Abstract
Traumatic spinal cord injury (SCI) results in direct and indirect damage to neural tissues, which results in motor and sensory dysfunction, dystonia, and pathological reflex that ultimately lead to paraplegia or tetraplegia. A loss of cells, axon regeneration failure, and time-sensitive pathophysiology make tissue repair difficult. Despite various medical developments, there are currently no effective regenerative treatments. Stem cell therapy is a promising treatment for SCI due to its multiple targets and reactivity benefits. The present review focuses on SCI stem cell therapy, including bone marrow mesenchymal stem cells, umbilical mesenchymal stem cells, adipose-derived mesenchymal stem cells, neural stem cells, neural progenitor cells, embryonic stem cells, induced pluripotent stem cells, and extracellular vesicles. Each cell type targets certain features of SCI pathology and shows therapeutic effects via cell replacement, nutritional support, scaffolds, and immunomodulation mechanisms. However, many preclinical studies and a growing number of clinical trials found that single-cell treatments had only limited benefits for SCI. SCI damage is multifaceted, and there is a growing consensus that a combined treatment is needed.
Keywords: spinal cord injury, stem cells, BM-MSCs, U-MSCs, AD-MSCs, NSCs, NPCs, ESCs, iPSCs, EVs
Introduction
Spinal cord injury (SCI) is a devastating injury that is a source of extensive psychological and economic burden for patients and healthcare systems1,2. It is estimated that SCI affects more than 1 million people in the United States alone, with approximately 17,000 new cases each year3. Current treatments include spinal decompression surgery, treatment for spasticity, and rehabilitation therapy. Despite some advances in clinical management that improve patient’s quality of life4,5, SCI recovery is very limited, and finding alternative treatments for paralysis remains a top priority.
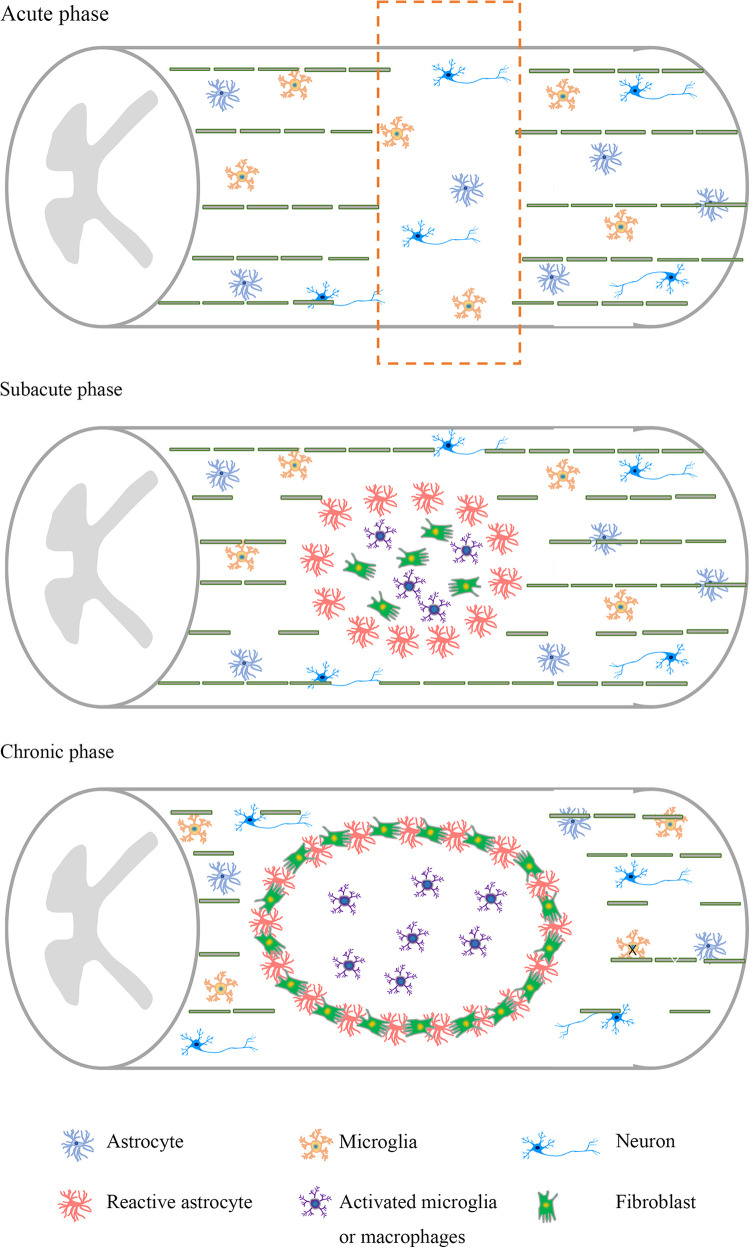
The time-sensitive and complex pathophysiology make it particularly difficult to investigate therapeutic targets for SCI6. After the initial mechanical injury, there are a series of secondary events that worsen the condition of patients7. The inflammatory response, gliosis hyperplasia, formation of an inhibitory environment8, and scar formation impede axonal regeneration and limit the potential for many therapeutic interventions (Fig. 1).
Figure 1.
Pathological characteristics of spinal cord injury at different stages. Neuronal apoptosis and axonal damage are abundant in the acute stage. At the subacute stage, there is a large loss of neurons, axons, and myelin. Activated astrocytes, activated microglia, and macrophages accumulate in the injury site. At the chronic stage, a glial scar and an injury cavity further develop, and the inhibitory microenvironment is formed.
Cell therapies exhibit neuroprotective and nerve regeneration potential in SCI with different targets and responses to stimuli, such as regulating inflammatory responses, providing nutritional support, and improving plasticity. With these excessive potential mechanisms, various cells from different tissue sources, including bone marrow mesenchymal stem cells (BM-MSCs), umbilical mesenchymal stem cells (U-MSCs), adipose-derived mesenchymal stem cells (AD-MSCs), neural stem cells (NSCs), neural progenitor cells (NPCs), embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and extracellular vesicles (EVs), were studied. Previous reviews discussed cell therapy for SCI, but there is a lack of systematic elucidation, such as the original function of these cells, the function of modified cells, and the effect of combined therapy. This review performed an up-to-date summary of the current research status, challenges, and prospects for stem cell therapy in SCI to provide an overview of this field9–13 (Table 1).
Table 1.
The Effects of Different Stem Cells on Spinal Cord Injury.
| Cell type | Effects |
|---|---|
| BM-MSCs | Secrete neurotrophic factors14
Promote axonal regeneration15 Reduce astroglial scarring density16 Reduce inflammatory reactions17 Reduce BSCB leakage18 Regulate autophagy19 Alleviate neuropathic pain20 Improve bladder compliance21 |
| U-MSCs | Protect neurons22
Inhibit glial scars23 Decrease reactive astrocytes24 Attenuate ischemic compromise of the spinal cord25 Alleviate allodynia and hyperalgesia26–28 Improve muscle tension, bladder function, and urine control29 Improve SSEP30 Alleviate neuropathic pain30 |
| AD-MSCs | Protect neurons31–34
Promote cell survival and tissue repair35 Suppress immune activity36 Secrete anti-inflammatory factors36 Activate angiogenesis37 Reduce the formation of cavities36 Improve sensory and motor functions37 Ameliorate erectile dysfunction31–34 |
| NSCs and NPCs | Increase neuroprotective cytokines38,39
Improve cell proliferation38 Increase myelination40 Modulate the inflammatory response41 Promote respiratory recovery42 |
| ESCs | Promote astrogliosis43,44
Enable axons to pass CSPG45 Support nodal architecture46,47 Attenuate neuropathic pain48 |
| iPSCs | Improve neurotrophic factor secretion49
Promote axonal sprouting50 Inhibit demyelination51,52 Promote synapse formation53 Inhibit glial scar50 Reduce lesion size54 Improve respiratory function54 |
| EVs derived from stem cells | Regulate axon regeneration55
Protect cells from apoptosis55 Inhibit the activation of astrocytes56 Inhibit inflammation57 Reduce injury size58 Protect the integrity of the BSCB59 |
AD-MSC: adipose-derived mesenchymal stem cell; BM-MSC: bone marrow mesenchymal stem cell; BSCB: blood-spinal cord barrier; CSPG: chondroitin sulfate proteoglycan; ESC: embryonic stem cell; EV: extracellular vesicle; iPSC: induced pluripotent stem cell; NPC: neural progenitor cell; NSC: neural stem cell; SSEP: somatosensory-evoked potential; U-MSC: umbilical mesenchymal stem cells.
Stem Cell Transplantation Strategy
Bone Marrow Mesenchymal Stem Cells
BM-MSCs are partially differentiated progenitor cells that are present in adult bone marrow and support sustained hematopoiesis and bone regeneration60. These cells were originally considered pluripotent, with the ability to differentiate into neurons and glial cells. However, additional studies showed that BM-MSC therapy primarily involved in cell fusion and transdifferentiation instead of cell differentiation. Early in vivo studies demonstrated that BM-MSC introduction into the lesion site of spinal cord contusion rats resulted in the formation of tissue bundles of astrocytes and neuronal predecessors15. The introduction of BM-MSCs to the injury site reduced inflammatory reactions17, astroglial scarring density16, and blood-spinal cord barrier (BSCB) leakage18; modulated astrogliosis; alleviated neuropathic pain; and improved the functional recovery of hindlimb movement, which may involve the matrix metalloproteinase (MMP) 2/STAT3 pathway61. Conditioned medium from MSCs exhibited a therapeutic effect on SCI and may regulate the autophagy- and survival-related proteins Olig 2 and HSP7019.
Further investigation of the BM-MSC intravenous graft model indicated that functional recovery was achieved via the expansion of neurotrophic factors, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and vascular endothelial growth factor (VEGF)14. NGF and BDNF are key regulators of neuronal differentiation, and VEGF is a key factor in the initiation and maintenance of angiogenesis and vasculogenesis induction62,63. Besides, BM-MSCs may be used as carriers due to their tropism to the injury sites and of interleukin-13 (IL-13), which is an inducer of the anti-inflammatory microglia/macrophage phenotype that significantly improved motor function recovery and decreased demyelination64.
