Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative diseases. Increasing studies have demonstrated the critical importance for redox proteins mediating neuronal protection in models of AD. This review briefly describes some of the risk factors contributing to AD, specifically highlighting the important roles of oxidative stress in the pathology of AD. Then this article concisely introduces the dysregulation and functions of two main redox enzymes, peroxiredoxins and glutaredoxins, in AD models. This review emphasizes the neuroprotective role of the third redox enzyme thioredoxin (Trx), an important multifunctional protein regulating cellular redox status. This commentary not only summarizes the alterations of Trx expression in AD patients and models, but also reviews the potential effects and mechanisms of Trx, Trx-related molecules and Trx-inducing compounds against AD. In conclusion, Trx has a potential neuroprotection in AD and may be very promising for clinical therapy of AD in the future.
Keywords: Alzheimer’s disease, oxidative stress, redox enzymes, thioredoxin, neuroprotection
Alzheimer’s Disease
Alzheimer's disease (AD) is the most common neurodegenerative disease with memory decline, cognitive dysfunction and personality changes (Calabrese et al., 2006). Neuropsychiatric symptoms, including depression, apathy and hallucinations, are also frequently observed in AD patients (Fakhoury, 2018). AD is the most common cause of dementia (James et al., 2014) and is a growing global health concern with huge implications for individuals and society (Lane et al., 2018). The pathological features of AD are the density and distribution of extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles (NFTs, composed of the microtubule-associated protein Tau and hyperphosphorylated Tau) in the central nervous system, both of which highly contribute to the neurodegenerative processes in AD (Grundke-Iqbal et al., 1986; Hanseeuw et al., 2019). In addition, microglial activation, associated astrogliosis, dystrophic neurites, synapse loss and cerebral amyloid angiopathy are also observed (Serrano-Pozo et al., 2011). Typical late-onset AD is likely to be driven by a complex interplay between genetic and environmental factors.
Aging and Genetic Factors of AD
AD is often divided into two types: sporadic AD and familial AD. Aging is the most important risk factor for sporadic AD (Albensi, 2019) because a great majority of patients with AD are over 65 years old (Sawda et al., 2017) and the probability of the disease increases with age (Alzheimer's Association, 2016). A recent study demonstrated that aging processes promoted the formation of NFTs (Gant et al., 2018). Thus, sporadic AD is usually referred to as late-onset AD. The etiology of sporadic AD is not clearly understood, but it has been associated with several genetic factors (Piaceri et al., 2013). More than 20 risk loci of AD were identified with the advent of next‐generation sequencing and genome-wide association studies. Among these genetic risk loci, apolipoprotein E4 (APOE4) is the strongest risk factor for sporadic AD (Xia et al., 2018). The APOE4 protein affects the primary neuropathological markers of AD, including Aβ plaques, NFTs and chronic gliosis (Flowers and Rebeck, 2020). APOE4 has the ability to bind β-amyloid and plays a role in Aβ conversion from monomeric and non-toxic forms to oligomers and fibrils (Castano et al., 1995). In addition, numerous studies have demonstrated that exposure to some environmental factors, such as xenobiotics and lifestyle stress, also contribute to the progression of sporadic AD (Aaseth et al., 2020; Madore et al., 2020; Shi et al., 2020).
Currently familial AD has been reported to associate with mutations in three major genes: Aβ precursor protein (APP), presenilin1 (PS1), and presenilin 2 (PS2), which induce abnormal of Aβ (Bertram et al., 2010; Dorszewska et al., 2016). So far more than 200 distinct disease-causing mutations in the three genes have been discovered (Hsu et al., 2020). It seems that the presence of the mutations in familial AD genes may lead to an anomalous overproduction of the Aβ peptide and its aggregation in most instances, and thus cause early-onset or familial AD (Carter et al., 1992; Tsubuki et al., 2003; Giau et al., 2019; Lin et al., 2020).
Oxidative Stress in AD
Oxidative stress has been recognized as a significant contributing factor in the development of neurodegenerative diseases. Increased production of reactive oxygen species (ROS), which are associated with disease-dependent loss of mitochondrial function, altered metal homeostasis and reduced antioxidant defense, directly affect synaptic activity and neurotransmission in neurons leading to cognitive dysfunction (Tonnies and Trushina, 2017). Oxidative stress is an important pathophysiological change in AD. Increasing evidences indicated that oxidative stress in the brain tissue significantly contributes to the pathology of AD (Jia et al., 2017). The deposition of Aβ is clarified to occur early in the progression of AD (Gordon et al., 2018). Prior to the formation of fibril Aβ, oligomeric Aβ, the toxic species of this peptide, could induce oxidative damage. The oxidation of lipids, proteins, and nucleic acids in neurons is a common pathological feature of AD (Pratico, 2008). Oxidative stress is in turn associated with amyloid-peptide oligomer inserted in the mitochondrial membranes, which will lead to more serious oxidative stress (Butterfield and Boyd-Kimball, 2020). Increased levels of oxidative stress not only increased APP expression and enhanced secretion of APP cleavage product sAPPβ (Muche et al., 2017), but also participated in the development of AD by promoting Aβ deposition, tau hyperphosphorylation, and the subsequent loss of synapses and neurons (Chen and Zhong, 2014). In the APP/PS1 double transgenic mouse model of AD, ROS levels in synaptosome were dramatically increased, which resulted in synaptic dysfunction during the development of AD (Kommaddi et al., 2019). Besides the oxidative stress in neurons, microglial activation-induced ROS production also play an imperative role in AD (Bhat et al., 2019). Production of ROS and MDA were significantly increased and the SOD activity was inhibited in Aβ treated microglia (Cui et al., 2019). Accumulating evidences have demonstrated that microglia can directly mediate synapse loss and exacerbate tau pathology (Hansen et al., 2018). Moreover, activated microglia can secrete toxic factors to directly or indirectly injure neurons (Liddelow et al., 2017).
These evidences above suggest that oxidative stress is an essential part of the pathological process of AD and is closely correlated with amyloid pathology by forming serious pathophysiological cycles, inducing mitochondrial dysfunction and promoting metal toxicity (Chen and Zhong, 2014). Oxidative stress is not only an essential pathological marker of AD, but also serves as a potential treatment target.
Microglial Activation and Neuroinflammation in AD
Microglia, the resident immune cells of the brain, are critical to immunity and homeostasis in the central nervous system. Being one of the first immune cells, microglia constitute the first line of cellular defense against invading pathogens and other types of brain injury (Fakhoury, 2018). Aβ could promote microglia activation leading to secretion of proinflammatory cytokines (Mosher and Wyss-Coray, 2014). Triggering receptor expressed in myeloid cells 2 (TREM2) is a microglial cell surface receptor central to proliferation, survival, and phagocytosis of microglia (Carmona et al., 2018). The number of mitochondria and ATP levels were less in TREM2−/− microglia from 5×FAD mice, suggesting that TREM2 plays an essential role in maintaining mitochondrial function and metabolic fitness of microglia (Ulland et al., 2017). Neuroinflammation plays a significant role in the development and pathology of AD (Agrawal and Jha, 2020). APP/PS1 mice showed increased expression of NOD-like receptor protein (NLRP3), IL-1β, and cleaved caspase 1 (Fang et al., 2019). Knockout of NLRP3 and caspase 1 significantly improved the phagocytosis of microglia, suggesting that activated NLRP3 in APP/PS1 mice contributes to the pathology of AD (Heneka et al., 2013). In addition, microglial production of apoptosis-associated speck-like protein containing a CARD (ASC) binds and cross-seeds extracellular Aβ (Venegas et al., 2017). Exposure to ASC-Aβ composites amplified the proinflammatory response, finally resulting in pyroptotic cell death (Friker et al., 2020).
Roles of Redox Enzymes in AD
Redox enzymes in cells provide protection against oxidative damage, such as scavenging ROS, maintaining intracellular redox balance, and regulating vital signaling events caused by ROS. These enzymes mainly include peroxiredoxins (Prxs), glutaredoxins (Grxs), and thioredoxins (Trxs) (Johnson et al., 2015).
