Abstract
Background:
Hypercoagulation is one of the striking features of COVID-19. Patients hospitalized with COVID-19 are at high risk for venous thromboembolism. However, it is unknown if the risk for venous thromboembolism persists after discharge.
Case Summary:
We report a case with pulmonary embolism 5 months after COVID-19. No risk factors for venous thrombosis have been identified.
Conclusion:
In COVID-19 related hospitalization, large studies are needed to identify the risk of venous thromboembolism after discharge.
Keywords: COVID-19, post discharge, venous thromboembolism, deep venous thrombosis, pulmonary embolism
Introduction
Coronavirus disease 2019 (COVID-19) is continuing its spread with more than 30 million confirmed cases around the globe. To date, the long-term sequelae of COVID-19 is unknown as we are still in the first months of the pandemic. Our experience with other coronaviruses suggests the potential for ongoing organ damage1 and this might be applicable to COVID-19. With this huge number of infected patients, the long-term impact of COVID-19 will cause a significant burden on health care system. Hypercoagulation is one of the remarkable features of COVID-19.2-4 Patients who are hospitalized with COVID-19 are at increased risk for venous thromboembolism. Incidence of venous thromboembolism in patients with COVID-19 is 20 to 40% in the intensive care unit (ICU)2-4 and 3 to 8% in non-ICU5,6 even when prophylactic anticoagulation is used. However, it is unknown if risk for venous thromboembolism continues after discharge. We report a case without risk factors for venous thromboembolism, developed pulmonary embolism 5 months after COVID-19. This case raises concern about the possibility of prolonged risk for venous thromboembolism in COVID-19.
Case Report
A 41-year-old male was admitted to the hospital because of chest pain. It started 2 days earlier and continued to worsen until the time of presentation. The pain is left-sided and nonradiating. He described the pain as sharp and stabbing. He noted that the pain worsens with respiration. He rated the pain at 6 on a scale of 0 to 10 (with 10 indicating the most severe pain). He has no fever, runny nose, sore throat, chills, palpitations, cough, shortness of breath, nausea, abdominal pain, diarrhea, joint pain, or rashes. He has no leg pain or swelling. 5 months ago, patient was diagnosed with COVID-19 through nasal swab nucleic acid test. He had cough and shortness of breath that lasted for 2 weeks and completely resolved after. Due to mild clinical disease coarse, the patient was isolated at home. He did not require oxygen supplement or hospital admission. The patient had no other medical history and took no medications.
He reported no recent travel or previous surgery. His family history included hypertension and heart disease in his mother. He had no previous history or family history of thromboembolic events. He did not smoke tobacco, drink alcohol, or use illicit drugs. He was exercising regularly. He had no risk factors for venous thromboembolism.
On examination, the temperature was 36.6°C, the blood pressure 138/64 mm Hg, the heart rate 118 beats per minute, the respiratory rate 21 breaths per minute, and the oxygen saturation 93% while the patient was breathing ambient air. The heart was tachycardic, with normal first (S1) and second (S2) heart sounds. There was no evidence of a heart murmur or rub. The lungs were clear on auscultation. There was no leg swelling. The remainder of the examination was normal. An electrocardiogram showed sinus tachycardia at a rate of 112 beats per minute and was otherwise normal.
The white-cell count, platelet count and hemoglobin level were normal as were levels of sodium, potassium, carbon dioxide, urea nitrogen, creatinine, and calcium. Troponin I and B-type natriuretic peptide level were normal. D-dimer level was elevated, 4.54 mg/L FEU (reference range, <0.50). Testing to detect SARS-CoV-2 infection was negative. other test results are summarized in Table 1. systemic inflammation markers like C-reactive protein (CRP), ferritin and lactate dehydrogenase (LDH) were not obtained.
Table 1.
