ABSTRACT
Objectives
HIV population is aging at an earlier age than those uninfected, requiring more non-HIV medications to treat noncommunicable diseases. In the context of chronic HIV infection, the next therapeutic change would be the polymedication control. This paper has the purpose of explore the attitudes of older people living with HIV toward deprescribing.
Material and methods
This was an observational, prospective and multicenter study conducted from March-April, 2018. People living with HIV (PLWH) on highly active antiretroviral therapy and older than 65 years were included. In addition to demographic and pharmacotherapeutic data, attitudes regarding deprescribing were collected through the “Revised Patients’ Attitudes Towards Deprescribing Questionnaire”.
Results
A total of 42 patients were included in this study. Regarding their attitudes in relation to deprescription, there were three statements with the most consensuses. The first (“I have a good understanding of the reasons I was prescribed each of my medicines”) had 91.9% consensus. The second and third questions showed 89.2% consensus in both cases; “Overall, I am satisfied with my current medicines” and “I like to be involved in making decisions about my medicines with my doctors”.
Conclusions
This study is the first to explore the beliefs and attitudes of older PLWH in relation to deprescription process. There are positive attitudes regarding medication knowledge but there also is a percentage of patients who had a negative opinion regarding deprescription. We must study and go deeper in our knowledge of techniques that could help us to better understand their preferences, in order to establish effective and successful deprescription strategies.
Key-words: older HIV, beliefs, attitudes, deprescription
RESUMEN
Objetivos
La población VIH envejece a edades más tempranas que la población no infectada, requiriendo más medicamentos para tratar el resto de enfermedades concomitantes. Por tanto, en el contexto de infección crónica por VIH, el siguiente desafío terapéutico sería el control de la polimedicación. Este trabajo tiene el propósito de explorar las actitudes y creencias de pacientes VIH de edad avanzada con respecto a la desprescripción.
Material y métodos
Se trata de un estudio observacional, prospectivo y multicéntrico realizado desde marzo hasta abril de 2018. Se incluyeron pacientes VIH en tratamiento con terapia antirretroviral mayores de 65 años. Además de datos demográficos y farmacoterapéuticos, se recogieron las actitudes y creencias en relación con la desprescripción a través del cuestionario “Revised Patients’ Attitudes Towards Deprescribing”.
Resultados
Se incluyeron un total de 42 pacientes. En relación a sus creencias y actitudes, se obtuvo mayor consenso en tres preguntas. La primera (“Entiendo las razones de por qué me han prescrito mis medicamentos”) tuvo un consenso del 91,9%. En la segunda (“En general, estoy satisfecho con mis medicamentos actuales”) y tercera pregunta (“Me gusta estar involucrado en la toma de decisiones sobre mis medicamentos”) se obtuvo un 89,2% de consenso en ambos casos.
Conclusiones
Este estudio es el primero en explorar las creencias y actitudes de pacientes VIH de edad avanzada en relación con la desprescripción. Se han encontrado actitudes po- sitivas con respecto al conocimiento de la medicación; también existe un porcentaje de pacientes con opinión negativa acerca de la desprescripción. Es necesario profundizar en el conocimiento de técnicas que nos puedan ayudar a entender mejor las preferencias de los pacientes, con el objetivo de establecer estrategias de desprescripción adecuadas y efectivas.
PALABRAS CLAVE: VIH edad avanzada, creencias, actitudes, desprescripción
INTRODUCTION
The world’s HIV population is successfully aging [1] at an earlier age than those uninfected, requiring more non-HIV medications to treat noncommunicable diseases (NCD) [2–4]. The Dutch cohort ATHENA [2] estimates that by 2030, 73% of HIV-infected patients will be over the age of 50, 84% will have at least one comorbidity, and 20% will receive ≥3 non-HIV medications.
Polypharmacy (used of 6 or more drugs) is an increasingly serious public health issue [5]. However, in recent years, the term of “excessive polypharmacy”, which describes the use of 10 or more drugs [6], has been introduced. In people living with HIV (PLWH), this is especially worrisome since in some studies the prevalence of polypharmacy and excessive polypharmacy was 56% and 10%, respectively [7]. In the context of chronic HIV infection, the next therapeutic change in these patients would be the polymedication control [8].
The effect of aging and polypharmacy could increase the risk of drug interactions risk and adverse events [8,9], the lack of adherence to HIV and non-HIV medication [8,10], the risk of hospitalizations [8,11], falls [8,12] and death [8,13]. Many of these adverse events could be potentially preventable. All of this leads to the need for greater attention to the aging HIV population, as they are more complex patients than the general population. In addition, it is necessary to establish comprehensive care processes for these patients from all areas, including the pharmacotherapeutic field, always keeping the patient as a priority. At the present time, there is limited knowledge of deprescription in older-PLWH (OPLWH). For this reason, it is necessary to carry out research on this type of intervention in the HIV-infected population.
The aim of this study was to explore the attitudes of OPLWH toward deprescribing. Additionally, we sought to understand whether clinical and demographic characteristics were associated with these attitudes.
