SOM, and CCK-8 have been verified for specificity and previously used to label neurons in the BLA and other structures (Apergis-Schoute, Pinto, & Pare, 2007; Hu, Zhang, Czeh, Flugge, & Zhang, 2010; Narushima et al., 2007; Riccio et al., 2014; Zhu et al., 2016). For each of the primary antibodies, before any double-labeling experiments were performed, we processed sections with the primary antibody omitted, but the appropriate secondary antibody present, and there was an absence of staining. In parallel with the double-labeling experiments, we tested for secondary antibody specificity. One of the two primary antibodies was omitted, but the tissue was processed with both secondary antibodies. We confirmed that there was no “crosstalk” between the red and green channels and that the secondary antibodies were specific.
3 | RESULTS
To determine whether CRF1 receptors are localized to specific inhibitory cell types, we first confirmed their presence within the BLA in general and on GABAergic interneurons specifically. CRF1 receptors, visualized with immunoperoxidase labeling, were distributed throughout the LA and BA (Figure 1a). Next, using dual immunofluorescence, we labeled tissue for CRF1 receptors and GAD67, a marker for GABAergic neurons in the BLA (Apergis-Schoute et al., 2007). Confocal microscopy revealed that CRF1 receptors were indeed located on GABAergic inhibitory neurons within the BLA (Figure 1b). Additionally, there were many neurons that expressed CRF1 receptors, but not GAD67, and were presumably excitatory principal neurons.
FIGURE 1.
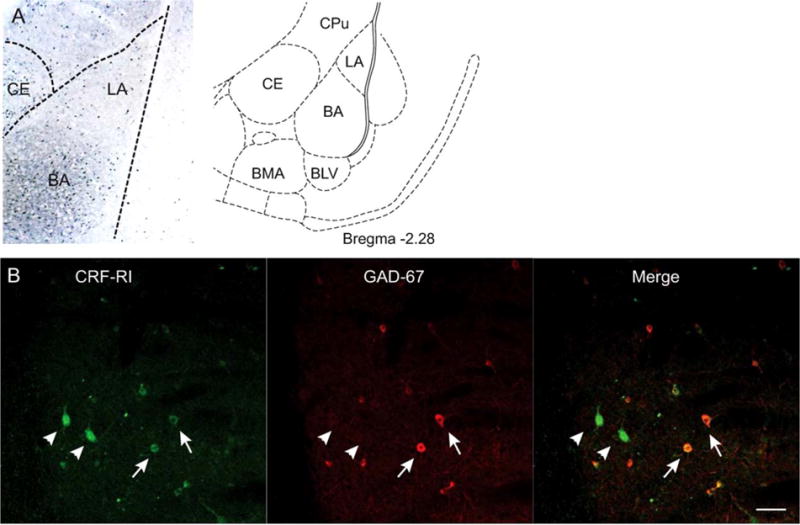
Localization of CRF1 receptors on GABAergic interneurons. (A) Left: distribution of CRF1 receptors in the amygdala. LA: lateral nucleus, BA: basal nucleus, CE: central nucleus. Right, samples were taken from the LA and BA. CPu: caudate putamen, BLV: ventral basolateral nucleus, BMA: basomedial nucleus. Adapted from Paxinos and Watson (1998). (B) Colocalization of CRF1 receptors (green) and GAD67 (red). Merging of the red and green channels indicates several somata expressing both GAD67 and CRF1 receptors (arrows). Several neurons expressing CRF1 receptors (arrowheads) do not express GAD67. Scale bar: 50 μm
We next made a quantitative assessment of the distribution of CRF1 receptors on three types of GABAergic interneurons: those expressing the calcium binding protein PV, those expressing SOM and those expressing CCK. We analyzed both left and right hemispheres of each brain, and sampled from both the LA and BA. Due to possible distribution differences of CRF1 receptors in the LA and BA (Kuhne et al., 2012), we analyzed these two areas separately.
