Abstract
Background
Chronic musculoskeletal pain (CMP) is the most common self-reported chronic pain condition. Current treatment for CMP is limited.
Methods
This was a two-phase study. In Phase 1, three auricular point acupressure (APA)-naïve participants were recruited to explore their experiences of APA and a smartphone app was developed based on their feedback. In Phase 2, a prospective longitudinal study was used to examine the effectiveness of the smartphone app to self-manage CMP.
Results
Phase 1 resulted in the successful development of the APA smartphone app. In Phase 2, after four weeks of APA, participants reported reduced pain intensity (30%), pain interference (35%), and disability (40%), as well as improved physical function (47%). The mean score for the participants’ perception of treatment efficacy was 4.94 (SD = 2.08, scale of 0–7) indicating that approximately 70% of participants rated global improvements with noticeable changes. The majority (88%, n = 22) of the participants were satisfied with the treatment: 32% [8] were very satisfied and 56% [n = 14] were somewhat satisfied. The average frequency of pressing APA seeds per day was 2.93 times (SD = 2.27, range 0–10) and 1.60 minutes per time (SD = 2.64, range 0–10); the participants were able to adhere to the suggested pressing time per day, although they only pressed the ear points about 53% of the suggested time.
Conclusion
It is feasible for individuals to learn APA from the smartphone app and successfully self-administer APA to manage their pain. Participants found the app useful and were satisfied with the information provided through the app.
Keywords: auricular point acupressure, musculoskeletal pain, smartphone application, self-management
Background
Chronic musculoskeletal pain (CMP) is the most common self-reported and clinically diagnosed chronic pain condition in the U.S.1–6 In 2016, 20% of American adults reported chronic pain, with 8% suffering from limitations in their activities of daily living.1 Chronic pain is the second most common reason for healthcare provider visits in the U.S.,5,7 with annual expenses over $635 billion, accounting for medical care costs, reduced disability-related productivity, and lost wages.8 CMP is described as the most common type of chronic pain occurring frequently in the joints and back,9 lasting beyond normal healing (3–6 months).8
Pharmacotherapy is currently the predominant treatment for chronic pain in U.S. medical practice;10–14 however, many analgesics are associated with numerous, potentially deleterious side effects (e.g., nonsteroidal anti-inflammatory drugs and their predilection for causing renal insufficiency, gastrointestinal bleeding, hypertension, congestive heart failure.)15–18 Opioids are also often prescribed due in part to the convenience of this approach (easy to prescribe), resulting in an abundance of opioid prescriptions dispensed in the U.S.19 Opioid analgesics are commonly overprescribed and are associated with risks of addiction, sleep-disordered breathing, delirium, and falls/fractures,18,20 leading to the current opioid crisis.21
Non‐pharmacological therapy and self‐management are recommended in evidence‐based guidelines for the treatment of CMP9,22 but these have barriers for implementation. Self‐management alone may be insufficient for effective, sustained pain management23 and needs to be supported with other treatment modalities for best results.24,25 Exercise during randomized clinical trials (RCTs)22,26 has been shown to be moderately effective for chronic pain, but long‐term adherence after the trials end is challenging.27 It has been demonstrated that even a simple physical intervention such as a walking program has waning efficacy over time.28 Multidisciplinary rehabilitation programs are time‐consuming, costly, and labor intensive, as with physical therapy; to achieve optimal outcomes, patients must carry forward the principles and practices taught in these programs.29 Further, other suggested treatments such as mindfulness‐based stress reduction,30–32 tai chi,33,34 yoga,35,36 cognitive behavioral therapy,32,37 and spinal manipulation38 have barriers to scale‐up, including patient and provider buy‐in, insurance coverages, and accessibility to care, limiting adherence to these evidence‐based guidelines.20,39–45 Even Acupuncture, which has supporting evidence to manage pain effectively,9 is hard to scale up because it involves complex assessments and individualized treatments by trained providers, representing barriers to widespread implementation. Although these various evidence‐based modalities are recommended in guidelines, challenges continue to exist as to how to best implement these in practice to enhance utilization.9