MATERIAL AND METHODS
Consent to participate: This study was approved by the Seville-Sur Ethics Committee. All participants provided written informed consent.
Patients. This was an observational, prospective and multicenter study (two centres participated) conducted from March-April, 2018. PLWH on highly active antiretroviral therapy (ART) and older than 65 years were included. All participants provided written informed consent. Patients were followed up by the Pharmaceutical Care Consultation of Viral Diseases from the Hospital Pharmacy Service. Patients participating in clinical trials, with a malign neoplastic or other systemic autoimmune disease as well as those who did not give their written consent were excluded.
The socio-demographic information and comorbidities were collected from the electronic medical record. HIV-related characteristics included route of acquisition, current CD4-T cell count, CD4/CD8 ratio, and ART regimen, among other were also evaluated.
Multimorbidity was defined as the presence of >3 NCD. NCD data records included in this study were: cardiovascular disease, hepatic diseases, metabolic and endocrine disorders (diabetes mellitus), central nervous system diseases, renal diseases (chronic renal failure), lung disease (chronic obstructive pulmonary diseases), neuropsychiatric diseases (depression, dementia, schizophrenia, bipolar disorder, psychosis), and bone disease (osteoporosis, bone fracture).
Regarding pharmacotherapeutic endpoints, we evaluated: type of concomitant medication prescribed, concomitant medication associated with fall risk (loop diuretics, antipsychotics, antidepressants, benzodiazepines, opioids, antiepileptics); ART regimen, polypharmacy, total medication adherence, and complexity index by Medication Regimen Complexity Index (MRCI) [14]. Patients were classified in low MRCI (<11) or high MRCI (≥11) [7], according to the total MRCI (MRCI ART plus concomitant medication). This score has previously been validated [15,16] and applicable to children and adults with HIV. Adherence to ART was measured with the Simplified Medication Adherence Questionnaire (SMAQ) [17] and hospital dispensing records [18]. Adherence to concomitant medication was measure with the Morisky-Green questionnaire (MMAS) [19] and electronic pharmacy dispensing records. For both types of treatment, the multi-interval adherence index will be used for the last 6 months of treatment.
We obtained the number of comorbidities and comedications for other chronic diseases (non-HIV drugs) from review of the medical history and electronic health prescriptions program of Andalusia and La Rioja Public Health System.
Based on the complexity index, the MRCI was calculated using a web tool of Colorado University [14] an adaptation of the score created by Martin et al. [15]. This validated tool includes 65 items grouped into three subgroups: dose forms, dosing frequencies, and additional instructions relevant to drug administration.
The Revised Patients’ Attitudes Towards Deprescribing (rPATD) Questionnaire (Versions for Older Adults and Caregivers) [20] contains 22 (older adults) and 19 (caregiver) questions, designed to assess individuals’ attitudes, beliefs, and experiences regarding their medications, and the potential withdrawal of 1 or more of their medications. The questionnaire has established face, content, criterion, construct, and internal validity, as well as test-retest reliability in Australian older adults [20]. The results are self-reported attitudes, are not medication spe- cific, and are hypothetical in relation to intentions. These statements had response categories of “strongly agree,” “agree,”, “I am not sure”, “disagree,” and “strongly disagree”. We used the Spanish version of rPATD, adapted by De Juan et al. [21].
Statistical analysis. Discrete variables were expressed as counts (percentage) and continuous variables as medians and interquartile ranges (IQRs) or means and standard deviation (SD). Differences in categorical variables were calculated using a two-sided likelihood ratio chi-square test or Fisher exact test, and the t-student test or Mann-Whitney nonparametric test were used for continuous variables, when appropriate. The adjustment or not to normality will be verified by means of the Kolmogorov-Smirnov or Shapiro-Wilk tests, according to the size of the groups.
Participant responses to the questions in the Medication Attitudes module are reported as proportions. We focused on three of the rating-scale statements as the main outcomes of interest as they had more consensuses in their answers. A 5-category scale from strongly disagree to strongly agree was employed. The responses to these questions were converted to a binary variable of agree (strongly agree or agree) and disagree (I am not sure, disagree or strongly disagree), which was then examined using logistic regression to assess unadjusted associations between the three main statements and respondents’ demographic and clinical characteristics. A logistic regression model was then used to assess the the likelihood of willingness to stop and wanting to reduce drugs use after adjusting for demographic and clinical characteristics. Data were analyzed using IBM SPSS Statistics version 20.0 software. The threshold for statistical significance was defined as p<0.05.
Ethics statement. This study was approved by the Seville-Sur Ethics Committee (reference 0628-N-18). All participants provided written informed consent.
RESULTS
A total of 42 patients were included in this study and 78.6% (n =33) were male. The median age was 70 years [IQR = 68-76]. Thirty-seven patients answered the survey themselves; in five cases, the caregiver provided the answer. The baseline features characteristics of the patients are shown in Table 1. Table 2 also showed the attitudes of the patients about the three most consensuses.
