Abstract
Purpose of Review
This review will summarize recent data defining the relationship between rheumatoid arthritis (RA) and the microbiome at mucosal sites throughout the body. It will highlight what is known, what is speculated, and current knowledge gaps regarding the microbiome in RA.
Recent Findings
An extensive relationship between the microbiome and immune cell function can influence RA-related inflammation and T cell and B cell biology. Studies are beginning to characterize microbial changes in individuals who are at risk for RA, which is a critical element needed to understand the influence of the microbiome on RA pathogenesis.
Summary
Expanding our understanding of the microbiome in RA beyond the bacteria at the gut and oral mucosae into the lung and urogenital surfaces, including viral and fungal components, and establishing the relationship across mucosal sites will be critical in future work. Importantly, approaches to manipulate the microbiome could lead to novel therapeutic and preventive strategies.
Keywords: Rheumatoid arthritis, Microbiome, Pathogenesis, Mucosal sites
Introduction
The human microbiome includes the extensive communities of microbes living symbiotically throughout the skin and mucosal sites of each individual. Advances in technologies over the past several decades have advanced our ability to better understand the beneficial as well as deleterious effects of the microbiome on human health and disease. It is now well established that the microbiome can directly and indirectly influence an individual’s immune system development and function. Microbes can directly influence proper development of the innate and adaptive immune system, the balance of Th17+ and T regulatory cells, and the development of secondary lymphoid structures [1–3]. In addition, metabolites generated by microbiota can affect mucosal immune cell function and epithelial barrier integrity [4]. Certain alterations of the human microbiome can also lead to the development of inflammation, activation of autoreactive T and B cells, and molecular mimicry (i.e., cross-reactivity of a microbial and human protein) [5]. As such, effects of the microbiome have been implicated in the pathogenesis of several autoimmune diseases, including rheumatoid arthritis (RA).
An important feature in the development of RA is that it develops in multiple phases, including a pre-clinical phase of systemic autoimmunity that precedes the onset of joint symptoms and inflammatory arthritis by several years [6]. Because of these multiple phases, microbiota could have an effect on RA development at one or multiple time points along the way (Fig. 1). For example, microbiota could contribute to or prevent the expansion of autoimmunity and inflammation during the pre-clinical or clinically classified phases of RA, or microbiota could influence transitions between these different phases. In addition, there are different mechanisms by which the microbiome could influence the development of RA at these different time points (Fig. 1). There are also multiple sites with distinct microbiomes that have each been associated with RA pathogenesis. This review will focus the microbiomes of the gut, lung, oral, and urogenital mucosal sites and data demonstrating their associations with RA.
Fig. 1.
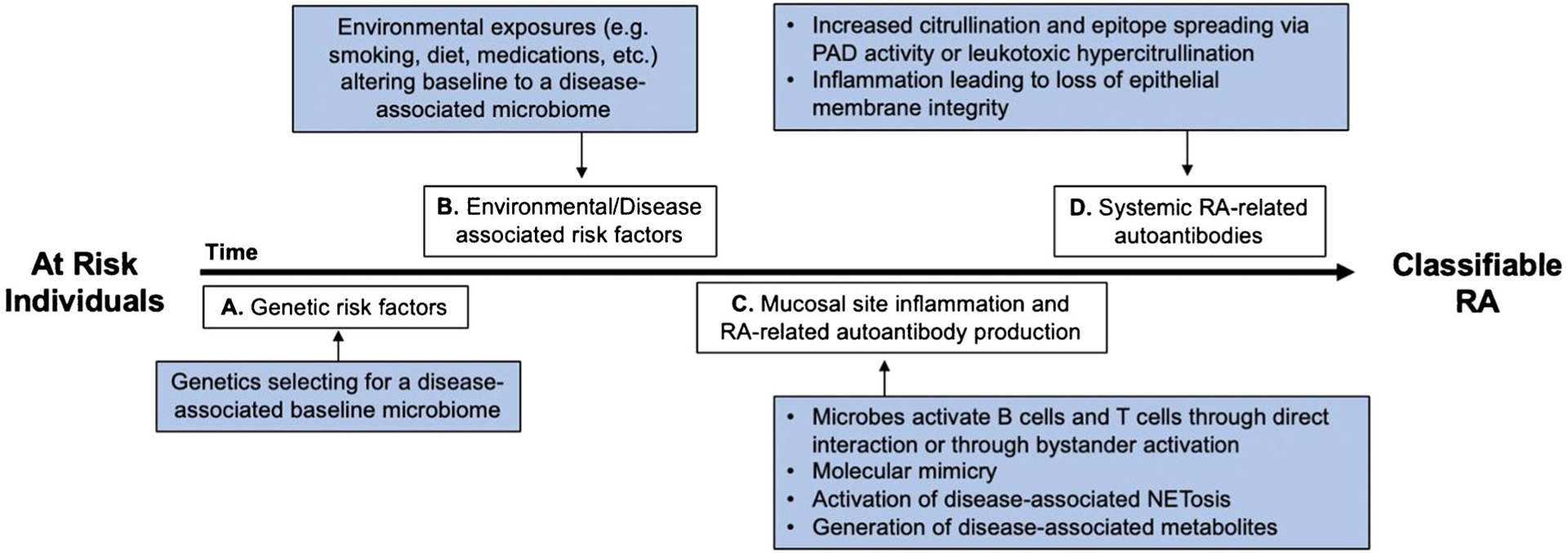
