Abstract
Background
The α1-adrenoreceptor antagonist prazosin has in many but not all studies been found to be effective for PTSD associated nightmares, hyperarousal symptoms, and total symptom severity. The particular efficacy of prazosin for nightmares and hyperarousal symptoms suggests there may be a subset of PTSD symptoms that are more tightly associated with an α1-adrenoreceptor mediated noradrenergic mechanism, but cross traditional diagnostic symptom clusters. However, the efficacy of prazosin for individual symptoms other than nightmares and sleep disruption has not previously been examined.
Methods
In a post hoc reanalysis of a previously published, randomized controlled trial of twice daily prazosin for PTSD, we examined the relative effect of prazosin on individual items of the CAPS for DSM-IV, and tested whether prazosin responsiveness predicted the partial correlation of the changes in symptom intensity at the level of individual subjects. Results were not adjusted for multiple comparisons.
Results
Prazosin showed the largest effect for distressing dreams, anhedonia, difficulty falling or staying asleep, difficulty concentrating, and hypervigilance. These items were also (a) of higher baseline severity in the underlying population, and (b) more related in how they fluctuated at the level of individual subjects. Covariance analysis did not support a clear cutoff between highly prazosin responsive items and those showing a smaller, not statistically significant response.
Conclusions
In this data set, twice daily prazosin substantially reduced not only nightmares and sleep disruption, but the majority of hyperarousal symptoms, with some evidence of efficacy for avoidance symptoms. The relationship of baseline symptom distribution to which symptoms showed significant response to prazosin reinforces the possibility that differences in a clinical trial’s participant populations may significantly influence trial outcome. The pattern of symptom endorsement at the level of individual subjects was consistent with prazosin-responsive items sharing a common pathophysiologic mechanism.
Keywords: nightmares, noradrenaline, prazosin, posttraumatic stress disorder, symptom clusters
Introduction
Prazosin, an antagonist of the α1 adrenoceptor (AR), has been found effective in reducing the symptoms of Posttraumatic Stress Disorder (PTSD) in six randomized controlled trials (RCTs),1–6 although a seventh RCT of prazosin for PTSD was negative.7 Prazosin’s use in PTSD was initially focused on recurrent distressing dreams (for simplicity, referred to here as ‘nightmares’).8 However, two small-sample crossover RCTs using a nighttime-only prazosin dose have also found significant improvement in the Clinician Administered PTSD Scale (CAPS) item addressing difficulty falling or staying asleep,3 total CAPS scores,1 and each of the DSM-IV CAPS clusters.1 Among the individual CAPS clusters, the effect size for the hyperarousal symptoms (cluster D, effect size 0.9; Table 1) was at least as large as that seen for the re-experiencing symptoms, the cluster that includes the nightmare item (cluster B, effect size 0.7).
Table 1.
Categorization of PTSD symptoms by DSM IV and DSM 5.
| DSM IV | DSM 5 | ||||
|---|---|---|---|---|---|
| B: Intrusive recollections/reexperiencing | B1 | recurrent and distressing recollections | B: Intrusive recollections/reexperiencing | B1 | recurrent involuntary intrusive memories |
| B2 | recurrent distressing dreams | B2 | recurrent distressing dreams | ||
| B3 | flashbacks | B3 | flashbacks | ||
| B4 | psychological distress at exposure to reminders | B4 | psychological distress at exposure to reminders | ||
| B5 | physiologic reactivity upon exposure to reminders | B5 | physiologic reactivity upon exposure to reminders | ||
| C: avoidance/numbing | C1 | avoiding thoughts, feelings or conversations | C: avoidance | C1 | avoiding thoughts, feelings or memories |
| C2 | avoiding activities, places or people | C2 | avoiding external reminders | ||
| C3 | inability to recall an important aspect | D: negative alterations in cognition/mood | D1 | inability to recall an important aspect | |
| – | D2 | negative beliefs about oneself, others, or the world | |||
| – | D3 | blame of self or others | |||
| – | D4 | persistent negative emotional state | |||
| C4 | decreased interest in significant activities | D5 | decreased interest in significant activities | ||
| C5 | detachment or estrangement | D6 | detachment or estrangement | ||
| C6 | restricted range of affect | D7 | absence of positive emotions | ||
| C7 | foreshoretened future | – | |||
| D: hyperarousal | D1 | insomnia | E: hyperarousal | E6 | sleep disturbance |
| D2 | irritability or anger | E1 | irritable or aggressive behavior | ||
| D3 | difficulty concentrating | E5 | problems with concentration | ||
| D4 | hypervigilance | E3 | hypervigilance | ||
| D5 | exaggerated startle | E4 | exaggerated startle | ||
| – | E2 | reckless or self-destructive behavior | |||
The first RCT to use a midmorning dose as well as a nighttime dose was carried out in a parallel group study in active duty service members (n = 32 prazosin, n = 35 placebo).5 With this regimen, significant reductions were seen in nightmares, the total CAPS score and the hyperarousal cluster, with smaller, not statistically significant numerical reductions in the re-experiencing and avoidance clusters. The pattern of the largest efficacy being spread between the hyperarousal cluster and particularly the nightmare item of the re-experiencing cluster is potentially consistent with preclinical findings connecting increased noradrenergic activity and hyperarousal symptoms,9,10 as well as empirical and basic science findings of a connection between noradrenergic activity and nightmares.11 However, the idea that prazosin may be particularly effective for a group of symptoms that are related to a noradrenergic mechanism of expression, and that these symptoms cross the boundaries of the symptom clusters as they are defined in both the DSM-IV and the DSM 5 (Table 1), raise the question of whether the pattern of symptoms that are the most responsive to prazosin may not be well-captured within the current diagnostic system.