Laboratory findings
| Variable | On admission | Reference ranges |
|---|---|---|
| Hematocrit, % | 46.3 | 38.9-49.7 |
| Hemoglobin, mg/dL | 15.1 | 13.3-17.1 |
| White blood cell count, K/CUMM | 9.5 | 3.5-10.6 |
| Differential count, K/CUMM | ||
| Neutrophils | 6.0 | 1.58-7.13 |
| Lymphocytes | 1.8 | 1.0-3.8 |
| Monocytes | 0.8 | 0.1-0.88 |
| Eosinophils | 0.0 | 0.0-0.6 |
| Basophils | 0.0 | 0.0-0.2 |
| Platelet count, K/CUMM | 229 | 150-450 |
| Prothrombin time, second(s) | 11.2 | 9.4-11.7 |
| Partial thromboplastin time, second(s) | 32.8 | 23.1-33.1 |
| International normalized ratio (INR) | 1.12 | 0.90-1.13 |
| D-Dimer, mg/L | 4.54 | 0.0-0.50 |
| Sodium, mMol/L | 139 | 136-145 |
| Potassium, mMol/L | 3.9 | 3.5-5.1 |
| Chloride, mMol/L | 103 | 98-107 |
| Bicarbonate, mMol/L | 26 | 21-31 |
| Anion gap, mMol/L | 10 | 5-15 |
| Glucose, mg/dL | 125 | 75-105 |
| Blood urea Nitrogen, mg/dL | 19 | 7-25 |
| Creatinine, mg/dL | 1.18 | 0.70-1.30 |
| Calcium, mg/dL | 9.6 | 8.6-10.8 |
| Magnesium, mg/dL | 2.3 | 1.6-3.0 |
| B-type natriuretic peptide, pg/mL | 5 | <101 |
| Troponin I, ng/L | 3 | 3-17 |
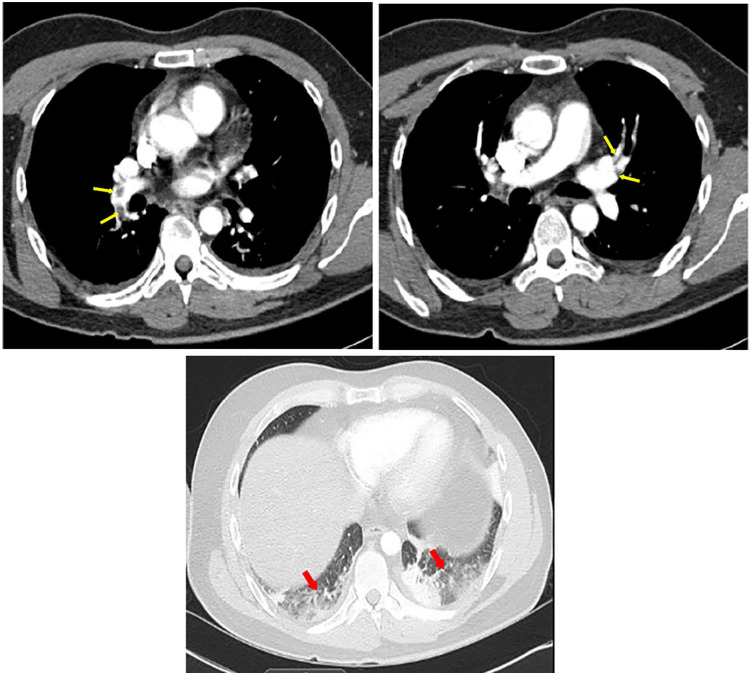
Radiography of the chest revealed atelectasis in right and left lower lobes (Red arrows). No evidence of pulmonary infiltrates or cardiomegaly (Figure 1). Computed tomographic (CT) angiography of the chest (Figure 2) revealed pulmonary embolism involved middle lobe and lower lobe branches of the right pulmonary artery as well as lower lobe and upper lobe branches of the left pulmonary artery (Yellow arrows). It also redemonstrated the atelectasis in right and left lower lobes (Red arrows). Intravenous infusions of heparin were administered, and the patient was admitted to the hospital.
Figure 1.