Potential mechanisms by which the microbiome influences different stages of RA development. a There are multiple genetic risk factors for RA, which could select for a disease-associated baseline microbiome. b There are multiple environmental risk factors associated with RA, which could each uniquely alter an individual’s baseline microbiome into a disease-associated microbiome. c Mucosal site inflammation and autoimmunity is demonstrated in individual’s at risk for RA during a pre-clinical period of RA. During this time, the microbiome can have multiple effects on the immune system, both directly and indirectly. d Systemic RA-related autoantibodies are present years prior the development of inflammatory arthritis. During this time, the microbiome can have effects to propagate RA-related autoimmunity
Gastrointestinal (GI) Microbiome
The link between GI dysbiosis (alterations in microbial ecology) and RA pathogenesis has been a topic of investigation for over 25 years [7]. The gut microbiome makes up the largest proportion of microbiota in the body, and localized changes in the gut microbiome can affect systemic inflammation and autoimmunity [8]. As such, understanding changes in the gut microbiome can be informative to understanding the overall pathogenesis of RA.
Bacterial Changes Associated with Established RA
Cohort studies have identified gut dysbiosis in patients with RA compared with controls, including a depletion of gram-negative bacteria and an expansion of gram-positive bacteria in RA patients. In particular, the gram-positive bacterial species Clostridium asparagiforme, Gordonibacter pamelaeae, Eggerthella lenta, and Lachnospiraceae bacterium were enriched in RA patients. Interestingly, these bacterial differences seemed to normalize when RA was treated [9]. Others have observed decreased overall bacterial diversity in the gut microbiome that is associated with disease duration and the presence of rheumatoid factor (RF) [10]. One gut microbe that has emerged to be of particular interest in RA pathogenesis is Prevotella copri (P. copri). In 2013, a study by Scher and colleagues demonstrated a significant expansion of P. copri in the stool of RA patients with untreated new-onset RA compared with chronic RA, healthy controls, and patients with psoriatic arthritis [11]. A subsequent study found that P. copri epitopes are presented in the synovial tissue and circulation of RA patients and could elicit Th1 and antibody responses in various subgroups of RA patients and not in healthy controls [12••]. In addition, Maeda and colleagues confirmed the increased abundance of P. copri in fecal samples of a subgroup of early RA [5]. Interestingly, transfer of these fecal samples from RA patients to SKG germ-free mice resulted in robust arthritis after mice were treated with zymosan [5]. Altogether, these data provide a strong link between gut dysbiosis and the pathophysiology of RA, although many questions remain: How does dysbiosis change during the different phases of RA development? Are the microbial changes causative or an effect of RA-related inflammation? and What is the specific mechanistic contribution of the microbiome to the development of RA in these stages?
The Gut Microbiome in Pre-clinical RA
Few studies have investigated the gut microbiome in the pre-clinical phase of RA, but a recent study by Nguyen and colleagues found that a history of chronic diarrhea was associated with an increased risk of developing RA [13]. Alpizar-Rodriguez and colleagues also investigated the stool microbiome of first-degree relatives (FDRs) of RA patients, and while they did not find differences in bacterial diversity between FDRs with and without systemic RA-related autoantibodies including RF and anti-citrullinated protein antibodies (ACPA), they observed an increased abundance in the family Prevotellaceace in FDRs with systemic RA-related autoimmunity who are representative of the pre-clinical phase of RA [14••].