The division of PTSD symptoms into distinct clusters has been controversial,12 with both the clusters themselves and some of the items within them changing substantially between DSM IV and DSM 5.13 Most attempts to organize PTSD symptoms into meaningful groupings have been carried out using different methods for factor analysis – a strategy that looks at whether certain symptoms cluster together across patients – as an indication that they are pathophysiologycally or functionally related.14
Another strategy that may be promising for revealing symptoms with related pathophysiology is to look at how individual PTSD symptoms change in response to pharmacologic interventions. Studies of the effects of fluoxetine15 and sertraline16 on individual PTSD symptoms have found largely broad-based efficacy, but with somewhat stronger efficacy for items pertaining to mood or cognitive aspects for fluoxetine, and psychological rather than somatic items for sertraline; in both studies, insomnia and nightmares were highlighted as items with absent or particularly weak effects. More recently, Stein et al. performed a factor analysis of pooled data from two large studies of venlafaxine versus placebo in PTSD,17 where they found that venlafaxine also was broadly effective for PTSD symptoms, without specificity for particular clusters. They postulated that looking at the pattern of symptom response to pharmacologic interventions with a more specific mechanisms of action might reveal more specific results.
In this study, we performed exploratory subgroup analyses using the data from Raskind et al.5 to examine the effect of the selective noradrenergic receptor antagonist prazosin on each of the 17 individual items in the CAPS-IV. We used these data to ask three basic question: First, does prazosin show an evenly distributed, nonspecific effect on PTSD symptoms, or a more specific pattern of effects? Second, can the variations in responsiveness of different symptoms be explained simply by the initial distribution of symptoms in the sample? And third, do the symptoms that are the most prazosin-responsive also co-vary at the level of individual subjects, consistent with a model where these symptoms are pathophysiologically linked?
Methods
Data
Data were extracted from a 15-week RCT of twice daily prazosin (n = 32) or placebo (n = 35) in 67 active-duty soldiers who met DSM-IV criteria for PTSD following combat deployments to Iraq and Afghanistan, which has been previously published (Table 2).5 Data extracted included the 17 CAPS individual item (sum of frequency and intensity scores, range: 0-8) and total (range: 0-136) scores for each participant at each week (0, 7, 11, and 15), group assignment, gender and use of antidepressant medication. No adjustments were made for multiple comparisons. All calculations were done using R in RStudio18,19 and the packages lme4,20 ggplot2,21 EnvStats,22 and boot.23,24
Table 2.
Demographic and clinical characteristics of sample.
| Prazosin (N = 32) | Placebo (N = 35) | |
|---|---|---|
| Age (years) | 30.0 ± 6.6 | 30.8 ± 6.5 |
| Education (years)* | 13.3 ± 1.9 | 13.0 ± 2.1 |
| Male | 26 (81%) | 31 (89%) |
| Race | ||
| African American | 4 (13%) | 5 (14%) |
| Asian | 1 (3%) | 0 |
| Caucasian | 21 (66%) | 21 (60%) |
| Hispanic | 5 (16%) | 3 (9%) |
| Native American | 0 | 2 (6%) |
| Other | 1 (3%) | 4 (11%) |
| Major depression | 11 (34%) | 15 (43%) |
| Maintenance Antidepressant | 10 (31%) | 13 (37%) |
*Missing value for 3 Veterans in each group.
Effect of Prazosin on Individual PTSD Symptoms
The effect of prazosin on individual PTSD symptoms was addressed via linear mixed effects models, with symptom as the dependent variable, and the predictor variables week, treatment group, and a week-by-treatment interaction term, along with terms to adjust for gender and use of antidepressant medication; subject was treated as a random effect. A significant (two-tailed p < 0.05) week-by-treatment interaction indicates a difference in rate of change from baseline between the prazosin and placebo group. Following the recommendation of Nakagawa & Cuthill,25 we defined effect size in clinically meaningful units as 15-week improvement in the prazosin group minus 15-week improvement in placebo (Improvement Beyond Placebo, IBP); i.e., the week-by-treatment interaction term multiplied by 15. Confidence intervals were calculated using the likelihood profile.