Genetic engineering of BM-MSCs is an encouraging method to enhance their therapeutic effect, such as the regulation of specific factors or proteins. Insulin-like growth factor 1 (IGF-1) is an important factor for maintaining the characteristics of NPCs. IGF-1 overexpression of BM-MSCs strengthens antioxidant reactions and improves basso mouse scale (BMS) scores65. Other approaches, such as modification of the microRNA-124 gene66, silencing the Nogo-66 receptor gene67, inhibition of tumor necrosis factor α (TNF-α)68, and overexpression of neurotrophin-3 (NT-3)69, the chemokine stromal-derived factor-170, and neurotrophic factor-derived glial cell (GDNF) genes71, exhibited better efficacy than original BM-MSCs in motor function and surrounding axon densities. The effects of individual cell transplantation are enhanced by cotransplantation with cells from other sources. These coupling strategies are primarily focused on MSCs and Schwann cells (SCs) because these cells regulate the microenvironment and improve the survival, differentiation, and proliferation of cotransplanted cells. Various studies reported that MSCs enhanced the effects of SCs72 and olfactory ensheathing cells (OECs) by decreasing cell apoptosis73.
A longitudinal study of BM-MSC-based treatment of cervical SCI patients expanded autologous BM-MSCs and introduced these cells via intradural injection. Improved upper limb motor function and magnetic resonance imaging (MRI) images were observed in 6 of 10 candidates 6 months after transplantation21. Six patients with complete SCI received autologous MSC and SC therapy, and the results showed improvements in american spinal cord injury association (ASIA) grade, bladder compliance, and axonal regeneration. Similarly, a patient with chronic SCI received MSC therapy, and neurological function and the ability to walk were improved20. However, a phase III clinical trial demonstrated that single MSC application was safe but had little therapeutic effect. This result may be related to the timing of MSC transplantation because the homing capacity of stem cells is not substantial in chronic SCI74. Because of the controversial reports on the extent of patient responses to BM-MSC therapies, the efficacy of BM-MSCs must be further confirmed75,76. Several trials are ongoing, and completion of these studies will provide needed information to initiate a larger investigation of the efficacy of BM-MSC therapies. Overall, BM-MSC therapy is beneficial for SCI recovery by improving the microenvironment of the injury site, enhancing nutritional support, modulating the inflammatory response, and alleviating BSCB leakage. Patients avoid immunoreaction by receiving autologous cell transplantation. Therefore, BM-MSCs have huge potential for SCI treatment due to their reduced immunogenicity and improved availability. However, the therapeutic effects, homing ability, survival, and proliferation of single-cell types are limited. Further studies should focus on these aspects and combinational therapy to improve the efficacy of BM-MSCs.
Umbilical MSCs
Recent studies investigated MSCs separated from umbilical cords and adipose tissue77,78. U-MSCs possess the ability to develop into a homogeneous population that expresses neural markers and develops neural phenotypic features79. An early study found that U-MSCs migrated into the injury site but not noninjured areas after transplantation80, which lays the foundation for their therapeutic effects. Previous studies demonstrated that U-MSCs protected neurons from apoptosis22, inhibited the formation of glial scars via regulation of MMP223, attenuated ischemic compromise of the spinal cord25, decreased reactive astrocytes24, improved motor function, and alleviated allodynia and hyperalgesia after SCI in animal experiments26–28. U-MSCs demonstrated a better effect for a wide dynamic range of neurons than BM-MSCs28. Park and colleagues found that transplanted U-MSCs exhibited a better effect 1 week after SCI than at 12 h and 2 weeks, which indicates a potential time point for the treatment of SCI81.
Wnt proteins are involved in neural precursor (NP) differentiation and axon development, and Wnt-3a plays important roles in spinal cord dorsal interneuron differentiation. To enhance the efficacy of U-MSCs, researchers established Wnt3a-secreting U-MSCs by gene modification, which showed a better therapeutic effect than primary U-MSCs in SCI rats. Rats that received Wnt3a-MSCs had increased motor function scores and elevated expression of axonal regeneration-related proteins, including choline acetyltransferase, growth-associated protein 43, and microtubule-associated protein 282. Cotransplantation may complement and synergize to improve single-cell therapies83. The cotransplantation of human U-MSCs and human NSCs exhibited the best efficacy compared to that of transplantation of hU-MSCs or hNSCs alone84.
U-MSCs improved motor function in the lower limb and expanded the atrophied spinal cord after injection into the subarachnoid, intradural, or extradural space of the spinal cord in patients with compressed fractures85. After U-MSC transplantation, 7 of 10 patients with thoracolumbar SCI had obvious improvements in movement, muscle tension, bladder function, and urine control compared to those of patients who received rehabilitation therapy alone29. The somatosensory-evoked potential (SSEP) and clinical manifestations of neuropathic pain of a patient with 2-year complete cervical SCI were significantly improved and alleviated 1 year after U-MSC transplantation, and the physiological function of myelinated large fibers was reflected by the SSEP30. U-MSCs are conveniently obtained because the umbilical cord is generally discarded. U-MSCs are obtained from umbilical blood, perivascular regions, and the umbilical vein subendothelium without ethical issues, and these cells are beneficial in the recovery of SCI via different mechanisms24. Further efforts are needed to fully assess the effectiveness of UC-MSC transplantation.
Adipose-derived MSCs
AD-MSCs and BM-MSCS share some similarities, such as morphology and cell surface antigen expression, but they differ in proliferation rates and multilineage capabilities86. Adipose tissue contains more somatic stem cells than bone marrow, which makes AD-MSCs a good candidate for MSCs, especially with adipose tissue availability87,88.
AD-MSC transplantation demonstrated satisfactory effects in chronic and acute SCI. Intravenous administration of AD-MSCs activates angiogenesis and upregulates ERK and Akt, which improves hindlimb motor function37. AD-MSCs also promote cell survival and tissue repair by increasing the expression of beta3-tubulin, BDNF, and ciliary neurotrophic factor (CNTF)35. AD-MSCs may protect neurons and ameliorate erectile dysfunction in rats with SCI31–34.
In addition to the direct effects, human adipose-derived stem cells transdifferentiate into neuron/motoneuron-like cells, which reduce the formation of cavities and suppress immune activity via the inhibition of astrocyte reactivation and secretion of anti-inflammatory factors36. Hypoxic preconditioning-treated AD-MSCs promoted cell survival and increased the expression of marker genes in DsRed-engineered neural stem cells, which enhanced the effect of the combined treatment of stem cells and gene therapy for SCI89.
Although AD-MSCs transplantation has been investigated in animal SCI models, large longitudinal clinical trials using stem cells derived from adipose tissue are lacking. Early studies investigating the safety of intravenous AD-MSCs showed no tumorigenicity or other adverse side effects. One study investigated the effects of autologous transplantation of AD-MSCs in 14 patients with SCI who underwent intrathecal transplantation. ASIA sensory and motor scores and electrophysiological evaluations, including MRI and electromyography, were used to determine the effect. After the intervention, 10 patients showed sensory improvement, but the size of the lesion visualized using MRI remained stable. None of the patients treated with AD-MSCs had serious adverse events90. Some barriers should be elucidated before clinical translation, such as standard protocols of cell generation, cell characteristics, and clear disclosure of the underlying mechanism, and larger experimental animals that are closer to humans should be used.
NSCs and NPCs
NSCs and NPCs are pluripotent cells that are isolated from the subventricular region of the ventricles and hippocampus of the brain and the ependymal region of the central canal of the spinal cord1,91–96. These cells are capable of differentiating into specific neuronal or glial cells, enhancing remyelination and providing nutritional support, which makes them suitable for cell transplantation therapy in SCI38.
NPCs primarily differentiate into oligodendrocytes97,98, increase myelination40, and improve hindlimb function. One study also demonstrated that transplantation of NPCs obtained from the subventricular zone promoted respiratory recovery after SCI, which did not work by differentiation42. NPC transplantation increased the expression of NGF, CNTF, BDNF, IGF-1, and GDNF, which are beneficial for SCI recovery39. NPCs also modulate the inflammatory response41 via inhibition of the secretion of reactive macrophages and T cells and neuroprotective cytokines99. Previous studies revealed that the transplantation of NPCs during the acute stage demonstrated better efficacy than during the subacute and chronic stages100, and transplantation in intact soft tissue may produce better efficacy than transplantation in the injury site during the subacute period101.
Modified NSCs may exhibit better therapeutic efficacy than naïve cells. Inhibition of leucine-rich repeat and immunoglobulin domain-containing protein (LINGO)-1 in NSCs facilitated neuronal differentiation and recovery in SCI rats102. Transplantation of recombinant NSCs with VEGF reduced transient receptor potential vanilloid (TRPV1), increased the release of neurotrophic factors, and promoted neuronal recovery103. NSCs with high expression of E-cadherin, a transmembrane adhesion protein, increased the survival of NSCs, decreased the release of inflammatory factors, and promoted functional recovery104. Overexpression of the antiapoptotic gene Bcl-XL105, upregulation of miR-124106, upregulation of NT-3107, or polarization toward a more oligodendrogenic fate108 also achieved better recovery. Mild hypothermia109 or hypoxia pretreated110 of NSCs showed a more favorable effect on SCI than untreated NSCs by improving cell proliferation and upregulating neurotrophic and growth factors. Combined with MSCs111, SCs112 and OECs113 also enhanced neuronal differentiation and cell survival, which further improved motor recovery.
A 2018 study demonstrated that perilesional intramedullary injections of NSCs were safe, but the dose should be verified114. Twelve amyotrophic lateral sclerosis patients received transplantation of human spinal cord–derived NPCs, and the results showed that NPC transplantation was safe, which initiated further clinical trials115,116. NPCs showed great potential for SCI treatment, but the functional recovery was limited. Quintessential combinational methods have raised much hope to enhance the efficacy of NPCs. However, rodents were generally used as subjects in previous studies, and some specific larger animals that are closer to humans should be used as experimental subjects to address the problems and move toward clinical translation117.
