Abstract
In silico analysis of six phytochemicals, flabelliferin, marmelosin, piperine, ocimin, curcumin and leucoanthocyanin, along with three drug compounds, nelfinavir, remdesivir and hydroxychloroquine, as positive control against drug targets of one SARS-CoV-2 viral protease, COVID-19 main protease (SARS CoV-2 3CLpro/Mpro), two coronavirus proteases, SARS-CoV main peptidase (SARS CoV Mpro), SARS-CoV main proteinase (SARS CoV 3CLpro), and one human cellular transmembrane serine proteinase (TMPRSS2), was carried out. Except leucoanthocyanin all other phytochemicals proved better than all three positive control drugs against SARS-CoV main peptidase, whereas, flabelliferin was found to be the potential inhibitor for SARS-CoV main proteinase out performing all the positive control drugs and phytochemicals. Amongst the compounds studied, the best inhibitor for COVID-19 main protease was nelfinavir followed by flabelliferin and ocimin. Flabelliferin was found to the best promising inhibitor of human cellular transmembrane serine proteinase, followed by nelfinavir, curcumin, piperine and marmelosin. The result on the inhibition of human cellular transmembrane serine proteinase against COVID-19 has a stable therapeutic advantage as mutation may quickly occur on viral drug targets. Hence, all the phytochemicals tested in the present study are the potential inhibitors of the all the four drug targets and can form a part of therapeutics against COVID-19 with further clinical studies.
Communicated by Ramaswamy H. Sarma
Keywords: COVID-19 main protease, SARS-CoV main peptidase, SARS-CoV main proteinase, TMPRSS2, drug likeliness
1. Introduction
From the beginning of this century, three variants of coronaviruses, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle-East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2 have caused high mortality pneumonia in humans. The recent pandemic caused by SARS-CoV-2 (COVID-19), was isolated and sequenced by Zhou et al. (2020). SARS-CoV-2 is a pleomorphic enveloped particle containing single-stranded (positive-sense) RNA associated with a nucleoprotein within a capsid comprised of matrix protein and the envelope bears club-shaped glycoprotein projections (Mousavizadeh & Ghasemi, 2020). SARS-CoV-2 has a similar genomic organization like other beta-corona viruses, consisting of a 5′-untranslated region (UTR), a replicase complex (orf1ab) encoding non-structural proteins (nsps), a spike protein (S) gene, envelope protein (E) gene, a membrane protein (M) gene, a nucleocapsid protein (N) gene, 3′-UTR, and several unidentified non-structural open reading frames (Zhu et al., 2020).
SARS-CoV-2 binds to human ACE2 (the angiotensin converting enzyme 2) by its spike and allows COVID-19 to enter and infect cells (Mousavizadeh & Ghasemi, 2020; Shang et al., 2020). For the virus to complete entry into the cell following this initial process, the spike protein has to be primed by an enzyme. Similar to SARS-CoV, SARS-CoV-2 uses a protease called transmembrane protease serine 2 (TMPRSS2) to complete this process (Guo et al., 2020; Hoffmann et al., 2020). The SARS-S/ACE2 interface is the determining factor for the infection, and the efficiency of ACE2 usage is the key for SARS-CoV transmissibility. The COVID-19 infection produces variety of responses and several abnormalities in human including cellular immune deficiency, coagulation activation, myocardia injury, hepatic and kidney injury, and secondary bacterial infection (Chen et al., 2020).
The replication of coronaviruses occurs in host cell cytoplasm mediated by the viral proteolytic enzymes viz. coronavirus main proteinase (Anand et al., 2003), coronavirus main peptidase (Lee et al., 2007) in general for coronavirus family and papain-like protease (PLpro) and 3 chymotrypsin-like cysteine protease (Astuti & Ysrafil, 2020) in particular for SARS CoV-2. Hence, these viral enzymes and the human cellular protease TMPRSS2 can act as the potential drug targets. There were studies on this line targeting either viral chymotrypsin-like cysteine protease (Anurag et al., 2020; Khaerunnisa et al., 2020; Sharma & Kaur, 2020; Xu et al., 2020), SARS coronavirus main protease (Tahir Ul Qamar et al., 2020), SARS coronavirus main peptidase (Khaerunnisa et al., 2020), or human transmembrane protease serine 2 (Hoffmann et al., 2020). However, so far all the four targets were not studied together.