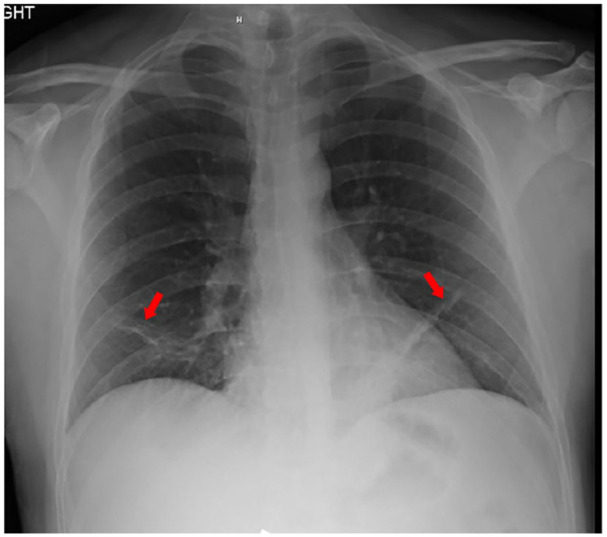
Radiography of the chest
Figure 2.
Computed tomographic (CT) angiography of the chest
Next day, patient’s tachycardia was resolved. Patient oxygen saturation was 96% on room air. Other vital signs remained stable. Patient was started on apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily and discharged home.
Discussion
We present a case with pulmonary embolism diagnosed 5 months after resolution of COVID-19. Risk factors for provoked PE other than history of COVID-19 have been excluded such as personal history of cancer or coagulation disorders or DVT/PE, immobilization, medications and surgery or trauma within the last 3 months. To our knowledge, only few cases of venous thromboembolism reported late after COVID-19 onset.7-11 However, our case is the first to report venous thromboembolism beyond the first 3 months of COVID-19 hospitalization or onset. These cases raise the following questions: Is COVID-19 an independent long-term risk factor for venous thromboembolism? How long this risk lasts? Is there a high-risk subgroup? How we identify this group? Large research studies are needed to address these questions and if we find a long-term risk for venous thromboembolism in COVID-19, clinical trials are needed to test the efficacy of extended post-discharge thromboprophylaxis.
In summary, this case suggests that the risk for venous thromboembolism in hospitalized patients with COVID-19 may persist after discharge. Large studies are needed to confirm this risk.
Reprints: No reprints will be ordered.
Patient consent to publish this case study was obtained.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhanad Taha  https://orcid.org/0000-0002-5867-1929
https://orcid.org/0000-0002-5867-1929
References
- 1. Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;s128:2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beckman M, Nyrén S, Kistner A. A case-report of widespread pulmonary embolism in a middle-aged male seven weeks after asymptomatic suspected COVID 19 infection. Thromb J. 2020;18:19. doi: 10.1186/s12959-020-00235-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Tano G, Moschini L, Loffi M, Testa S, Danzi GB. Late pulmonary embolism after COVID-19 pneumonia despite adequate rivaroxaban treatment. Eur J Case Rep Intern Med. 2020;7:001790. doi: 10.12890/2020_001790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vechi HT, Maia LR, Alves MDM. Late acute pulmonary embolism after mild Coronavirus Disease 2019 (COVID-19): a case series. Rev Inst Med Trop Sao Paulo. 2020;62:e63. doi: 10.1590/S1678-9946202062063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujikura K, Fontes JD, Taub CC. Saddle pulmonary embolism and thrombus-in-transit straddling the patent foramen ovale 28 days after COVID symptom onset. Echocardiography. 2020;37:1296-1299.s [DOI] [PubMed] [Google Scholar]
- 11. Karolyi M, Pawelka E, Omid S, et al. Late onset pulmonary embolism in young male otherwise healthy COVID-19 patients. Eur J Clin Microbiol Infect Dis. Published online September 23, 2020. doi: 10.1007/s10096-020-04044-x [DOI] [PMC free article] [PubMed] [Google Scholar]