Embryonic Stem Cells
ESCs are multipotent stem cells that are capable of differentiating into new cell types in the body. ESCs differentiate into neurons and glial cells to replace nonfunctional cells or tissues in SCI118,119. However, their undifferentiated form is rarely used due to the risk of tumorigenicity. Previous studies demonstrated that ESC transplantation was effective for SCI recovery120–122. ESCs transfected with cell adhesion molecule L1, which promotes neuronal survival and neurite sprouting, had promising potential for SCI treatment122.
ESC-derived definitive neural stem cells express myelin basic protein46,123, support nodal architecture, and display multilayer myelination in SCI animal models46,47. Human embryonic stem cell–derived oligodendrocytes43,124 or oligodendrocyte progenitor cells43,44 and motoneuron progenitors promote astrogliosis and enhance motor recovery. ESC-derived neural lineage cells enable axons to pass through chondroitin sulfate proteoglycan (CSPG), which is a tremendous barrier to axonal regeneration, and exhibit therapeutic potential for SCI treatment. The expression of nerve glial antigen 2 and MMP945 is involved in this process. Transplantation of GABAergic neurons derived from mouse ESCs attenuated neuropathic pain and increased the paw withdrawal threshold and vocalization threshold48.
A clinical study in 2014 showed that human ESC-derived oligodendrocyte progenitor cell transplantation was safe for SCI patients125,126. Another two studies in 2016 demonstrated that SCI patients had restored body functions after intervention with human ESCs127, and there were no serious complications. However, the pluripotency of ESCs may result in tumor formation due to their considerable proliferative ability. There may be genetic changes during the cell culture process128. Therefore, it is critical to optimize the differentiation protocol to decrease tumor occurrence and control cell populations to match the different recovery requirements in SCI patients129.
Induced Pluripotent Stem Cells
There is significant controversy about ESCs due to their origin. iPSCs, which share the same pluripotent characteristics as ESCs, may neutralize this problem. iPSCs are generated from reprogrammed somatic cells12,130–132, which are separated from accessible tissue, such as autologous skin, which avoids ethical issues, allows autologous cell transplantation, and prevents rejection.
NPs derived from a clone of human iPSCs led to restoration of the injury site133. IPSCs-derived neural stem/progenitor cells (iPSC-NS/PCs) inhibited demyelination51,52 and promoted synapse formation53 and neurotrophic factor secretion, which improved functional recovery in common marmosets after SCI without tumor formation49. Researchers found that only spinal cord-type NPCs from human iPSCs exhibited efficacy, compared to that with forebrain-type NPCs from human iPSCs, which indicates the importance of the regional identity134. A comparative study demonstrated that iPSC-NPs exhibited the best effect due to their strong graft survival, glial scar inhibition, and axonal sprouting enhancement compared to those of BM-MSCs and NPs derived from an immortalized spinal fetal cell line (SPC-01)50. Different transplantation regions may lead to different effects, and researchers found that intraspinal implantation (cells present in the tissue) may produce better long-term efficacy than intrathecal implantation (paracrine only mechanism)135.
Modified human iPSC-derived astrocytes reduced lesion size and morphological denervation of respiratory phrenic motor neurons and improved respiratory function54. Similarly, γ-secretase inhibitors promoted iPSC-derived NPCs maturation and increased neuronal commitment via regulation of the NOTCH signaling pathway136.
A case report demonstrated that NSCs derived from iPSCs obtained from a healthy 86-year-old male differentiated into neurons and glia, and axons extended long distances and formed synapses after cell transplantation137. Another study suggested that the iCaspase9 gene alleviated adverse events after iPSC-derivative transplantation138. Another study demonstrated that hydrogels modified with an RGD peptide and platelet-derived growth factor (PDGF-A) promoted cell survival and differentiation and reduced teratoma formation139. However, there are opposite results that human iPSC-derived NPCs do not provide beneficial results for SCI therapy. Some of these studies had limitations with graft survival or time to transplant140,141. The tumorigenesis of iPSCs and the prohibitively high cost–benefit for developing treatments142 hinder the clinical translation143. It is crucial to develop optimized solutions, including standard protocols for collecting cells, the ideal time for cell delivery, and the safe and effective routes of administration in clinical treatment.
EVs Derived From Stem Cells
EVs have come into the spotlight in recent years because of their satisfying therapeutic potential. They are small vesicles (100–1,000 nm) secreted from a variety of cells and have a lipid bilayer membrane. EVs work as cell communication messengers by carrying nucleic acids, proteins, and lipids144,145. EVs are not a single type of vesicle but consist of ectosomes, microvesicles, and exosomes. Exosomes, with diameters of 50–150 nm, are remarkable carriers with low immunogenicity and high biocompatibility146, which protect their cargo from degradation and maintain their biological activity147.
EVs exhibit robust chemotaxis to the injury site and cooperate with neurons. Recent studies reported that MSC-57 and NSC-derived55 EVs inhibited inflammation, protected cells from apoptosis and reduced injury size, and the mechanism may involve autophagy55 and the microRNA-21-5p/FasL gene axis58. Lankford et al. found that exosomes accumulated in the injury sites of the spinal cord and spleen after IV injection148,149. Other studies demonstrated that exosomes derived from BM-MSCs were primarily incorporated in microglial cells, downregulated nuclear factor kappa-B150, protected the integrity of the BSCB49, inhibited the activation of A1 astrocytes56, and played a protective role in rats after SCI.
Exosomes derived from gene-modified stem cells showed more therapeutic potential than exosomes derived from native stem cells. For example, exosomes derived from miR-133b-modified adipose-derived stem cells regulated axon regeneration and improved neurological function after SCI55. Phosphatase and tensin homolog (PTEN) exists in neurons and axons, and it plays an inhibitory role in the growth of axons. Therefore, suppression of PTEN in MSC-derived exosomes showed desirable therapeutic effects on SCI151,152. Similarly, the downregulated expression of phosphatase and tensin homolog pseudogene 1 (PTENP1) in exosomes derived from differentiated P12 cells and MSCs promoted neuronal survival and functional recovery by regulating the expression of miR-19b and miR-21153. There was an obvious decrease in miR-544 expression after SCI, and exosomes derived from miR-445-modified MSCs improved functional recovery in rats after SCI154. MiR-126 loaded in MSC-derived exosomes enhanced angiogenesis, inhibited inflammation, and had an encouraging effect on SCI155. Similarly, miR-21 deficiency in exosomes derived from MSCs also displayed desirable effects156. Iron oxide nanoparticles (IONPs) carried by exosome-mimetic nanovesicles (NVs), which were derived from IONP-treated MSCs, enhanced NV homing capacity and further promoted the therapeutic potential of NVs in SCI157. Since few studies demonstrated the pathophysiology of EVs in SCI, further studies are needed to identify the molecular mechanism and related signaling pathways of the therapeutic effects of EVs. Some nontargeted EVs have also been reported158, and normalizing the isolation and acquisition of EVs is paramount before translating this therapeutic method to SCI patients clinically159.
Other Combinatorial Methods
Neuroprotection
Neuroprotective drugs aim to minimize pathological damage and preserve neural tissue. Only methylprednisolone has been clinically proven to provide benefits post-SCI, but it also brings some risks, including gastrointestinal bleeding, wound infection, and thromboembolism160. However, increasingly promising neuroprotective interventions are under investigation (e.g., chondroitinase161,162, alginate scaffolds163, TNF-α antagonists, anti-Nogo antibodies, minocycline, and Lavandula angustifolia extract164). These interventions may be used before or during cell transplantation to create a microenvironment that improves stem cell efficacy. Therapeutic hypothermia in combination with cell therapy has been successfully used for neuroprotection. Hypothermia lowers the basal metabolic rate and reduces inflammation to provide synergistic action in SCI165. Minocycline synergistically improved the anti-inflammatory effects of MSCs166. Although the initial results are encouraging, additional work is needed to optimize the efficacy of combination treatment. The combination of BM-MSC transplantation and propofol injection effectively improved neuroprotection167, increased horseradish peroxidase-positive nerve fibers, and shortened the latencies of SSEPs and motor-evoked potentials in the hindlimb168. Zhang et al. injected NSCs into the tibial nerve and investigated the effect of lithium chloride (LiCl) on the survival of neurons and axons. They found that LiCl promoted NSC differentiation, and this combinational therapy increased the regeneration of axons in the tibial nerve and decreased the formation of glial scars169. Electroacupuncture170, folic acid, substance P171, and granulocyte-macrophage colony-stimulating factor172 also exhibited synergetic effects by improving NSC proliferation and neuron survival in SCI rats.
Biomaterials
Although stem cell therapy has gained momentum in the field of SCI therapy, it has room for improvement. Biological material use is an encouraging approach for cell therapy by bridging the lesion cavity, replacing damaged extracellular matrices, and integrating the host tissue and transplanted cells. Matrigel is primarily composed of laminin, collagen IV, heparan sulfate proteoglycans, and growth factors that support cell survival and differentiation173; increase neuronal markers; decrease fibrosis, astrogliosis markers, and inflammatory factors174; and enhance behavioral recovery in SCI animals. Hydrogels possess a three-dimensional (3D) network structure that provides the benefits regarding electrostatic forces, steric hindrance, and entanglement. These gels are injected or implanted directly because of their soft texture. Laminin-coated hydrogel enhanced the viability of IPSC-NPs and promoted host axon and astrocyte growth in the lesion site175. Ischemia and hypoxia following the primary injury may exacerbate the pathological process of SCI and extremely impede functional recovery after SCI. To address the ischemia and hypoxia in SCI, prevascularized nerve conduits based on the stem cell sheet were designed and implanted in the injury spinal cord, which exhibited satisfactory potential176. Self-assembling peptides form 3D nanofibers via self-assembly after direct injection to the injury site, act as structural framework, and regulate the microenvironment. The use of NPCs with the self-assembling peptide QL6 reduced cystic cavity formation and inflammation and enhanced synaptic connections by reducing astrogliosis and CSPG, which improved forelimb function in a cervical injury SCI model177. A previous study reported that chondroitinase ABC (ChABC) enhanced the therapeutic effect of NPCs in SCI, but the ChABC delivery efficiency was unsatisfactory. Nori et al. manufactured NPCs biased toward an oligodendrogenic fate and upgraded the ChABC delivery system via a crosslinked methylcellulose biomaterial, and this combinatorial therapy promoted oligodendrocyte differentiation, remyelination, and synaptic connectivity178. An N-cadherin-modified linearly ordered collagen scaffold promoted the migration and differentiation of endogenous neural/progenitor stem cells and produced a desirable therapeutic effect in rats after SCI179. The collagen microchannel scaffold and paclitaxel-liposome combination induced neuronal differentiation of NSCs and growth of neurons and axons, which exhibited great potential for SCI treatment180. Other scaffolds, such as silk fibroin combined with neurotrophic factors181,182, fibrin scaffolds containing growth factors183, polycistronic delivery of IL-10 and NT-3184, also promoted the differentiation, proliferation, and viability of transplanted cells, which has desirable therapeutic potential for SCI treatment.