Table 1.
Caracteristics of older adults
| Respondent Characteristics | Total (n=42) |
|---|---|
| Age (years); n (%) | |
| < 70 | 20 (47.6) |
| ≥70 | 22 (52.4) |
| Sex; n (%) | |
| Men | 33 (78.6) |
| Women | 9 (21.4) |
| Nationality; n (%) | |
| Spanish | 39 (92.9) |
| Others | 3 (7.1) |
| Education; n (%) | |
| Being able to read but no studies/primary studies | 37 (88.1) |
| High school/completed university studies | 5 (11.9) |
| Patient/caregiver relationship (if caregiver survey); n (%) | |
| Son/daughter | 4 (80) |
| Other relationship | 1 (20) |
| Patient’s Residence; n (%) | |
| Unaccompanied home | 12 (28.6) |
| Home with relatives/ Nursing homes | 30 (71.4) |
| Acquisition risk factor; n (%) | |
| Sexual | 37 (88.1) |
| Parenteral | 5 (11.9) |
| Viral load; n (%) | |
| Undetectable (< 50 copies/mL) | 41 (97.6) |
| Detectable (> 50 copies/mL) | 1 (2.4) |
| CD4 (cell/µL); n (%) | |
| < 200 cell/µL | 5 (11.9) |
| ≥200 cell/µL | 37 (88.1) |
| CD4/CD8; n (%) | |
| < 0.7 | 22 (52.4) |
| ≥0.7 | 20 (47.6) |
| Viral Hepatopathies; n (%) | |
| Yes | 7 (16.7) |
| No | 35 (83.3) |
| Dyslipemia; n (%) | |
| Yes | 31 (73.8) |
| No | 11 (26.2) |
| Central nervous system diseases; n (%) | |
| Yes | 8 (19.1) |
| No | 34 (80.9) |
| Cardiovascular risk; n (%) | |
| Yes | 18 (42.9) |
| No | 24 (57.1) |
| Hypertension; n (%) | |
| Yes | 19 (45.2) |
| No | 23 (54.8) |
| Respondent Characteristics | Total (n=42) |
| Diabetes mellitus; n (%) | |
| Yes | 28 (66.7) |
| No | 14 (33.3) |
| Other comorbidities (total number); n (%) | |
| < 1 | 10 (23.8) |
| ≥1 | 32 (76.2) |
| Total number of comorbidities; n (%) | |
| < 3 | 11 (26.2) |
| ≥3 | 31 (73.8) |
| Pluripathological patient (3 or more diseases) | |
| N, (%) | 32 (76.2) |
| Type of concomitant medication prescribed; n (%) | |
| Antiulcerous /antiacids | 19 (45.2) |
| Psychoactive drugs | 10 (23.8) |
| Hypolipemics | 28 (66.7) |
| Antihypertensive and cardiovascular risk drugs | 29 (69.0) |
| Antidiabetics | 12 (28.6) |
| Concomitant medication associated with fall risk; n (%) | |
| Yes | 19 (45.2) |
| No | 23 (54.8) |
| Type of ART therapy; n (%) | |
| Two NRTI plus NNRTI | 8 (19.1) |
| Two NRTI plus b/PI | 5 (11.9) |
| Two NRTI plus INSTI | 18 (42.8) |
| Others | 11 (26.2) |
| ART situation; n (%) | |
| Naive | 4 (9.5) |
| Rescue | 9 (21.4) |
| Multifailure | 29 (69.1) |
| Therapy type; n (%) | |
| Monotherapy | 2 (4.7) |
| Bitherapy | 8 (19.1) |
| Triple-therapy | 32 (76.2) |
| Polymedicated patients (≥6 drugs, including ART) | |
| N, (%) | 20 (47.6) |
| Total medication adherence | |
| N, (%) | 31 (73.8) |
| Complexity index by MRCI; n (%) | |
| Low MRCI (< 11) | 18 (42.9) |
| High MRCI (≥11) | 24 (57.1) |
NRTI; nucleoside reverse transcriptase inhibitors, NNRTI; non-nucleoside reverse transcriptase inhibitor, b/PI; boosted protease inhibitor, INSTI; integrase strand transfer inhibitor, MRCI; Medication Regimen Complexity Index
Table 2.