As on 10th June 2020 more than 7 million people were infected and more than 0.4 million people succumbed to COVID-19 worldwide. Though, the count of the infected is on upward trend, there is no clinically proven preventive or therapeutic measures are available as on date. The in-silico methods are cost-effective initial approach for identifying appropriate ligands and potential inhibitors against SARS-CoV-2.Studies have indicated the potentiality of anti-viral drugs like protease inhibitors lopinavir/ritonavir, nucleoside analogues, neuraminidase inhibitors, remdesivir, umifenovir, tenofovir disoproxil (TDF), and lamivudine (3TC), etc. for the treatment of COVID-19 infected patients (Lu, 2020). Xu et al. (2020) has screened 1903 approved drugs by homology modeling, molecular docking and binding free energy calculation and suggested nelfinavir as potential inhibitor against SARS CoV-2. The screening of clinically approved drugs with the druggable target SARS-CoV-2 helicase protein suggested that vapreotide as a choice of drug for wet lab studies to inhibit the infection of SARS-CoV-2 (Borgio et al., 2020). Another in-silico approach identified one potent ligand against the envelope protein and one potent ligand against nucleocapsid phosphoprotein of SARS-CoV-2. These two ligands are the added targets for the development of drugs against SARS –CoV-2. Using molecular docking it was evidenced that statins as efficient SARS-CoV-2 Mpro inhibitors (Reiner et al., 2020). Based on the similarity identified using in slico analysis, the nsp13, the helicase protein of SARS-CoV-2 with SARS- and MERS-Nsp13, Habtemariam et al. (2020) proposed this nonstructural protein also an important drug target.
Though in light of recent evidences on the safety and efficacy of hydroxychloroquine as a treatment for COVID-19 patients, the Executive Group of the Solidarity Trial of World Health Organization (WHO) decided to withhold the use of the hydroxychloroquine (HCQ), the final decision is expected in mid-June. Hence, as on date, HCQ forms one of the potential drugs for treatment of COVID-19. The in vitro studies showed the 7 times increased efficacy of hydroxychloroquine (Yao et al., 2020) compared to chloroquine (Wang et al., 2020) despite very similar chemical properties. The drug nelfinavir was shown to inhibit the SARS- dCoV replication (Yamamoto et al., 2004) and the in silico analysis has shown it be a highly potential inhibitor of SARS-CoV-2 main protease (Mothay & Ramesh, 2020), whereas, remdesivir was shown to be a potent antiviral drug for SARS-CoV-2 virus in in vitro studies (Lu, 2020; Wang et al., 2020). Studies have indicated the potentiality of anti-viral drugs like protease inhibitors lopinavir/ritonavir, nucleoside analogues, neuraminidase inhibitors, remdesivir, umifenovir, tenofovir disoproxil (TDF), and lamivudine (3TC), etc. for the treatment of COVID-19 infected patients (Lu, 2020).
The present study explores the phytochemicals such as flabelliferin B from Borassus flabellifer, marmelosin from Aegle marmelos, ocimin from Curcuma caesia, piperine from Piper nigrum, curcumin from Curcuma longa and leucoanthocyanin from Phyllanthus emblica as potential drugs for COVID-19 as these plants and their principal phyto constituents are being used in traditional Indian medicine. These phytochemical were selected as Phyllanthus emblica (Jain et al., 2015), Aegle marmelos (Baliga et al., 2012), Curcuma caesia (Deeki et al., 2019; Venugopal et al., 2017), Borassus flabellifer (Jamkhande et al., 2016), piperine (Chopra et al., 2016), and curcumin (Zahedipour et al., 2020) are shown to possess one or other medicinal properties like immune modulatory, antitussive, thrombolytic, anti-inflammatory, cytoprotective, anti-microbial, anti viral, which may reduce the symptoms of COVID-19.
The phytochemicals were analyzed along with available drugs nelfinavir, hydroxychloroquine and remdesivir as positive control against the major viral proteins viz., chymotrypsin-like cysteine protease, main peptidase, main proteinase and the human TMPRSS2 as potential drug targets using homology modeling, molecular docking and binding free energy calculation. By far this is the first comprehensive in silico analysis involving all the four drug targets for COVID-19.
2. Materials and methods
2.1. Target preparation
The 3 D structures of COVID-19 main protease (6LU7.pdb), SARS-CoV main peptidase (2GTB.pdb), SARS-CoV main proteinase (3M3V.pdb) and Type II transmembrane serine proteinase (2OQ5.pdb) were retrieved from RCSB Protein Data Bank (https://www.rcsb.org/).
2.2. Phytochemical retrieval
Chemical, structural details and canonical SMILES of the ligand molecules were obtained from PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Using canonical SMILES, the PDB structures of the compound were obtained by an online tool named CORINA. Basic details of the phytochemicals used in the study are given in Table 1.
Table 1.