It remains clear that additional studies of individuals at risk for RA are needed to better understand the role of the microbiome in disease development, but it is also important to consider what is known about gut dysbiosis and risk factors for RA. For example, HLA-DRB1 risk alleles, which are associated with increased RA risk, are also associated with a distinct gut microbiome [15], potentially suggesting that genetic modulation of immune responses to gut microbes could be a mediating factor in the genetic risk associated with RA. In addition, age, smoking, and diet are risk factors for RA development [16, 17], and each can have direct effects on the makeup of the gut microbiome [18, 19]. Importantly, understanding how gut microbes play a role in the breach of immune tolerance and lead to a systemic autoimmune response can lead researchers to develop novel targeted strategies for RA management and prevention.
Potential Mechanisms by Which the Gut Microbiome Affects RA
There are several challenges in human microbiome studies that limit the ability to thoroughly understand the mechanisms by which gut dysbiosis in RA could contribute to disease pathogenesis. Despite known differences between human RA and murine arthritis models, murine models are often needed to provide such mechanistic insight. Experiments in murine models dating back 10+ years have established important connections between murine GI microbiome composition and arthritis development. Mouse models of inflammatory arthritis, including the K/BxN and interleukin(IL)-1 receptor antagonist knockout (ILRn−/−) models, have significantly reduced disease when in germ-free environments [20, 21]. In the germ-free K/BxN mouse model, the reduction in arthritis correlated with reduced autoantibodies, and both disease and autoantibodies increased when the mice were mono-colonized with segmented filamentous bacteria (SFB) [20], which were directed by T follicular helper cell (Tfh) differentiation in Peyer’s patches (PPs) that then migrated to the spleen [22]. Similarly, mono-colonization of germ-free ILRn−/− mice with Lactobacillus bifidus induced rapid arthritis development. Conventionally housed ILRn−/− mice develop intestinal dysbiosis characterized by a significant expansion of Helicobacter and a reduction in Prevotella as they develop arthritis [21], which is associated with increased Th1 and Th17 immune responses compared with germ-free ILRn−/− mice [21]. Finally, work by our lab and others demonstrated that microbial depletion with broad-spectrum antibiotics ameliorates arthritis in the collagen-induced arthritis (CIA) model [23, 24]. We also found that early antibiotic treatment led to reduced autoantibody production, while later treatment resulted alterations in autoantibody glycosylation, supporting the hypothesis that the microbiome may influence arthritis development differently during the different phases of disease [24]. Further supporting our observations that changes in microbiota lead to changes in B cell activity, recent reports have shown that specific microbiota-derived metabolites can activate the aryl hydrocarbon receptor on regulatory B cell populations, significantly attenuating murine arthritis [25•]. Together, findings from these models are highly suggestive that bacterial changes in the gut during the stages of RA development have the potential for modulation of systemic immunity relevant to RA.
Lung Microbiome
It is well established that there is a strong relationship between the lung and RA. Lung disease is one of the most common extra-articular manifestations of RA and is clinically apparent in up to 70% of patients [26–28]. The cornerstone of the mucosal origins hypothesis is that RA begins with inflammation and autoantibody production at mucosal sites [6], and studies of the lung mucosa have most strongly supported this hypothesis [29–33]. While the factors that lead to inflammation and RA-related autoantibody generation in the lung are likely multifactorial, effects of the lung microbiome could play a key role.
Associations of the Lung Microbiome and RA
Despite strong links between RA and the lung, few studies have characterized the lung microbiome in RA. In established RA, Scher and colleagues compared the lung microbiome using bronchoalveolar lavage (BAL) fluid between treatment naïve RA patients, patients with sarcoidosis, and healthy controls [34]. The lung microbiome in RA had less diversity compared with healthy controls, but the microbial lung dysbiosis in RA was similar to sarcoidosis despite the absence of self-reported or clinically apparent lung disease in the RA patients. With no published studies in pre-clinical RA, it remains unknown how the lung microbiome might influence the early stages of RA pathogenesis.
As mentioned above, smoking is a strong risk factor for RA. It can also induce major changes to the lung microbiome [35–37]. However, studies have not yet addressed smoking-related changes to the lung microbiome in established RA or individuals with systemic RA-related autoimmunity. It is of interest that aberrant neutrophil extracellular traps (NETs) have been linked to RA pathogenesis. NET formation is a process during which neutrophils release their chromatin in complex with nuclear and cytoplasmic proteins [38]. Enhanced NET formation has been demonstrated in the peripheral blood and synovial fluid of patients with established RA [39], and NETs can express RA-related citrullinated proteins that have been associated with ACPA generation in RA [40]. Our group has previously reported that subjects at risk for RA have increased levels of NET remnants in the lung, measured in induced sputum [30, 41]. Because microbes are a common trigger of NET formation [38], microbial-induced NET formation could provide a potential mechanistic link between the lung and RA. However, further studies are needed to better elucidate the lung microbiome’s role in RA pathogenesis.