Prxs in AD
Prxs are a type of selenium independent antioxidant enzymes that can protect organisms from oxidative damage caused by ROS. Accumulating literatures have reported that the expression of the six subtypes of Prxs is changed in AD. Prx1 and Prx4 were significantly decreased in postmortem brains of AD compared to normal subjects along with higher levels of protein oxidation (Majd and Power, 2018). 2-D gel electrophoresis and mass spectrometry analysis also revealed that Prx2 exists in a more oxidized state in AD brains than in age-matched controls brains (Cumming et al., 2007). The loss of Prx3 was identified in the hippocampal mitochondria of APP/PS1 transgenic mice (Choi et al., 2014). Prx5 was increased in the N2a-APPswe cell model of AD and Prx5 showed a higher level in the brain of APP transgenic mouse than that in a nontransgenic mouse (Park et al., 2017). Changes in Prx expression are closely associated with protection from neuronal death. Overexpression of Prx1 attenuated Aβ1-42-induced cell death (Oku et al., 2017). Prx4 protected HT-22 hippocampal neurons against Aβ oligomer-mediated apoptosis by inhibiting endoplasmic reticulum stress (Kam et al., 2019). Prx5 is upregulated in both the cytoplasm and mitochondria, protecting cells from Aβ oligomer-mediated oxidative stress by eliminating intracellular and mitochondrial ROS (Park et al., 2017). Importantly, the induced Prx5 expression by the Aβ oligomer played a key role in regulating both the activation of Ca2+-mediated calpain (Park et al., 2017) and ERK-Drp1-induced mitochondrial fragmentation (Kim et al., 2016). Prx6 was reported to have the potential to promote cognitive improvement in APP/PS1 double-mutant transgenic mice (Yun et al., 2016). A most recent study demonstrated that Prx6 regulated the protective response of astrocytes toward Aβ plaques (Pankiewicz et al., 2020).
Grxs in AD
Grxs, a type of small thiol/disulfide oxidoreductases, are important for the regulation of cellular protein thiol redox homeostasis through catalyzing the reduction of disulfide bonds in target proteins (Verma et al., 2020). The expression and redox state of Grxs were well studied in AD models and patients. The expression of synaptic Grx1 levels was significantly reduced in APP/PS1 double transgenic mice (Kommaddi et al., 2019). Grx1 and Grx2 expression in the axonal area of hippocampus CA1 of AD patients also showed a significant decrease compared to the controls, with no difference in the neuronal cell bodies (Arodin et al., 2014). An increase of oxidized Grx1 was observed in the frontal cortex and hippocampal CA1 regions from one AD brain and Aβ treatment also resulted in the oxidation of Grx1 and the activation of ASK1 cascade in SH-SY5Y cells (Akterin et al., 2006). Grx1 overexpression rescued the decreased viability of SH-SY5Y cells treated with Aβ (Akterin et al., 2006). Overexpressing Grx1 in the brains of APP/PS1 mice also restored memory recall after contextual fear conditioning (Kommaddi et al., 2019), suggesting that increasing Grx1 levels may be potential for the treatment of AD.
Trxs in AD
Trx System
Trx is an essential redox balance regulator in mammalian cells and is induced by various factors, including oxidation, radiation, ultraviolet rays, viral infections and ischemia/reperfusion (Zhou et al., 2020), as well as by both chronic and acute stress (Jia et al., 2014; Jia et al., 2016). Trx-1 and Trx-2 are the two primary forms, respectively distributing in cytoplasm and mitochondria, that associate with their respective reductases, TrxR1 and TrxR2 (McBean et al., 2017). Trx, TrxR and NADPH constitute the Trx system, which has been reported to have various biological activities. Trx can directly scavenge singlet oxygen (Das and Das, 2000) and reduce exposed protein disulfides (Masutani et al., 2004). Trx couples with Prx to scavenge ROS, such as directly converting H2O2 to H2O (Granger and Kvietys, 2015). Our previous studies demonstrated that Trx exerted protective effects in oxidative stress, morphine and methamphetamine addiction, autoimmune disease, cerebral ischemic damage and cancers (Chen et al., 2014; Zeng, Zhou, et al., 2014; Zeng et al., 2015; Jia et al., 2019; L. Yang et al., 2020; Zeng et al., 2020). Trx was reported to improve the learning and memory ability of Parkinsonism mice (X. Zhang et al., 2018) and has neuroprotective roles through inhibiting endoplasmic reticulum stress-mediated neural apoptosis in celluler and mouse models of Parkinson’s disease (Zeng, Jia, et al., 2014). Importantly, increasing evidences have demonstrated that Trx also plays neuroprotection in AD.
Expression of Trxs in AD
It has been reported that Trx-1 can be released to the cerebrospinal fluid of AD patients and that the levels of Trx-1 in cerebrospinal fluid are significantly increased in the early stages of AD in comparison to mild cognitive impairment (MCI) (Arodin et al., 2014). Interestingly, the expression of Trx in the plasma from AD patients is also increased, which may provide a defense mechanism against oxidative stress (Cornelius et al., 2013; Arodin et al., 2014). Though Trx expression was increased in peripheral tissues, it’s clarified that Trx is reduced in the brain AD patients and animal models. Trx-1 levels are decreased in the brain in amnestic mild cognitive impairment, a transition stage between normal aging and AD (Di Domenico et al., 2010). A decrease in expression Trx-1 was observed in the frontal cortex and hippocampal CA1 regions from the brain of one AD patient (Akterin et al., 2006). Similarly, Trx-2 expression in hippocampus tissues of AD patients is also markedly reduced (Arodin et al., 2014). Though Trx expression was demonstrated a general decrease in the amygdala and hippocampus of AD brains, TrxR levels were significantly increased in these AD brain regions (Lovell et al., 2000). Interestingly, TrxR is decreased in the frontal cortex (Venkateshappa et al., 2012).
The data from animal and cellular models of AD agree with what is seen in the tissue from patient with AD. In APP/PS1 double transgenic mice, the synaptic Trx levels were also significantly reduced (Kommaddi et al., 2019). ApoE4, the major genetic risk factor for AD, disrupted lysosomal integrity and increased the release of Cathepsin D into the cytoplasm, which could decrease the Trx-1 levels both in human primary cortical neurons and neuroblastoma cells and in the hippocampus of ApoE4 targeted replacement mice and subsequently result in the activation of ASK1 pathway (Persson et al., 2017). These results suggest that the downregulation of Trx-1 is involved in the toxicity caused by ApoE4. Aβ1-42-treated mice showed a reduction of Trx, as well as its transcription factor nuclear factor-E2-related factor 2 (Nrf2) in hippocampal neurons (Duan and Si, 2019) (Table 1).
Table 1.
The Expression Redox State of Trx in AD Models and Patients.
| Subjects | Expression and redox status of Trx | References |
|---|---|---|
| AD patients | ↑Trx-1 in cerebrospinal fluid | Arodin et al., 2014 |
| ↑Trx-1 in the plasma | Arodin et al., 2014; Cornelius et al., 2013 | |
| ↓Trx-1 in the brain | Di Domenico et al., 2010 | |
| ↓Trx-1 in the frontal cortex and hippocampal CA1 regions | Akterin et al., 2006 | |
| ↓Trx-2 in hippocampus tissues | Arodin et al., 2014 | |
| APP/PS1 mice | ↓Synaptic Trx | Kommaddi et al., 2019 |
| Mice expressing ApoE4, SH-SY5Y/human primary cortical neurons treated with recombinant ApoE4 | ↓Trx-1 | Persson et al., 2017 |
| Aβ1-42-treated mice | ↓Trx-1 in hippocampal neurons | Duan and Si, 2019 |
| SH-SY5Y cells treated with Aβ1-42 | ↑Oxidation of Trx-1 | Akterin et al.,2006 |
| Primary cerebral cortical neurons and HT22 hippocampal cells from Aβ1-42-treated mice | ↑Oxidation of Trx-1 | Wang, Xu, et al., 2019 |
Note:↑, increase; ↓, decrease.
The redox status of Trx also changed in AD models. Similar to Grx1, Aβ treatment also resulted in the oxidation of Trx-1 and the activation of the ASK1 cascade in SH-SY5Y cells (Akterin et al., 2006). Wang et al. found that Aβ treatment may increase protein levels of Thioredoxin-interacting protein (TXNIP, an endogenous inhibitor of Trx), which subsequently inhibits Trx reducing capability and enhances the oxidative modification of protein cysteines in the active site of the protein, but with no change of the Trx protein levels (Y. Wang, Wang, et al., 2019). What’s more, the expression pattern of Trx-1 and Trx-2 was shown to be correspondingly altered in hippocampus tissue sections from AD patients compared to controls (Arodin et al., 2014). Immunohistochemical staining of Trx1 revealed that only cytosolic localization was observed in hippocampus CA1 of AD patients, whereas Trx-1 in the control sections was observed to translocate to the nucleus, with no difference in the expressing levels between control and AD patients. In contrast, Trx-2 expression was dramatically decreased in the AD groups (Table 1).
Based on the above proofs, it is generally acknowledged that the intracellular antioxidant defense system in AD is impaired. The changes of Trx expression and/or redox state in the brain are associated with AD progress.