Many kinds of biomaterial scaffolds have been used to deliver MSCs to damaged spinal cords. Unlike NPCs, MSCs likely provide nutritional support, promote axonal regeneration and angiogenesis, and reduce inflammation. Modified biodegradable chitin conduits in combination with BM-MSC transplantation improved the microenvironment for MSCs, prevented scar formation, and promoted recovery after right spinal cord hemisection injury185. Superparamagnetic iron oxide labeling of BM-MSCs coupled with magnetic guidance offers a promising avenue for the clinical treatment of SCI by enhancing the homing efficiency of cells186. AD-MSCs encapsulated in a fibrin matrix, which is a biopolymer that simulates the natural microenvironment, inhibited injury cavity expansion, increased tissue retention, and promoted recovery of function and structure187. However, some previous studies demonstrated that some biomaterials stimulated a disadvantageous microenvironment in the lesion site, such as a proinflammatory milieu188. Other tissue engineering scaffolds, such as acellular spinal cord scaffolds189, polycaprolactone190, 3D gelatin methacrylate hydrogels191, and 3D fibrin-based scaffolds192, enhanced axonal regeneration and tissue remodeling and improved the therapeutic effect of stem cells. In general, the use of biological materials is a promising combination approach for SCI cell therapy by improving cell implantation, delivering certain factors, promoting neural marker expression and axonal regeneration, inhibiting the inflammatory response, and contacting the injured central nervous system (CNS) tissue.
Challenges and Prospects
Stem cells have neuroregenerative and neuroprotective effects in SCI cell therapy. Cell-based therapies in SCI have different mechanisms in functional recovery, such as immunomodulation, cell replacement nutrition, and scaffold support. However, stem cell therapies present particular safety concerns. First, cell therapy–related immunotoxicity, immunogenicity, and tumorigenicity are often discussed in preclinical studies. Second, limited cell survival and limited integration were common obstacles in previous studies with different experimental designs, including cell number, timing of treatment193, and strategies of transplantation194. Third, it is important to ensure the genetic stability, generation consistency, and storage safety162 of stem cells195. The quality and repeatability of stem cell transplantation are critical to clinical translation. Small differences in cell origin and growth conditions may have a significant impact on the outcomes196,197. Fourth, the mechanism of the effects and biological properties should be further investigated to guide the clinical application187. Finally, small sample size, limited supervision, and poor quality are the common problems of most registered clinical trials that hinder the development of stem cell therapy198. Standard protocols are difficult to confirm due to the heterogeneity of the injury type and level, the particular time of treatment, and the different number of transplanted cells.
Encouraging preclinical studies, coupled with publicity, led to early clinical deployment, but the results were mixed. One specific type of stem cell achieves only a limited therapeutic effect. Therefore, many researchers are committed to enhancing the efficacy of stem cells. The use of genetic engineering technology, cell coupling, combinational therapy with neuroprotective agents, trophic factors, biomaterials, and rehabilitation may help improve the therapeutic effectiveness of stem cells in heterogeneous patient populations. Research is needed to optimize their use.
Conclusion
Although cell therapy offers important promise for SCI treatment, there are many obstacles to clinical translation. These obstacles include suitable cell types and sources, cell survival, quality and repeatability of stem cells and optimal transplantation dosage and timing. There are endogenous differences between experimental animals and humans, and much work should be completed before clinical transformation. Each type of stem cell has unique benefits. Previous studies already focused on how to enhance the efficacy of stem cells and made positive achievements. Future treatments may use a variety of novel strategies to address the problems of SCI.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Liyi Huang  https://orcid.org/0000-0001-7888-9186
https://orcid.org/0000-0001-7888-9186
References
- 1. Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current options for cell therapy in spinal cord injury. Trends Mol Med. 2017;23(9):831–849. [DOI] [PubMed] [Google Scholar]
- 2. Bhat IA, Sivanarayanan TB, Somal A, Pandey S, Bharti MK, Panda BS, Indu B, Verma M, Anand J, Sonwane A, Kumar GS, et al. An allogenic therapeutic strategy for canine spinal cord injury using mesenchymal stem cells. J Cell Physiol. 2019;234(3):2705–2718. [DOI] [PubMed] [Google Scholar]
- 3. Center NSCIS. Spinal cord injury (SCI) 2016 facts and figures at a glance. J Spinal Cord Med. 2016;39(4):493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dvorak MF, Noonan VK, Fallah N, Fisher CG, Finkelstein J, Kwon BK, Rivers CS, Ahn H, Paquet J, Tsai EC, Townson A, et al. The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: an observational Canadian cohort study. J Neurotrauma. 2015;32(9):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson JR, Forgione N, Fehlings MG. Emerging therapies for acute traumatic spinal cord injury. CMAJ. 2013;185(6):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu D, Li X, Xiao Z, Yin W, Zhao Y, Tan J, Chen B, Jiang X, Dai J. Different functional bio-scaffolds share similar neurological mechanism to promote locomotor recovery of canines with complete spinal cord injury. Biomaterials. 2019;214:119230. [DOI] [PubMed] [Google Scholar]
- 7. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15–26. [DOI] [PubMed] [Google Scholar]
- 8. Fehlings MG, Hawryluk GW. Scarring after spinal cord injury. J Neurosurg Spine. 2010;13(2):165–167.discussion 167–168. [DOI] [PubMed] [Google Scholar]
- 9. Kanno H, Pearse DD, Ozawa H, Itoi E, Bunge MB. Schwann cell transplantation for spinal cord injury repair: its significant therapeutic potential and prospectus. Rev Neurosci. 2015;26(2):121–128. [DOI] [PubMed] [Google Scholar]
- 10. Li L, Adnan H, Xu B, Wang J, Wang C, Li F, Tang K. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: a systematic review and meta-analysis. Eur Spine J. 2015;24(5):919–930. [DOI] [PubMed] [Google Scholar]
- 11. Oliveri RS, Bello S, Biering-Sorensen F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: systematic review with meta-analyses of rat models. Neurobiol Dis. 2014;62:338–353. [DOI] [PubMed] [Google Scholar]
- 12. Khazaei M, Siddiqui AM, Fehlings MG. The Potential for ips-derived stem cells as a therapeutic strategy for spinal cord injury: opportunities and challenges. J Clin Med. 2014;4(1):37–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stenudd M, Sabelstrom H, Frisen J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015;72(2):235–237. [DOI] [PubMed] [Google Scholar]
- 14. Cizkova D, Rosocha J, Vanicky I, Jergova S, Cizek M. Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol. 2006;26(7–8):1167–1180. [DOI] [PubMed] [Google Scholar]
- 15. Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matyas JJ, Stewart AN, Goldsmith A, Nan Z, Skeel RL, Rossignol J, Dunbar GL. Effects of bone-marrow-derived msc transplantation on functional recovery in a rat model of spinal cord injury: comparisons of transplant locations and cell concentrations. Cell Transplant. 2017;26(8):1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han D, Wu C, Xiong Q, Zhou L, Tian Y. Anti-inflammatory mechanism of bone marrow mesenchymal stem cell transplantation in rat model of spinal cord injury. Cell Biochem Biophys. 2015;71(3):1341–1347. [DOI] [PubMed] [Google Scholar]
- 18. Matsushita T, Lankford KL, Arroyo EJ, Sasaki M, Neyazi M, Radtke C, Kocsis JD. Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp Neurol. 2015;267:152–164. [DOI] [PubMed] [Google Scholar]
- 19. Tsai MJ, Liou DY, Lin YR, Weng CF, Huang MC, Huang WC, Tseng FW, Cheng H. Attenuating spinal cord injury by conditioned medium from bone marrow mesenchymal stem cells. J Clin Med. 2018;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kakabadze Z, Kipshidze N, Mardaleishvili K, Chutkerashvili G, Chelishvili I, Harders A, Loladze G, Shatirishvili G, Kipshidze N, Chakhunashvili D, Chutkerashvili K. Phase 1 trial of autologous bone marrow stem cell transplantation in patients with spinal cord injury. Stem Cells Int. 2016;2016:6768274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park JH, Kim DY, Sung IY, Choi GH, Jeon MH, Kim KK, Jeon SR. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70(5):1238–1247,discussion 1247. [DOI] [PubMed] [Google Scholar]
- 22. Dasari VR, Veeravalli KK, Tsung AJ, Gondi CS, Gujrati M, Dinh DH, Rao JS. Neuronal apoptosis is inhibited by cord blood stem cells after spinal cord injury. J Neurotrauma. 2009;26(11):2057–2069. [DOI] [PubMed] [Google Scholar]
- 23. Veeravalli KK, Dasari VR, Tsung AJ, Dinh DH, Gujrati M, Fassett D, Rao JS. Human umbilical cord blood stem cells upregulate matrix metalloproteinase-2 in rats after spinal cord injury. Neurobiol Dis. 2009;36(1):200–212. [DOI] [PubMed] [Google Scholar]
- 24. Hu SL, Luo HS, Li JT, Xia YZ, Li L, Zhang LJ, Meng H, Cui GY, Chen Z, Wu N, Lin JK, et al. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit Care Med. 2010;38(11):2181–2189. [DOI] [PubMed] [Google Scholar]
- 25. Judas GI, Ferreira SG, Simas R, Sannomiya P, Benicio A, da Silva LF, Moreira LF. Intrathecal injection of human umbilical cord blood stem cells attenuates spinal cord ischaemic compromise in rats. Interact Cardiovasc Thorac Surg. 2014;18(6):757–762. [DOI] [PubMed] [Google Scholar]