Auricular point acupuncture/acupressure (APA) is a method derived from Traditional Chinese Medicine (TCM). In the 1950’s, Dr. Paul Nogier, a well-known French Neurosurgeon, theorized that the ear represents an inverted fetus within the womb as a somatotopic map representing reflex parts of the human body.46,47 In other words, the ear is a microsystem based on reflexology; acupoints for the entire body are represented on the inner and outer ear lobes.48,49 By stimulating specific auricular points, corresponding parts of the body that are symptomatic can be treated by a stimulus pressed on the ear acupoints.48,49 The stimulation of the auricular point can be performed by acupuncture needles, pellets/seeds, or electric stimulation.48,50
Acupuncture, a well-known treatment in TCM, is performed by inserting sterile needles into specific acupoints; it is considered safe when performed by experienced practitioners.48,49 However, the widespread application of acupuncture to manage pain is limited by the paucity of trained acupuncturists, inadequate or lack of insurance coverage, need for patients to travel frequently to the acupuncture clinic,51 fear of pain caused by needle insertion,52 and the potential for infection.51
On the other hand, APA is a non-invasive procedure where acupuncture-like stimulations are done to ear points using small pellets or seeds instead of needles. We have plenty of supporting evidence on the efficacy of APA to self-manage pain.53–65 Comparing APA to sham (placebo) APA, our studies demonstrated: (1) rapid and sustained significant effect: APA resulted in ≥38% rapid pain relief among participants at three minutes post-APA;66,67 >44% pain relief and >28% improved physical function at follow-up after a 4-week APA treatment;53–59 (2) reduction of medication use: After 4 weeks of APA, ≥60% of participants reported less use of pain medications54,63; and (3) significant impact on physiological measures: APA controls pain through blocking pro-inflammatory cytokines (IL-1β, IL-2)60–62 and modulating nerve sensitivity.62 No adverse effects from APA have been reported.68,69
Despite the long use of traditional Chinese Medicine in Asia and Europe, APA is relatively new in the U.S. and has not been widely used, made available, or researched as extensively as other pain treatments. From our multiple studies,53–65 we received an overwhelming number of requests from former study participants for further APA treatment, and they expressed their desire to incorporate APA into their pain self-management plans. Therefore, the purpose of this study was to develop a smartphone application (app) to help increase the use of and access to APA and then evaluate the feasibility and preliminary efficacy of this APA smartphone app. We are guided by Lorig’s conceptualization of self-management, grounded in Bandura’s theory of self-efficacy70 and the National Institute of Nursing Research (NINR) at the National Institutes of Health (NIH)71 where patients are able take control of their care as active participants.72 The key 2011 Institute of Medicine report on pain emphasized that the majority of pain care needs to occur through self-management.8
Methods
This study was conducted in two phases. In Phase 1 (developmental phase), three APA naïve participants were recruited to explore their experiences in self-administering APA. Taking their input into account, the APA smartphone app was developed to act as a self-guided tool to self-administer APA to manage pain. In Phase 2, thirty participants were enrolled to complete a baseline visit (first time point or T1) where they answered a set of questionnaires. The APA smartphone app was installed into their phones and they received instructions on how to use the app to administer APA for 4 weeks. Immediately after the four-week intervention period (second time point or T2), participants returned for a post-intervention visit to complete the same questionnaires as in the baseline visit and a qualitative interview about their experiences with APA and the smartphone app. Participants then completed a follow-up over the phone after 1-month of completion of the intervention (third time point or T3).
Design
Phase 1
A purposive sample (APA naïve participants) was used to select three adult participants (one male and two females, mean age = 62 years) with CMP who were enrolled into this study. They received the first APA treatment provided by a research team member with background and expertise in APA and instructions were given on how to self-administer APA at home (including identifying ear points, placing the seed/tapes on the ear, and stimulating the ear points). An APA kit was provided (contains probe, seeds/tapes, and forceps). After four weeks of self-administered APA, participants were interviewed to share their experiences with the research team on practicing APA and barriers to self-administration.