Neuroprotection of Trxs in AD
Some studies have demonstrated that Trx plays neuroprotective roles in animal and cellular models of AD. An interesting study revealed that the rats with higher levels of the Trx mRNA and protein in the hippocampus acted better in the Morris water maze, suggesting that a deficit of Trx might play an important role in the impaired spatial learning and memory of AD rats (X. H. Yang et al., 2012). This is consistent with another report in which transgenic overexpression of Trx-1 ameliorated the learning and memory deficits in the MPTP-induced Parkinson’s disease model in mice (X. Zhang et al., 2018). Interestingly, overexpression of mitochondrial TrxR-2 markedly decreased the expression of Aβ peptide and inhibited its deposits in a transgenic elegans model of AD (Cacho-Valadez et al., 2012), which may be related to the reduction of oxidized mitochondrial Trx-2 by TrxR-2. Overexpressing Trx-1 also showed neuroprotection against Aβ1-42-induced neurotoxicity in SH-SY5Y cells (Akterin et al., 2006). Consistently, exogenous administration of Trx exerted remarkable neuroprotection against the neurotoxicity of Aβ in primary cultures of fetal rat cortical neurons (Ju et al., 2005). The neurotoxicity of ApoE4 was similarly counteracted by overexpression of Trx-1 in SH-SY5Y cells (Persson et al., 2017). Downregulation of Trx contributed to the increased susceptibility of neurons to oxidative stress (Ding et al., 2008). Trx-1 played a critical regulatory role in inhibiting caspase-6 activation and nuclear invagination (an important player in the pathophysiology of neurodegenerative diseases). In cell-free nuclei preparation and purified enzymatic assays, oxidized Trx-1 increased the enzymatic activity of caspase-6 and the cleavage of lamin-B1, while reduced Trx-1 showed opposite effects (Islam et al., 2019). Aβ-resistant PC12 cells displayed higher levels of Trx and TrxR, two enzymes critical for maintaining the activity of Prx with reduced cysteine (Cumming et al., 2007). Inhibition of TrxR activity could interrupt autophagy flux by induction of lysosomal deficiency and promoted apoptosis (Nagakannan et al., 2016). These studies suggested that the enhancement of endogenous Trx may provide promising therapy strategies.
Neuroprotection of Other Trx-Related Molecules in AD
Thioredoxin-80 (Trx80), a truncated form of Trx-1, is reported to be secreted from monocytes displaying cytokine activity (Pekkari and Holmgren, 2004). Trx80, localizing mainly to neurons, was dramatically decreased in AD brains and Trx80 levels in cerebrospinal fluid correlated with those of the classical AD biomarkers Aβ1-42 and total Tau (Gil-Bea et al., 2012). Though it loses its reductive capacity when Trx-1 is cleaved to Trx80 (Pekkari and Holmgren, 2004), Trx80 inhibited Aβ1-42 aggregation and its neurotoxic effects, suggesting that Trx80 deficit might participate in the pathogenesis of AD (Gil-Bea et al., 2012). The C4b-binding protein interacts with other amyloidogenic proteins, such as Aβ peptide (Trouw et al., 2008). Trx80 has been described to bind to C4BP, forming aggregate deposits in human brains (King et al., 2012). In a Drosophila melanogaster model, overexpression of Trx80 inhibited Aβ1-42 accumulation in the brain and restored the life span and locomotor activity via promoting autophagosome formation(Gerenu et al., 2019). Trx-mimetic peptides, containing the canonical -Cys-X-X-Cys- or -Cys-X-Cys- motif of the Trx-active site, could mimic and enhance the cellular activity of Trx (Cohen-Kutner et al., 2013). Trx-mimetic peptides represent a new family of potent and selective redox compounds that could act as potential candidates for the prevention and treatment of oxidative stress-related diseases (Bachnoff et al., 2011). The Trx-mimetic peptide could improve cognitive function in a mouse model of mild traumatic brain injury (Baratz-Goldstein et al., 2016). Trx-mimetic peptide, Ac-Cys-Pro-Cys-amide, prevented the expression of TXNIP, inhibited the JNK/p38MAPK-mediated neuronal apoptosis and attenuated the neuro-inflammatory processes (Cohen-Kutner et al., 2014). These data suggest that Trx mimetic peptides may become beneficial for preventing neurological disorders such as AD.
Neuroprotection of Trx Inducers in AD
Besides Trx and Trx-related molecules showing neuroprotection in AD, numerous studies have demonstrated that Trx inducers also have the potential against AD, suggesting Trx inducers may be much more potential for AD treatment.
Resveratrol
It’s well-known that some polyphenolic compounds play neuroprotective effects in AD models (Masci et al., 2015). Resveratrol, a group of compounds called polyphenols extracted from plants, has been indicated its promising use clinically for oxidative-related diseases, such as diabetes, cardiovascular diseases, ischemic brain injury, neurodegenerative diseases (Bonnefont-Rousselot, 2016; Y. Gao et al., 2018; Zhao et al., 2018; Izquierdo et al., 2019). Although resveratrol at high concentrations has been reported to have a potential to diminish Trx-1 expression, promote Trx-1 oxidation and alter its subcellular location, low-dose resveratrol could significantly upregulate the expression of Trx-1 (Thirunavukkarasu et al., 2007; Feng and Zhang, 2019). It has been reported that resveratrol may be a potential therapy for AD (X. Wang et al., 2018). Resveratrol is a well-known activator of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2). Resveratrol attenuates the Aβ1-42-induced cytotoxicity by upregulating heme oxygenase-1 (HO-1) via the PI3K/Akt/Nrf2 pathway in PC12 cells (Hui et al., 2018). Under the oxidative condition, Nrf2 is critical for Trx-1 expression because the promoter of the Trx-1 gene contains an antioxidant response element (ARE) (Im et al., 2012). A growing body of evidences have demonstrated that TXNIP plays an essential role in the activation of NLRP3 inflammasome in various acute and chronic diseases (Nasoohi et al., 2018). TXNIP-NLRP3 inflammasome may contribute to the pathogenesis of AD and other age-related dementias (L. Li et al., 2019). The protection of resveratrol treatment against Aβ-induced activation of TXNIP-NLRP3 inflammasome may at least in part due to its upregulatory effect of Trx expression (Feng and Zhang, 2019) (Table 2).
Table 2.
Studies of Representative Compounds Related to Trx Against AD.
| Compound | Treatment | Species | Model | Protective mechanisms | References |
|---|---|---|---|---|---|
| Resveratrol | 200 mg/kg·per day) for 8 weeks, oral gavage | Mice | overexpressing APP | ↑learning and memory, ↑hUC-MSCs engraftment and neurogenesis, ↓neural apoptosis in the hippocampus | X. Wang et al., 2018 |
| 10, 20, 40 μM | PC12 cells | Aβ1-42 | ↑the cell viability of PC12 cells, ↓the production of MDA and ROS, ↑expression of SOD and GSH, activates PI3K/Akt/Nrf2/HO-1 signaling pathway | Hui et al., 2018 | |
| 10, 50 nM | BV-2 microglial cells | Aβ1-42 | ↓Aβ-induced proliferation and activation of BV-2 cells, ↓the release of proinflammatory cytokines, IL-6 and TNF-α, IL-1β, ↓ TXNIP/Trx/NLRP3 pathway | Feng and Zhang, 2019 | |
| Salidroside | 30 mg/kg orally once daily for 3 consecutive months | Mice | APPswe/PS1ΔE9 | ↑learning and memory, ↑SOD and GSH in hippocampal tissue, ↓MDA and nitrate in the hippocampus, and the apoptosis of hippocampal neurons, ↓IL-6 and TNF-α | Q. Li et al., 2018 |
| 0.3 mg/ml with free access for 2 months | Mice | APP/PS1 | ↑locomotor activity, ↓soluble and insoluble Aβ levels, ↑expression of PSD95, NMDAR1, and CaMK II, ↑PI3K/Akt/mTOR signaling | H. Wang et al., 2020 | |
| 20, 40 mg/kg orally daily for 28 days from day 15th of d-gal injection | Rats | d-galactose | ↑cognitive function, ↓TNF-α, IL-6) and IL-1β, ↓TXNIP, ↑Trx, ↓Bax and caspase-9, ↑Bcl-2, ↓SIRT1/NF-κB pathway | J. Gao et al., 2016 | |
| 50 and 100 μM | SH-SY5Y cells | Aβ25-35 | ↓loss of cell viability and apoptosis, ↑Trx, HO-1 and PrxI, ↑mitochondrial membrane potential, ↓phosphorylation of JNK and p38 MAP kinase | L. Zhang et al., 2010 | |
| 50 μM | PC12 cells | Aβ1-42 | ↓Aβ1-42-induced cytotoxicity and mitochondria-mediated apoptotic pathways, ↑ ERK1/2 and AKT signaling pathways | Liao et al., 2019 | |
| Estrogen | 250 nM pre-treated for 24 h | SH-SY5Y cells | Aβ1-42 | ↓Aβ neurotoxicity and ASK1 activation, ↑Trx-1 expression | Mateos et al., 2012 |
| 100 nM co-treated for 24 h | SH-SY5Y cells | Aβ1-42 | ↑cell viability and protein level of Trx, ↓ROS production, cell apoptosis, ΔΨm, and the protein levels of PERK, IREα, and TXNIP | Pan et al., 2020 | |
| Dl-NBP | 40 and 80 mg/kg orally once daily for 3 months | Mice | SAMP8 | ↑cognitive function and synaptic plasticity | Lv et al., 2018 |
| 20 mg/kg oral gavage once daily for 5 months | Mice | APP/PS1 | ↑Trx, ↓TXNIP/NLRP3 | Wang et al., 2019 | |
| 20, 60 mg/kg oral gavage once daily for 5 months | Mice | APP/PS1 | ↑learning and memory and synaptic plasticity, ↓soluble Aβ and Aβoligomer in the mouse brain, ↑CREB and Nrf2 | Wang et al., 2016 | |
| 1 and 10 μM | Rat primary astrocytes | Aβ1-42 | ↓Aβ-induced activation of astrocytes and the upregulation of proinflammatory molecules, IκBα degradation and NF-κB translocation | H. M. Wang et al., 2013 |
Note:↑, increase; ↓, decrease.