Relationship of Measured Response to Prazosin Versus Symptom Severity at Baseline and Following Placebo Treatment
We examined whether the difference in efficacy across individual CAPS items suggests a specificity in how individual symptoms respond to prazosin, versus whether these differences are better explained by (a) a lower baseline mean score for some items versus others (which limits room for improvement); (b) a higher response to placebo for some items versus others; or (c) a combination of these two, where the baseline frequency of symptoms plus their response to placebo together resulted in insufficient room for a pharmacologic intervention to result in any further change. We first quantified the relationship between the effect of prazosin observed for a given item (i.e., IBP) and the mean rating at baseline for that item using Pearson’s product-moment correlation based on the n = 17 CAPS items. We additionally used Pearson’s product-moment correlation to quantify the relationship between IBP and the predicted mean score at 15-weeks for the placebo group (a measure of how much room for improvement is left in the placebo group after 15 weeks of treatment).
Pairwise Covariance Analysis
To investigate whether symptoms that are the most prazosin-responsive co-vary more with each other than with the other symptoms, we computed the change from baseline (i.e., slope) for each participant for each of the 17 CAPS items and looked at pairwise correlations of slopes between CAPS items. Thus, each pairwise correlation coefficient represents the degree to which change in one item is correlated with change in the second item. The correlation coefficients were calculated either unadjusted; adjusted for the items’ baseline values and the participants’ baseline values for total CAPS score; or adjusted for the items’ baseline values, the participants’ baseline values for total CAPS score, and the participants’ change from baseline (slope) in total CAPS score. Adjusted correlations were computed by fitting a linear model with item slope as the response variable and adjustment variables (item baseline value and baseline total CAPS score; or item baseline value, baseline total CAPS score, and change in total CAPS score) as predictor variables, computing the residuals from this model, and computing the correlation between residuals for a pair of items. As the goal of the analysis was to ask whether pairs of two highly prazosin-responsive items covaried more than pairs of one highly prazosin-responsive item and one non-highly prazosin responsive item, as would be expected if the highly prazosin-responsive items are pathophysiologically related, we began by identifying the items with both statistically and clinically significant improvement relative to placebo (the “highly prazosin responsive” group). Pairwise correlation coefficients were then calculated separately for (1) pairs where both items were in the highly prazosin responsive group, and (2) pairs where one item was in the highly prazosin responsive group, and the other was not. We then computed the mean correlation over all pairwise correlations separately within groups (1) and (2), and then compared these two average correlations. The 95% confidence interval for the mean correlation within a group and the confidence interval for the difference in mean correlations between groups were computed via bias-corrected and accelerated bootstrapping24 with 5,000 resamples. For each bootstrap: subjects were resampled (stratified by treatment group), unadjusted and adjusted pairwise correlations between item slopes were computed, and then the mean correlation was computed for groups (1) and (2), along with the difference in the means.
A secondary analysis further divided the non-prazosin responsive group into those with intermediate IBPs (the “intermediate group”) and those with the lowest IBPs (the “lowest prazosin responsivity” group). Pairwise correlation coefficients were then calculated separately for (1) pairs where both items were in the highly prazosin responsive group, (2) pairs where one item was in the highly prazosin responsive group and one item was in the intermediate group, and (3) pairs where one item was in the highly prazosin responsive group and one was in the lowest prazosin responsive group. Finally, the above procedures were also repeated separately for the participants in the prazosin group alone and in the placebo group alone.
Results
1. The effect of prazosin was not evenly distributed among symptoms or symptom clusters
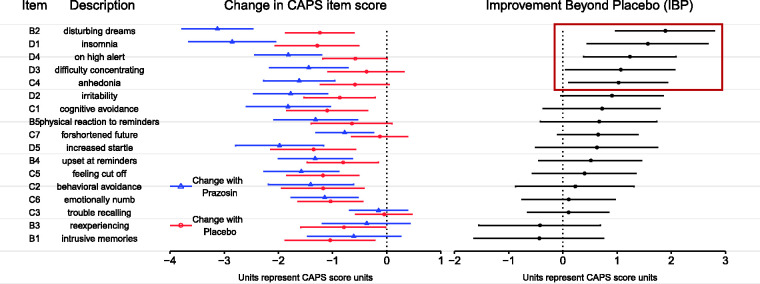
Although the 15-week improvement in the prazosin group was larger than that of the placebo group for 15 of the 17 items, there was substantial variation in the magnitude of difference shown across items (Figure 1 and Supplemental Table 1). For the five items with the largest difference between improvement on prazosin and improvement on placebo (Improvement Beyond Placebo [IBP]), this difference was both greater than one unit on the CAPS standardized rating scale (0-8), and was statistically significantly different from zero using a two-tailed test at p < .05 (not adjusted for multiple comparisons). Thus, this group of five items was used for later analyses that examined the relationships between the most highly prazosin responsive items and the other items. Three of the five items in this group, and one of the two items with trends towards significance, were from the hyperarousal cluster, while of the four items from the reexperiencing cluster besides the nightmare item, only physiologic reactivity upon exposure to reminders was even in the top half by IBP. Thus, these results were not consistent with prazosin having a distributed, nonspecific effect on PTSD symptoms. Instead, they showed a substantial degree of variation in the efficacy across symptoms, and a tendency for hyperarousal items to show higher evidence of efficacy and reexperiencing items other than the nightmare item to shower lower evidence of efficacy.