List of phytochemicals and drug molecules used for docking studies.
| phytochemical | Canonical smiley | Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| Curcumin | COC1=C(C=CC(=C1)C=CC(=O)CC(=O)C=CC2=CC(=C(C=C2)O)OC)O | C21H20O6 | 368.4 |
| Ocimin | COC1=CC=C(C=C1)C=CCCC=CC2=CC=C(C=C2)OC | C20H22O2 | 294.4 |
| Flabelliferin B | CC(=O)C1=CC2(C(CC1O)C3(CCC4C(CCCC4(C3CC2OC(=O)C)C)(C)C)C)C | C27H42O4 | 430.6 |
| Marmelosin | CC(=CCOC1=C2C(=CC3=C1OC=C3)C=CC(=O)O2)C | C16H14O4 | 270.28 |
| Leucoanthocyanin | C1=C(C=C(C(=C1O)O)O)C2C(C(C3=C(C=C(C=C3O2)O)O)O)O | C15H14O8 | 322.27 |
| Piperine | C1CCN(CC1)C(=O)C=CC=CC2=CC3=C(C=C2)OCO3 | C17H19NO3 | 285.34 |
| Remdesivir (Positive Control) |
CCC(CC)COC(=O)C(C)NP(=O)(OCC1C(C(C(O1)(C#N)C2=CC=C3N2N=CN=C3N)O)O)OC4=CC=CC=C4 | C27H35N6O8P | 602.6 |
| Hydroxychloroquine (Positive Control) |
CCN(CCCC(C)NC1=C2C=CC(=CC2=NC=C1)Cl)CCO | C18H26ClN3O | 335.9 |
| Nelfinavir (Positive Control) |
CC1=C(C=CC=C1O)C(=O)NC(CSC2=CC=CC=C2)C(CN3CC4CCCCC4CC3C(=O)NC(C)(C)C)O.CS(=O)(=O)O | C32H45N3O4S | 567.8 |
2.3. Active site prediction
The predicted active sites for COVID-19 main protease and SARS-CoV main peptidase were derived from Khaerunnisa et al. (2020) and SARS-CoV main proteinase was derived from Tahir Ul Qamar et al. (2020). Active site of the TMPRSS2 protein molecules was identified (Supplementary File. 1) using the online tool called Computed Atlas for Surface Topography of Proteins (CASTp) (Tian et al., 2018).
2.4. Grid generation and molecular docking
All the selected phytochemicals were docked into the binding site of the three viral protein and one human cell protein using AutoDock 4.2 following the procedure described by (Morris et al., 1998). AutoDock version 4.2 was used for protein optimization, by removing water and other atoms, and then adding a polar hydrogen group. The grid-based method was done to allow rapid evaluation of the binding energy of conformation.
Grid was generated with AutoDock 4.2 and the target proteins were embedded in the grid. The XYZ coordinates were adjusted while setting grid for enclosing the active residues. It ensured the accurate binding of proteins with ligand molecules. The grid spacing was given as 0.375 A° and the XYZ dimension as 60 × 60 × 60 for all proteins. The grid was centered on the active site and XYZ-coordinates of the macromolecules were as follows, for 6LU7 − 14.252 A°,17.529 A°,64.042 A°, for 2GTB 20.131 A°, −8.576 A°, 12.749 A°, for 3M3V −5.935 A°,2.785 A°,8.9 A° and for 2OQ5 − 1.263 A°, 18.552 A°,23.665 A°. All other parameters were kept as default for docking.
2.5. Post dock analysis and drug likeness
pKCSM is a novel approach in prediction of pharmacokinetics properties like absorption, distribution, metabolism, excretion, and toxicity and it provides a rapid and easy, early evaluation of compounds (Pires et al., 2015). Each promising molecule was submitted on an open access online tool http://biosig.unimelb.edu.au/pkcsm/prediction. It is a graph-based signature to develop predictive models of central ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) properties for drug development.