APA Treatment Protocol
Detailed information about the APA treatment protocol has been described in our previous publications.53,58,73–75 The ear points to treat pain include: corresponding ear points (specific ear points corresponding to participant’s body pain locations on both sides of the ears, front and back), and Shenmen and Subcortex points known for alleviating stress and pain. The protocol included how to locate ear points corresponding to the body part in pain, how to tape the seeds on the identified ear points, and how to press the ear points to achieve pain relief.76,77 Participants were instructed to evenly press the tapes and seeds covering each ear point without rubbing (to avoid skin damage or movement of the seed from the ear point) for three minutes, three times daily (nine minutes total), even if they were not experiencing pain. The optimal pressure was considered to have been achieved when the participant felt localized tingling or mild discomfort. The overall treatment duration was four weekly cycles. Each weekly cycle included five days of wearing the taped seeds and two days without seeds. Participants were instructed to remove the tape/seeds at the end of the 5th day, let the ear points rest for two days, and re-apply after 2 days. This cycle minimized the risk of allergic reactions to the tape and allowed the ear points to recover and restore sensitivity prior to the next treatment. After four weeks of APA treatment, they were interviewed about their experiences about the APA treatment.
Development of the APA Smartphone App
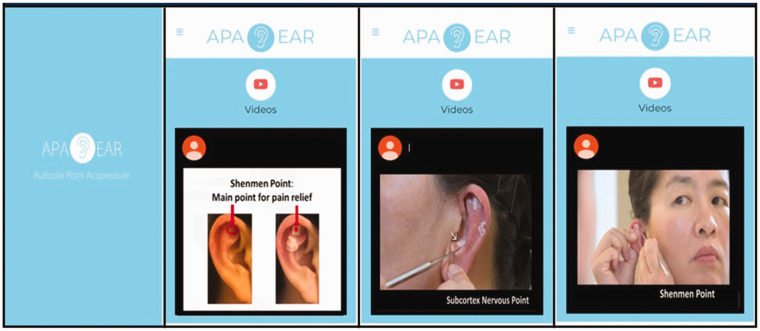
All three participants reacted extremely positively from the rapid pain relief they received after stimulating their ear points and found it was not difficult to self-administer APA based on their in-person training and instructions provided. One participant was able to walk and flex their back much better after about 5 minutes of the treatment. One participant did mention that it took her several times to find the right ear points for treatment. Based on participant feedback from the APA experiences, videos of the APA protocol were developed. The videos were reviewed by the three participants and edited for clarity. It took two editing sessions to finalize the videos. Figure 1 demonstrates the APA treatment protocol video for low back pain. The final videos included:
Figure 1.
Auricular Point Acupressure Smartphone App for Musculoskeletal Pain.
A. Instructional Video (∼5 min Long)
The instructional video included: (1) the theoretical background of APA, (2) a breakdown of the APA kit, and (3) a brief introduction about the APA treatment protocol.
B. Demonstration Video (∼3 min Long)
The demonstration video included: (1) how to use the probe to find the corresponding ear points based on the body area of pain, (2) how to tape the seeds on the identified ear points, and (3) how to stimulate the ear points.
The app was made available at the app store (Android or iOS) after the participants enrolled in the study. Once downloaded, individuals could access the videos as many time as they liked.
Phase 2
To examine the feasibility and assess the preliminary efficacy of the APA smartphone app as a self-guided tool to self-administer APA, a prospective, longitudinal study (single group, open trial design) was conducted. After baseline data were collected (T1), participants installed the phone app and received instructions on how to use the app to administer APA for 4 weeks and self-manage their pain. After the four-week intervention period, participants returned for an immediate post-intervention visit (T2), and then completed a 1-month follow-up post-intervention (T3) over the phone. STRICA guidelines were used in reporting our findings based on the study design.78
Participants and Study Setting
Inclusion criteria: Participants were eligible if they: (1) were 18 years or older, (2) were able to read and write English, (3) had CMP for at least 3 months, (4) reported an average intensity of pain ≥ 4 on an 11-point numerical pain scale for the previous week, (5) were smartphone users, and (6) were able to apply pressure to the seeds taped on their ears. Participants were excluded if they had any allergy to latex (tapes used on ear points). This study was conducted at the Johns Hopkins Medicine (JHM) Study Office.