Salidroside
Salidroside, an active ingredient extracted from traditional Chinese medicine (Rhodiola rosea L.), could protect against oxidative stress-induced cell apoptosis (L. Zhang et al., 2007). In a APPswe/PS1ΔE9 model, administration of Salidroside attenuated the memory and learning impairment of AD mice (Q. Li et al., 2018). In this study, it was demonstrated that the effects of Salidroside administration on AD mice were, at least partially, via suppression of oxidative damage, inflammation and apoptosis in hippocampal neurons (Q. Li et al., 2018). A recent study suggested that Salidroside may protect the damaged synapses of the neurons in the APP/PS1 mice via decreasing both the soluble and insoluble Aβ levels, increasing the expression of PSD95, NMDAR1, and calmodulin-dependent protein kinase II, and upregulating PI3K/Akt/mTOR signaling (H. Wang et al., 2020). The protection of Salidroside in AD models may be related to the induction of Trx after Salidroside treatment. In a d-galactose-induced rat model of AD, administration of Salidroside suppressed inflammation via inhibiting Sirt1/NF-κB signaling pathway (J. Gao et al., 2016). The treatment of Salidroside could induce Trx expression and inhibit TXNIP activation in the hippocampal neurons (J. Gao et al., 2015). What’s more, Salidroside inhibited d-galactose-induced mitochondria-mediated apoptosis. It has been reported that Salidroside upregulated Trx expression and further protected SH-SY5Y cells against Aβ25-35-induced oxidative stress and the activation of JNK/p38MAPK cascade (L. Zhang et al., 2010). Liao et al demonstrated that Salidroside prevented PC12 cells from Aβ1-42-induced neurotoxicity and apoptosis through activating the ERK1/2 and Akt signaling pathways (Liao et al., 2019) (Table 2).
Estrogen
Given that the risk for AD is associated with age-related loss of ovarian hormones in women (Marongiu, 2019; Torromino et al., 2020), estrogen replacement therapy is considered for AD treatment. It has been clarified that treatment with low dose estrogen elevated significantly the protein levels of Trx-1 (Campos et al., 2014). Estrogen-mediated protection against Aβ toxicity is dependent on the induction of Trx-1 through the cGMP/protein kinase (PKG) signaling pathway (Chiueh et al., 2003), which in turn promotes the activation of Akt and the inhibition of ASK1 (Mateos et al., 2012). Considering that Aβ increases the expression of TXNIP (Y. Wang, Wang, et al., 2019), the upregulation of Trx-1 by estrogen can further inhibit TXNIP-induced oxidative stress by Aβ and activate AMPK signaling(Table 2) (Pan et al., 2020). Prospective and case-control studies have demonstrated that estrogen replacement therapy can effectively reduce cognitive deficits in some but not all postmenopausal women(Qin et al., 2020).
Dl-3-n-butylphthalide
The antioxidant Dl-3-n-butylphthalide (Dl-NBP) is a natural antioxidant used for cerebral ischemia treatment in China (F. Wang, Ma, et al., 2016; Xu et al., 2019). Some studies revealed that Dl-NBP plays neuroprotective roles in lipopolysaccharide and rotenone models Parkinson’s disease respectively through reducing oxidative stress and inhibiting microglial activation (Xiong et al., 2012; Chen et al., 2018). In SAMP8 mice, oral administration of Dl-NBP for 3 months significantly alleviated cognitive impairment via improving synaptic plasticity of hippocampal neurons, suggesting that Dl-NBP may be a potential drug candidate for the treatment of cognitive impairment in AD (Lv et al., 2018). What’s more, there is also an evidence that Dl-NBP may have the potential to inhibit Aβ aggregation (Qiang et al., 2017). Dl-NBP blocked the interaction between TXNIP and NLRP3 and inhibited the activation of NLRP3 inflammasome, and then ameliorated neuronal apoptosis though increasing Trx expression in the APP/PS1 mouse brains (C. Y. Wang, Xu, et al., 2019). Dl-NBP-induced upregulation of Trx may be involved in the enhancement of CREB and Nrf2, two of transcription factors of Trx, in the AD mouse model (C. Y. Wang, Wang, et al., 2016). In cultured rat primary astrocytes, Dl-NBP was reported to attenuate the Aβ-induced inflammatory responses via inhibiting the NF-κB signaling pathway (H. M. Wang et al., 2013) (Table 2). These studies indicate that the neuroprotection of Dl-NBP against AD is closely related to the induction of Trx.
Conclusions
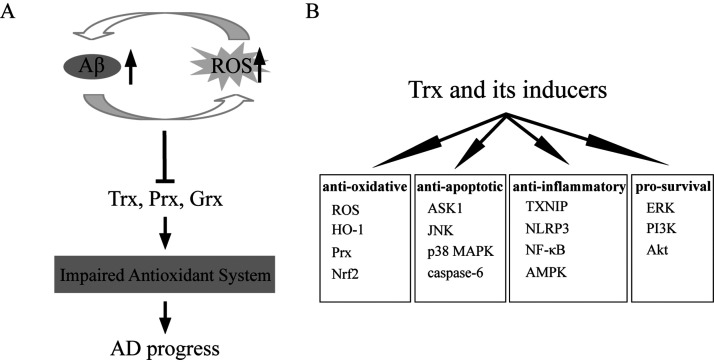
In conclusion, oxidative stress has been recognized as a significant contributing factor in the development of AD. Aβ deposition induces oxidative stress, which in turn accelerates Aβ deposition and the formation of fibril Aβ. The vicious circle of Aβ deposition and oxidative stress leads to impaired intracellular antioxidant defenses, further neurons death and ultimate AD progress. Decrease of content or loss of function of redox enzymes usually sensitizes cultured cells or brain neurons of AD patients and animals to oxidative stress, further leading to increased cell death. Currently, there are no established treatments that slow the progression of AD. As presented in this review, increasing studies have demonstrated that the redox enzymes play important roles in regulating the oxidative state in AD patients and models. In vitro and in vivo experiments have proven that enhancement of endogenous Trx expression and administration of exogenous Trx inducers play neuroprotective roles in AD, including inhibition of oxidative stress, inflammation (TXNIP, NLRP3, NF-κB), and apoptosis (ASK1/JNK/p38MAPK, caspase-6) and activation of pro-survival signaling pathways (PI3K/Akt and ERK) (Figure 1). These findings suggest that increasing the expressions and enhancing the activity of Trx may provide a strategy to slow the progression of AD. Thus, therapeutic approaches aimed at enhancing catalytic redox activity will provide a promising and exciting new avenue for the treatment of AD. Further studies are needed to clarify the detailed molecular mechanisms underlying how Trx functions in AD. In addition, the roles of other members of the Trx system, TrxR and TXNIP, in AD should also be further investigated deeply.
Figure 1.