The effect of prazosin was largest for symptoms with high mean severity at baseline and at end of study in the placebo group
Figure 1.
Effect of prazosin on individual PTSD symptoms as described by CAPS for DSM-IV individual item scores (range: 0–8). Change in CAPS item scores over 15 weeks, as calculated by a linear mixed effects model, are given by treatment group with 95% confidence intervals, adjusted for gender and use of antidepressant medications. The difference between the effect of prazosin and the effect of placebo, termed Improvement Beyond Placebo (IBP), is presented in the final column with 95% confidence intervals. Items in the dark red box represent those with an IBP > 1, all of which were also items for which the effect of prazosin was statistically significantly different from that of placebo with a p < .05, uncorrected for multiple comparisons; this group is termed the “highly prazosin responsive group” in text.
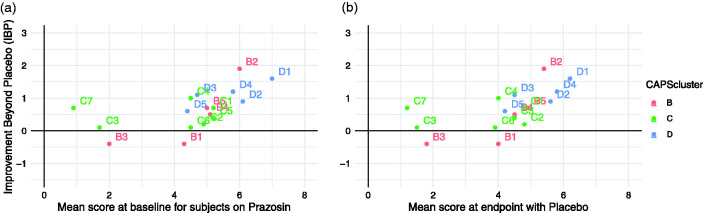
We next examined the relationship between IBP and the distribution of the individual symptoms’ initial severity at baseline, and final severity in the placebo group – in other words, how much symptom severity was “available” for the study drug to show improvement against. We found a strong positive correlation between IBP and both baseline level of symptom endorsement (Figure 2(a); R = 0.57) and residual symptoms after placebo (Figure 2(b); R = 0.59), consistent with the possibility that at least some of the difference in which symptoms responded to prazosin was related to the prevalence of that symptom in the population, and the degree to which it remained at an elevated level even after treatment with placebo.
2. Pairs of items that are both highly prazosin responsive covary more than pairs of items where only one item is highly prazosin responsive
Figure 2.
(a) Improvement beyond placebo (IBP; defined as 15-week change from baseline for prazosin minus 15-week change from baseline for placebo, based on linear mixed effect model), as a function of the mean rating for that item at baseline. (b) IBP as a function of the mean final item rating in the placebo group (based on linear mixed effects model), representing the amount of room “left” for an effect. Error bars indicate 95% confidence interval.
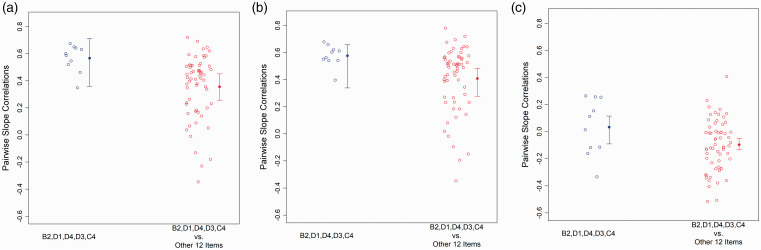
As described above, pairwise correlation coefficients (PCC) were used to quantify the degree to which the change in symptom intensity over time covaried for individual pairs of CAPS items (symptoms). When unadjusted PCC for pairs of items that are both highly prazosin responsive were compared to PCC for pairs of items where only one was highly prazosin responsive (Figure 3(a)), the mean PCC for the pairs of items where both were highly prazosin responsive (0.57 ± 0.1 SD) was significantly larger than that for pairs where only one was highly prazosin responsive (0.36 ± 0.23; 95% CI for difference: [0.07, 0.34]). Although this difference is consistent with the highly prazosin responsive items being pathophysiologically linked, it could also be related to differences in the baseline severity of these items, or in the total severity of symptoms in participants for whom these items are rated more highly. To reduce the risk of such statistical confounding, PCC were also calculated with adjustment for both each individual’s total CAPS and the individual item scores at baseline (Figure 3(b)). Using these adjusted values, the mean for pairs where both items were highly prazosin-responsive (0.58 ± 0.08) remained significantly larger than that for pairs where only one item was highly prazosin responsive (0.41 ± 0.24; 95% CI for difference: [0.04, 0.25]).
Figure 3.