3. Results and discussion
3.1. Phytochemicals Vs cellular transmembrane serine proteinase (TMPRSS2)
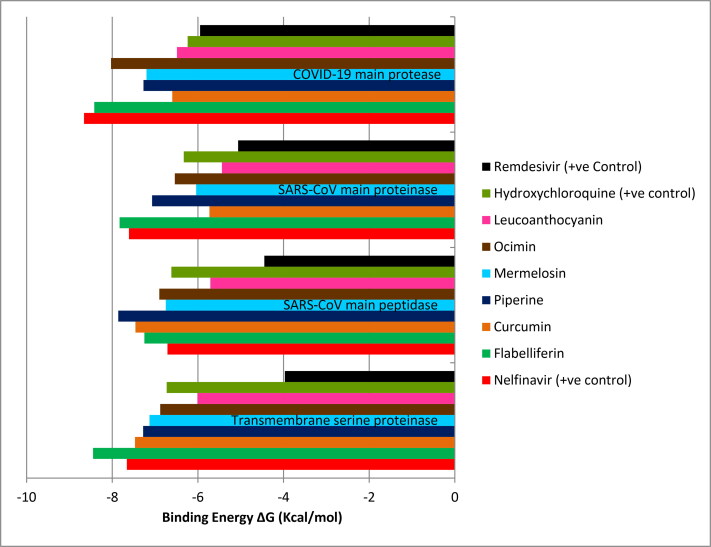
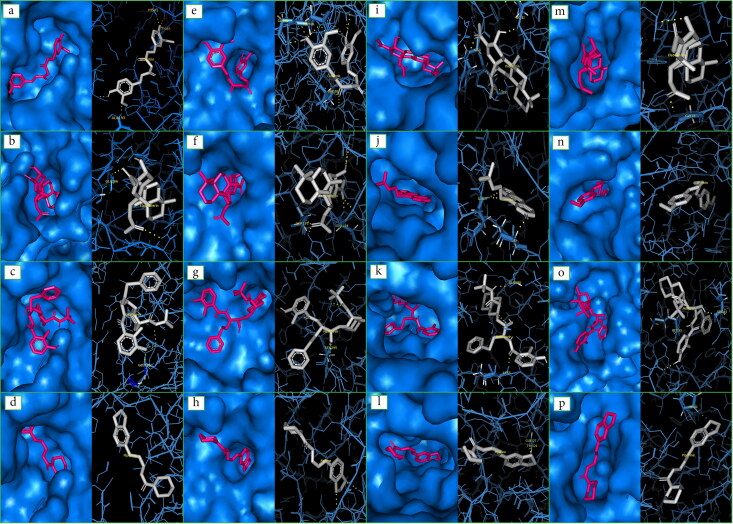
Results of docking analysis revealed that all the phytochemicals tested showed negative binding energy on the target protein TMPRSS2. Hence, all phytochemical can be given equal importance as potential drugs. The phytochemical flabelliferin from Borassus flabellifer had the highest binding energy of (-) 8.45 kcal/mol with TMPRSS2 (Table 2a and Figure 1) than the all three positive control drugs and formed strong hydrogen bonds with SER195: HG and SER214:HN (Figure 2). Molecular interaction studies revealed that phytochemicals, curcumin (- 7.47 kcal/mol), marmelosin (- 7.13 kcal/mol) and leucoanthocyanin (- 6.01 kcal/mol) apart from showing negative binding energy, they had more than one active residue sites. Curcumin (GLY148:O; ASP147:HN; SER195: HG) and leucoanthocyanin (ASP189:OD1; ASP147:HN; SER195: OG) had three and marmelosin (SER195: OG SER214:HN) had two active residue sites. So far, no plant derived molecule has been suggested as a potential inhibitor of TMRSS2. However, Hoffman et al. (2020) has reported Camostat mesylate, as a clinically approved TMPRSS2 inhibitor to block SARS-CoV-2 entry to human cells, indicating its therapeutic potential as a drug against COVID-19.
Table 2a.
Interaction of phytochemicals with Cellular transmembrane serine proteinase and COVID-19 main protease.
| Phyto chemical | Drug Targets |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cellular transmembrane serine proteinase |
COVID-19 main protease |
|||||||||
| Binding energy | Ligand efficiency | Inhibition constant | h-bond | Residue (h-bond) |
Binding energy | Ligand efficiency | Inhibition constant | h-bond | Residue (h-bond) |
|
| Nelfinavir | −7.66 | −0.19 | 2.41 | 1 | SER195: HG | −8.66 | −0.22 | 451.98 | 2 | SER144: OG MET165:O |
| Flabelliferin | −8.45 | −0.27 | 635.97 | 2 | SER195: HG SER214:HN | −8.42 | −0.27 | 678.08 | 1 | GLY138:HN |
| Curcumin | −7.47 | −0.28 | 3.36 | 3 | GLY148:O ASP147:HN SER195: HG | −6.6 | −0.24 | 14.63 | 1 | CYS145:HN |
| Piperine | −7.28 | −0.35 | 4.59 | 0 | −7.27 | −0.35 | 4.71 | 1 | HIS41:HE2 | |
| Marmelosin | −7.13 | −0.36 | 5.97 | 2 | SER195: OG SER214:HN | −7.2 | −0.36 | 5.3 | 1 | GLU166:HN |
| Ocimin | −6.88 | −0.31 | 9.01 | 0 | −8.03 | −0.37 | 1.31 | 0 | ||
| Leuco anthocyanin | −6.01 | −0.26 | 39.03 | 3 | ASP189:OD1 ASP147:HN SER195: OG | −6.49 | −0.28 | 17.41 | 5 | HIS164:HN MET165:HN TYR161: OH MET165:O SER144: OG |
| Hydroxy chloroquine | −6.73 | −0.29 | 11.68 | 3 | GLU218:O GLY216:HN GLY216:O | −6.24 | −0.27 | 26.77 | 2 | GLY138:O PHE140:HN |
| Remdesivir | −3.97 | −0.09 | 1.22 | 0 | −5.95 | −0.14 | 43.65 | 1 | GLU166:O | |
Figure 1.