Statistical results of the univariate analysis
| Respondent characteristics | “I have a good understanding of the reasons I was prescribed each of my medicines” (n=37) | p |
OR (95% CI) |
“Overall, I am satisfied with my current medicines” (n=37) | p |
OR (95% CI) | “I like to be involved in making decisions about my medicines with my doctors” (n=37) | p |
OR (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disagree n (%) |
Agree n (%) |
Disagree n (%) |
Agree n (%) |
Disagree n (%) |
Agree n (%) |
||||||||||||
| Cardiovascular risk; n (%) | |||||||||||||||||
| No | 0 (0.0) | 23 (62.2) | 0.047 | 1.273 (0.968-1.673) | 2 (5.4) | 21 (56.8) | 0.625 | - | 2 (5.4) | 21 (56.8) | 0.625 | - | |||||
| Yes | 3 (8.1) | 11 (29.7) | 2 (5.4) | 12 (32.4) | - | 2 (5.4) | 12 (32.4) | - | |||||||||
| Hypertension; n (%) | |||||||||||||||||
| No | 1 (2.7) | 17 (46.0) | 1.000 | - | 1 (2.7) | 17 (46.0) | 0.604 | - | 1 (2.7) | 17 (46.0) | 0.604 | - | |||||
| Yes | 2 (5.4) | 17 (46.0) | - | 3 (8.1) | 16 (43.2) | - | 3 (8.1) | 16 (43.2) | - | ||||||||
| Diabetes mellitus; n (%) | |||||||||||||||||
| No | 3 (8.1) | 21 (56.8) | 0.538 | - | 3 (8.1) | 21 (56.8) | 1.000 | - | 3 (8.1) | 21 (56.8) | 0.276 | - | |||||
| Yes | 0 (0.0) | 13 (35.1) | - | 1 (2.7) | 12 (32.4) | - | 0 (0.0) | 13 (35.1) | - | ||||||||
| Type of concomitant medication prescribed; n (%) | |||||||||||||||||
| Antiulcerous /antiacids | 2 (5.4) | 14 (37.8) | 0.568 | - | 3 (8.1) | 13 (35.1) | 0.296 | - | 3 (8.1) | 13 (35.1) | 0.296 | - | |||||
| Psychoactive drugs | 1 (2.7) | 7 (18.9) | 0.530 | - | 1 (2.7) | 7 (18.9) | 1.000 | - | 1 (2.7) | 7 (18.9) | 1.000 | - | |||||
| Hypolipemics | 1 (2.7) | 22 (59.5) | 0.544 | - | 3 (8.1) | 20 (54.1) | 1.000 | - | 3 (8.1) | 20 (54.1) | 1.000 | - | |||||
| Antihypertensive and cardiovascular risk drugs | 3 (8.1) | 22 (59.5) | 0.537 | - | 4 (10.8) | 21 (56.8) | 0.282 | - | 4 (10.8) | 21 (56.8) | 0.282 | - | |||||
| Antidiabetics | 1 (2.7) | 10 (27.0) | 1.000 | - | 2 (5.4) | 9 (24.3) | 0.567 | - | 1 (2.7) | 10 (27.0) | 1.000 | - | |||||
| Type of ART therapy; n (%) | |||||||||||||||||
| Two NRTI plus NNRTI | 0 (0.0) | 7 (18.9) | 0.188 | - | 1 (2.7) | 6 (16.2) | 0.607 | - | 0 (0.0) | 7 (18.9) | 0.428 | - | |||||
| Two NRTI plus b/PI | 1 (2.7) | 4 (10.8) | - | 0 (0.0) | 5 (13.5) | - | 0 (0.0) | 5 (13.5) | - | ||||||||
| Two NRTI plus INSTI | 0 (0.0) | 15 (40.5) | - | 1 (2.7) | 14 (37.8) | - | 3 (8.1) | 12 (32.4) | - | ||||||||
| Others | 2 (5.4) | 8 (21.6) | - | 2 (5.4) | 8 (21.6) | - | 1 (2.7) | 9 (24.3) | - | ||||||||
| Cardiovascular risk; n (%) | |||||||||||||||||
| No | 0 (0.0) | 23 (62.2) | 0.047 | 1.273 (0.968-1.673) | 2 (5.4) | 21 (56.8) | 0.625 | - | 2 (5.4) | 21 (56.8) | 0.625 | - | |||||
| Yes | 3 (8.1) | 11 (29.7) | 2 (5.4) | 12 (32.4) | - | 2 (5.4) | 12 (32.4) | - | |||||||||
| Hypertension; n (%) | |||||||||||||||||
| No | 1 (2.7) | 17 (46.0) | 1.000 | - | 1 (2.7) | 17 (46.0) | 0.604 | - | 1 (2.7) | 17 (46.0) | 0.604 | - | |||||
| Yes | 2 (5.4) | 17 (46.0) | - | 3 (8.1) | 16 (43.2) | - | 3 (8.1) | 16 (43.2) | - | ||||||||
| Diabetes mellitus; n (%) | |||||||||||||||||
| No | 3 (8.1) | 21 (56.8) | 0.538 | - | 3 (8.1) | 21 (56.8) | 1.000 | - | 3 (8.1) | 21 (56.8) | 0.276 | - | |||||