In addition, the lung microbiome could influence the pathogenesis of RA-associated interstitial lung disease (RA-ILD). Established and emerging data have found that increased bacterial burden and perturbations within the lung microbiome are associated with development, progression, and mortality in various ILDs [42, 43], including idiopathic pulmonary fibrosis (IPF) that shares clinical and genetic features with RA-ILD [44–47]. One of the strongest genetic risk factors for IPF—a gain of function promoter variant in the MUC5B gene—is also highly associated with the development of RA-ILD [48, 49]. Of interest, MUC5B overexpression in the distal airway epithelium results in mucin overproduction, ciliary dysfunction, and a compromised host defense—all of which have the potential to alter the microbial community within the lung [50].
Oral Microbiome
In addition to the lung and gut, the oral microbiome has also been linked to RA pathogenesis. In particular, specific bacteria within the oral mucosa, Porphyromonas gingivalis (Pg) and Aggregatibacter actinomycetemcomitans (Aa), have been associated with RA pathogenesis via mechanisms of citrullinated protein production. The following section will further discuss data linking the oral microbiome and RA.
Periodontitis and RA
Periodontitis (PD), one of the most common oral diseases in the adult population, develops in response to bacterial biofilms that form on the surface of tooth enamel. Supporting evidence in multiple epidemiologic studies suggest an association between PD and RA [51–54]. PD has been associated with increased systemic autoantibody production and disease activity in RA [55, 56], and treatment of PD has been shown to improve markers of systemic inflammation and RA disease activity [57, 58]. Despite these correlations, the high incidence of PD in the general population and its poorly defined diagnostic criteria have made it difficult to fully understand its relationship with RA.
Specific Periodontal Microbes and RA
Specific periodontal microorganisms, namely, Pg and Aa, have been closely investigated in an attempt to better understand the interplay between RA and PD. Pg is a gram-negative anaerobic bacteria often termed the keystone microorganism in the development of PD [59]. Pg is unique in that is uses an intrinsic bacterial-peptidylarginine deiminase (PAD) that can citrullinate both host and bacterial proteins, creating potential citrullinated antigen targets for ACPA. Various animal models have described a link between Pg and the development of arthritis [60–63]. Oral inoculation of Pg in rats lead to sero-positive (anti-CCP2+) erosive arthritis [61], and using a CIA model, treatment of Pg after oral inoculation resulted in reduced severity and incidence of arthritis [62]. In epidemiologic human studies, antibody levels to Pg positively correlate with serum ACPA levels, markers of systemic inflammation, and RA disease activity [64–66]. However, variability is seen across studies, and this may be reflective of the heterogeneous antibody testing against several different Pg antigens [67]. In addition to serological profiling, detection of Pg within gingival samples using PCR and high throughput sequencing provides another means of assessment. Unfortunately, results have been inconsistent with studies having found both an increased and decreased abundance of Pg within the oral microbiome in RA [9, 68–70].
It has also been suggested that increased Pg could be independent of RA and instead explained by other variables such as more advanced PD and smoking [68, 70]. In addition, a recent study by Tong and colleagues compared the salivary microbiome of individuals with pre-clinical RA-related auto-immunity (i.e., serum ACPA+ without arthritis) to those with established RA and healthy controls. While the overall microbial composition was similar in the RA and control groups, the ACPA+ individuals exhibited decreased overall microbial diversity with significantly decreased Pg abundance [71•]. These findings do not preclude an association of Pg with the pathogenesis of RA but do suggest that the mechanisms involved are complex and multifactorial.
Similar to Pg, Aa is also a gram-negative bacteria. It is primarily associated with a form of PD known as aggressive PD, and its expression of leukotoxin-A, a pore-forming protein, plays the most significant role in pathogenesis of PD. Relevant to RA, Konig and colleagues found that Aa-associated leukotoxin-A activates endogenous PAD within neutrophils leading to hypercitrullination within periodontal tissues ultimately forming a citrullinome of similar makeup to that seen in the synovium of RA [72]. Antibodies to leukotoxin-A were also prevalent in RA and associated with ACPA and RF [72]. While these data are intriguing, further studies are needed to better characterize this relationship as these findings in RA patients have not yet been reproduced by other groups [73].