Interaction of phytochemicals and drug targets based on binding energy.
Figure 2.
Docking images of SARS-CoV main peptidase with a. Curcumin, b. Flabelliferin, c. Nelfinavir, d. Piperine; Cellular transmembrane serine proteinase with e. Curcumin, f. Flabelliferin, g. Nelfinavir, h. Piperine; SARS-CoV main proteinase with i. Flabelliferin, j. Marmelosin, k. Nelfinavir, l. Piperine; COVID-19 main protease with m. Flabelliferin, n. Ocimin, o. Nelfinavir, p. Piperine.
3.2. Phytochemicals Vs COVID-19 main protease
Similarly, all phytochemicals had shown negative binding energy on the target protein COVID-19 main protease. Flabelliferin showed highest binding energy (Table 2a and Figure 1) amongst all phytochemicals analyzed, and it was higher than two positive control drugs (HCQ and remdesivir). The drug nelfinavir had shown slightly higher binding energy (-8.66 kcal/mol) than flabelliferin (-8.42 kcal/mol) followed by ocimin (-8.03 kcal/mol), piperine (-7.27 kcal/mol), marmelosin (-7.2 kcal/mol), and curcumin (-6.6 kcal/mol). Similar trend was observed by (Khaerunnisa et al., 2020), where nelfinavir was having more binding affinity than the curcumin with COVID-19 main protease. Xu et al. (2020) indicated that among tested drugs nelfinavir was identified as the best potential inhibitor against COVID-19 Mpro. Apart from good binding energy (-6.49 kcal/mol) leucoanthocyanin had highest number (5) of active residue sites (HIS164:HN; MET165:HN; TYR161: OH; MET165:O; SER144: OG).
3.3. Phytochemicals Vs SARS-CoV main peptidase
Here again all the phytochemicals had shown negative binding energy on the target protein (Table 2b and Figure 1). Except leucoanthocyanin all the phytochemicals performed better than all the three positive control drugs. Amongst them, piperine, curcumin and flabelliferin found to be better drug compounds. Curcumin (CYS85:O; HIS41:HN; ALA193:HN), marmelosin (ARG40:HH22; ASP187:HN; ARG40: HE) had three each and leucoanthocyanin (ASP187:O; THR175:OG1; VAL186:O; GLU166:HN; CYS85:O) had five active residue sites. Similar study was made with plant molecules by (Khaerunnisa et al., 2020) targeting 2GTB.
Table 2b.
Interaction of phytochemicals with SARS-CoV main peptidase and SARS-CoV main proteinase.
| Phytochemical | Drug Targets |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV main peptidase |
SARS-CoV main proteinase |
|||||||||
| Binding energy | Ligand efficiency | Inhibition constant | h-bond | Residue (h-bond) | Binding energy | Ligand efficiency | Inhibition constant | h-bond | residue (h-bond) | |
| Nelfinavir | −6.71 | −0.17 | 12.13 | 1 | GLU166:HN | −7.61 | −0.25 | 1.81 | 2 | GLY143:HNG LU166:HN |
| Flabelliferin | −7.25 | −0.23 | 4.83 | 1 | GLU166:HN | −7.83 | −0.25 | 1.81 | 2 | GLY143:HN GLU166:HN |
| Curcumin | −7.46 | −0.28 | 3.38 | 3 | CYS85:O HIS41:HN ALA193:HN | −5.73 | −0.21 | 62.95 | 2 | GLU166:O GLU166;HN |
| Piperine | −7.86 | −0.37 | 1.73 | 0 | −7.07 | −0.34 | 6.56 | 2 | PHE140:HN HIS172:HE2 | |
| Marmelosin | −6.75 | −0.34 | 11.26 | 3 | ARG40:HH22 ASP187:HN ARG40:HE | −6.04 | −0.3 | 37.37 | 2 | GLU166:HN GLN189:HN |
| Ocimin | −6.9 | −0.31 | 8.81 | 1 | ALA193:HN | −6.54 | −0.3 | 16.08 | 2 | THR190:O GLN192:HN |
| Leucoanthocyanin | −5.71 | −0.25 | 65.26 | 5 | ASP187:O THR175:OG1 VAL186:O GLU166:HN CYS85:O | −5.44 | −0.24 | 102.87 | 5 | GLU166:O TYR54:OH ALA46:O GLU166:HN MET49:HN |
| Hydroxyl chloroquine | −6.62 | −0.29 | 14.16 | 1 | VAL186:HN | −6.33 | −0.28 | 22.86 | 1 | THR24:O |
| Remdesivir | −4.45 | −0.11 | 545.67 | 3 | CYS145:HN GLU166:O GLU166:HN | −5.06 | −0.12 | 197.05 | 3 | CYS44:O ASP187:O GLN189:HN |
3.4. Phytochemicals Vs SARS-CoV main proteinase
All the selected phytochemicals showed negative binding energy and flabelliferin (-7.83 kcal/mol) was showing the highest binding energy followed by piperine (-7.07 kcal/mol). Among the drugs nelfinavir (-7.61 kcal/mol) showed the highest binding energy. However, flabelliferin was better than the nelfinavir (Table 2b and Figure 1). All phytochemicals were having more than one active residue sites viz. leucoanthocyanin had five and all other phytochemicals had two each. Tahir Ul Qamar et al. (2020) screened medicinal plant library and found some nine phytochemicals from traditional Chinese medicinal plant having potential inhibitory properties against SARS-CoV main proteinase.