The Neuroprotective Roles of Trx and Its Inducers in AD. (A) Aβ deposition induces oxidative stress (ROS), which in turn accelerates Aβ deposition and the formation of fibril Aβ. Both of Aβ deposition and ROS induction lead to compromised antioxidant activity of the cells, which further promote the AD progress. (B) Trx and its inducers plays the anti-oxidative, anti-apoptotic, anti-inflammatory and pro-survival roles in AD models.
Footnotes
Author Contributions: X-SZ and J-JJ designed the article contents. J-JJ and X-SZ wrote the original paper. X-SZ, G-TX and Z-QW revised the paper. All authors reviewed the paper and approved the submitted version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (31600837), Natural Science Foundation of Henan Province (202300410332) and Research Foundation for Advanced Talents of Jiaxing University (CD70520018).
ORCID iD: Xiansi Zeng https://orcid.org/0000-0002-1495-701X
References
- Aaseth J., Buha A., Wallace D. R., Bjorklund G. (2020). Xenobiotics, trace metals and genetics in the pathogenesis of tauopathies. Int J Environ Res Public Health, 17, 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal I., Jha S. (2020). Mitochondrial dysfunction and Alzheimer's disease: Role of microglia. Front Aging Neurosci, 12, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akterin S., Cowburn R. F., Miranda-Vizuete A., Jimenez A., Bogdanovic N., Winblad B., Cedazo-Minguez A. (2006). Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ, 13, 1454–1465. [DOI] [PubMed] [Google Scholar]
- Albensi B. C. (2019). Dysfunction of mitochondria: Implications for Alzheimer's disease. Int Rev Neurobiol, 145, 13–27. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. (2016). 2016 Alzheimer's disease facts and figures. Alzheimers Dement, 12, 459–509. [DOI] [PubMed] [Google Scholar]
- Arodin L., Lamparter H., Karlsson H., Nennesmo I., Bjornstedt M., Schroder J., Fernandes A. P. (2014). Alteration of thioredoxin and glutaredoxin in the progression of Alzheimer's disease. J Alzheimers Dis, 39, 787–797. [DOI] [PubMed] [Google Scholar]
- Bachnoff N., Trus M., Atlas D. (2011). Alleviation of oxidative stress by potent and selective thioredoxin-mimetic peptides. Free Radic Biol Med, 50, 1355–1367. [DOI] [PubMed] [Google Scholar]
- Baratz-Goldstein R., Deselms H., Heim L. R., Khomski L., Hoffer B. J., Atlas D., Pick C. G. (2016). Thioredoxin-mimetic-peptides protect cognitive function after mild traumatic brain injury (mTBI). PLoS One, 11, e0157064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L., Lill C. M., Tanzi R. E. (2010). The genetics of Alzheimer disease: back to the future. Neuron, 68, 270–281. [DOI] [PubMed] [Google Scholar]
- Bhat S. A., Sood A., Shukla R., Hanif K. (2019). AT2R activation prevents microglia pro-inflammatory activation in a NOX-dependent manner: Inhibition of PKC activation and p47(phox) phosphorylation by PP2A. Mol Neurobiol, 56, 3005–3023. [DOI] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D. (2016). Resveratrol and cardiovascular diseases. Nutrients, 8, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield D. A., Boyd-Kimball D. (2020). Mitochondrial oxidative and nitrosative stress and Alzheimer disease. Antioxidants (Basel ), 9, 818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacho-Valadez B., Munoz-Lobato F., Pedrajas J. R., Cabello J., Fierro-Gonzalez J. C., Navas P., Swoboda P., Link C. D., Miranda-Vizuete A. (2012). The characterization of the Caenorhabditis elegans mitochondrial thioredoxin system uncovers an unexpected protective role of thioredoxin reductase 2 in beta-amyloid peptide toxicity. Antioxid Redox Signal, 16, 1384–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V., Sultana R., Scapagnini G., Guagliano E., Sapienza M., Bella R., Kanski J., Pennisi G., Mancuso C., Stella A. M., Butterfield D. A. (2006). Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxid Redox Signal, 8, 1975–1986. [DOI] [PubMed] [Google Scholar]
- Campos C., Sartorio C. L., Casali K. R., Fernandes R. O., Llesuy S., da Rosa Araujo A. S., Bello-Klein A., Rigatto K. V. (2014). Low-dose estrogen is as effective as high-dose treatment in rats with postmenopausal hypertension. J Cardiovasc Pharmacol, 63, 144–151. [DOI] [PubMed] [Google Scholar]
- Carmona S., Zahs K., Wu E., Dakin K., Bras J., Guerreiro R. (2018). The role of TREM2 in Alzheimer's disease and other neurodegenerative disorders. Lancet Neurol, 17, 721–730. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Desmarais E., Bellis M., Campion D., Clerget-Darpoux F., Brice A., Agid Y., Jaillard-Serradt A., Mallet J. (1992). More missense in amyloid gene. Nat Genet, 2, 255–256. [DOI] [PubMed] [Google Scholar]
- Castano E. M., Prelli F., Wisniewski T., Golabek A., Kumar R. A., Soto C., Frangione B. (1995). Fibrillogenesis in Alzheimer's disease of amyloid beta peptides and apolipoprotein E. Biochem J, 306(Pt 2), 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zeng X., Luo F., Lv T., Zhou X., Bai J. (2014). The decreased expression of thioredoxin-1 in brain of mice with experimental autoimmune myasthenia gravis. Neuromuscul Disord, 24, 726–735. [DOI] [PubMed] [Google Scholar]
- Chen Y., Jiang M., Li L., Ye M., Yu M., Zhang L., Ge B., Xu W., Wei D. (2018). DL3nbutylphthalide reduces microglial activation in lipopolysaccharide induced Parkinson's disease model mice. Mol Med Rep, 17, 3884–3890. [DOI] [PubMed] [Google Scholar]
- Chen Z., Zhong C. (2014). Oxidative stress in Alzheimer's disease. Neurosci Bull, 30, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiueh C., Lee S., Andoh T., Murphy D. (2003) Induction of antioxidative and antiapoptotic thioredoxin supports neuroprotective hypothesis of estrogen. Endocrine, 21, 27–31. [DOI] [PubMed] [Google Scholar]
- Choi K. J., Kim M. J., Je A. R., Jun S., Lee C., Lee E., Jo M., Huh Y. H., Kweon H. S. (2014). Three-dimensional analysis of abnormal ultrastructural alteration in mitochondria of hippocampus of APP/PSEN1 transgenic mouse. J Biosci, 39, 97–105. [DOI] [PubMed] [Google Scholar]
- Cohen-Kutner M., Khomsky L., Trus M., Aisner Y., Niv M. Y., Benhar M., Atlas D. (2013). Thioredoxin-mimetic peptides (TXM) reverse auranofin induced apoptosis and restore insulin secretion in insulinoma cells. Biochem Pharmacol, 85, 977–990. [DOI] [PubMed] [Google Scholar]
- Cohen-Kutner M., Khomsky L., Trus M., Ben-Yehuda H., Lenhard J. M., Liang Y., Martin T., Atlas D. (2014). Thioredoxin-mimetic peptide CB3 lowers MAPKinase activity in the Zucker rat brain. Redox Biol, 2, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C., Trovato Salinaro A., Scuto M., Fronte V., Cambria M. T., Pennisi M., Bella R., Milone P., Graziano A., Crupi R., Cuzzocrea S., Pennisi G., Calabrese V. (2013). Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: role of vitagenes. Immun Ageing, 10, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B., Zhang S., Wang Y., Guo Y. (2019). Farrerol attenuates beta-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line. Biomed Pharmacother, 109, 112–119. [DOI] [PubMed] [Google Scholar]
- Cumming R. C., Dargusch R., Fischer W. H., Schubert D. (2007). Increase in expression levels and resistance to sulfhydryl oxidation of peroxiredoxin isoforms in amyloid beta-resistant nerve cells. J Biol Chem, 282, 30523–30534. [DOI] [PubMed] [Google Scholar]
- Das K. C., Das C. K. (2000). Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: redox independent functions. Biochem Biophys Res Commun, 277, 443–447. [DOI] [PubMed] [Google Scholar]
- Di Domenico F., Sultana R., Tiu G. F., Scheff N. N., Perluigi M., Cini C., Butterfield D. A. (2010). Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res, 1333, 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Gao J., Zhu Z., Xiong Y., Liu J. (2008). Mitochondrial dysfunction enhances susceptibility to oxidative stress by down-regulation of thioredoxin in human neuroblastoma cells. Neurochem Res, 33, 43–50. [DOI] [PubMed] [Google Scholar]