Urogenital Microbiome
The urogenital microbiome, primarily consisting of microbes in the lower genital tract, is generally less well studied than microbiomes of the gut, lung, and oral mucosae. In women, the female genital tract microbiome is uniquely characterized by low diversity and is predominated by a community of Lactobacillus species [74]. Microbes of the urogenital tract have the potential to influence autoimmunity and RA in similar manners as those described for the gut, lung, and oral mucosae, but the unique characteristics of the female genital tract microbiome raise the possibility that they could also play a potential role in sex differences in RA.
Urogenital Microbiome in RA
There is limited data exploring the urogenital microbiome in RA. Older literature suggested a link between RA and Proteus mirabilis (Pm) [75–77]. These data included the identification of higher anti-Pm antibody levels in RA patients compared with controls, higher rates of Pm isolation from the urine of RA patients, and sequence homology between amino acid motifs that bind the shared epitope and Pm. However, these studies were only performed in individuals with established RA and do not include individuals in the pre-clinical phase of RA, making it difficult to distinguish whether this association is related to the development of RA or a consequence of RA. Sandberg and colleagues found that urogenital tract infections were associated with a decreased risk of developing RA in a Swedish population-based case-control study [78], but the contribution of urogenital microbial dysbiosis in the absence of overt infection has not been studied related to risk of developing RA.
When considering the potential role of the urogenital microbiome in RA, it is important to consider that women develop RA approximately 3 times more often than men [79]. Yet, the etiology of this sex difference is unknown. Hypotheses include immunologic effects of sex hormones and pregnancy as well as genetic or environmental risk factors disproportionately effecting women. However, an additional area that should be explored in more detail is the effect of the vaginal microbiome on inflammation and autoimmunity in the development of RA, particularly given that dysbiosis in the vaginal microbiome can trigger RA-associated inflammatory cytokines (e.g., IL-1β, IL-6, IL-8, and TNFα) as well as Th1 induction of naïve CD4+ T cells [80, 81].
Future Directions
Over the past several years, data continue to strengthen the support that the microbiome plays a role in RA pathogenesis. However, it continues to be a challenge to understand if this is a direct or indirect relationship, is the relationship causal or simply the result of a change induced by RA-related inflammation, and is microbial dysbiosis necessary or sufficient for RA development. More research in this area is clearly needed, in large part because of the tremendous opportunity for treatments and prevention if we can fully understand the microbiome’s role in RA.
There are many important future areas of research needed in this area. Foremost is understanding the mechanisms of disease development associated with microbiota in RA and where in the timeline of RA development they have an effect (Fig. 1). Such discoveries can be challenging when studying human subjects and will likely require large longitudinal cohorts. As highlighted in this review, multiple mucosal sites are suggested to play a role in RA, and some studies have begun to simultaneously study more than one mucosal site in RA. However, this approach will be imperative going forward not only to understand whether it is a microbial-driven process at a single site or an additive effect at multiple mucosal sites that contributes to RA but also to elucidate the contribution of immune cross-talk between different mucosal sites and how mucosal sites could ultimately influence joint inflammation. There has also been identification of a circulating microbiome in RA and other autoimmune diseases [82], and understanding whether it is a marker of mucosal dysbiosis, a marker of mucosal epithelial integrity, or if it has its own unique contribution to RA pathogenesis will be important. Moreover, microbiome studies to date in RA have largely focused on bacteria. As technologies advance, it will be critical to also understand the effects of viral and fungal microorganisms on inflammation and autoimmunity in RA. Lastly, in addition to understanding the role of microbes on the development of RA, it is becoming clear that a patient’s microbiome can also have an effect on metabolism and effectiveness of the medications used to treat their RA (Reviewed in [83]), and understanding this in more detail could change our approach to RA management.
Conclusions
In summary, the human microbiome is a key factor in developing and maintaining the host immune response. Various disturbances in the microbiome at multiple mucosal sites have been associated with distinct effects on the immune system that have been associated with RA. Future studies are needed to better define microbial changes in the pathogenesis of RA, including exactly how and when they influence the development of RA. Importantly, such findings can better inform whether targeting the microbiome could have a beneficial effect on RA treatment and even prevention of joint disease in RA.