3.5. Pkcsm drug likeliness test
The ADMED properties of all the tested phytochemicals were shown in Table 3. The Caco2 permeability for all the phytochemicals except curcumin and leucoanthocyanin were high and ranged from 0.733 for flabelliferin to 1.687 for ocimin. Intestinal absorption percentage was high for all the phytochemicals tested. When interaction of phytochemicals and P-glycoprotein was assessed, only marmelosin was found to be not binding to P-glycoprotein. VDss value, which indicates the total volume of drug uniformly distributed in the body, was within the range for flabelliferin, piperine and marmelosin and it was low for curcumin. It was found to be high for ocimin and very high for leucoanthocyanin. Blood Brain Barrier (BBB) permeability was within range for flabelliferin, piperine, marmelosin and curcumin. Whereas, leucoanthocyanin was found below the low limit and ocimin was found above the high limit. As far as interaction with
Table 3.
Drug likeliness of phytochemicals.
| Lead molecules | Water solubility (log mol/L) | Caco2 permeability (Log Papp in 10-6 cm/s) | Intestinal absorption (human-%) | P-glycoprotein substrate (yes/no) | VDss (human) (logL/kg) | BBB permeability (log BB) | CYP2D6 substrate (yes/no) | AMES toxicity |
Hepato toxicity | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nelfinavir | −3.894 | 0.693 | 70.888 | yes | 0.563 | −0.522 | no | no | yes | |
| Flabelliferin B | −5.978 | 0.733 | 95.661 | yes | −0.056 | −0.352 | no | no | no | |
| Ocimin | −6.216 | 1.687 | 93.091 | yes | 0.685 | 0.595 | no | no | yes | |
| Piperine | −3.464 | 1.596 | 94.444 | yes | 0.158 | −0.102 | no | no | yes | |
| Marmelosin | −3.831 | 1.383 | 97.755 | no | 0.147 | 0.176 | no | yes | no | |
| Curcumin | −4.01 | −0.093 | 82.19 | yes | −0.215 | −0.562 | no | no | no | |
| Leucoanthocyanin | −2.916 | −0.059 | 51.018 | yes | 1.721 | −1.185 | no | no | no | |
CYP2D6 substrate none of the phytochemicals showed positive interaction. Further, except marmelosin none of the phytochemicals exhibited AMES toxicity and only ocimin and piperine showed hepatotoxicity.
4. Conclusion
COVID-19 has created the worldwide cataclysm and from the date of its emergence all the stakeholders trying to identify for a clinically proven drug. So, it is the need of the hour to find efficient molecule to manage this pandemic. In the present study we explored the potential phytochemicals as possible drugs through cheminformatics platform. The homology modeling, molecular docking and binding free energy calculation of six phytochemicals namely flabelliferin, marmelosin, piperine, ocimin, curcumin and leucoanthocyanin along with three drug compounds viz. nelfinavir, remdesivir and hydroxychloroquine positive control against drug targets of one SARS-CoV-2 viral proteases,COVID-19 main protease, two SARS CoV enzymes, SARS-CoV main peptidase, SARS-CoV main proteinase and one human cellular transmembrane serine proteinase had revealed that all the phytochemicals tested can be considered as potential drugs, since, all the phytochemicals exhibited negative binding energy with all the drug targets tested. It was found that all the six phytochemicals were better than remdesivir against all the drug targets. In case of SARS-CoV main peptidase except leucoanthocyanin all other phytochemicals proved better than all three positive control drugs. Whereas, flabelliferin was found to be the best inhibitor of SARS-CoV main proteinase, better than all the positive control drugs. The best inhibitor for COVID-19 main protease was nelfinavir followed by flabelliferin and ocimin. As far as inhibition of human cellular protease transmembrane serine proteinase (TMPRSS2), flabelliferin was found to the best followed by nelfinavir, curcumin, piperine and marmelosin.