| Yes | 0 (0.0) | 13 (35.1) | - | 1 (2.7) | 12 (32.4) | - | 0 (0.0) | 13 (35.1) | - | ||||||||
| Type of concomitant medication prescribed; n (%) | |||||||||||||||||
| Antiulcerous /antiacids | 2 (5.4) | 14 (37.8) | 0.568 | - | 3 (8.1) | 13 (35.1) | 0.296 | - | 3 (8.1) | 13 (35.1) | 0.296 | - | |||||
| Psychoactive drugs | 1 (2.7) | 7 (18.9) | 0.530 | - | 1 (2.7) | 7 (18.9) | 1.000 | - | 1 (2.7) | 7 (18.9) | 1.000 | - | |||||
| Hypolipemics | 1 (2.7) | 22 (59.5) | 0.544 | - | 3 (8.1) | 20 (54.1) | 1.000 | - | 3 (8.1) | 20 (54.1) | 1.000 | - | |||||
| Antihypertensive and cardiovascular risk drugs | 3 (8.1) | 22 (59.5) | 0.537 | - | 4 (10.8) | 21 (56.8) | 0.282 | - | 4 (10.8) | 21 (56.8) | 0.282 | - | |||||
| Antidiabetics | 1 (2.7) | 10 (27.0) | 1.000 | - | 2 (5.4) | 9 (24.3) | 0.567 | - | 1 (2.7) | 10 (27.0) | 1.000 | - | |||||
| Type of ART therapy; n (%) | |||||||||||||||||
| Two NRTI plus NNRTI | 0 (0.0) | 7 (18.9) | 0.188 | - | 1 (2.7) | 6 (16.2) | 0.607 | - | 0 (0.0) | 7 (18.9) | 0.428 | - | |||||
| Two NRTI plus b/PI | 1 (2.7) | 4 (10.8) | - | 0 (0.0) | 5 (13.5) | - | 0 (0.0) | 5 (13.5) | - | ||||||||
| Two NRTI plus INSTI | 0 (0.0) | 15 (40.5) | - | 1 (2.7) | 14 (37.8) | - | 3 (8.1) | 12 (32.4) | - | ||||||||
| Others | 2 (5.4) | 8 (21.6) | - | 2 (5.4) | 8 (21.6) | - | 1 (2.7) | 9 (24.3) | - | ||||||||
| Therapy type; n (%) | |||||||||||||||||
| Triple-therapy | 0 (0.0) | 27 (73.0) | 0.015 | 1.429 (0.952-2.143) | 2 (5.4) | 25 (67.6) | - | 3 (8.1) | 24 (64.9) | - | |||||||
| Monotherapy | 3 (8.1) | 7 (18.9) | 0 (0.0) | 2 (5.4) | 0.327 | - | 0 (0.0) | 2 (5.4) | 0.874 | - | |||||||
| Bitherapy | 2 (5.4) | 6 (16.2) | - | 1 (2.7) | 7 (18.9) | - | |||||||||||
| Polymedicated patients (≥6 drugs, including ART) | |||||||||||||||||
| N, (%) | 2 (5.4) | 14 (37.8) | 0.568 | - | 4 (10.8) | 12 (32.4) | 0.028 | 1.333 (1.005-1.769) | 3 (8.1) | 13 (35.1) | 0.296 | - | |||||
| Complexity index by MRCI; n (%) | |||||||||||||||||
| Low MRCI (< 11) | 1 (2.7) | 16 (43.2) | 1.000 | - | 0 (0.0) | 17 (46.0) | 0.109 | - | 1 (2.7) | 16 (43.2) | 0.609 | - | |||||
| High MRCI (≥11) | 2 (5.4) | 18 (48.7) | - | 4 (10.8) | 16 (43.2) | - | 3 (8.1) | 17 (46.0) | - | ||||||||
OD; Odds ratio, Confidence Interval; CI, NRTI; nucleoside reverse transcriptase inhibitors, NNRTI; non-nucleoside reverse transcriptase inhibitor, b/PI; boosted protease inhibitor, INSTI; integrase strand transfer inhibitor, MRCI; Medication Regimen Complexity Index
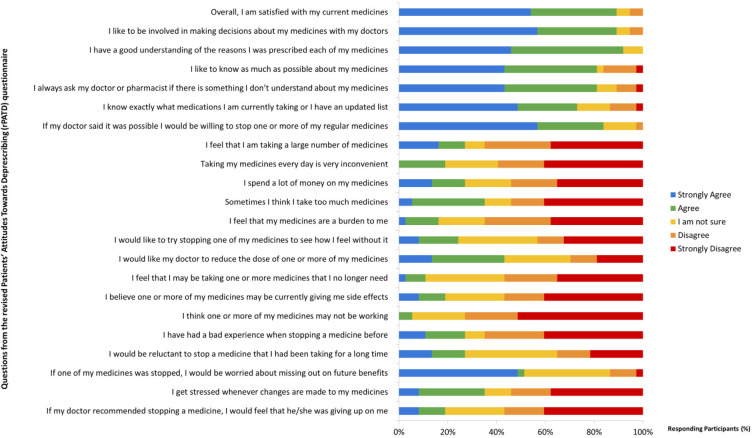
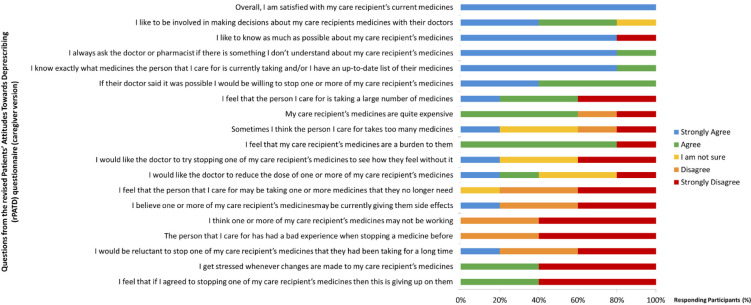
Regarding their beliefs and attitudes in relation to deprescription reported by the own patients, the results were heterogeneous. The answer to each question asked to both patients and caregivers are shown in Figure 1 and 2, respectively. Three were the statements with the most consensuses. The first (“I have a good understanding of the reasons I was prescribed each of my medicines”) had 91.9% consensus (agree or strongly agree). The second and third questions showed 89.2% consensus (agree or strongly agree) in both cases; “Overall, I am satisfied with my current medicines” and “I like to be involved in making decisions about my medicines with my doctors”.