Footnotes
Conflict of Interest MKD has received research support from Pfizer Inc. on studies related to RA. All other authors have declared that no conflict of interest exists.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15(3):374–81. 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D, Kim YG, Seo SU, Kim DJ, Kamada N, Prescott D, et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat Med. 2016;22(5):524–30. 10.1038/nm.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15. 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheum. 2016;68(11):2646–61. 10.1002/art.39783. [DOI] [PubMed] [Google Scholar]
- 6.Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol. 2018;14(9):542–57. 10.1038/s41584-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eerola E, Mottonen T, Hannonen P, Luukkainen R, Kantola I, Vuori K, et al. Intestinal flora in early rheumatoid arthritis. Br J Rheumatol. 1994;33(11):1030–8. 10.1093/rheumatology/33.11.1030. [DOI] [PubMed] [Google Scholar]
- 8.De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195(1):74–85. 10.1111/cei.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895–905. 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Wright K, Davis JM, Jeraldo P, Marietta EV, Murray J, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8(1):43 10.1186/s13073-016-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.••.Pianta A, Arvikar S, Strle K, Drouin EE, Wang Q, Costello CE, et al. Evidence of the Immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheum. 2017;69(5):964–75. 10.1002/art.40003 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a potential mechanism by which P. copri peptides could contribute to RA pathogenesis through stimulation of Th1 responses in a subgroup of RA patients.
- 13.Nguyen Y, Mariette X, Salliot C, Gusto G, Boutron-Ruault MC, Seror R. Chronic diarrhoea and risk of rheumatoid arthritis: findings from the French E3N-EPIC cohort study. Rheumatology (Oxford). 2020. 10.1093/rheumatology/keaa133. [DOI] [PubMed] [Google Scholar]
- 14.••.Alpizar-Rodriguez D, Lesker TR, Gronow A, Gilbert B, Raemy E, Lamacchia C, et al. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann Rheum Dis. 2019;78(5):590–3. 10.1136/annrheumdis-2018-214514 [DOI] [PubMed] [Google Scholar]; This is one of the first studies to characterize the gut microbiome in first degree relatives of those with RA in addition to a subset with preclinical RA, thereby opening the door to better understand the microbiota as a potential trigger of RA in humans.
- 15.Asquith M, Sternes PR, Costello ME, Karstens L, Diamond S, Martin TM, et al. HLA alleles associated with risk of Ankylosing spondylitis and rheumatoid arthritis influence the gut microbiome. Arthritis Rheum. 2019;71(10):1642–50. 10.1002/art.40917. [DOI] [PubMed] [Google Scholar]
- 16.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 17.Johansson K, Askling J, Alfredsson L, Di Giuseppe D, group Es. Mediterranean diet and risk of rheumatoid arthritis: a population-based case-control study. Arthritis Res Ther. 2018;20(1):175 10.1186/s13075-018-1680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Yun Y, Kim SJ, Lee EJ, Chang Y, Ryu S, et al. Association between cigarette smoking status and composition of gut microbiota: population-based cross-sectional study. J Clin Med. 2018;7(9): 282 10.3390/jcm7090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–27. 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogier R, Ederveen THA, Boekhorst J, Wopereis H, Scher JU, Manasson J, et al. Aberrant intestinal microbiota due to IL-1 receptor antagonist deficiency promotes IL-17- and TLR4-dependent arthritis. Microbiome. 2017;5(1):63 10.1186/s40168-017-0278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng F, Klinger CN, Felix KM, Bradley CP, Wu E, Tran NL, et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s patch T follicular helper cells. Immunity. 2016;44(4):875–88. 10.1016/j.immuni.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietrosimone KM, Jin M, Poston B, Liu P. Collagen-Induced Arthritis: A model for Murine Autoimmune Arthritis. Bio Protoc. 2015;5(20):e1626 10.21769/bioprotoc.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jubair WK, Hendrickson JD, Severs EL, Schulz HM, Adhikari S, Ir D, et al. Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheum. 2018;70(8):1220–33. 10.1002/art.40490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.•.Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31(4):837–51 e10. 10.1016/j.cmet.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that supplementation of a known microbial-derived metabolite, butyrate, in mice significantly reduced arthritis by driving regulatory B cell populations and suppressing B cell and plasmablast differentiation.