PV+ and SOM+ interneurons are two distinct subpopulations of calbindin-positive interneurons comprising roughly 55% of GABAergic neurons within the BLA (McDonald & Mascagni, 2002). We examined the distribution of CRF1 receptors on PV+ interneurons using nine animals. We analyzed two sections per hemisphere for a total of 36 samples. Using paired-sample t-tests, we found no statistical difference between samples taken from the LA and BA for either the total number of PV+ cells (p = .97) or the number of PV+ neurons co-expressing CRF1 receptors (p = .79). We therefore summed the counts for each LA and BA together to describe the neurons of each BLA. Across all animals, we counted an average of 24.2 ± 2.6 PV+ neurons per BLA, of which 18.1 ± 2.3 were doubly labeled for CRF1 receptors (Figure 2a).
FIGURE 2.
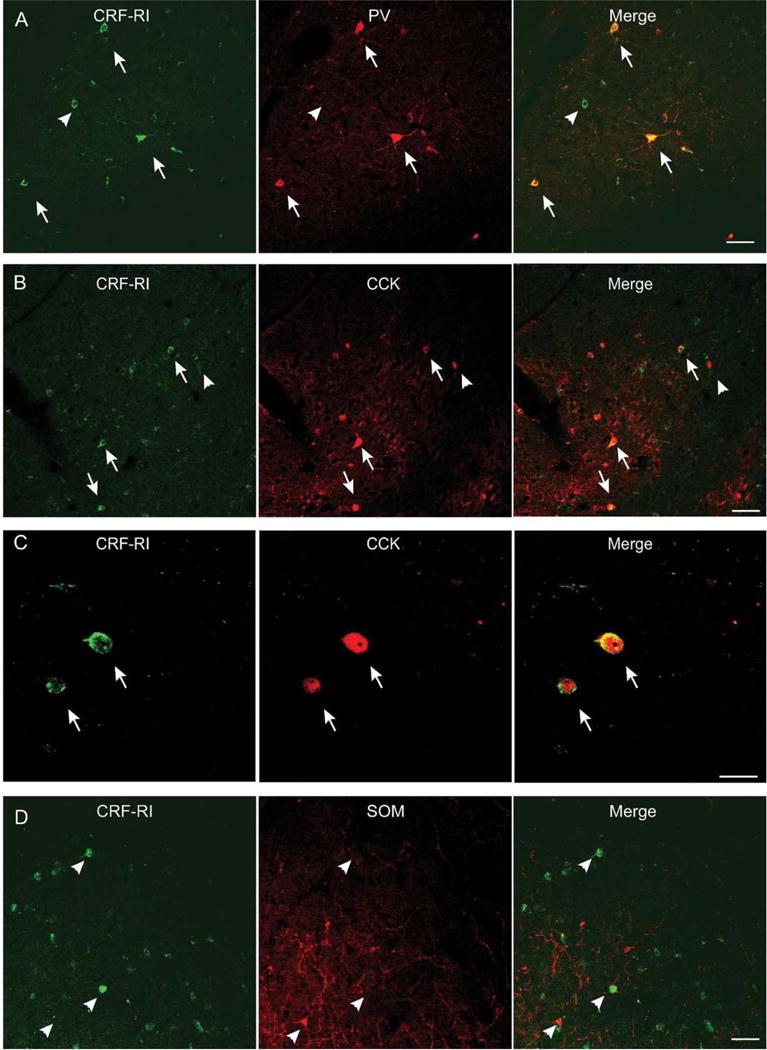
Localization of CRF1 receptors on three different GABAergic populations within the BLA. (A) Colocalization of CRF1 receptors (green) and the calcium-binding protein PV (red). Many somata showed dual-labeling (arrows), but many CRF1 receptor-containing neurons did not express PV (arrowhead). Scale bar: 50 μm. (B) Colocalization of CRF1 receptors (green) and the peptide CCK (red). Many somata showed dual labeling (arrows). Some CCK+ neurons did not express CRF1 receptors (arrowhead). Scale bar: 50 μm. (C) Higher magnification revealed two neurons expressing both CRF1 receptors (green) and CCK (red). Scale bar: 20 μm. (D) Colocalization of CRF1 receptors (green) and the peptide SOM (red). The majority of SOM neurons do not express CRF1 receptors (arrowheads). Scale bar: 50 μm
We next examined the distribution of CRF1 receptors on CCK-containing interneurons using seven animals. Again, paired sample t-tests found no difference between samples taken from the LA and BA for either the total number of CCK1 neurons (p = .44) or the number of CCK1 neurons expressing CRF1 receptors (p = .28). As above, we summed the counts for each LA and BA together. Across all animals, we counted an average of 61.1 ± 8.9 CCK1 neurons per BLA, of which 35.1 ± 7.5 were double labeled for CRF1 receptors (Figure 2b,c).