Figure 1.
Older VIH+ adults’ attitudes toward their medications and deprescribing
Figure 2.
Caregivers’ attitudes toward their care medications and deprescribing
Other statement with broad consensus and special relevance to the issue of attitudes towards deprescription was “If my doctor said it was possible, I would be willing to stop one or more of my regular medicines”; 83.8% consensus (agree or strongly agree). The last interesting statement having a high consensus showed 73% consensus (strongly disagree or disagree); “I think one or more of my medicines may not be working”.
In the caregiver´s questionnaire, we can highlight four statements, all of them 100% consensus (agree or strongly agree): “Overall, I am satisfied with my care recipient’s current medicines”; “I always ask the doctor, pharmacist or other healthcare professional if there is something I don’t understand about my care recipient’s medicines”; “I know exactly what medicines the person that I care for is currently taking and/or I have an up-to-date list of their medicines”; and “If their doctor said it was possible I would be willing to stop one or more of my care recipient’s medicines”. On the other hand, 80% of caregivers (agree or strongly agree) felt that “My care recipient’s medicines are a burden to them”.
The results of the univariate analysis were heterogeneous, so we did not perform a multivariate analysis subsequently. Relevant results were shown in Table 2. We could argument, on the basis of the univariate analysis that, on the question “I have a good understanding of the reasons I was prescribed each of my medicines”, people with cardiovascular disease seemed to be more in agreement with this issue (p= 0.048; [95% Confidence Interval (CI) 0.968-1.673]. The same happened with patients with triple-therapy in comparison with mono or bi-therapy [both considered as Less-Drug Regime (LDR)], p= 0.015; [95% CI 0.952-2.143]. Regarding question “Overall, I am satisfied with my current medicines”, having no central nervous system pathology was positively related to being satisfied with treatment, p= 0.026; [95% CI 0.005-0.693]. In contrast, patients with central nervous system pathology were 16.8 times more likely to be dissatisfied with their treatment. Patients who were not polymedicated were 1.33 times more likely to say that they are satisfied with their medication, p= 0.028; [95% CI 1.005-1.769].
DISCUSSION
We explored the attitudes of OPLWH toward deprescribing. These patients showed positive attitudes regarding their medication knowledge and concerned to be properly informed about medications they were taking. Something similar are reported by caregivers. However, regarding to the discomfort of taking medication, there was no clear consensus, showing that not all patients were uncomfortable taking their medication. By contrast, there was a higher percentage of caregivers who thought that the medicines taken by the person they care for, were a burden on them. Also, they felt that the person they care for was taking too many medicines. It is important to keep in mind that the opinions about therapy of the patients differ from the opinions of the people who take care of them. Without a doubt this will force us to establish a therapeutic consensus with our patients.
In terms of key questions about deprescription, there were a percentage of patients who had a negative opinion regarding these items. This situation could imply a negative attitude towards deprescription because not all patients were receptive to it. In this sense, will be necessary to explain the benefits that deprescription could have for some of the patients [22]. The process of identifying attitudes and beliefs in relation to the deprescription has been evaluated in elderly and polymedicated patients [23]. To our knowledge, no study had evaluated it in OPLWH. In Switzerland, Zechmann et al. [23] performed an exploratory analysis that included, among others, questions about deprescribing. The authors observed that 18 of 19 patients fully trusted their general practitioner, 17 of 19 had participated in shared-decision-making processes even before this study, and 8 of 19 perceived polypharmacy as a substantial burden. They identified patient involvement in deprescribing and coordination of care as key issues for deprescribing among older multimorbid patients with polypharmacy.