- 26.Ascherman DP. Interstitial lung disease in rheumatoid arthritis. Curr Rheumatol Rep. 2010;12(5):363–9. 10.1007/s11926-010-0116-z. [DOI] [PubMed] [Google Scholar]
- 27.Brito Y, Glassberg MK, Ascherman DP. Rheumatoid arthritis-associated interstitial lung disease: current concepts. Curr Rheumatol Rep. 2017;19(12):79 10.1007/s11926-017-0701-5. [DOI] [PubMed] [Google Scholar]
- 28.Spagnolo P, Lee JS, Sverzellati N, Rossi G, Cottin V. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheum. 2018;70(10):1544–54. 10.1002/art.40574. [DOI] [PubMed] [Google Scholar]
- 29.Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–54. 10.1002/art.38066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheum. 2017;69(6):1165–75. 10.1002/art.40066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheum. 2014;66(1):31–9. 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- 32.Reynisdottir G, Olsen H, Joshua V, Engstrom M, Forsslund H, Karimi R, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. 2016;75(9):1722–7. 10.1136/annrheumdis-2015-208216. [DOI] [PubMed] [Google Scholar]
- 33.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61. 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scher JU, Joshua V, Artacho A, Abdollahi-Roodsaz S, Ockinger J, Kullberg S, et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome. 2016;4(1):60 10.1186/s40168-016-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C, Shi G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J Transl Med. 2019;17(1):225 10.1186/s12967-019-1971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187(10):1067–75. 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mammen MJ, Sethi S. COPD and the microbiome. Respirology. 2016;21(4):590–9. 10.1111/resp.12732. [DOI] [PubMed] [Google Scholar]
- 38.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 39.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol. 2017;2(10):eaag3358 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demoruelle MK, Bowers E, Lahey LJ, Sokolove J, Purmalek M, Seto NL, et al. Antibody responses to citrullinated and noncitrullinated antigens in the sputum of subjects with rheumatoid arthritis and subjects at risk for development of rheumatoid arthritis. Arthritis Rheum. 2018;70(4):516–27. 10.1002/art.40401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384(9944):691–702. 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salisbury ML, Han MK, Dickson RP, Molyneaux PL. Microbiome in interstitial lung disease: from pathogenesis to treatment target. Curr Opin Pulm Med. 2017;23(5):404–10. 10.1097/MCP.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014;2(7):548–56. 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molyneaux PL, Cox MJ, Wells AU, Kim HC, Ji W, Cookson WO, et al. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res. 2017;18(1): 29 10.1186/s12931-017-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199(9):1127–38. 10.1164/rccm.201809-1650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong X, Su F, Xu X, Xu H, Yang T, Xu Q, et al. Alterations to the lung microbiome in idiopathic pulmonary fibrosis patients. Front Cell Infect Microbiol. 2019;9:149 10.3389/fcimb.2019.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. 2018;379(23):2209–19. 10.1056/NEJMoa1801562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–12. 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Q, Wang Y, Qu D, Yu J, Yang J. The possible pathogenesis of idiopathic pulmonary fibrosis considering MUC5B. Biomed Res Int. 2019;2019:9712464–12. 10.1155/2019/9712464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5(4):218–24. 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 52.Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81(2):223–30. 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- 53.Fuggle NR, Smith TO, Kaul A, Sofat N. Hand to mouth: a systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front Immunol. 2016;7:80 10.3389/fimmu.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheum. 2014;66(5):1090–100. 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Correa JD, Fernandes GR, Calderaro DC, Mendonca SMS, Silva JM, Albiero ML, et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep. 2019;9(1):8379 10.1038/s41598-019-44674-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eriksson K, Fei G, Lundmark A, Benchimol D, Lee L, Hu YOO, et al. Periodontal health and oral microbiota in patients with rheumatoid arthritis. J Clin Med. 2019;8(5):630 10.3390/jcm8050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaur S, Bright R, Proudman SM, Bartold PM. Does periodontal treatment influence clinical and biochemical measures for rheumatoid arthritis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):113–22. 10.1016/j.semarthrit.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Silvestre FJ, Silvestre-Rangil J, Bagan L, Bagan JV. Effect of non-surgical periodontal treatment in patients with periodontitis and rheumatoid arthritis: a systematic review. Med Oral Patol Oral Cir Bucal. 2016;21(3):e349–54. 10.4317/medoral.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–25. 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung H, Jung SM, Rim YA, Park N, Nam Y, Lee J, et al. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PLoS One. 2017;12(11):e0188698 10.1371/journal.pone.0188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Courbon G, Rinaudo-Gaujous M, Blasco-Baque V, Auger I, Caire R, Mijola L, et al. Porphyromonas gingivalis experimentally induces periodontis and an anti-CCP2-associated arthritis in the rat. Ann Rheum Dis. 2019;78(5):594–9. 10.1136/annrheumdis-2018-213697. [DOI] [PubMed] [Google Scholar]
- 62.Lubcke PM, Ebbers MNB, Volzke J, Bull J, Kneitz S, Engelmann R, et al. Periodontal treatment prevents arthritis in mice and methotrexate ameliorates periodontal bone loss. Sci Rep. 2019;9(1): 8128 10.1038/s41598-019-44512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marchesan JT, Gerow EA, Schaff R, Taut AD, Shin SY, Sugai J, et al. Porphyromonas gingivalis oral infection exacerbates the development and severity of collagen-induced arthritis. Arthritis Res Ther. 2013;15(6):R186 10.1186/ar4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arvikar SL, Collier DS, Fisher MC, Unizony S, Cohen GL, McHugh G, et al. Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R109 10.1186/ar4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.•.Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, van der Woude D, et al. Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol. 2010;37(6):1105–12. 10.3899/jrheum.091323 [DOI] [PubMed] [Google Scholar]; This study found that after oral inoculation of Pg, treatment with oral antibiotics prevented development of arthritis in CIA mice to the same degree as oral methotrexate.