Finally, we examined the distribution of CRF1 receptors on SOM+ neurons using six animals. Due to low SOM+ neuron counts per sample (3.0 on average, with as few as 1 SOM+ cell in some cases), we analyzed three images per area (e.g., left LA) and summed the numbers for that area for each brain. No differences were found between samples taken from the LA and BA for either the number of SOM+ neurons (p = .38) or the number of SOM+ neurons doubly labeled for CRF1 receptors (p = .24). We adjusted the neuron counts to account for the increased sampling, in order to directly compare across cell types. Across all animals, we counted an average of 5.9 ± 0.3 SOM+ neurons per BLA, of which 2.1 ± 0.3 were positive for CRF1 receptors (Figure 2d).
We converted colocalization counts to percentages, for each BLA to compare CRF-1 receptor expression across interneuron subtypes (Figure 3). A one-way ANOVA across all three groups found a significant main effect of group (F(2,43) = 16.7; p < .001). Tukey’s post hoc tests found significant differences between percentage colocalization of CRF1 receptors with PV and CCK (p < .05), CCK and SOM (p < .05) and PV and SOM (p < .001).
FIGURE 3.
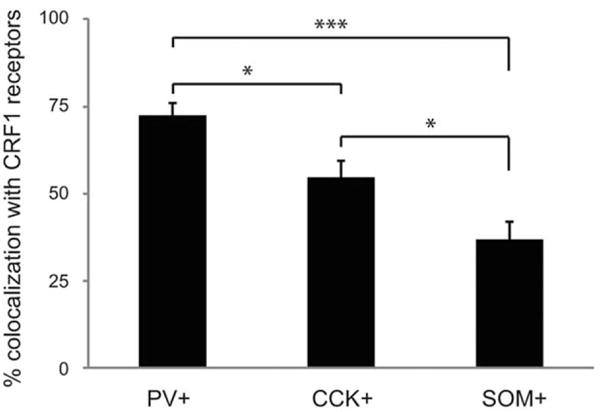
Percentage colocalization with CRF1 receptors for each of the three interneuron subtypes: PV, CCK, and SOM. *p < .05, ***p < .001
4 | DISCUSSION
An analysis of the distribution of CRF1 receptors on three different GABAergic interneuron subtypes revealed that these receptors are preferentially located on PV+ neurons, and to a much lesser extent on SOM1 neurons. In addition, roughly half of CCK+ interneurons express CRF1 receptors.