Covariance of change from baseline in individual symptoms when both symptoms were identified as “highly prazosin responsive” or when only one symptom was identified as highly prazosin responsive and the other was not, quantified as pairwise correlation coefficients (PCC). (a) The mean PCC for the pairs of items where both were highly prazosin responsive (0.57 ± 0.1 SD) was significantly larger than that for pairs where only one was highly prazosin responsive (0.36 ± 0.23; 95% CI for difference: [0.07, 0.34]). (b) Adjusting for baseline symptom scores and total CAPS score at baseline, the mean for pairs where both items were highly prazosin-responsive (0.58 ± 0.08) remained significantly larger than that for pairs where only one item was highly prazosin responsive (0.41 ± 0.24; 95% CI for difference: [0.04, 0.25]). (c) Adjusting for baseline symptom scores, total CAPS score at baseline, and change in total CAPS, the mean for pairs where both items are highly prazosin responsive (0.03 ± 0.21) remained larger than that for pairs where only one item was in this group (−0.10 ± 0.19), but the difference was no longer significant (95% CI for difference: [−0.03, 0.23]). However, the difference between groups was significant when only participants who received placebo were analyzed (see text and Supplemental Figure 1F).
An additional, alternative explanation of the observed relationships is that the larger PCC values are a result of the items having been selected for having a significant response to treatment, such that any tendency for a particular individual to have a larger response to treatment across all symptoms, might result in the symptoms with the largest response having larger covariance. To address this risk, we completed two further analysis. First, we recalculated PCC values adjusting now for each individual’s total CAPS at baseline, individual item scores at baseline, and change in total CAPS (Figure 3(c)). Using these further adjusted PCC values, the mean for pairs where both items are highly prazosin responsive (0.03 ± 0.21) remained larger than that for pairs where only one item was in this group (−0.10 ± 0.19), but the difference was no longer significant (95% CI for difference: [−0.03, 0.23]). This suggests that at least some of the observed pattern may have been due to the statistical confounding regarding the selection of items.
Second, we repeated the entire set of analyses separately for participants who received placebo and for participants who received prazosin. If the observed relationships were due to the higher likelihood of covariance in items identified as prazosin responsive, we would expect that we would continue to see similar or even increased PCC in the group that received prazosin, but would not see this effect in the group that received placebo. However, while the direction of the effect remained the same in all comparisons made, we found that in the group of individuals who received prazosin, the effect was not significant for the unadjusted comparison (Supplemental Figure 1A, means 0.50 ± 0.14 vs 0.32 ± 0.30, 95% CI for difference [−0.01, 0.35]), when PCC were adjusted for baseline item and total CAPS severity scores (Supplemental Figure 1B, means 0.49 ± 0.14 and 0.35 ± 0.33, 95% CI for difference [0.00, 0.29]) and when additionally adjusted for change in total CAPS (Supplemental Figure 1C, means −0.03 ± 0.31 and −0.10 ± 0.29. 95% CI of difference [−0.08, 0.25]). In contrast, in the group of individuals who received placebo, the original finding was preserved, with significant differences seen in the means for the unadjusted PCC (Supplemental Figure 1D, means 0.59 ± 0.13 and 0.39 ± 0.21, 95% CI of difference [0.09, 0.33]), PCC adjusted for baseline item and total CAPS scores (Supplemental Figure 1E, means 0.62 ± 0.11 and 0.44 ± 0.20, 95% CI of difference [0.10, 0.26]), and PCC additionally adjusted for change in total CAPS (Supplemental Figure 1F, means 0.12 ± 0.24, −0.08 ± 0.18, 95% CI for difference [0.07, 0.36]).
Finally, as the specific threshold to consider a symptom highly prazosin responsive was arbitrarily chosen, as a secondary analysis we also repeated the analyses dividing the non-highly prazosin responsive group into two groups, again based on IBP: those with medium prazosin responsivity and those with lowest prazosin responsivity. We then compared the means for the unadjusted PCC (Supplemental Figure 2A) when both items were in the highly prazosin responsive group to those when one item was in the highly prazosin responsive group and one was in the medium responsivity group (mean in later group 0.45 ± 0.16, 95% CI of difference [-0.04, 0.24]); when one item was in the highly prazosin responsive group and one was in the lowest prazosin responsivity group (mean in later group 0.22 ± 0.26, 95% CI of difference [0.21, 0.52]). The difference in mean PCC when one item was in the highly prazosin responsive group and one was in the medium responsivity group was also significantly different from the mean PCC when one item was in the highly prazosin responsive group and one was in the lowest responsivity group (95% CI of difference [0.13, 0.33]). As with the two-group analyses, these findings were similar when using PCC adjusted for item and total symptoms severity at baseline (Supplemental Figure 2B) and were similar direction but no longer statistically significant when using PCC additionally adjusted for change in total CAPS (Supplemental Figure 2C). These findings are consistent with items that are more prazosin responsive being pathophysiologically linked, but suggest that the specific division into highly prazosin responsive items and items with medium responsivity does not capture a pathophysiologically relevant distinction.