It is worth highlighting the role of clinical pharmacist in the area of deprescription. Clear examples could be found in the studies of Whitman et al. [24], Blanco et al. [22] and McNicholl et al. [25]. Of all of them, Blanco et al. [22] specifically focus on HIV-infected persons and concluded that the deprescribing process shared by professionals and patients definitively would improve management of treatment in this population, and this was what our current article wanted to highlight. In this sense, Cooley et al. [26] showed that medication management capabilities in PLWH over 50 years was reduced compared to seronegative patients of the same age range. In this study, OPLWH scored lower on the “Medication Management Test-Revised” and had a higher pill burden.
An interesting aspect to be evaluated was if clinical and demographic characteristics were associated with these attitudes. Unfortunately, the results were not conclusive. There were only statistically significant differences that defined the subgroup of patients with cardiovascular disease as more likely to have a good knowledge of the medications they take. Something similar happened in patients on triple-therapy vs. monotherapy or bitherapy, possibly because triple-therapy treatments usually come in a single-tablet regimen. Also, not having central nervous system pathology seemed to be related to being satisfied with the treatment. The same happened for non-polymedicated patients; probably because of they had to take a lower number of medications.
The main limitation of this study could be the small number of patients, which could limit our analysis, and the loss of information intrinsically related to the dichotomization of the indices that were used. However, this was a multicenter study which contained a representative target population. A relevant aspect of our study was the successfully identification of attitudes and believes about the deprescribing concept in OPLWH. We reckoned that HIV-specialist clinical team interventions must be carried out more frequently and intensively in these current patients, with a methodology focused in their needs and individual characteristics but not only on the prescribed medication. This underlines the importance of an effective patient-focused care model to closely monitor high risk-patients. Future efforts would be necessary to validate the most tailored prospective strategies to improve deprescription process.
CONCLUSIONS
This study is the first to explore the beliefs and attitudes of OPWH in relation to deprescription process. There are positive attitudes regarding medication knowledge and many patients who make no effort to take medication. However, there is a percentage of patients who had a negative opinion regarding deprescription. This situation could involve a regressive attitude towards deprescription, as not all patients would be receptive to it. Also, it is important to note that caregivers’ responses to questions associated with deprescribing are sometimes very different from those of patients without caregivers. We need to assess in more detail our patients’ attitudes towards deprescription, as it helps the process itself to be successful. A more predisposed patient will be able to achieve the pharmacotherapeutic objectives proposed by the healthcare professional in a more efficient way. Thus, we must study and go deeper in our knowledge of techniques that could help us to better understand their preferences, in order to establish effective and successful deprescription strategies.
ACKNOWLEDGEMENTS
The authors would like to thank De Juan Roldán JI et al. for their work in adapting the Spanish version of the questionnaire.
FUNDING
None to declare.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Trickey A, May MT, Vehreschild JJ, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–56. DOI: 10.1016/S2352-3018(17)30066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem AV., et al. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect Dis. 2015;15(7):810–8. DOI: 10.1016/S1473-3099(15)00056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gomez FJ, Rabanaque-Hernandez MJ. Prevalence of concomitant medications in older HIV+ patients and comparison with general population. HIV Clin Trials. 2015;16(3):117–24. DOI: 10.1179/1528433614Z.0000000012 [DOI] [PubMed] [Google Scholar]
- 4.Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gómez FJ, Com-paired-Turlán V, Rabanaque-Hernández MJ. Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clin Interv Aging. 2016;11:1149–57. DOI: 10.2147/CIA.S108072\ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupo de expertos de la Secretaría del Plan Nacional sobre el sida y SEGG. Documento de consenso sobre edad avanzada e infección por el virus de la inmunodeficiencia humana. 2015. [Cited September 19 2020]. Available from: https://www.segg.es/media/descargas/Documento-de-edad-avanzada-y-VIH.pdf
- 6.Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Cumming RG, Handelsman DJ, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin. Pharmacol. Ther. 2012;91(3):521–8. DOI: 10.1038/clpt.2011.258 [DOI] [PubMed] [Google Scholar]