Evidence suggests that PV+ neurons modulate both anxiety and fear learning. In general, disrupting GABAergic neurotransmission with GABAA receptor antagonists leads to increases in anxiety-like behavior (Quirk & Gehlert, 2003). Methods for reducing anxiety such as environmental enrichment, as well as administration of anxiogenic drugs increase the number of PV+ neurons in the BLA (Hale et al., 2010; Urakawa et al., 2013). In fear learning paradigms, PV+ neurons exhibit increased firing rates during the presentation of an auditory CS cue, presumably through direct sensory input from the auditory thalamus and cortex (Sah, Faber, Lopez De Armentia, & Power, 2003; Woodson, Farb, & Ledoux, 2000). Optogenetic activation of PV+ neurons during the CS correlates with enhanced learning and stronger auditory responses in principal neurons (Wolff et al., 2014). A few recent studies suggest that PV+ neurons might play a role in fear extinction. Extinction training silences a large proportion of BLA neurons that are active during fear conditioning (Herry et al., 2008; Repa et al., 2001). These silenced neurons exhibit greater perisomatic GAD67 and PV staining than neurons that are not silenced, suggesting a proliferation of GABAergic synapses from PV+ neurons on to silenced excitatory cells (Trouche, Sasaki, Tu, & Reijmers, 2013).
Given the differential distribution of CRF1 receptors on PV+ and SOM1 neurons (72 vs. 37%), CRF might produce its anxiogenic effects by modulating BLA neuron activity in a cell type specific matter. PV+ interneurons exhibit high firing rates and preferentially form synapses at the perisomatic region of their target cells, thus controlling neuronal activity and spike output (Muller et al., 2006; Woodruff & Sah, 2007). It is currently unknown how CRF receptor activation on PV+ neurons within the BLA affects the activity of PV+ neurons themselves or their targets. However, chronic (5 day) stimulation of CRF receptors in the BLA reduces spontaneous and evoked IPSPs (Rainnie et al., 2004). In the central nucleus of the amygdala, CRF administration enhances GABAergic synaptic transmission via activation of CRF1 receptors (Nie et al., 2004). Within the BLA, it appears that the CRF system and GABAergic systems modulate each other. CRF overexpression leads to changes in GABA receptor subtype expression and sensitivity (Vinkers et al., 2012). GABAergic disinhibition or CRF excitation both result in increased excitability of neurons within the amygdala (Sajdyk, Schober, Gehlert, & Shekhar, 1999). CRF receptor activation increases the excitability of BLA neurons by reducing the slow after-hyperpolarization (Rainnie et al., 1992).
A second possibility is that CRF modulates the activity of CCK+ neurons in the BLA. These neurons can be one of two types. There are large, multipolar neurons that express CCK+, some of which also express calbindin. Smaller CCK+ neurons exhibit various amounts of colocalization with calretinin and VIP. Together, they constitute over 40% of GABAergic interneurons within the BLA, although the majority of CCK+ neurons are the smaller type (Mascagni & McDonald, 2003). Based on the size of the CCK+ neurons labeled in this study, it appears that both large and small types express CRF1 receptors. Future studies should determine the co-expression of CRF1 receptors and CCK with either calretinin or calbindin to more thoroughly describe this population of interneurons.
Here, we used a polyclonal antibody directed against the C-terminal octapeptide fragment of CCK, which, when released from neurons acts at CCK receptors. Exogenous administration of the CCK receptor agonist pentagastrin provokes anxiety-like behavior in animals (Dauge & Lena, 1998) and panic attacks in humans (Bradwejn, 1993). Interestingly, pentagastrin administered prior to extinction training impairs fear extinction learning (Chhatwal et al., 2009). The same pattern of results is found when CRF or CRF receptors agonists are infused into the BLA: animals show increased levels of anxiety (Rainnie et al., 2004) and impaired fear extinction (Abiri et al., 2014). An interaction between these two peptidergic systems in modulating anxiety has been previously demonstrated: chronic stimulation of CRF1 receptors results in both an increase in CCK mRNA levels in the amygdala and higher anxiety-like behavior in the open field and elevated plus maze (Sherrin et al., 2009). Moreover, CCK release induces long-duration compound postsynaptic potentials in BLA neurons. These responses are enhanced by CRF (Chung & Moore, 2009a,b). This suggests that activation of CRF1 receptors on CCK+ neurons would potentiate the effects of CCK release within the BLA. Here, we found that 54.5% of CCK+ neurons also expressed CRF1 receptors. Given the similar behavioral effects of CRF and CCK receptor agonist administration on anxiety-like behaviors and fear extinction, it is possible that CRF might be interacting with the CCK system to influence both behaviors.