Discussion
Both clinical and pre-clinical research support an important role for noradrenergic dysregulation in the pathophysiology of PTSD (for review, see Hendrickson and Raskind8). The available research also suggests that there may be heterogeneity in which symptoms are mediated primarily by α1 ARs (blocked by prazosin), versus which are mediated by β ARs (blocked by propranolol).26 Furthermore, some symptoms may be minimally related to changes in noradrenergic signaling, or only secondarily related (e.g., if avoidance of reminders is mediated in part by physiologic arousal in response to reminders, and physiologic arousal is mediated by noradrenaline). An examination of how individual PTSD symptoms respond to specific pharmacologic intervention is important for both immediate clinical care and for our understanding of the underlying pathophysiology.
In this post hoc analysis, unadjusted for multiple comparisons, we found that evidence that prazosin shows evidence of significantly reducing the majority of DSM-IV hyperarousal symptoms, including the items addressing disrupted sleep, difficulty with concentration, and hypervigilance; a trend towards efficacy for irritability was also seen. Significant reductions were also seen in nightmares and diminished interest in activities, with a trend towards efficacy for foreshortened future. This suggests that for a population with high baseline prevalence of these symptoms, these symptoms are those most likely to respond to prazosin.
The correlation of efficacy for a given symptom with that symptom’s baseline prevalence leaves the question open, however, of whether this pattern of efficacy might change in a population with a different distribution of baseline symptoms. This issue is well known in pain research.27 This type of effect may contribute to the frequent inconsistency in psychiatric research of the results of RCTs,28 including of prazosin.7 These results also raise the question of whether prazosin would be effective for these symptoms in a population that does not meet criteria for PTSD, such as those with anxiety.
We were also interested in why prazosin might be more effective for some symptoms than for others – specifically, whether these data provide support for the idea of there being a subset of symptoms that are more closely linked to either disruptions in the noradrenergic system in general or are more dependent on α1 AR signaling in particular. Our data provided potential support for this idea in several ways. First, in contrast to the findings of Stein et al.,17 we found significant variability in the degree of response of different symptoms to a 15-week titration of prazosin, with greater efficacy for symptoms in the hyperarousal cluster than symptoms in other clusters. This distribution of efficacy is consistent with previous findings suggesting a closer connection between noradrenergic mechanisms and sleep11,29 and hyperarousal symptoms9,10 in PTSD. Although it is not possible with this data set to disambiguate the relative contribution of the baseline prevalence of these symptoms to this pattern, this could be done in future work looking at studies done in populations with different initial distributions of symptoms.
Second, although the high baseline levels of nightmares in this study population were directly selected for as part of the inclusion criteria, there were no inclusion criteria that directly selected for the high baseline scores on items in the hyperarousal cluster, and this distribution of baseline symptoms was not seen in the earlier venlafaxine analysis.17 One possible explanation for this difference in baseline symptom distribution is that hyperarousal symptoms may simply be more prevalent in active duty soldiers than in a civilian sample. Alternatively, the pattern could also be produced if there were a relationship between the pathophysiology of nightmares and the other symptoms most effectively addressed by prazosin, such that the selection of those with high baseline levels of nightmares resulted in the selection of a population with high baseline levels of particularly hyperarousal symptoms.
Third, we hypothesized that if the reason some symptoms were more responsive to prazosin than others was that they were most directly linked to increased signaling through the α1 AR, and noradrenergic signaling often increases or decreases generally throughout then brain, then these symptoms might be expected to fluctuate together at the level of individuals, as well. To test this hypothesis, we used partial correlation coefficients (PCC) to quantify the extent to which any given pair of symptoms covaried across the population of participants. Consistent with our hypothesis, we found that pairs of symptoms where both symptoms were highly prazosin responsive covaried significantly more than pairs of symptoms where only one symptom was highly prazosin responsive.
The PCC analysis has substantial limitations. Specifically, it is also possible that this type of a finding could be simply a statistical artifact of the highly prazosin responsive items having higher baseline severity and greater total change, particularly in the participants who received prazosin rather than placebo. Supporting this possibility, we found that the higher degree of covariance between pairs of symptoms where both were highly prazosin responsive versus where only one was highly prazosin responsive was no longer statistically significant when the PCCs were adjusted for both baseline symptom severity and the change in total symptom severity. Surprisingly, however, when we performed these analyses separately for participants who had received prazosin versus participants who had received placebo, we found that the higher covariance of symptoms when both symptoms were in the highly prazosin responsive group was not only still present in the participants who had received placebo, but remained statistically significant when the analysis was adjusted for baseline symptom severity and change in total symptom severity. This finding is less consistent with the higher degree of covariance between highly prazosin responsive items being a statistical artifact of how the symptoms were chosen, but is consistent with the original hypothesis that these symptoms are more likely to covary because they are pathophysiologically related, even in the absence of pharmacologic perturbation by prazosin.