- 26. Cui B, Li E, Yang B, Wang B. Human umbilical cord blood-derived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp Ther Med. 2014;7(5):1233–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dasari VR, Spomar DG, Li L, Gujrati M, Rao JS, Dinh DH. Umbilical cord blood stem cell mediated downregulation of fas improves functional recovery of rats after spinal cord injury. Neurochem Res. 2008;33(1):134–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaner T, Karadag T, Cirak B, Erken HA, Karabulut A, Kiroglu Y, Akkaya S, Acar F, Coskun E, Genc O, Colakoglu N. The effects of human umbilical cord blood transplantation in rats with experimentally induced spinal cord injury. J Neurosurg Spine. 2010;13(4):543–551. [DOI] [PubMed] [Google Scholar]
- 29. Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hua R, Li P, Wang X, Yang J, Zheng P, Niu X, Li Y, An Y. Evaluation of somatosensory evoked potential and pain rating index in a patient with spinal cord injury accepted cell therapy. Pain Physician. 2016;19(4):E659–E666. [PubMed] [Google Scholar]
- 31. Albayrak O, Sener TE, Ersahin M, Ozbas-Turan S, Ekentok C, Tavukcu HH, Cevik O, Cetinel S, Ertas B, Sener G. Mesenchymal stem cell therapy improves erectile dysfunction in experimental spinal cord injury. Int J Impot Res. 2020;32(3):308–316. Epub 2019 Jul 4. [DOI] [PubMed] [Google Scholar]
- 32. Kim Y, Lee SH, Kim WH, Kweon OK. Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs. J Vet Sci. 2016;17(1):123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oh JS, Park IS, Kim KN, Yoon DH, Kim SH, Ha Y. Transplantation of an adipose stem cell cluster in a spinal cord injury. Neuroreport. 2012;23(5):277–282. [DOI] [PubMed] [Google Scholar]
- 34. Escalhao CCM, Ramos IP, Hochman-Mendez C, Brunswick THK, Souza SAL, Gutfilen B, Dos Santos Goldenberg RC, Coelho-Sampaio T. Safety of allogeneic canine adipose tissue-derived mesenchymal stem cell intraspinal transplantation in dogs with chronic spinal cord injury. Stem Cells Int. 2017;2017:3053759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aras Y, Sabanci PA, Kabatas S, Duruksu G, Subasi C, Erguven M, Karaoz E. The Effects of adipose tissue-derived mesenchymal stem cell transplantation during the acute and subacute phases following spinal cord injury. Turk Neurosurg. 2016;26(1):127–139. [DOI] [PubMed] [Google Scholar]
- 36. Gao S, Guo X, Zhao S, Jin Y, Zhou F, Yuan P, Cao L, Wang J, Qiu Y, Sun C, Kang Z, et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019;10(8):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohta Y, Hamaguchi A, Ootaki M, Watanabe M, Takeba Y, Iiri T, Matsumoto N, Takenaga M. Intravenous infusion of adipose-derived stem/stromal cells improves functional recovery of rats with spinal cord injury. Cytotherapy. 2017;19(7):839–848. [DOI] [PubMed] [Google Scholar]
- 38. Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 2011;28(8):1611–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–129. [DOI] [PubMed] [Google Scholar]
- 40. Amemori T, Romanyuk N, Jendelova P, Herynek V, Turnovcova K, Prochazka P, Kapcalova M, Cocks G, Price J, Sykova E. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res Ther. 2013;4(3):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sankavaram SR, Hakim R, Covacu R, Frostell A, Neumann S, Svensson M, Brundin L. Adult neural progenitor cells transplanted into spinal cord injury differentiate into oligodendrocytes, enhance myelination, and contribute to recovery. Stem Cell Reports. 2019;12(5):950–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandhu MS, Ross HH, Lee KZ, Ormerod BK, Reier PJ, Fuller DD. Intraspinal transplantation of subventricular zone-derived neural progenitor cells improves phrenic motor output after high cervical spinal cord injury. Exp Neurol. 2017;287(Pt 2):205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cloutier F, Siegenthaler MM, Nistor G, Keirstead HS. Transplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regen Med. 2006;1(4):469–479. [DOI] [PubMed] [Google Scholar]
- 44. Kerr CL, Letzen BS, Hill CM, Agrawal G, Thakor NV, Sterneckert JL, Gearhart JD, All AH. Efficient differentiation of human embryonic stem cells into oligodendrocyte progenitors for application in a rat contusion model of spinal cord injury. Int J Neurosci. 2010;120(4):305–313. [DOI] [PubMed] [Google Scholar]
- 45. Vadivelu S, Stewart TJ, Qu Y, Horn K, Liu S, Li Q, Silver J, McDonald JW. NG2+ progenitors derived from embryonic stem cells penetrate glial scar and promote axonal outgrowth into white matter after spinal cord injury. Stem Cells Transl Med. 2015;4(4):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salewski RP, Mitchell RA, Shen C, Fehlings MG. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015;24(1):36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iwai H, Shimada H, Nishimura S, Kobayashi Y, Itakura G, Hori K, Hikishima K, Ebise H, Negishi N, Shibata S Habu S, et al. Allogeneic neural stem/progenitor cells derived from embryonic stem cells promote functional recovery after transplantation into injured spinal cord of nonhuman primates. Stem Cells Transl Med 2015;4(7):708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hwang I, Hahm SC, Choi KA, Park SH, Jeong H, Yea JH, Kim J, Hong S. Intrathecal transplantation of embryonic stem cell-derived spinal GABAergic neural precursor cells attenuates neuropathic pain in a spinal cord injury rat model. Cell Transplant. 2016;25(3):593–607. [DOI] [PubMed] [Google Scholar]
- 49. Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7(12):e52787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruzicka J, Machova-Urdzikova L, Gillick J, Amemori T, Romanyuk N, Karova K, Zaviskova K, Dubisova J, Kubinova S, Murali R, Sykova E, et al. A comparative study of three different types of stem cells for treatment of rat spinal cord injury. Cell Transplant 2017;26(4):585–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Salewski RP, Buttigieg J, Mitchell RA, van der Kooy D, Nagy A, Fehlings MG. The generation of definitive neural stem cells from PiggyBac transposon-induced pluripotent stem cells can be enhanced by induction of the NOTCH signaling pathway. Stem Cells Dev. 2013;22(3):383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salewski RP, Mitchell RA, Li L, Shen C, Milekovskaia M, Nagy A, Fehlings MG. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Transl Med. 2015;4(7):743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, Sugai K, Nishimura S, Iwai H, Isoda M, Shibata S, et al. Grafted human ips cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Reports. 2016;6(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li K, Javed E, Scura D, Hala TJ, Seetharam S, Falnikar A, Richard JP, Chorath A, Maragakis NJ, Wright MC, Lepore AC. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp Neurol. 2015;271:479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rong Y, Liu W, Wang J, Fan J, Luo Y, Li L, Kong F, Chen J, Tang P, Cai W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10(5):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, Zhou Z, Zhou Z, Xu T, Jiang T, Yang S. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of a1 neurotoxic reactive astrocytes. J Neurotrauma 2019;36(3):469–484. [DOI] [PubMed] [Google Scholar]
- 57. Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. [DOI] [PubMed] [Google Scholar]
- 58. Zhou X, Chu X, Yuan H, Qiu J, Zhao C, Xin D, Li T, Ma W, Wang H, Wang Z, Wang D. Mesenchymal stem cell derived EVs mediate neuroprotection after spinal cord injury in rats via the microRNA-21-5p/FasL gene axis. Biomed Pharmacother. 2019;115:108818. [DOI] [PubMed] [Google Scholar]
- 59. Lu Y, Zhou Y, Zhang R, Wen L, Wu K, Li Y, Yao Y, Duan R, Jia Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front Neurosci. 2019;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21(9):1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim C, Kim HJ, Lee H, Lee H, Lee SJ, Lee ST, Yang SR, Chung CK. Mesenchymal stem cell transplantation promotes functional recovery through MMP2/STAT3 related astrogliosis after spinal cord injury. Int J Stem Cells. 2019;12(2):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–580. [DOI] [PubMed] [Google Scholar]
- 63. Liu F, Xuan A, Chen Y, Zhang J, Xu L, Yan Q, Long D. Combined effect of nerve growth factor and brainderived neurotrophic factor on neuronal differentiation of neural stem cells and the potential molecular mechanisms. Mol Med Rep. 2014;10(4):1739–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dooley D, Lemmens E, Vangansewinkel T, Le Blon D, Hoornaert C, Ponsaerts P, Hendrix S. Cell-Based delivery of interleukin-13 directs alternative activation of macrophages resulting in improved functional outcome after spinal cord injury. Stem Cell Reports. 2016;7(6):1099–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Allahdadi KJ, de Santana TA, Santos GC, Azevedo CM, Mota RA, Nonaka CK, Silva DN, Valim CXR, Figueira CP, Dos Santos WLC, do Espirito Santo RF, et al. IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res Ther. 2019;10(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Song JL, Zheng W, Chen W, Qian Y, Ouyang YM, Fan CY. Lentivirus-mediated microRNA-124 gene-modified bone marrow mesenchymal stem cell transplantation promotes the repair of spinal cord injury in rats. Exp Mol Med. 2017;49(5):e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li Z, Zhang Z, Zhao L, Li H, Wang S, Shen Y. Bone marrow mesenchymal stem cells with Nogo-66 receptor gene silencing for repair of spinal cord injury. Neural Regen Res. 2014;9(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peng RJ, Jiang B, Ding XP, Huang H, Liao YW, Peng G, Cheng Q, Xi J. Effect of TNF-alpha inhibition on bone marrow-derived mesenchymal stem cells in neurological function recovery after spinal cord injury via the wnt signaling pathway in a rat model. Cell Physiol Biochem. 2017;42(2):743–752. [DOI] [PubMed] [Google Scholar]