- 66.Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9(1):38–42. 10.1016/j.intimp.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Banuelos E, Mukherjee A, Darrah E, Andrade F. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Clin Med. 2019;8(9):1309 10.3390/jcm8091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beyer K, Zaura E, Brandt BW, Buijs MJ, Brun JG, Crielaard W, et al. Subgingival microbiome of rheumatoid arthritis patients in relation to their disease status and periodontal health. PLoS One. 2018;13(9):e0202278 10.1371/journal.pone.0202278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mikuls TR, Walker C, Qiu F, Yu F, Thiele GM, Alfant B, et al. The subgingival microbiome in patients with established rheumatoid arthritis. Rheumatology (Oxford). 2018;57(7):1162–72. 10.1093/rheumatology/key052. [DOI] [PubMed] [Google Scholar]
- 70.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64(10):3083–94. 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.•.Tong Y, Zheng L, Qing P, Zhao H, Li Y, Su L, et al. Oral microbiota perturbations are linked to high risk for rheumatoid arthritis. Front Cell Infect Microbiol. 2019;9:475 10.3389/fcimb.2019.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]; This is one of the first studies to characterize the gingival microbiome in preclinical RA providing insight into how bacteria could have different effects at different stages of RA development.
- 72.Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8(369): 369ra176 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volkov M, Dekkers J, Loos BG, Bizzarro S, Huizinga TWJ, Praetorius HA, et al. Comment on “Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis”. Sci Transl Med. 2018;10(433):eaan8349 10.1126/scitranslmed.aan8349. [DOI] [PubMed] [Google Scholar]
- 74.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6): e37818 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebringer A, Ptaszynska T, Corbett M, Wilson C, Macafee Y, Avakian H, et al. Antibodies to proteus in rheumatoid arthritis. Lancet. 1985;2(8450):305–7. 10.1016/s0140-6736(85)90352-6. [DOI] [PubMed] [Google Scholar]
- 76.Ebringer A, Cunningham P, Ahmadi K, Wrigglesworth J, Hosseini R, Wilson C. Sequence similarity between HLA-DR1 and DR4 subtypes associated with rheumatoid arthritis and proteus/serratia membrane haemolysins. Ann Rheum Dis. 1992;51(11):1245–6. 10.1136/ard.51.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson C, Thakore A, Isenberg D, Ebringer A. Correlation between anti-proteus antibodies and isolation rates of P. mirabilis in rheumatoid arthritis. Rheumatol Int. 1997;16(5):187–9. 10.1007/BF01330294. [DOI] [PubMed] [Google Scholar]
- 78.Sandberg ME, Bengtsson C, Klareskog L, Alfredsson L, Saevarsdottir S. Recent infections are associated with decreased risk of rheumatoid arthritis: a population-based case-control study. Ann Rheum Dis. 2015;74(5):904–7. 10.1136/annrheumdis-2014-206493. [DOI] [PubMed] [Google Scholar]
- 79.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–82. 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Teijlingen NH, Helgers LC, Zijlstra-Willems EM, van Hamme JL, Ribeiro CMS, Strijbis K, et al. Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells. J Reprod Immunol. 2020;138:103085 10.1016/j.jri.2020.103085. [DOI] [PubMed] [Google Scholar]
- 81.Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep. 2017;7(1):11974 10.1038/s41598-017-12198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammad DBM, Hider SL, Liyanapathirana VC, Tonge DP. Molecular characterization of circulating microbiome signatures in rheumatoid arthritis. Front Cell Infect Microbiol. 2019;9:440 10.3389/fcimb.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manasson J, Blank RB, Scher JU. The microbiome in rheumatology: where are we and where should we go? Ann Rheum Dis. 2020;79(6):727–33. 10.1136/annrheumdis-2019-216631. [DOI] [PubMed] [Google Scholar]