Study Instruments
Brief Pain Inventory (BPI)79
The subscale of BPI on “pain severity/intensity” in the last 7 days was used. The subscale has 4 items assessing pain at its “worst,” “least,” “average,” and “now (current),” with range of scores from 0 (“no pain”) to 10 (“pain as bad as you can imagine”). Higher scores indicate that patients have more severe pain. The BPI also includes pain medication use, percentage of perceived pain relief by pain medication, and front and back body diagrams for participants to mark their pain locations. BPI has established reliability and validity cited in over 400 publications.79 To evaluate the magnitude of change in pain intensity, pain relief was defined as a reduction of 30% in pain intensity. This is considered to be a “moderate clinically important difference” by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)80 and was used to define a significant effect of APA.33
Roland-Morris Disability Questionnaire (RMDQ)81
This 24-item measure was used to assess the impact of CMP on daily functioning. Participants were asked to rate yes or no on the statement related to their physical function. The scores ranged from 0 (no disability) to 24 (maximum disability). RMDQ is a reliable, valid, and sensitive measure, demonstrating substantial construct validity.81,82
Pain Catastrophizing Scale (PCS)83
This 13-item, self-report measure was used to evaluate exaggerated and negative interpretations of pain. Participants were asked to reflect on past painful experiences and to indicate to which degree they experienced each of the following when feeling pain: rumination (4 items), magnification (3 items), or helplessness (6 items). The PCS features a 0–4 Likert scale (scores ranged from 0 to 52) from “not at all” to “all the time.” A higher PCS score indicated stronger pain catastrophizing.
Fear-Avoidance Beliefs Questionnaire (FABQ)84
A valid and reliable, modified form of the FABQ84 was used that focuses on patients’ beliefs about how physical activity affected their pain (4 items).The FABQ features a 0–6 Likert type scale from 0 (completely disagree) to 6 (completely agree), with scores ranging from 0 to 24. Higher scores indicated elevated fear-avoidance.
PROMIS 2985
The subscales of “Physical Function” (4-item), “Anxiety” (4-item), “Depression” (4-item), and “Fatigue” (4-item) in the PROMIS 29 were used. The scores for each subscale ranged from 4 to 20, with higher scores indicating greater symptoms. PROMIS-29 has established reliability and validity86,87 and is widely used in the U.S.
Patient Global Impression of Change (PGIC)88
The PGIC was used to measure the impression of change after completion of this 2-phase study. The PGIC scores reflected a patient's belief about the efficacy of a treatment. PGIC is a 7-point scale depicting a patient's rating of overall improvement ranging from “very much improved” (1) to “very much worse” (7). PGIC has established psychometric properties to identify clinically significant changes in patients’ subjective outcome measures.88
Treatment Satisfaction89
This is a one-item scale and was used to assess participants’ satisfaction with the treatment. The scores ranged from 1 (“completely satisfied”), 2 (“somewhat satisfied”), to 3 (“not satisfied”). This satisfaction survey has been used effectively in our previous study on low back pain with APA.89
APA Practice89
This is measured using a two-item, open-ended scale to collect how participants practiced their APA. The scale asks “How many times did you press the seed per day” and “How long did you press the seeds each time.” APA adherence to practice was defined by the number of participants who were able to follow at least two-thirds of the suggested pressing time (at least two times/day, two minutes/time).55,89,90 This measure was used succesfully in our previous study on low back pain.55,89,90
Demographic and Health History Data
A questionnaire was used to collect demographic information (e.g., age, marital status, educational level, employment, estimated income), pain location, and pain medications used.
Procedure
We promoted the study information and recruited participants by using online study flyers distributed at outpatient clinics at JHM Johns Hopkins Pain Treatment Center, referrals from physicians, and social media advertisement websites (e.g., Craigslist, Inc.). This study was approved by the Insitutional Review Board (IRB) at JHM. After consents were obtained and baseline data were collected, participants installed the APA smartphone app on their phones and received the APA kit. They then self-administered APA for four weeks to manage their pain using the APA smartphone app as a self-management guide or tool to practice APA. Study outcomes were assessed at baseline (T1), immediately post-intervention (T2), and at the 1-month follow-up after the intervention (T3). Data were collected using the Research Electronic Data Capture (REDCap), which is a secure data management system approved by the IRB as compliant with HIPAA security policies. A brief interview was conducted at T2 to explore participants’ experiences about the app. The interviews were recorded so that transcripts could be reviewed for analysis.
Data Analyses
Based on the nature of this study (small sample size), descriptive analyses (means and standard deviations) were used to examine the outcome measures. Data analyses were based on the participants who completed all of the data assessments (n = 25, 83%). Effect size as standardized differences in mean between two means (Cohen's d) was calculated; this would inform the sample sizes for future randomized controlled trials. All data analyses were performed using SPSS 25©. For the interview data, transcripts of audio-recordings were analyzed using conventional content analysis.91 Common suggestions and concerns were extracted to address issues with the feasibility of the APA smartphone app.