- 69. Stewart AN, Kendziorski G, Deak ZM, Bartosek NC, Rezmer BE, Jenrow K, Rossignol J, Dunbar GL. Transplantation of mesenchymal stem cells that overexpress NT-3 produce motor improvements without axonal regeneration following complete spinal cord transections in rats. Brain Res. 2018;1699:19–33. [DOI] [PubMed] [Google Scholar]
- 70. Stewart AN, Matyas JJ, Welchko RM, Goldsmith AD, Zeiler SE, Hochgeschwender U, Lu M, Nan Z, Rossignol J, Dunbar GL. SDF-1 overexpression by mesenchymal stem cells enhances GAP-43-positive axonal growth following spinal cord injury. Restor Neurol Neurosci. 2017;35(4):395–411. [DOI] [PubMed] [Google Scholar]
- 71. Shahrezaie M, Mansour RN, Nazari B, Hassannia H, Hosseini F, Mahboudi H, Eftekhary M, Kehtari M, Veshkini A, Ahmadi Vasmehjani A, Enderami SE. Improved stem cell therapy of spinal cord injury using GDNF-overexpressed bone marrow stem cells in a rat model. Biologicals. 2017;50:73–80. [DOI] [PubMed] [Google Scholar]
- 72. Oraee-Yazdani S, Hafizi M, Atashi A, Ashrafi F, Seddighi AS, Hashemi SM, Seddighi A, Soleimani M, Zali A. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: safety and possible outcome. Spinal Cord. 2016;54(2):102–109. [DOI] [PubMed] [Google Scholar]
- 73. Ge L, Liu K, Liu Z, Lu M. Co-transplantation of autologous OM-MSCs and OM-OECs: a novel approach for spinal cord injury. Rev Neurosci. 2016;27(3):259–270. [DOI] [PubMed] [Google Scholar]
- 74. Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A Phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 2016;78(3):436–447; discussion 447. [DOI] [PubMed] [Google Scholar]
- 75. Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, Park HS. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11(5–6):913–922. [DOI] [PubMed] [Google Scholar]
- 76. Sykova E, Homola A, Mazanec R, Lachmann H, Konradova SL, Kobylka P, Padr R, Neuwirth J, Komrska V, Vavra V, Stulík J, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15(8–9):675–687. [DOI] [PubMed] [Google Scholar]
- 77. Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332(2):370–379. [DOI] [PubMed] [Google Scholar]
- 78. Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–1026. [PubMed] [Google Scholar]
- 79. Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7(11):581–588. [DOI] [PubMed] [Google Scholar]
- 80. Saporta S, Kim JJ, Willing AE, Fu ES, Davis CD, Sanberg PR. Human umbilical cord blood stem cells infusion in spinal cord injury: engraftment and beneficial influence on behavior. J Hematother Stem Cell Res. 2003;12(3):271–278. [DOI] [PubMed] [Google Scholar]
- 81. Park SS, Byeon YE, Ryu HH, Kang BJ, Kim Y, Kim WH, Kang KS, Han HJ, Kweon OK. Comparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotrophin-3. Cell Transplant. 2011;20(11–12):1867–1880. [DOI] [PubMed] [Google Scholar]
- 82. Seo DK, Kim JH, Min J, Yoon HH, Shin ES, Kim SW, Jeon SR. Enhanced axonal regeneration by transplanted Wnt3a-secreting human mesenchymal stem cells in a rat model of spinal cord injury. Acta Neurochir (Wien). 2017;159(5):947–957. [DOI] [PubMed] [Google Scholar]
- 83. Hu JG, Wang XF, Deng LX, Liu NK, Gao X, Chen JH, Zhou FC, Xu XM. Cotransplantation of glial restricted precursor cells and Schwann cells promotes functional recovery after spinal cord injury. Cell Transplant. 2013;22(12):2219–2236. [DOI] [PubMed] [Google Scholar]
- 84. Sun L, Wang F, Chen H, Liu D, Qu T, Li X, Xu D, Liu F, Yin Z, Chen Y. Co-Transplantation of human umbilical cord mesenchymal stem cells and human neural stem cells improves the outcome in rats with spinal cord injury. Cell Transplant. 2019;28(7):893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kang KS, Kim SW, Oh YH, Yu JW, Kim KY, Park HK, Song CH, Han H. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy. 2005;7(4):368–373. [DOI] [PubMed] [Google Scholar]
- 86. Danisovic L, Varga I, Polak S, Ulicna M, Hlavackova L, Bohmer D, Vojtassak J. Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. Gen Physiol Biophys. 2009;28(1):56–62. [PubMed] [Google Scholar]
- 87. De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. [DOI] [PubMed] [Google Scholar]
- 88. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. [DOI] [PubMed] [Google Scholar]
- 89. Oh JS, Ha Y, An SS, Khan M, Pennant WA, Kim HJ, Yoon DH, Lee M, Kim KN. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neurosci Lett. 2010;472(3):215–219. [DOI] [PubMed] [Google Scholar]
- 90. Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39(6):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Emgard M, Piao J, Aineskog H, Liu J, Calzarossa C, Odeberg J, Holmberg L, Samuelsson EB, Bezubik B, Vincent PH, Falci SP, et al. Neuroprotective effects of human spinal cord-derived neural precursor cells after transplantation to the injured spinal cord. Exp Neurol. 2014;253:138–145. [DOI] [PubMed] [Google Scholar]
- 92. Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. [DOI] [PubMed] [Google Scholar]
- 94. Parr AM, Kulbatski I, Zahir T, Wang X, Yue C, Keating A, Tator CH. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience. 2008;155(3):760–770. [DOI] [PubMed] [Google Scholar]
- 95. Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci. 1999;19(9):3287–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen MS, Lin HK, Chiu H, Lee DC, Chung YF, Chiu IM. Human FGF1 promoter is active in ependymal cells and dopaminergic neurons in the brains of F1B-GFP transgenic mice. Dev Neurobiol. 2015;75(3):232–248. [DOI] [PubMed] [Google Scholar]
- 97. Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El Beheiry H, Morshead C, Fehlings MG. Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. J Neurosci. 2007;27(13):3416–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mothe AJ, Tator CH. Transplanted neural stem/progenitor cells generate myelinating oligodendrocytes and Schwann cells in spinal cord demyelination and dysmyelination. Exp Neurol. 2008;213(1):176–190. [DOI] [PubMed] [Google Scholar]
- 99. Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7(5):395–406. [DOI] [PubMed] [Google Scholar]
- 100. Liu Y, Zhou Y, Zhang C, Zhang F, Hou S, Zhong H, Huang H. Optimal time for subarachnoid transplantation of neural progenitor cells in the treatment of contusive spinal cord injury. Neural Regen Res. 2013;8(5):389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Piltti KM, Salazar DL, Uchida N, Cummings BJ, Anderson AJ. Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem Cells Transl Med. 2013;2(3):204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chen N, Cen JS, Wang J, Qin G, Long L, Wang L, Wei F, Xiang Q, Deng DY, Wan Y. Targeted inhibition of leucine-rich repeat and immunoglobulin domain-containing protein 1 in transplanted neural stem cells promotes neuronal differentiation and functional recovery in rats subjected to spinal cord injury. Crit Care Med. 2016;44(3):e146–e157. [DOI] [PubMed] [Google Scholar]
- 103. Zeng Y, Han H, Tang B, Chen J, Mao D, Xiong M. Transplantation of recombinant vascular endothelial growth factor (vegf)189-neural stem cells downregulates transient receptor potential vanilloid 1 (trpv1) and improves motor outcome in spinal cord injury. Med Sci Monit. 2018;24:1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen D, Hu S, Liu J, Li S. E-cadherin regulates biological behaviors of neural stem cells and promotes motor function recovery following spinal cord injury. Exp Ther Med. 2019;17(3):2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee SI, Kim BG, Hwang DH, Kim HM, Kim SU. Overexpression of Bcl-XL in human neural stem cells promotes graft survival and functional recovery following transplantation in spinal cord injury. J Neurosci Res. 2009;87(14):3186–3197. [DOI] [PubMed] [Google Scholar]
- 106. Xu W, Li P, Qin K, Wang X, Jiang X. miR-124 regulates neural stem cells in the treatment of spinal cord injury. Neurosci Lett. 2012;529(1):12–17. [DOI] [PubMed] [Google Scholar]
- 107. Kusano K, Enomoto M, Hirai T, Tsoulfas P, Sotome S, Shinomiya K, Okawa A. Transplanted neural progenitor cells expressing mutant NT3 promote myelination and partial hindlimb recovery in the chronic phase after spinal cord injury. Biochem Biophys Res Commun. 2010;393(4):812–817. [DOI] [PubMed] [Google Scholar]
- 108. Nagoshi N, Khazaei M, Ahlfors JE, Ahuja CS, Nori S, Wang J, Shibata S, Fehlings MG. Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing. Stem Cells Transl Med. 2018;7(11):806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang D, Liang J, Zhang J, Liu S, Sun W. Mild hypothermia combined with a scaffold of NgR-silenced neural stem cells/Schwann cells to treat spinal cord injury. Neural Regen Res. 2014;9(24):2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fan WL, Liu P, Wang G, Pu JG, Xue X, Zhao JH. Transplantation of hypoxic preconditioned neural stem cells benefits functional recovery via enhancing neurotrophic secretion after spinal cord injury in rats. J Cell Biochem. 2018;119(6):4339–4351. [DOI] [PubMed] [Google Scholar]
- 111. Oh JS, Kim KN, An SS, Pennant WA, Kim HJ, Gwak SJ, Yoon DH, Lim MH, Choi BH, Ha Y. Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transplant. 2011;20(6):837–849. [DOI] [PubMed] [Google Scholar]
- 112. Niapour A, Karamali F, Nemati S, Taghipour Z, Mardani M, Nasr-Esfahani MH, Baharvand H. Cotransplantation of human embryonic stem cell-derived neural progenitors and schwann cells in a rat spinal cord contusion injury model elicits a distinct neurogenesis and functional recovery. Cell Transplant. 2012;21(5):827–843. [DOI] [PubMed] [Google Scholar]