- Dorszewska J., Prendecki M., Oczkowska A., Dezor M., Kozubski W. (2016). Molecular basis of familial and sporadic Alzheimer's disease. Curr Alzheimer Res, 13, 952–963. [DOI] [PubMed] [Google Scholar]
- Duan Q., Si E. (2019). MicroRNA-25 aggravates Abeta1-42-induced hippocampal neuron injury in Alzheimer's disease by downregulating KLF2 via the Nrf2 signaling pathway in a mouse model. J Cell Biochem 120, 15891–15905. [DOI] [PubMed] [Google Scholar]
- Fakhoury M. (2018). Microglia and astrocytes in Alzheimer's disease: Implications for therapy. Curr Neuropharmacol 16, 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang E. F., Hou Y., Palikaras K., Adriaanse B. A., Kerr J. S., Yang B., Lautrup S., Hasan-Olive M. M., Caponio D., Dan X., Rocktaschel P., Croteau D. L., Akbari M., Greig N. H., Fladby T., Nilsen H., Cader M. Z., Mattson M. P., Tavernarakis N., Bohr V. A. (2019). Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat Neurosci, 22, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Zhang L. (2019). Resveratrol suppresses abeta-induced microglial activation through the TXNIP/TRX/NLRP3 signaling pathway. DNA Cell Biol, 38, 874–879. [DOI] [PubMed] [Google Scholar]
- Flowers S. A., Rebeck G. W. (2020). APOE in the normal brain. Neurobiol Dis 136, 104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friker L. L., Scheiblich H., Hochheiser I. V., Brinkschulte R., Riedel D., Latz E., Geyer M., Heneka M. T. (2020). beta-Amyloid clustering around ASC fibrils boosts its toxicity in microglia. Cell Rep, 30, 3743 e3746–3754 e3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant J. C., Kadish I., Chen K. C., Thibault O., Blalock E. M., Porter N. M., Landfield P. W. (2018). Aging-related calcium dysregulation in rat entorhinal neurons homologous with the human entorhinal neurons in which Alzheimer's disease neurofibrillary tangles first appear. J Alzheimers Dis, 66, 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., He H., Jiang W., Chang X., Zhu L., Luo F., Zhou R., Ma C., Yan T. (2015). Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer's disease. Behav Brain Res, 293, 27–33. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhou R., You X., Luo F., He H., Chang X., Zhu L., Ding X., Yan T. (2016). Salidroside suppresses inflammation in a D-galactose-induced rat model of Alzheimer's disease via SIRT1/NF-kappaB pathway. Metab Brain Dis, 31, 771–778. [DOI] [PubMed] [Google Scholar]
- Gao Y., Fu R., Wang J., Yang X., Wen L., Feng J. (2018). Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway. Pharm Biol, 56, 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerenu G., Persson T., Goikolea J., Calvo-Garrido J., Loera-Valencia R., Pottmeier P., Santiago C., Poska H., Presto J., Cedazo-Minguez A. (2019). Thioredoxin-80 protects against amyloid-beta pathology through autophagic-lysosomal pathway regulation. Mol Psychiatry. Advance online publication. 10.1038/s41380-019-0521-2 [DOI] [PubMed] [Google Scholar]
- Giau V. V., Bagyinszky E., Youn Y. C., An S. S. A., Kim S. (2019). APP, PSEN1, and PSEN2 mutations in Asian patients with early-onset Alzheimer disease. Int J Mol Sci, 20, 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Bea F., Akterin S., Persson T., Mateos L., Sandebring A., Avila-Carino J., Gutierrez-Rodriguez A., Sundstrom E., Holmgren A., Winblad B., Cedazo-Minguez A. (2012). Thioredoxin-80 is a product of alpha-secretase cleavage that inhibits amyloid-beta aggregation and is decreased in Alzheimer's disease brain. EMBO Mol Med, 4, 1097–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B. A., et al. (2018). Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer's disease: A longitudinal study. Lancet Neurol, 17, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., Kvietys P. R. (2015). Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol, 6, 524–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A, 83, 4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw B. J., Betensky R. A., Jacobs H. I. L., Schultz A. P., Sepulcre J., Becker J. A., Cosio D. M. O., Farrell M., Quiroz Y. T., Mormino E. C., Buckley R. F., Papp K. V., Amariglio R. A., Dewachter I., Ivanoiu A., Huijbers W., Hedden T., Marshall G. A., Chhatwal J. P., Rentz D. M., Sperling R. A., Johnson K. (2019). Association of amyloid and tau with cognition in preclinical Alzheimer disease: A longitudinal study. JAMA Neurol, 76, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. V., Hanson J. E., Sheng M. (2018). Microglia in Alzheimer's disease. J Cell Biol, 217, 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T. C., Gelpi E., Halle A., Korte M., Latz E., Golenbock D. T. (2013). NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature, 493, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S., Pimenova A. A., Hayes K., Villa J. A., Rosene M. J., Jere M., Goate A. M., Karch C. M. (2020). Systematic validation of variants of unknown significance in APP, PSEN1 and PSEN2. Neurobiol Dis, 139, 104817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Y., Chengyong T., Cheng L., Haixia H., Yuanda Z., Weihua Y. (2018). Resveratrol attenuates the cytotoxicity induced by amyloid-beta1-42 in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem Res 43, 297–305. [DOI] [PubMed] [Google Scholar]
- Im J. Y., Lee K. W., Woo J. M., Junn E., Mouradian M. M. (2012). DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet, 21, 3013–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. I., Nagakannan P., Ogungbola O., Djordjevic J., Albensi B. C., Eftekharpour E. (2019). Thioredoxin system as a gatekeeper in caspase-6 activation and nuclear lamina integrity: Implications for Alzheimer's disease. Free Radic Biol Med, 134, 567–580. [DOI] [PubMed] [Google Scholar]
- Izquierdo V., Palomera-Avalos V., Lopez-Ruiz S., Canudas A. M., Pallas M., Grinan-Ferre C. (2019). Maternal resveratrol supplementation prevents cognitive decline in senescent mice offspring. Int J Mol Sci, 20, 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James B. D., Leurgans S. E., Hebert L. E., Scherr P. A., Yaffe K., Bennett D. A. (2014). Contribution of Alzheimer disease to mortality in the United States. Neurology, 82, 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. J., Geng W. S., Wang Z. Q., Chen L., Zeng X. S. (2019). The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharmacol 84, 453–470. [DOI] [PubMed] [Google Scholar]
- Jia J. J., Zeng X. S., Li K., Ma L. F., Chen L., Song X. Q. (2016). The expression of thioredoxin-1 in acute epinephrine stressed mice. Cell Stress Chaperones, 21, 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. J., Zeng X. S., Song X. Q., Zhang P. P., Chen L. (2017). Diabetes mellitus and Alzheimer's disease: The protection of epigallocatechin-3-gallate in streptozotocin injection-induced models. Front Pharmacol, 8, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. J., Zeng X. S., Zhou X. S., Li Y., Bai J. (2014). The induction of thioredoxin-1 by epinephrine withdraws stress via interaction with beta-arrestin-1. Cell Cycle, 13, 3121–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. M., Wilson-Delfosse A. L., Chen S. G., Mieyal J. J. (2015). The roles of redox enzymes in Parkinson's disease: Focus on glutaredoxin. Ther Targets Neurol Dis, 2, e790. [PMC free article] [PubMed] [Google Scholar]
- Ju T. C., Chen S. D., Liu C. C., Yang D. I. (2005). Protective effects of S-nitrosoglutathione against amyloid beta-peptide neurotoxicity. Free Radic Biol Med, 38, 938–949. [DOI] [PubMed] [Google Scholar]
- Kam M. K., Lee D. G., Kim B., Lee H. S., Lee S. R., Bae Y. C., Lee D. S. (2019). Peroxiredoxin 4 ameliorates amyloid beta oligomer-mediated apoptosis by inhibiting ER-stress in HT-22 hippocampal neuron cells. Cell Biol Toxicol, 35, 573–588. [DOI] [PubMed] [Google Scholar]
- Kim B., Park J., Chang K. T., Lee D. S. (2016). Peroxiredoxin 5 prevents amyloid-beta oligomer-induced neuronal cell death by inhibiting ERK-Drp1-mediated mitochondrial fragmentation. Free Radic Biol Med, 90, 184–194. [DOI] [PubMed] [Google Scholar]