Results
Feasibility of Recruitment
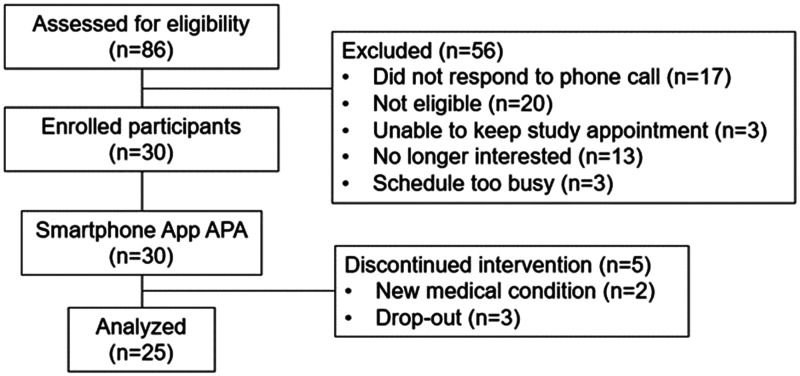
For recruitment, we received 31 referrals from healthcare providers and 55 inquiries from individuals who learned of our study from our advertisements. Fifty-six participants were excluded for various reasons (Figure 2). Of the 30 remaining who were enrolled in this study, two dropped out because of new medical conditions unrelated to APA; one dropped out because of a very busy schedule, and two failed to schedule their post-intervention visit. Consequently, 25 participants completed this study (83% retention rate).
Figure 2.
Participant Recruitment Flowchart.
Participants’ Characteristics
Table 1 presents the demographic characteristics of 30 participants. The average age of the participants was 54 years (SD = 12.49, ranging from 22 to 68). Among the 30 participants, the majority were women (n = 25, 83%), Whites (n = 15, 50%), and had college degrees or more (n = 23, 77%). The average multisite body pain locations were 2.9 (SD = 1.18, range 1–5). These pain locations frequently included the back (83%), neck (73%), knee (47%), finger (40%), and foot (40%). Approximately 43% of the participants took medications to treat their pain.
Table 1.
Characteristics of the Participants (N = 30).
| Variable | N (%) |
|---|---|
| Age | |
| Mean (SD) (Range) | 54.0 (12.49) (22–68) |
| Gender | |
| Male | 5 (17) |
| Female | 25 (83) |
| Race/ethnicity (n) | |
| White | 15 (50) |
| Black/African American | 12 (40) |
| More than one race | 3 (1) |
| Marital status (n)* | |
| Currently married | 9 (30) |
| Divorced | 4 (13) |
| Widowed | 4 (13) |
| Never married | 10 (34) |
| Separated | 1 (3) |
| Living with a partner | 2 (7) |
| Employment situation (n)* | |
| Working (full time) | 13 (43) |
| Working (part time) | 4 (13) |
| Not employed | 11 (37) |
| Retired | 2 (7) |
| Education level (n)* | |
| High school | 2 (7) |
| Some college | 5 (16) |
| College graduate | 9 (30) |
| Post-graduate degree | 14 (47) |
| Estimated income before taxes (n)* | |
| Less than $10,000 | 3 (10) |
| $10,000 to $19,999 | 8 (27) |
| $20,000 to $39,999 | 5 (17) |
| $40,000 to $59,000 | 4 (13) |
| $60,000 to $100,000 | 7 (23) |
| More than $100,000 | 3 (10) |
| Current pain medication use (n) | |
| Yes | 13 (43) |
| No | 17 (57) |
| Current sleep medication use (n) | |
| Yes | 3 (10) |
| No | 27 (90) |
| Number of pain locations | |
| Mean (SD) (Range) | 2.9 (1.18) (1–5) |
| Back | 25 (83) |
| Neck | 22 (73) |
| Knee | 14 (47) |
| Finger | 12 (40) |
| Shoulder | 6 (20) |
| Foot | 12 (40) |
| Hip | 6 (20) |
| Leg | 4 (13) |
*n varies due to missing data.