- 113. Wang G, Ao Q, Gong K, Zuo H, Gong Y, Zhang X. Synergistic effect of neural stem cells and olfactory ensheathing cells on repair of adult rat spinal cord injury. Cell Transplant. 2010;19(10):1325–1337. [DOI] [PubMed] [Google Scholar]
- 114. Levi AD, Okonkwo DO, Park P, Jenkins AL, 3rd, Kurpad SN, Parr AM, Ganju A, Aarabi B, Kim D, Casha S, Fehlings MG, et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery. 2018;82(4):562–575. [DOI] [PubMed] [Google Scholar]
- 115. Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, Polak M, Bordeau J, Sakowski SA, Glass JD. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75(3):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells 2012;30(6):1144–1151. [DOI] [PubMed] [Google Scholar]
- 117. Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio EA, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S, Huie JR, et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nature Medicine. 2018;24(4):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Koch P, Opitz T, Steinbeck JA, Ladewig J, Brustle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U S A. 2009;106(9):3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R, Stice SL. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells. 2006;24(1):125–138. [DOI] [PubMed] [Google Scholar]
- 120. Guarino AT, McKinnon RD. Reprogramming cells for brain repair. Brain Sci 2013;3(3):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee TH. Functional effect of mouse embryonic stem cell implantation after spinal cord injury. J Exerc Rehabil. 2013;9(2):230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cui YF, Xu JC, Hargus G, Jakovcevski I, Schachner M, Bernreuther C. Embryonic stem cell-derived L1 overexpressing neural aggregates enhance recovery after spinal cord injury in mice. PLoS One. 2011;6(3):e17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rowland JW, Lee JJ, Salewski RP, Eftekharpour E, van der Kooy D, Fehlings MG. Generation of neural stem cells from embryonic stem cells using the default mechanism: in vitro and in vivo characterization. Stem Cells Dev. 2011;20(11):1829–1845. [DOI] [PubMed] [Google Scholar]
- 124. Alsanie WF, Niclis JC, Petratos S. Human embryonic stem cell-derived oligodendrocytes: protocols and perspectives. Stem Cells Dev. 2013;22(18):2459–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shroff G, Dhanda Titus J, Shroff R. A review of the emerging potential therapy for neurological disorders: human embryonic stem cell therapy. Am J Stem Cells. 2017;6(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- 126. Scott CT, Magnus D. Wrongful termination: lessons from the Geron clinical trial. Stem Cells Transl Med. 2014;3(12):1398–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shroff G. Human embryonic stem cell therapy in chronic spinal cord injury: a retrospective study. Clin Transl Sci. 2016;9(3):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Narva E, Autio R, Rahkonen N, Kong L, Harrison N, Kitsberg D, Borghese L, Itskovitz-Eldor J, Rasool O, Dvorak P, Hovatta O, et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat Biotechnol. 2010;28(4):371–377. [DOI] [PubMed] [Google Scholar]
- 129. Marichal N, Garcia G, Radmilovich M, Trujillo-Cenoz O, Russo RE. Spatial domains of progenitor-like cells and functional complexity of a stem cell niche in the neonatal rat spinal cord. Stem Cells. 2012;30(9):2020–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 131. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 132. Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. [DOI] [PubMed] [Google Scholar]
- 133. Romanyuk N, Amemori T, Turnovcova K, Prochazka P, Onteniente B, Sykova E, Jendelova P. Beneficial effect of human induced pluripotent stem cell-derived neural precursors in spinal cord injury repair. Cell Transplant. 2015;24(9):1781–1797. [DOI] [PubMed] [Google Scholar]
- 134. Kajikawa K, Imaizumi K, Shinozaki M, Shibata S, Shindo T, Kitagawa T, Shibata R, Kamata Y, Kojima K, Nagoshi N, Matsumoto M, et al. Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol Brain. 2020;13(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Amemori T, Ruzicka J, Romanyuk N, Jhanwar-Uniyal M, Sykova E, Jendelova P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther. 2015;6:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Okubo T, Iwanami A, Kohyama J, Itakura G, Kawabata S, Nishiyama Y, Sugai K, Ozaki M, Iida T, Matsubayashi K, Matsumoto M, et al. Pretreatment with a gamma-secretase inhibitor prevents tumor-like overgrowth in human iPSC-derived transplants for spinal cord injury. Stem Cell Reports. 2016;7(4):649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LS, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014;83(4):789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Itakura G, Kawabata S, Ando M, Nishiyama Y, Sugai K, Ozaki M, Iida T, Ookubo T, Kojima K, Kashiwagi R, Yasutake K, et al. Fail-safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem Cell Reports 2017;8(3):673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fuhrmann T, Tam RY, Ballarin B, Coles B, Elliott Donaghue I, van der Kooy D, Nagy A, Tator CH, Morshead CM, Shoichet MS. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23–36. [DOI] [PubMed] [Google Scholar]
- 140. Pomeshchik Y, Puttonen KA, Kidin I, Ruponen M, Lehtonen S, Malm T, Akesson E, Hovatta O, Koistinaho J. Transplanted human induced pluripotent stem cell-derived neural progenitor cells do not promote functional recovery of pharmacologically immunosuppressed mice with contusion spinal cord injury. Cell Transplant. 2015;24(9):1799–1812. [DOI] [PubMed] [Google Scholar]
- 141. Nutt SE, Chang EA, Suhr ST, Schlosser LO, Mondello SE, Moritz CT, Cibelli JB, Horner PJ. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp Neurol. 2013;248:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fischer I, Dulin JN, Lane MA. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat Rev Neurosci. 2020;21(7):366–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lopez-Serrano C, Torres-Espin A, Hernandez J, Alvarez-Palomo AB, Requena J, Gasull X, Edel MJ, Navarro X. Effects of the post-spinal cord injury microenvironment on the differentiation capacity of human neural stem cells derived from induced pluripotent stem cells. Cell Transplant. 2016;25(10):1833–1852. [DOI] [PubMed] [Google Scholar]
- 144. Torralba D, Baixauli F, Villarroya-Beltri C, Fernandez-Delgado I, Latorre-Pellicer A, Acin-Perez R, Martin-Cofreces NB, Jaso-Tamame AL, Iborra S, Jorge I, González-Aseguinolaza G, et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat Commun. 2018;9(1):2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. [DOI] [PubMed] [Google Scholar]
- 146. Wang X, Botchway BOA, Zhang Y, Yuan J, Liu X. Combinational treatment of bioscaffolds and extracellular vesicles in spinal cord injury. Frontiers in Molecular Neuroscience. 2019;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Taylor DD, Gercel-Taylor C. Exosome platform for diagnosis and monitoring of traumatic brain injury. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652):20130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lankford KL, Arroyo EJ, Nazimek K, Bryniarski K, Askenase PW, Kocsis JD. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018;13(1):e0190358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. All AH, Bazley FA, Gupta S, Pashai N, Hu C, Pourmorteza A, Kerr C. Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PLoS One. 2012;7(10):e47645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhao C, Zhou X, Qiu J, Xin D, Li T, Chu X, Yuan H, Wang H, Wang Z, Wang D. Exosomes derived from bone marrow mesenchymal stem cells inhibit complement activation in rats with spinal cord injury. Drug Des Devel Ther 2019;13:3693–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, Popovtzer R, Offen D, Levenberg S. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog sirna repairs complete spinal cord injury. ACS nano. 2019;13(9):10015–10028. [DOI] [PubMed] [Google Scholar]
- 152. Kang J, Zhang C, Zhi Z, Wang Y, Liu J, Wu F, Xu G. Stem-like cells of various origins showed therapeutic effect to improve the recovery of spinal cord injury. Artif Cells Nanomed Biotechnol. 2020;48(1):627–638. [DOI] [PubMed] [Google Scholar]
- 153. Wang Z, Song Y, Han X, Qu P, Wang W. Long noncoding RNA PTENP1 affects the recovery of spinal cord injury by regulating the expression of miR-19b and miR-21. J Cell Physiol. 2019;235(4):3634–3645. [DOI] [PubMed] [Google Scholar]
- 154. Li C, Li X, Zhao B, Wang C. Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Arch Physiol Biochem. 2020:126(4):369–375. [DOI] [PubMed] [Google Scholar]
- 155. Huang JH, Xu Y, Yin XM, Lin FY. Exosomes derived from mir-126-modified mscs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience. 2019;424:133–145. [DOI] [PubMed] [Google Scholar]
- 156. Ji W, Jiang W, Li M, Li J, Li Z. miR-21 deficiency contributes to the impaired protective effects of obese rat mesenchymal stem cell-derived exosomes against spinal cord injury. Biochimie. 2019;167:171–178. [DOI] [PubMed] [Google Scholar]
- 157. Kim HY, Kumar H, Jo MJ, Kim J, Yoon JK, Lee JR, Kang M, Choo YW, Song SY, Kwon SP, Hyeon T, et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18(8):4965–4975. [DOI] [PubMed] [Google Scholar]
- 158. Osier N, Motamedi V, Edwards K, Puccio A, Diaz-Arrastia R, Kenney K, Gill J. Exosomes in Acquired Neurological Disorders: New Insights into Pathophysiology and Treatment. Mol Neurobiol. 2018;55(12):9280–9293. [DOI] [PubMed] [Google Scholar]
- 159. Patel DB, Santoro M, Born LJ, Fisher JP, Jay SM. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv. 2018;36(8):2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Evaniew N, Noonan VK, Fallah N, Rivers CS, Dvorak MF. Methylprednisolone for the treatment of patients with acute spinal cord injuries: response. J Neurotrauma 2016;33(10):975–976. [DOI] [PubMed] [Google Scholar]