Another important limitation of this analysis is that the binary division of symptoms into “highly prazosin responsive” and not highly prazosin responsive was made using the relatively arbitrary cutoff of statistical significance of the difference in mean change in the prazosin versus placebo groups. Even if there is a group of symptoms which are both more highly prazosin responsive and which share a common underlying pathophysiology, the symptoms identified here as highly prazosin responsive may inappropriately exclude symptoms that were for example of lower baseline severity in the population or for other reason missed the threshold for statistical significance. Further, there may not be a binary division – symptoms, which are clinically defined, may vary in the degree to which they reflect noradrenergically driven pathophysiology. To explore the extent to which the division used for highly prazosin responsive symptoms appears to reflect a biologically driven division, we also repeated the analyses dividing the non-highly prazosin responsive group into a medium prazosin responsivity group and a lowest prazosin responsivity group. We found that pairs of symptoms where one item was in the highly prazosin responsive group and one in the medium responsivity group covaried significantly more than pairs of symptoms when one item was in the highly prazosin responsive group and one was in the lowest responsivity group, suggesting the specific division between highly prazosin responsive symptoms and symptoms with medium responsivity does not correspond to a clear biologic division, and that many of the symptoms that displayed an intermediate prazosin responsivity may also share a common underlying pathophysiology.
There are a variety of potential mechanisms that could produce the observed pattern of a group of symptoms showing both more prazosin-responsivity and more association with each other in their degree of change at the level of individuals. First, there may be multiple neurotransmitter systems disrupted, with some symptoms being more related to noradrenergic dysregulation than others. This possibility would be consistent with evidence for both noradrenergic and serotonergic disruptions in PTSD.30 Second, different symptoms may depend on different parts of the noradrenergic signaling pathways for their expression,8 with the α1 AR blockade of prazosin blocking only a subset of noradrenergically modulated symptoms. Similarly, previously published analysis of the same trial examined here has found evidence suggesting that heterogeneity in the role of α1 AR upregulation in PTSD symptom production may be associated with who is more responsive to prazosin.31 Finally, the distribution of symptom responses seen here could also result if there were a direct connection between noradrenergic dysregulation and some symptoms, such as hyperarousal symptoms, while other symptoms, such avoidance and decreased engagement, were secondary, learned responses to the experience of the initial, more directly noradrenergic symptoms. In this situation, you might see much more coherent and consistent decreases in directly produced symptoms, while the change in secondary symptoms might be more gradual and variable.
These analyses have important limitations. They are post hoc analyses, based on a relatively small data set. The statistical issue of false positives associated with performing post hoc subgroup analyses in clinical trials is well documented.32–36 However, as Alosh et al.37 point out, in many instances subgroup analyses were critical for discovering new treatments or revising the population for treatment use. We therefore present our findings as suggestions for hypotheses to test in future clinical trials. It will be particularly important to test whether these findings are consistent in other affected populations, or whether a change in selection criteria, military service status, or baseline symptom distribution significantly change the results. In addition, a larger sample would allow for meaningful clustering and factor analyses.
Conclusions
Despite methodological limitations, these results demonstrate the potential utility of item covariance as a novel strategy for identifying pathophysiologically-related symptoms in clinical trial data, and lend support to the presence of a pathophysiologically-related grouping of PTSD symptoms that crosses the diagnostic symptom clustering as defined in both the DSM-IV and DSM 5.
Supplemental Material
Supplemental material, sj-pdf-1-css-10.1177_2470547020979780 for The Relative Effects of Prazosin on Individual PTSD Symptoms: Evidence for Pathophysiologically-Related Clustering by Rebecca C. Hendrickson, Steven P. Millard, Kathleen F. Pagulayan, Elaine R. Peskind and Murray A. Raskind in Chronic Stress
Acknowledgements
The authors are grateful to the active duty soldiers who participated in the original clinical trial, and to Jane Shofer for her assistance with earlier drafts of this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Raskind is a paid advisory board member of Pfizer Laboratories, Merck, and Takeda Pharmaceuticals. Dr. Peskind is a paid advisory board member for Lilly, Takeda, Merck, and Avanir pharmaceuticals. All other authors report no financial relationships with commercial interests.
The views expressed are those of the authors and do not reflect the official policy of the Department of Veterans Affairs or the U.S. Government.