The result on the inhibition of human cellular transmembrane serine proteinase (TMPRSS2) against COVID-19 has a stable therapeutic advantage as mutation may quickly occur on viral drug targets. Since, our study targeted viral enzyme and host cellular protein and found the phytochemicals inhibiting both the proteases, we expect adopting them on the part of therapeutic protocol against COVID-19 will have a combined and pyramiding benefit. Hence, all the phytochemicals tested in the present study in general and flabelliferin, piperine and ocimin in particular are the potential inhibitors of the all the four drug targets and can form a part of therapeutics against COVID-19 after further cheminformatics and clinical studies as their ADMET parameters are highly suitable as drugs. In addition, these compounds can also act in prophylactic measures as they are from edible plants, do not posses any toxicity and fared well in the drug likeliness test.
Supplementary Material
Acknowledgements
SM acknowledges Central University of Tamil Nadu, PU, MF &SS acknowledge Head, Division of Crop Improvement & Biotechnology & Dr. Nirmal Babu, Former Director ICAR – Indian Institute of Spices Research for the support. We also thank the anonymous reviewers for their suggestions which helped to improve the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anand, K., Ziebuhr, J., Wadhwani, P., Mesters, J. R., & Hilgenfeld, R. (2003). Coronavirus Main Proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science (New York, N.Y.).), 300(5626), 1763–1767. 10.1126/science.1085658 [DOI] [PubMed] [Google Scholar]
- Anurag, A., Nem Kumar, J., Neeraj, K., & Giriraj, T. K. (2020). Molecular docking study to identify potential inhibitor of covid-19 main protease enzyme: An in-silico approach. ChemRxiv. 10.26434/chemrxiv.12170904.v1 [DOI] [Google Scholar]
- Astuti, I. & Ysrafil, (2020). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes & Metabolic Syndrome, 14(4), 407–412. 10.1016/j.dsx.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga, M. S., Thilakchand, K. R., Rai, M. P., Rao, S., & Venkatesh, P. (2012). Aegle marmelos (L.) Correa (Bael) and its phytochemicals in the treatment and prevention of cancer. Integrative Cancer Therapies, 12(3), 187–196. 10.1177/1534735412451320 [DOI] [PubMed] [Google Scholar]
- Borgio, J. F., Alsuwat, H. S., Al Otaibi, W. M., Ibrahim, A. M., Almandil, N. B., Al Asoom, L. I., Salahuddin, M., Kamaraj, B., & AbdulAzeez, S. (2020). State-of-the-art tools unveil potent drug targets amongst clinically approved drugs to inhibit helicase in SARS-CoV-2. Archives of Medical Science : Ams, 16(3), 508–518. 10.5114/aoms.2020.94567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., Qiu, Y., Wang, J., Liu, Y., Wei, Y., Xia, J., Yu, T., Zhang, X., & Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet), 395(10223), 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, B., Dhingra, A. K., Kapoor, R. P., & Prasad, D. N. (2016). Piperine and its various physicochemical and biological aspects: A review. Open Chemistry Journal, 3(1), 75–96. 10.2174/1874842201603010075 [DOI] [Google Scholar]
- Deeki, T. L., Manivannan, S., Upadhyaya, S., & Roy, B. G. (2019). Targeted metabolic profiling of Black turmeric (Curcuma caesia Roxb.). Research Journal of Biotechnology, 14(9), 24–28. [Google Scholar]
- Guo, Y.-R., Cao, Q.-D., Hong, Z.-S., Tan, Y.-Y., Chen, S.-D., Jin, H.-J., Tan, K.-S., Wang, D.-Y., & Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Military Medical Research, 7(1), 11 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam, S., Nabavi, S. F., Banach, M., Berindan-Neagoe, I., Sarkar, K., Sil, P. C., & Nabavi, S. M. (2020). Should we try SARS-CoV-2 helicase inhibitors for COVID-19 therapy? Archives of Medical Research, 51(7), 733–735. 10.1016/j.arcmed.2020.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., Schiergens, T. S., Herrler, G., Wu, N.-H., Nitsche, A., Müller, M. A., Drosten, C., & Pöhlmann, S. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, R., Pandey, R., Mahant, R. N., & Rathore, D. S. (2015). A review on medicinal importance of Emblica officinalis. International Journal of Pharmaceutical Sciences and Research, 6(1), 72-84. 10.13040/IJPSR.0975-8232.6(1).72-84 [DOI] [Google Scholar]
- Jamkhande, P. G., Suryawanshi, V. A., Kaylankar, T. M., & Patwekar, S. L. (2016). Biological activities of leaves of ethnomedicinal plant, Borassus flabellifer Linn. (Palmyra palm): An antibacterial, antifungal and antioxidant evaluation. Bulletin of Faculty of Pharmacy, Cairo University, 54(1), 59–66. 10.1016/j.bfopcu.2016.01.002 [DOI] [Google Scholar]