- 161. Sarveazad A, Babahajian A, Bakhtiari M, Soleimani M, Behnam B, Yari A, Akbari A, Yousefifard M, Janzadeh A, Amini N, Agah S, et al. The combined application of human adipose derived stem cells and Chondroitinase ABC in treatment of a spinal cord injury model. Neuropeptides. 2017;61:39–47. [DOI] [PubMed] [Google Scholar]
- 162. Ahuja CS, Mothe A, Khazaei M, Badhiwala JH, Gilbert EA, van der Kooy D, Morshead CM, Tator C, Fehlings MG. The eading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9(12):1509–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hosseini SM, Sharafkhah A, Koohi-Hosseinabadi O, Semsar-Kazerooni M. Transplantation of neural stem cells cultured in alginate scaffold for spinal cord injury in rats. Asian Spine J. 2016;10(4):611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Yaghoobi K, Kaka G, Mansouri K, Davoodi S, Sadraie SH, Hosseini SR. Lavandula angustifolia extract improves the result of human umbilical mesenchymal Wharton’s Jelly stem cell transplantation after contusive spinal cord injury in wistar rats. Stem Cells Int 2016;2016:5328689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wang D, Zhang J. Effects of hypothermia combined with neural stem cell transplantation on recovery of neurological function in rats with spinal cord injury. Mol Med Rep. 2015;11(3):1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Chen D, Zeng W, Fu Y, Gao M, Lv G. Bone marrow mesenchymal stem cells combined with minocycline improve spinal cord injury in a rat model. Int J Clin Exp Pathol. 2015;8(10):11957–11969. [PMC free article] [PubMed] [Google Scholar]
- 167. Wang YX, Sun JJ, Zhang M, Hou XH, Hong J, Zhou YJ, Zhang ZY. Propofol injection combined with bone marrow mesenchymal stem cell transplantation better improves electrophysiological function in the hindlimb of rats with spinal cord injury than monotherapy. Neural Regen Res. 2015;10(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhou YJ, Liu JM, Wei SM, Zhang YH, Qu ZH, Chen SB. Propofol promotes spinal cord injury repair by bone marrow mesenchymal stem cell transplantation. Neural Regen Res. 2015;10(8):1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhang LQ, Zhang WM, Deng L, Xu ZX, Lan WB, Lin JH. Transplantation of a peripheral nerve with neural stem cells plus lithium chloride injection promote the recovery of rat spinal cord injury. Cell Transplant. 2018;27(3):471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Liu Z, Ding Y, Zeng YS. A new combined therapeutic strategy of governor vessel electro-acupuncture and adult stem cell transplantation promotes the recovery of injured spinal cord. Curr Med Chem 2011;18(33):5165–5171. [DOI] [PubMed] [Google Scholar]
- 171. Kim KT, Kim HJ, Cho DC, Bae JS, Park SW. Substance P stimulates proliferation of spinal neural stem cells in spinal cord injury via the mitogen-activated protein kinase signaling pathway. Spine J. 2015;15(9):2055–2065. [DOI] [PubMed] [Google Scholar]
- 172. Kim HJ, Oh JS, An SS, Pennant WA, Gwak SJ, Kim AN, Han PK, Yoon DH, Kim KN, Ha Y. Hypoxia-specific GM-CSF-overexpressing neural stem cells improve graft survival and functional recovery in spinal cord injury. Gene Ther. 2012;19(5):513–521. [DOI] [PubMed] [Google Scholar]
- 173. Wang J, Chu R, Ni N, Nan G. The Effect of Matrigel as scaffold material for neural stem cell transplantation for treating spinal cord injury. Sci Rep. 2020;10(1):2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Park SS, Lee YJ, Lee SH, Lee D, Choi K, Kim WH, Kweon OK, Han HJ. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy. 2012;14(5):584–597. [DOI] [PubMed] [Google Scholar]
- 175. Ruzicka J, Romanyuk N, Jirakova K, Hejcl A, Janouskova O, Machova LU, Bochin M, Pradny M, Vargova L, Jendelova P. The Effect of iPS-derived neural progenitors seeded on laminin-coated phema-moetacl hydrogel with dual porosity in a rat model of chronic spinal cord injury. Cell Transplantation 2019;28(4):400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Fan Z, Liao X, Tian Y, Xuzhuzi X, Nie Y. A prevascularized nerve conduit based on a stem cell sheet effectively promotes the repair of transected spinal cord injury. Acta Biomater. 2020;101:304–313. [DOI] [PubMed] [Google Scholar]
- 177. Zweckberger K, Ahuja CS, Liu Y, Wang J, Fehlings MG. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater. 2016;42:77–89. [DOI] [PubMed] [Google Scholar]
- 178. Nori S, Khazaei M, Ahuja CS, Yokota K, Ahlfors JE, Liu Y, Wang J, Shibata S, Chio J, Hettiaratchi MH, Führmann T, et al. Human oligodendrogenic neural progenitor cells delivered with chondroitinase abc facilitate functional repair of chronic spinal cord injury. Stem Cell Reports. 2018;11(6):1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Liu W, Xu B, Xue W, Yang B, Fan Y, Chen B, Xiao Z, Xue X, Sun Z, Shu M, Zhang Q, et al. A functional scaffold to promote the migration and neuronal differentiation of neural stem/progenitor cells for spinal cord injury repair. Biomaterials. 2020;243:119941. [DOI] [PubMed] [Google Scholar]
- 180. Li X, Fan C, Xiao Z, Zhao Y, Zhang H, Sun J, Zhuang Y, Wu X, Shi J, Chen Y, Dai J. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/beta-catenin signaling for spinal cord injury repair. Biomaterials. 2018;183:114–127. [DOI] [PubMed] [Google Scholar]
- 181. Ji WC, Li M, Jiang WT, Ma X, Li J. Protective effect of brain-derived neurotrophic factor and neurotrophin-3 overexpression by adipose-derived stem cells combined with silk fibroin/chitosan scaffold in spinal cord injury. Neurol Res. 2020;42(5):361–371. [DOI] [PubMed] [Google Scholar]
- 182. Tang S, Liao X, Shi B, Qu Y, Huang Z, Lin Q, Guo X, Pei F. The effects of controlled release of neurotrophin-3 from PCLA scaffolds on the survival and neuronal differentiation of transplanted neural stem cells in a rat spinal cord injury model. PLoS One. 2014;9(9):e107517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010;6(20):5127–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Smith DR, Dumont CM, Park J, Ciciriello AJ, Guo A, Tatineni R, Cummings BJ, Anderson AJ, Shea LD. Polycistronic delivery of IL-10 and NT-3 promotes oligodendrocyte myelination and functional recovery in a mouse spinal cord injury model. Tissue Eng Part A. 2020;26(11–12):672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Xue F, Wu EJ, Zhang PX, Li-Ya A, Kou YH, Yin XF, Han N. Biodegradable chitin conduit tubulation combined with bone marrow mesenchymal stem cell transplantation for treatment of spinal cord injury by reducing glial scar and cavity formation. Neural Regen Res. 2015;10(1):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Zhang RP, Xu C, Liu Y, Li JD, Xie J. Visual bone marrow mesenchymal stem cell transplantation in the repair of spinal cord injury. Neural Regen Res. 2015;10(3):404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mukhamedshina YO, Akhmetzyanova ER, Kostennikov AA, Zakirova EY, Galieva LR, Garanina EE, Rogozin AA, Kiassov AP, Rizvanov AA. Adipose-derived mesenchymal stem cell application combined with fibrin matrix promotes structural and functional recovery following spinal cord injury in rats. Front Pharmacol. 2018;9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Spejo AB, Chiarotto GB, Ferreira ADF, Gomes DA, Ferreira RS, Jr, Barraviera B, Oliveira ALR. Neuroprotection and immunomodulation following intraspinal axotomy of motoneurons by treatment with adult mesenchymal stem cells. J Neuroinflammation. 2018;15(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Liu J, Chen J, Liu B, Yang C, Xie D, Zheng X, Xu S, Chen T, Wang L, Zhang Z, Zhang Z, et al. Acellular spinal cord scaffold seeded with mesenchymal stem cells promotes long-distance axon regeneration and functional recovery in spinal cord injured rats. J Neurol Sci. 2013;325(1–2):127–136. [DOI] [PubMed] [Google Scholar]
- 190. Zhou X, Shi G, Fan B, Cheng X, Zhang X, Wang X, Liu S, Hao Y, Wei Z, Wang L, Feng S. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int J Nanomedicine. 2018;13:6265–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Fan L, Liu C, Chen X, Zou Y, Zhou Z, Lin C, Tan G, Zhou L, Ning C, Wang Q. Directing Induced Pluripotent Stem Cell Derived Neural Stem Cell Fate with a Three-Dimensional Biomimetic Hydrogel for Spinal Cord Injury Repair. ACS Appl Mater Interfaces. 2018;10(21):17742–17755. [DOI] [PubMed] [Google Scholar]
- 192. Montgomery A, Wong A, Gabers N, Willerth SM. Engineering personalized neural tissue by combining induced pluripotent stem cells with fibrin scaffolds. Biomater Sci. 2015;3(2):401–413. [DOI] [PubMed] [Google Scholar]
- 193. Zholudeva LV, Lane MA. Transplanting cells for spinal cord repair: who, what, when, where and why? Cell Transplant. 2019;28(4):388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ide C, Kanekiyo K. Points regarding cell transplantation for the treatment of spinal cord injury. Neural Regen Res. 2016;11(7):1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Nori S, Okada Y, Nishimura S, Sasaki T, Itakura G, Kobayashi Y, Renault-Mihara F, Shimizu A, Koya I, Yoshida R, Kudoh J, et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Reports. 2015;4(3):360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24(2):462–471. [DOI] [PubMed] [Google Scholar]
- 197. McEwen KR, Leitch HG, Amouroux R, Hajkova P. The impact of culture on epigenetic properties of pluripotent stem cells and pre-implantation embryos. Biochem Soc Trans 2013;41(3):711–719. [DOI] [PubMed] [Google Scholar]
- 198. Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA. 2012;307(17):1838–1847. [DOI] [PubMed] [Google Scholar]