PV+ and SOM+ neurons are nonoverlapping populations of calbindin-expressing GABAergic neurons in the BLA (Spampanato et al., 2011). Compared to PV+ and CCK+ neurons, we observed relatively fewer SOM+ neurons. Indeed, SOM-expressing neurons constitute only 11–18% of the GABAergic population in the BLA (McDonald & Mascagni, 2002). In contrast with CCK, evidence suggests that the neuropeptide SOM has potent anxiolytic properties (Yeung, Engin, & Treit, 2011; Yeung & Treit, 2012). Many SOM neurons also express neuropeptide Y, but the SOM and CCK neuropeptides are expressed in separate populations (McDonald, 1989). Here, we found that 36.7% of SOM neurons express CRF receptors, suggesting that modulation of the CRF system more strongly affects PV and CCK neurons than SOM neurons.
The exact source of CRF peptide in the BLA remains unclear. There are CRF positive cell bodies in the CE and in the BNST (Van Pett et al., 2000), but these areas do not project to the BLA (Pitkanen, Savander, & LeDoux, 1997). It is possible that increases in extracellular CRF in the CE are volume-conducted to the BLA. Other possible sources include the parabrachial nucleus, which contains CRF+ neurons (Pomrenze et al., 2015), projects to the BLA (Bernard, Alden, & Besson, 1993) and may relay sensory information about aversive unconditioned stimuli (Jasmin, Burkey, Card, & Basbaum, 1997).
One mechanism for fear inhibition and fear extinction involves prefrontal–amygdala projections that recruit clusters of intercalated cells (ITC), clusters of GABAergic neurons along the border between the BLA and CE (Busti et al., 2011). After extinction, ITC cells limit excitatory transmission between the BLA and CE by directly inhibiting CE neurons (Likhtik, Popa, Apergis-Schoute, Fidacaro, & Pare, 2008). It is therefore important to note that in this study, we did not include ITC neurons in our analysis of GABAergic interneurons. Although we did not analyze whether ITC neurons express CRF1 receptors, there is some evidence that these neurons express the CRF peptide itself (Moga & Gray, 1985).
Subpopulations of GABAergic interneurons within the BLA differ based on their peptidergic content and their electrophysiological properties (Mascagni, Muly, Rainnie, & McDonald, 2009; Woodruff & Sah, 2007). There is growing evidence that these subpopulations differentially contribute to anxiety-like behavior and associative learning (Wolff et al., 2014). Here we report that CRF1 receptors are expressed on the majority of PV interneurons, on roughly half of CCK interneurons, and on fewer SOM neurons. How activation of these receptors on specific neuronal populations translates to changes in behavior will add to our understanding of how modulation of the CRF system contributes to anxiety-related psychological disorders.
Acknowledgments
This work was supported by NIH grant MH-095032-01 to E.P.B and by the Amgen Scholars Summer Research Program at Columbia University.