- Khaerunnisa, S., Kurniawan, H., Awaluddin, R., & Suhartati, S. (2020). Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints, 10.20944/preprints202003.0226.v1 [DOI]
- Lee, T. W., Cherney, M. M., Liu, J., James, K. E., Powers, J. C., Eltis, L. D., & James, M. N. G. (2007). Crystal structures reveal an induced-fit binding of a substrate-like Aza-peptide epoxide to SARS coronavirus main peptidase. Journal of Molecular Biology, 366(3), 916–932. 10.1016/j.jmb.2006.11.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H. (2020). Drug treatment options for the 2019-new coronavirus (2019-nCoV). BioScience Trends, 14(1), 69–71. 10.5582/bst.2020.01020 [DOI] [PubMed] [Google Scholar]
- Morris, G. M., Goodsell, D. S., Halliday, R. S., Huey, R., Hart, W. E., Belew, R. K., & Olson, A. J. (1998). Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. Journal of Computational Chemistry, 19(14), 1639–1662. [Google Scholar]
- Mothay, D., & Ramesh, K. V. (2020). Binding site analysis of potential protease inhibitors of COVID-19 using AutoDock. VirusDisease, 31, 194-199. 10.1007/s13337-020-00585-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavizadeh, L., & Ghasemi, S. (2020). Genotype and phenotype of COVID-19: Their roles in pathogenesis. Journal of Microbiology, Immunology and Infection. 10.1016/j.jmii.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires, D. E. V., Blundell, T. L., & Ascher, D. B. (2015). pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. Journal of Medicinal Chemistry, 58(9), 4066–4072. 10.1021/acs.jmedchem.5b00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner, Ž., Hatamipour, M., Banach, M., Pirro, M., Al-Rasadi, K., Jamialahmadi, T., Radenkovic, D., Montecucco, F., & Sahebkar, A. (2020). Statins and the COVID-19 main protease: In silico evidence on direct interaction. Archives of Medical Science: AMS, 16(3), 490–496. 10.5114/aoms.2020.94655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, J., Wan, Y., Luo, C., Ye, G., Geng, Q., Auerbach, A., & Li, F. (2020). Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences, 117(21), 11727–11734. 10.1073/pnas.2003138117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. D., & Kaur, I. (2020, April). Molecular docking studies on Jensenone from eucalyptus essential oil as a potential inhibitor of COVID 19 corona virus infection. http://arxiv.org/abs/2004.00217
- Tahir Ul Qamar, M., Alqahtani, S. M., Alamri, M. A., & Chen, L. L. (2020). Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. Journal of Pharmaceutical Analysis, 10(4), 313–319. 10.1016/j.jpha.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, W., Chen, C., Lei, X., Zhao, J., & Liang, J. (2018). CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Research, 46(W1), W363–W367. 10.1093/nar/gky473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal, A., Rinu, K. A., & Joseph, D. (2017). Medicinal properties of black turmeric: A review. Innoriginal International Journal of Science, 4(3), 2–5. [Google Scholar]
- Wang, M., Cao, R., Zhang, L., Yang, X., Liu, J., Xu, M., Shi, Z., Hu, Z., Zhong, W., & Xiao, G. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research, 30(3), 269–271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., Peng, C., Shi, Y., Zhu, Z., Mu, K., Wang, X., & Zhu, W. (2020). Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. BioRxiv, 10.1101/2020.01.27.921627 [DOI] [Google Scholar]
- Yamamoto, N., Yang, R., Yoshinaka, Y., Amari, S., Nakano, T., Cinatl, J., Rabenau, H., Doerr, H. W., Hunsmann, G., Otaka, A., Tamamura, H., Fujii, N., & Yamamoto, N. (2004). HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochemical and Biophysical Research Communications, 318(3), 719–725. 10.1016/j.bbrc.2004.04.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, X., Ye, F., Zhang, M., Cui, C., Huang, B., Niu, P., Liu, X., Zhao, L., Dong, E., Song, C., Zhan, S., Lu, R., Li, H., Tan, W., & Liu, D. (2020). In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 71(15), 732–739. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedipour, F., Hosseini, S. A., Sathyapalan, T., Majeed, M., Jamialahmadi, T., Al‐Rasadi, K., Banach, M., & Sahebkar, A. (2020). Potential effects of curcumin in the treatment of COVID-19 infection. Phytotherapy Research), 34(11), 2911–2920. 10.1002/ptr.6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P., Yang, X.-L., Wang, X.-G., Hu, B., Zhang, L., Zhang, W., Si, H.-R., Zhu, Y., Li, B., Huang, C.-L., Chen, H.-D., Chen, J., Luo, Y., Guo, H., Jiang, R.-D., Liu, M.-Q., Chen, Y., Shen, X.-R., Wang, X., … Shi, Z.-L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 588(7836), E6–E6. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G. F., & Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.