- 7.Morillo-Verdugo R, Robustillo-Cortés MA, Abdel-Kader Martín L, Álvarez de Sotomayor Paz M, Lozano de León Naranjo F, Almeida González CV. Determination of a cutoff value for medication regimen complexity index to predict polypharmacy in HIV+ older patient. Rev Esp Quimioter. 2019;32(5):458–64. PMid: [PMC free article] [PubMed] [Google Scholar]
- 8.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: Polypharmacy. Drugs Aging. 2013;30(8):613-28. DOI: 10.1007/s40266-013-0093-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dequito AB, Mol PGM, van Doormaal JE, Zaal RJ, van den Bemt PMLA, Haaijer-Ruskamp FM, et al. Preventable and non-preventable adverse drug events in hospitalized patients: A prospective chart review in the Netherlands. Drug Saf. 2011;34(11):1089–100. DOI: 10.2165/11592030-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 10.Reeve E, Wiese MD. Benefits of deprescribing on patients’ adherence to medications. Int J Clin Pharm. 2014;36(1):26-9. DOI: 10.1007/s11096-013-9871-z [DOI] [PubMed] [Google Scholar]
- 11.Payne RA, Abel GA, Avery AJ, Mercer SW, Roland MO. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br. J. Clin. Pharmacol. 2014;77(6):1073–82. DOI: 10.1111/bcp.12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner JP, Jamsen KM, Shakib S, Singhal N, Prowse R, Bell JS. Poly-pharmacy cut-points in older people with cancer: how many medications are too many? Support Care Cancer. 2016;24(4):1831–40. DOI: 10.1007/s00520-015-2970-8 [DOI] [PubMed] [Google Scholar]
- 13.Jyrkkä J, Enlund H, Korhonen MJ, Sulkava R, Hartikainen S. Polypharmacy status as an indicator of mortality in an elderly population. Drugs Aging. 2009;26(12):1039–48. DOI: 10.2165/11319530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14.George J, Phun YT, Bailey MJ, Kong DCM, Stewart K. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369-76. DOI: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 15.Martin S, Wolters PL, Calabrese SK, Toledo-Tamula MA, Wood LV, Roby G, et al. The antiretroviral regimen complexity index: A novel method of quantifying regimen complexity. J. Acquir. Immune Defic. Syndr. 2007;45(5):535–44. DOI: 10.1097/qai.0b013e31811ed1f1 [DOI] [PubMed] [Google Scholar]
- 16.Manzano-García M, Pérez-Guerrero C, Álvarez de Sotomayor Paz M, Robustillo-Cortés M de las A, Almeida-González CV, Morillo-Verdugo R. Identification of the Medication Regimen Complexity Index as an Associated Factor of Nonadherence to Antiretroviral Treatment in HIV Positive Patients. Ann. Pharmacother. 2018;52(9):862–7. DOI: 10.1177/1060028018766908 [DOI] [PubMed] [Google Scholar]
- 17.Knobel H, Alonso J, Casado JL, Collazos J, González J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: The GEEMA study. AIDS. 2002. 8;16(4):605–13. DOI: 10.1097/00002030-200203080-00012 [DOI] [PubMed] [Google Scholar]
- 18.Tsai KT, Chen JH, Wen CJ, Kuo HK, Lu IS, Chiu LS, et al. Medication adherence among geriatric outpatients prescribed multiple medications. Am J Geriatr Pharmacother. 2011;10(1):61–8. DOI: 10.1016/j.amjopharm.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 19.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med. Care. 1986;24(1):67–74. DOI: 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 20.Reeve E, Low LF, Shakib S, Hilmer SN. Development and Validation of the Revised Patients’ Attitudes Towards Deprescribing (rPATD) Questionnaire: Versions for Older Adults and Caregivers. Drugs Aging. 2016;33(12):913–28. DOI: 10.1007/s40266-016-0410-1 [DOI] [PubMed] [Google Scholar]
- 21.de Juan Roldán JI, Gavilán Moral E, García Ruiz A. Estudio de validación al castellano del cuestionario Revised Patients’ Attitudes Towards Deprescribing (rPATD) para evaluar las actitudes de los pacientes hacia la deprescripción. 2017. XXXVII Congreso semFYC – Madrid. [Cited September 19 2020]. Available from: https://www.comunicacionescongresosemfyc.com/comunicacion/estudio-de-validacion-al-castellano-del-cuestionario-patients-attitudes-towards-deprescribing-para-evaluar-las-actitudes-de-los-pacientes-hacia-la-deprescripcion-oral [DOI] [PubMed]
- 22.Blanco JR, Morillo R, Abril V, Escobar I, Bernal E, Folguera C, et al. Deprescribing of non-antiretroviral therapy in HIV-infected patients. Eur J Clin Pharmacol. 2020;76(3):305-318. DOI: 10.1007/s00228-019-02785-z [DOI] [PubMed] [Google Scholar]
- 23.Zechmann S, Trueb C, Valeri F, Streit S, Senn O, Neuner-Jehle S. Barriers and enablers for deprescribing among older, multimorbid patients with polypharmacy: An explorative study from Switzerland. BMC Fam Pract. 2019;20(1):64 DOI: 10.1186/s12875-019-0953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitman A, DeGregory K, Morris A, Mohile S, Ramsdale E. Pharmacist-led medication assessment and deprescribing intervention for older adults with cancer and polypharmacy: a pilot study. Support Care Cancer. 2018;26(12):4105–13. DOI: 10.1007/s00520-018-4281-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNicholl IR, Gandhi M, Hare CB, Greene M, Pierluissi E. A Pharmacist-Led Program to Evaluate and Reduce Polypharmacy and Potentially Inappropriate Prescribing in Older HIV-Positive Patients. Pharmacotherapy. 2017;37(12):1498–506. DOI: 10.1002/phar.2043 [DOI] [PubMed] [Google Scholar]
- 26.Cooley SA, Paul RH, Ances BM. Medication management abilities are reduced in older persons living with HIV compared with healthy older HIV-controls. J Neurovirol. 2020;26:264–269 DOI: 10.1007/s13365-020-00827-2 [DOI] [PMC free article] [PubMed] [Google Scholar]