References
- Abiri D, Douglas CE, Calakos KC, Barbayannis G, Roberts A, Bauer EP. Fear extinction learning can be impaired or enhanced by modulation of the CRF system in the basolateral nucleus of the amygdala. Behavioural Brain Research. 2014;271:234–239. doi: 10.1016/j.bbr.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Duvarci S, Popa D, Pare D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apergis-Schoute J, Pinto A, Pare D. Muscarinic control of long-range GABAergic inhibition within the rhinal cortices. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:4061–4071. doi: 10.1523/JNEUROSCI.0068-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Hormones & Behavior. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: A Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. Journal of Comprehensive Neurology. 1993;392:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bradwejn J. Neurobiological investigations into the role of cholecystokinin in panic disorder. Journal of Psychiatry & Neuroscience. 1993;18:178–188. [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Fisher LA, Spiess J, Rivier C, Rivier J, Vale W. Corticotropin-releasing factor: Actions on the sympathetic nervous system and metabolism. Endocrinology. 1982;111:928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, Ferraguti F. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:5131–5144. doi: 10.1523/JNEUROSCI.6100-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. The Journal of Comparative Neurology. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- Chung L, Moore SD. Neuropeptides modulate compound postsynaptic potentials in basolateral amygdala. Neuroscience. 2009a;164:1389–1397. doi: 10.1016/j.neuroscience.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Chung L, Moore SD. Cholecystokinin excites interneurons in rat basolateral amygdala. Journal of Neurophysiology. 2009b;102:272–284. doi: 10.1152/jn.90769.2008. [DOI] [PubMed] [Google Scholar]
- Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiology & Behavior. 2004;82:751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Research. 1995;675:297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Valentino RJ. Corticotropin-releasing factor neurotransmission in locus coeruleus: a possible site of antidepressant action. Brain Research Bulletin. 1994;35:581–587. doi: 10.1016/0361-9230(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Dauge V, Lena I. CCK in anxiety and cognitive processes. Neuroscience and Biobehavioral Reviews. 1998;22:815–825. doi: 10.1016/s0149-7634(98)00011-6. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Annals of the New York Academy of Sciences. 1990;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Gafford G, Jasnow AM, Ressler KJ. Grin1 receptor deletion within CRF neurons enhances fear memory. PloS One. 2014;9:e111009. doi: 10.1371/journal.pone.0111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Johnson PL, Westerman AM, Abrams JK, Shekhar A, Lowry CA. Multiple anxiogenic drugs recruit a parvalbumin-containing subpopulation of GABAergic interneurons in the basolateral amygdala. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:1285–1293. doi: 10.1016/j.pnpbp.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. The Journal of Pharmacology and Experimental Therapeutics. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Vale EA, Lapsansky J, Behan DP, McClure LV, Ling N, Schulteis G. Enhancement of performance in multiple learning tasks by corticotropin-releasing factor-binding protein ligand inhibitors. Peptides. 1997;18:711–716. doi: 10.1016/s0196-9781(97)00120-4. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Mou L, Ressler KJ. In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Translational Psychiatry. 2012;2:e181. doi: 10.1038/tp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. The European Journal of Neuroscience. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. Journal of Psychiatric Research. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czeh B, Flugge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35:1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Card JP, Basbaum AI. Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat. Journal of Neuroscience. 1997;17:3751–3765. doi: 10.1523/JNEUROSCI.17-10-03751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Kozicz T, Richter J, Veening JG, Olivier B, Roubos EW. Corticotropin-releasing factor, urocortin 1, and their receptors in the mouse spinal cord. The Journal of Comparative Neurology. 2007;502:973–989. doi: 10.1002/cne.21347. [DOI] [PubMed] [Google Scholar]
- Kuhne C, Puk O, Graw J, Hrabe de Angelis M, Schutz G, Wurst W, Deussing JM. Visualizing corticotropin-releasing hormone receptor type 1 expression and neuronal connectivities in the mouse using a novel multifunctional allele. The Journal of Comparative Neurology. 2012;520:3150–3180. doi: 10.1002/cne.23082. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. Journal of Neurophysiology. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Research. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, Muly EC, Rainnie DG, McDonald AJ. Immunohistochemical characterization of parvalbumin-containing interneurons in the monkey basolateral amygdala. Neuroscience. 2009;158:1541–1550. doi: 10.1016/j.neuroscience.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Research. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Research Bulletin. 