- King B. C., Nowakowska J., Karsten C. M., Kohl J., Renstrom E., Blom A. M. (2012). Truncated and full-length thioredoxin-1 have opposing activating and inhibitory properties for human complement with relevance to endothelial surfaces. J Immunol, 188, 4103–4112. [DOI] [PubMed] [Google Scholar]
- Kommaddi R. P., Tomar D. S., Karunakaran S., Bapat D., Nanguneri S., Ray A., Schneider B. L., Nair D., Ravindranath V. (2019). Glutaredoxin1 diminishes amyloid beta-mediated oxidation of F-actin and reverses cognitive deficits in an Alzheimer's disease mouse model. Antioxid Redox Signal, 31, 1321–1338. [DOI] [PubMed] [Google Scholar]
- Lane C. A., Hardy J., Schott J. M. (2018). Alzheimer's disease. Eur J Neurol, 25, 59–70. [DOI] [PubMed] [Google Scholar]
- Li L., Ismael S., Nasoohi S., Sakata K., Liao F. F., McDonald M. P., Ishrat T. (2019). Thioredoxin-interacting protein (TXNIP) associated NLRP3 inflammasome activation in human Alzheimer's disease brain. J Alzheimers Dis, 68, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang J., Li Y., Xu X. (2018). Neuroprotective effects of salidroside administration in a mouse model of Alzheimer's disease. Mol Med Rep, 17, 7287–7292. [DOI] [PubMed] [Google Scholar]
- Liao Z. L., Su H., Tan Y. F., Qiu Y. J., Zhu J. P., Chen Y., Lin S. S., Wu M. H., Mao Y. P., Hu J. J., Yu E. Y. (2019). Salidroside protects PC-12 cells against amyloid beta-induced apoptosis by activation of the ERK1/2 and AKT signaling pathways. Int J Mol Med, 43, 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., Bennett M. L., Munch A. E., Chung W. S., Peterson T. C., Wilton D. K., Frouin A., Napier B. A., Panicker N., Kumar M., Buckwalter M. S., Rowitch D. H., Dawson V. L., Dawson T. M., Stevens B., Barres B. A. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Kapoor A., Gu Y., Chow M. J., Peng J., Zhao K., Tang D. (2020). Contributions of DNA damage to Alzheimer's disease. Int J Mol Sci, 21, 1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell M. A., Xie C., Gabbita S. P., Markesbery W. R. (2000). Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer's disease brain. Free Radic Biol Med, 28, 418–427. [DOI] [PubMed] [Google Scholar]
- Lv C., Ma Q., Han B., Li J., Geng Y., Zhang X., Wang M. (2018). Long-term DL-3-n-butylphthalide treatment alleviates cognitive impairment correlate with improving synaptic plasticity in SAMP8 mice. Front Aging Neurosci, 10, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore C., Yin Z., Leibowitz J., Butovsky O. (2020). Microglia, lifestyle stress, and neurodegeneration. Immunity, 52, 222–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majd S., Power J. H. T. (2018). Oxidative stress and decreased mitochondrial superoxide dismutase 2 and peroxiredoxins 1 and 4 based mechanism of concurrent activation of AMPK and mTOR in Alzheimer's disease. Curr Alzheimer Res, 15, 764–776. [DOI] [PubMed] [Google Scholar]
- Marongiu R. (2019). Accelerated ovarian failure as a unique model to study peri-menopause influence on Alzheimer's disease. Front Aging Neurosci, 11, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masci A., Mattioli R., Costantino P., Baima S., Morelli G., Punzi P., Giordano C., Pinto A., Donini L. M., d'Erme M., Mosca L. (2015). Neuroprotective effect of brassica oleracea sprouts crude juice in a cellular model of Alzheimer's disease. Oxid Med Cell Longev, 2015, 781938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani H., Bai J., Kim Y. C., Yodoi J. (2004). Thioredoxin as a neurotrophic cofactor and an important regulator of neuroprotection. Mol Neurobiol, 29, 229–242. [DOI] [PubMed] [Google Scholar]
- Mateos L., Persson T., Katoozi S., Gil-Bea F. J., Cedazo-Minguez A. (2012). Estrogen protects against amyloid-beta toxicity by estrogen receptor alpha-mediated inhibition of Daxx translocation. Neurosci Lett, 506, 245–250. [DOI] [PubMed] [Google Scholar]
- McBean G. J., Lopez M. G., Wallner F. K. (2017). Redox-based therapeutics in neurodegenerative disease. Br J Pharmacol, 174, 1750–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher K. I., Wyss-Coray T. (2014). Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol 88, 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muche A., Arendt T., Schliebs R. (2017). Oxidative stress affects processing of amyloid precursor protein in vascular endothelial cells. PLoS One, 12, e0178127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakannan P., Iqbal M. A., Yeung A., Thliveris J. A., Rastegar M., Ghavami S., Eftekharpour E. (2016). Perturbation of redox balance after thioredoxin reductase deficiency interrupts autophagy-lysosomal degradation pathway and enhances cell death in nutritionally stressed SH-SY5Y cells. Free Radic Biol Med, 101, 53–70. [DOI] [PubMed] [Google Scholar]
- Nasoohi S., Ismael S., Ishrat T. (2018). Thioredoxin-interacting protein (TXNIP) in cerebrovascular and neurodegenerative diseases: Regulation and implication. Mol Neurobiol 55, 7900–7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku Y., Murakami K., Irie K., Hoseki J., Sakai Y. (2017). Synthesized Abeta42 caused intracellular oxidative damage, leading to cell death, via lysosome rupture. Cell Struct Funct, 42, 71–79. [DOI] [PubMed] [Google Scholar]
- Pan Q., Guo K., Xue M., Tu Q. (2020). Estrogen protects neuroblastoma cell from amyloid-beta 42 (Abeta42)-induced apoptosis via TXNIP/TRX axis and AMPK signaling. Neurochem Int, 135, 104685. [DOI] [PubMed] [Google Scholar]
- Pankiewicz J. E., Diaz J. R., Marta-Ariza M., Lizinczyk A. M., Franco L. A., Sadowski M. J. (2020). Peroxiredoxin 6 mediates protective function of astrocytes in Abeta proteostasis. Mol Neurodegener, 15, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Kim B., Chae U., Lee D. G., Kam M. K., Lee S. R., Lee S., Lee H. S., Park J. W., Lee D. S. (2017). Peroxiredoxin 5 decreases beta-amyloid-mediated cyclin-dependent kinase 5 activation through regulation of Ca(2+)-mediated calpain activation. Antioxid Redox Signal, 27, 715–726. [DOI] [PubMed] [Google Scholar]
- Pekkari K., Holmgren A. (2004). Truncated thioredoxin: Physiological functions and mechanism. Antioxid Redox Signal, 6, 53–61. [DOI] [PubMed] [Google Scholar]
- Persson T., Lattanzio F., Calvo-Garrido J., Rimondini R., Rubio-Rodrigo M., Sundstrom E., Maioli S., Sandebring-Matton A., Cedazo-Minguez A. (2017). Apolipoprotein E4 elicits lysosomal cathepsin D release, decreased thioredoxin-1 levels, and apoptosis. J Alzheimers Dis, 56, 601–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaceri I., Nacmias B., Sorbi S. (2013). Genetics of familial and sporadic Alzheimer's disease. Front Biosci (Elite Ed), 5, 167–177. [DOI] [PubMed] [Google Scholar]
- Pratico D. (2008). Oxidative stress hypothesis in Alzheimer's disease: A reappraisal. Trends Pharmacol Sci, 29, 609–615. [DOI] [PubMed] [Google Scholar]
- Qiang X., Li Y., Yang X., Luo L., Xu R., Zheng Y., Cao Z., Tan Z., Deng Y. (2017). DL-3-n-butylphthalide-edaravone hybrids as novel dual inhibitors of amyloid-beta aggregation and monoamine oxidases with high antioxidant potency for Alzheimer's therapy. Bioorg Med Chem Lett, 27, 718–722. [DOI] [PubMed] [Google Scholar]
- Qin Y., An D., Xu W., Qi X., Wang X., Chen L., Chen L., Sha S. (2020). Estradiol replacement at the critical period protects hippocampal neural stem cells to improve cognition in APP/PS1 mice. Front Aging Neurosci, 12, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawda C., Moussa C., Turner R. S. (2017). Resveratrol for Alzheimer's disease. Ann N Y Acad Sci, 1403, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A., Frosch M. P., Masliah E., Hyman B. T. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med, 1, a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Pilozzi A. R., Huang X. (2020). Exposure of CuO nanoparticles contributes to cellular apoptosis, redox stress, and Alzheimer's abeta amyloidosis. Int J Environ Res Public Health, 17, 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirunavukkarasu M., Penumathsa S. V., Koneru S., Juhasz B., Zhan L., Otani H., Bagchi D., Das D. K., Maulik N. (2007). Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med, 43, 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnies E., Trushina E. (2017). Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers Dis, 57, 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torromino G., Maggi A., De Leonibus E. (2020). Estrogen-dependent hippocampal wiring as a risk factor for age-related dementia in women. Prog Neurobiol, 197, 101895. [DOI] [PubMed] [Google Scholar]