The investigators have adhered to the policies for protection of human participants as prescribed in 45 CFR 46.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Veterans Affairs (VA) Clinical Sciences Research and Development Service Career Development Award IK2CX001774 (RCH); VA Northwest Network MIRECC (RCH, MAR, ERP, SPM, KAP); VA Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment (RCH); and the U.S. Army Medical Research and Materiel Command, Fort Detrick, Maryland. The funding sources had no input in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
ORCID iD: Rebecca C. Hendrickson https://orcid.org/0000-0003-2377-823X
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003; 160(2):371–373. [DOI] [PubMed] [Google Scholar]
- 2.Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007; 61(8):928–934. [DOI] [PubMed] [Google Scholar]
- 3.Taylor FFB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008; 63(6):629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012; 72(2):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013; 170(9):1003–1010. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014; 69(4):235–242. [DOI] [PubMed] [Google Scholar]
- 7.Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for Post-Traumatic stress disorder in military veterans. N Engl J Med. 2018; 378(6):507–517. [DOI] [PubMed] [Google Scholar]
- 8.Hendrickson RC, Raskind MA. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp Neurol. 2016; 284(Pt B):181–195. [DOI] [PubMed] [Google Scholar]
- 9.Strawn JR, Geracioti TD. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008; 25(3):260–271. [DOI] [PubMed] [Google Scholar]
- 10.Charney DDS, Deutch AAY, Krystal JJH, et al. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry. 1993; 50(4):294–305. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014; 10:679–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McSweeney LB, Koch EI, Saules KK, et al. Exploratory factor analysis of diagnostic and statistical manual. 5th edition, criteria for posttraumatic stress disorder J Nerv Ment Dis. 2016; 204:9–14. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 14.Yufik T, Simms LJ. A meta-analytic investigation of the structure of posttraumatic stress disorder symptoms. J Abnorm Psychol. 2010; 119(4):764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meltzer-Brody S, Connor KM, Churchill E, et al. Symptom-specific effects of fluoxetine in post-traumatic stress disorder. Int Clin Psychopharmacol. 2000; 15(4):227–231. [DOI] [PubMed] [Google Scholar]
- 16.Davidson JRT, Landerman LR, Farfel GM, et al. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002; 32(4):661–670. [DOI] [PubMed] [Google Scholar]
- 17.Stein DJ, Rothbaum BO, Baldwin DS, et al. A factor analysis of posttraumatic stress disorder symptoms using data pooled from two venlafaxine extended-release clinical trials. Brain Behav. 2013; 3(6):738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Development Core Team. R: A Language End Environment for Statistical Computing. Vienna, Austria: R Development Core Team; 2017. http://www.r-project.org [Google Scholar]
- 19.RStudio Team. RStudio: Integrated Development for R. Boston, MA: RStudio Team; 2018. http://www.rstudio.com/ [Google Scholar]
- 20.Bates D, Maechler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1):1–48.
- 21.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer, 2016.
- 22.Millard SP. EnvStats: An R Package for Environmental Statistics New York: Springer; 2013. [Google Scholar]
- 23.Canty A, Ripley B. boot: Bootstrap R (S-Plus) Functions.
- 24.Davison A, Hinkley D. Bootstrap Methods and Their Applications. Cambridge, England: Cambridge University Press, 1997. [Google Scholar]
- 25.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007; 82(4):591–605. [DOI] [PubMed] [Google Scholar]
- 26.McCall JGG, Al-Hasani R, Siuda ERR, et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015; 87(3):605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhardwaj P, Yadav RK. Measuring pain in clinical trials: Pain scales, endpoints, and challenges. Int J Clin Exp Physiol. 2015; 2(3):151–156. [Google Scholar]
- 28.Marder SR, Laughren T, Romano SJ. Why are innovative drugs failing in phase III? Am J Psychiatry. 2017; 174(9):829–831. [DOI] [PubMed] [Google Scholar]
- 29.Mellman TA, Kumar A, Kulick-Bell R, et al. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995; 38(3):174–179. [DOI] [PubMed] [Google Scholar]
- 30.Southwick SM, Krystal JH, Bremner JD, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997; 54(8):749–758. [DOI] [PubMed] [Google Scholar]
- 31.Raskind MA, Millard SP, Petrie EC, et al. Higher pretreatment blood pressure is associated with greater PTSD symptom reduction in soldiers treated with prazosin. Biol Psychiatry. 2016; 80(10):736–742. [DOI] [PubMed] [Google Scholar]
- 32.Pocock SJ, Assmann SE, Enos LE, et al. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002; 21(19):2917–2930. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007; 357(21):2189–2194. [DOI] [PubMed] [Google Scholar]
- 34.Burke JF, Sussman JB, Kent DM, et al. Three simple rules to ensure reasonably credible subgroup analyses. BMJ. 2015; 351:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alosh M, Fritsch K, Huque M, et al. Statistical considerations on subgroup analysis in clinical trials. Stat Biopharm Res. 2015; 7(4):286–303. [Google Scholar]
- 36.Alosh M, Huque MF, Bretz F, et al. Tutorial on statistical considerations on subgroup analysis in confirmatory clinical trials. Stat Med. 2017; 36(8):1334–1360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-css-10.1177_2470547020979780 for The Relative Effects of Prazosin on Individual PTSD Symptoms: Evidence for Pathophysiologically-Related Clustering by Rebecca C. Hendrickson, Steven P. Millard, Kathleen F. Pagulayan, Elaine R. Peskind and Murray A. Raskind in Chronic Stress