1992;28:179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of calbindin-D (28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Research. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Gray TS. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. The Journal of Comparative Neurology. 1985;241:275–284. doi: 10.1002/cne.902410304. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. The Journal of Comparative Neurology. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry. 1988;21:76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberst AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neuroscience. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Messing RO. A transgenic rat for investigating the anatomy and function of corticotropin releasing factor circuits. Frontiers in Neuroscience. 2015;9:487. doi: 10.3389/fnins.2015.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Annals of the New York Academy of Sciences. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. Journal of Neuroscience Research. 1998;54:507–521. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Fernhout BJ, Shinnick-Gallagher P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. The Journal of Pharmacology and Experimental Therapeutics. 1992;263:846–858. [PubMed] [Google Scholar]
- Rajbhandari AK, Baldo BA, Bakshi VP. Predator stress-induced CRF release causes enduring sensitization of basolateral amygdala norepinephrine systems that promote PTSD-like startle abnormalities. Journal of Neuroscience. 2015;35:14270–14285. doi: 10.1523/JNEUROSCI.5080-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neuroscience. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Riccio A, Li Y, Tsvetkov E, Gapon S, Yao GL, Smith KS, Clapham DE. Decreased anxiety-like behavior and Gaq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. Journal of Neuroscience. 2014;34:3653–3667. doi: 10.1523/JNEUROSCI.2274-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: A translational research perspective. Hormones & Behavior. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: Dependence on glucocorticoid receptor activation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiological Reviews. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behavioural Brain Research. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: A cellular and molecular perspective. Trends in Neurosciences. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Sherrin T, Todorovic C, Zeyda T, Tan CH, Wong PT, Zhu YZ, Spiess J. Chronic stimulation of corticotropin-releasing factor receptor 1 enhances the anxiogenic response of the cholecystokinin system. Molecular Psychiatry. 2009;14:291–307. doi: 10.1038/sj.mp.4002121. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with post-embedding GABA and glutamate immunocytochemistry. The Journal of Comparative Neurology. 1994;342:232–248. doi: 10.1002/cne.903420207. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Cat intraamygdaloid inhibitory network: ultrastructural organization of parvalbumin-immunoreactive elements. The Journal of Comparative Neurology. 1998;391:164–179. doi: 10.1002/(sici)1096-9861(19980209)391:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. The Journal of Comparative Neurology. 2000;416:496–508. [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology. 2011;60:765–773. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:8909–8915. doi: 10.1523/JNEUROSCI.20-23-08909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Sasaki JM, Tu T, Reijmers LG. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 2013;80:1054–1065. doi: 10.1016/j.neuron.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- Urakawa S, Takamoto K, Hori E, Sakai N, Ono T, Nishijo H. Rearing in enriched environment increases parvalbumin-positive small neurons in the amygdala and decreases anxiety-like behavior of male rats. BMC Neuroscience. 2013;14:13. doi: 10.1186/1471-2202-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. The Journal of Comparative Neurology. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Hendriksen H, van Oorschot R, Cook JM, Rallipalli S, Huang S, Groenink L. Lifelong CRF overproduction is associated with altered gene expression and sensitivity of discrete GABA(A) and mGlu receptor subtypes. Psychopharmacology (Berlin) 2012;219:897–908. doi: 10.1007/s00213-011-2423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor sub-types in the dorsal raphe nucleus. Biological Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, Luthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson W, Farb CR, Ledoux JE. Afferents from the auditory thalamus synapse on inhibitory interneurons in the lateral nucleus of the amygdala. Synapse. 2000;38:124–137. doi: 10.1002/1098-2396(200011)38:2<124::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Yeung M, Engin E, Treit D. Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology. 2011;216:557–567. doi: 10.1007/s00213-011-2248-x. [DOI] [PubMed] [Google Scholar]
- Yeung M, Treit D. The anxiolytic effects of somatostatin following intra-septal and intra-amygdalar microinfusions are reversed by the selective sst2 antagonist PRL2903. Pharmacology, Biochemistry, and Behavior. 2012;101:88–92. doi: 10.1016/j.pbb.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Zhu XN, Liu XD, Zhuang H, Henkemeyer M, Yang JY, Xu NJ. Amygdala EphB2 signaling regulates glutamatergic neuron maturation and innate fear. Journal of Neuroscience. 2016;36:10151–10162. doi: 10.1523/JNEUROSCI.0845-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