- Trouw L. A., Nielsen H. M., Minthon L., Londos E., Landberg G., Veerhuis R., Janciauskiene S., Blom A. M. (2008). C4b-binding protein in Alzheimer's disease: binding to Abeta1-42 and to dead cells. Mol Immunol, 45, 3649–3660. [DOI] [PubMed] [Google Scholar]
- Tsubuki S., Takaki Y., Saido T. C. (2003). Dutch, Flemish, Italian, and Arctic mutations of APP and resistance of Abeta to physiologically relevant proteolytic degradation. Lancet, 361, 1957–1958. [DOI] [PubMed] [Google Scholar]
- Ulland T. K., Song W. M., Huang S. C., Ulrich J. D., Sergushichev A., Beatty W. L., Loboda A. A., Zhou Y., Cairns N. J., Kambal A., Loginicheva E., Gilfillan S., Cella M., Virgin H. W., Unanue E. R., Wang Y., Artyomov M. N., Holtzman D. M., Colonna M. (2017). TREM2 maintains microglial metabolic fitness in Alzheimer's disease. Cell, 170, 649 e613–663 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas C., Kumar S., Franklin B. S., Dierkes T., Brinkschulte R., Tejera D., Vieira-Saecker A., Schwartz S., Santarelli F., Kummer M. P., Griep A., Gelpi E., Beilharz M., Riedel D., Golenbock D. T., Geyer M., Walter J., Latz E., Heneka M. T. (2017). Microglia-derived, A. S.C specks cross-seed amyloid-beta in Alzheimer's disease. Nature, 552, 355–361. [DOI] [PubMed] [Google Scholar]
- Venkateshappa C., Harish G., Mahadevan A., Srinivas Bharath M. M., Shankar S. K. (2012). Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer's disease. Neurochem Res, 37, 1601–1614. [DOI] [PubMed] [Google Scholar]
- Verma A., Ray A., Bapat D., Diwakar L., Kommaddi R. P., Schneider B. L., Hirsch E. C., Ravindranath V. (2020). Glutaredoxin 1 downregulation in the substantia nigra leads to dopaminergic degeneration in mice. Mov Disord, 35, 1843–1853. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Wang Z. Y., Xie J. W., Wang T., Wang X., Xu Y., Cai J. H. (2016). Dl-3-n-butylphthalide-induced upregulation of antioxidant defense is involved in the enhancement of cross talk between CREB and Nrf2 in an Alzheimer's disease mouse model. Neurobiol Aging, 38, 32–46. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Xu Y., Wang X., Guo C., Wang T., Wang Z. Y. (2019). Dl-3-n-butylphthalide Inhibits NLRP3 inflammasome and mitigates Alzheimer's-like pathology via Nrf2-TXNIP-TrX axis. Antioxid Redox Signal, 30, 1411–1431. [DOI] [PubMed] [Google Scholar]
- Wang F., Ma J., Han F., Guo X., Meng L., Sun Y., Jin C., Duan H., Li H., Peng Y. (2016). DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci Rep, 6, 19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li Q., Sun S., Chen S. (2020). Neuroprotective effects of salidroside in a mouse model of Alzheimer's disease. Cell Mol Neurobiol, 40, 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. M., Zhang T., Huang J. K., Sun X. J. (2013). 3-N-butylphthalide (NBP) attenuates the amyloid-beta-induced inflammatory responses in cultured astrocytes via the nuclear factor-kappaB signaling pathway. Cell Physiol Biochem, 32, 235–242. [DOI] [PubMed] [Google Scholar]
- Wang X., Ma S., Yang B., Huang T., Meng N., Xu L., Xing Q., Zhang Y., Zhang K., Li Q., Zhang T., Wu J., Yang G. L., Guan F., Wang J. (2018). Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer's disease. Behav Brain Res, 339, 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Bharti V., Zhou H., Hoi V., Tan H., Wu Z., Nagakannan P., Eftekharpour E., Wang J. F. (2019). Upregulation of thioredoxin-interacting protein in brain of amyloid-beta protein precursor/presenilin 1 transgenic mice and amyloid-beta treated neuronal cells. J Alzheimers Dis, 72, 139–150. [DOI] [PubMed] [Google Scholar]
- Xia X., Jiang Q., McDermott J., Han J. J. (2018). Aging and Alzheimer's disease: Comparison and associations from molecular to system level. Aging Cell, 17, e12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong N., Huang J., Chen C., Zhao Y., Zhang Z., Jia M., Zhang Z., Hou L., Yang H., Cao X., Liang Z., Zhang Y., Sun S., Lin Z., Wang T. (2012). Dl-3-n-butylphthalide, a natural antioxidant, protects dopamine neurons in rotenone models for Parkinson's disease. Neurobiol Aging, 33, 1777–1791. [DOI] [PubMed] [Google Scholar]
- Xu Z. Q., Zhou Y., Shao B. Z., Zhang J. J., Liu C. (2019). A systematic review of neuroprotective efficacy and safety of DL-3-N-butylphthalide in ischemic stroke. Am J Chin Med, 47, 507–525. [DOI] [PubMed] [Google Scholar]
- Yang L., Guo N., Fan W., Ni C., Huang M., Bai L., Zhang L., Zhang X., Wen Y., Li Y., Zhou X., Bai J. (2020). Thioredoxin-1 blocks methamphetamine-induced injury in brain through inhibiting endoplasmic reticulum and mitochondria-mediated apoptosis in mice. Neurotoxicology, 78, 163–169. [DOI] [PubMed] [Google Scholar]
- Yang X. H., Liu H. G., Liu X., Chen J. N. (2012). Thioredoxin and impaired spatial learning and memory in the rats exposed to intermittent hypoxia. Chin Med J (Engl), 125, 3074–3080. [PubMed] [Google Scholar]
- Yun H. M., Jin P., Park K. R., Hwang J., Jeong H. S., Kim E. C., Jung J. K., Oh K. W., Hwang B. Y., Han S. B., Hong J. T. (2016). Thiacremonone potentiates anti-oxidant effects to improve memory dysfunction in an APP/PS1 transgenic mice model. Mol Neurobiol, 53, 2409–2420. [DOI] [PubMed] [Google Scholar]
- Zeng X. S., Geng W. S., Wang Z. Q., Jia J. J. (2020). Morphine addiction and oxidative stress: The potential effects of thioredoxin-1. Front Pharmacol, 11, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. S., Jia J. J., Kwon Y., Wang S. D., Bai J. (2014). The role of thioredoxin-1 in suppression of endoplasmic reticulum stress in Parkinson disease. Free Radic Biol Med, 67, 10–18. [DOI] [PubMed] [Google Scholar]
- Zeng X. S., Jia J. J., Ma L. F. (2015). Gensenoside Rb1 protects rat PC12 cells from oxidative stress-induced endoplasmic reticulum stress: the involvement of thioredoxin-1. Mol Cell Biochem, 410, 239–246. [DOI] [PubMed] [Google Scholar]
- Zeng X. S., Zhou X. S., Luo F. C., Jia J. J., Qi L., Yang Z. X., Zhang W., Bai J. (2014). Comparative analysis of the neuroprotective effects of ginsenosides Rg1 and Rb1 extracted from Panax notoginseng against cerebral ischemia. Can J Physiol Pharmacol, 92, 102–108. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yu H., Sun Y., Lin X., Chen B., Tan C., Cao G., Wang Z. (2007). Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol, 564, 18–25. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yu H., Zhao X., Lin X., Tan C., Cao G., Wang Z. (2010). Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int, 57, 547–555. [DOI] [PubMed] [Google Scholar]
- Zhang X., Bai L., Zhang S., Zhou X., Li Y., Bai J. (2018). Trx-1 ameliorates learning and memory deficits in MPTP-induced Parkinson's disease model in mice. Free Radic Biol Med, 124, 380–387. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Song W., Wang Z., Wang Z., Jin X., Xu J., Bai L., Li Y., Cui J., Cai L. (2018). Resveratrol attenuates testicular apoptosis in type 1 diabetic mice: Role of Akt-mediated Nrf2 activation and p62-dependent Keap1 degradation. Redox Biol, 14, 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wang C., Wu J., Fukunaga A., Cheng Z., Wang J., Yamauchi A., Yodoi J., Tian H. (2020). Anti-allergic and anti-inflammatory effects and molecular mechanisms of thioredoxin on respiratory system diseases. Antioxid Redox Signal, 32, 785–801. [DOI] [PubMed] [Google Scholar]