Feasibility of APA Practice
The results indicated that the average frequency of pressing the seeds per day among the participants was 2.96 times (SD = 2.27, range 0–10), at 1.6 minutes per time (SD = 2.64, range 0–10). Two participants indicated their ear was sore after pressing and one participant had sensitivity to the tapes used. Among 13 participants who used pain medications, 77% (n = 10) reported that they took less pain medications during the intervention and follow-up phase, and 88% (n = 22) indicated they will refer APA to their family or friends.
APA Treatment Outcomes
Pain Intensity, Interference, Disability, and Physical Function
After 4 weeks of APA treatment, pain intensity decreased by 35% (T2) at immediate post-APA and was maintained by 30% at 1-month follow-up (T3) compared to baseline (T1) (Table 2). Cohen’s effect size (d = 0.96) suggested a high improvement in the decrease of pain intensity. Pain interference also decreased by 35% at T2 and was closely maintained at 29% at T3 compared to baseline. Cohen’s effect size (d = 0.35) suggested a moderate improvement in decreased pain interference. Based on the score from the RMDQ, participants had decreased (39%) disability at T2, which continued to decrease to 40% at the 1-month follow-up (T3). Cohen’s effect size (d = 0.69) suggested a moderate improvement in reducing disability. The score from the PROMIS subscale of physical function showed improved function (14%) at T2, which dramatically improved (47%) at T3. Cohen’s effect size (d = 1.30) suggested a moderate improvement in physical function.
Table 2.
Smartphone Auricular Point Acupressure App Outcomes (n = 25).
| Pre-InterventionMean (SD) | Post-InterventionMean (SD) | Change (Post-Pre)% | Cohen’s d (effect size) | 1M Follow-UpMean (SD) | Change (1 M-Pre)% | Cohen’s d (effect size) | |
|---|---|---|---|---|---|---|---|
| Pain Intensity | 7.2 (1.80) | 4.7 (2.86) | 35% | 1.06 | 5.0 (2.67) | 30% | 0.96 |
| Pain Interference | 19.9 (18.74) | 13.0 (13.29) | 35% | 0.42 | 14.2 (13.46) | 29% | 0.35 |
| Physical Function | |||||||
| Function (PROMIS-29) | 11.3 (5.23) | 9.6 (3.61) | 14% | 0.36 | 6.0 (2.32) | 47% | 1.30 |
| Function (RMDQ) | 9.1 (6.12) | 5.5 (5.06) | 39% | 0.63 | 5.4 (4.25) | 40% | 0.69 |
| Psychological function | |||||||
| PCS | 12.4 (10.43) | 10.4 (9.91) | 16% | 0.19 | 10.2 (9.45) | 18% | 0.22 |
| Fear | 14.2 (12.00) | 10.3 (9.59)) | 27% | 0.36 | 10.3 (9.89)) | 27% | 0.35 |
| Depression (PROMIS-29) | 6.7 (3.47) | 6.5 (3.17) | 4% | 0.08 | 5.6 (2.62) | 16% | 0.34 |
| Anxiety (PROMIS-29) | 7.5 (3.88) | 7.0 (2.90) | 6% | 0.13 | 7.2 (3.30) | 4% | 0.07 |
| Other function | |||||||
| Sleep (PROMIS-29) | 11.4 (2.53) | 10.9 (2.55) | 4% | 0.20 | 10.6 (1.86) | 7% | 0.35 |
| Fatigue (PROMIS-29) | 12.1 (5.89) | 11.2 (5.33) | 8% | 0.17 | 10.5 (4.98) | 13% | 0.29 |
RMDQ= Roland-Morris Disability Questionnaire, PCS=Pain Catastrophizing scale, Effect size uses change to either post-intervention or 1 M-follow-up from pre-intervention as an estimate of change in control condition.
Pain Catastrophizing and Fear-Avoidance Beliefs
As shown in Table 2, participants reported a lower pain catastrophizing score (16%) at T2 and maintained a similar decreased magnitude (18%) at T3 compared to baseline. Fear-avoidance beliefs decreased to 27% at T2 and maintained the same magnitude at T3 compared to baseline.
Anxiety, Depression, Sleep Quality, and Fatigue (PROMIS 29)
As shown in Table 2, the changes in these scores using the PROMIS 29 subscales were modest at both data time points (T2 and T3) compared to baseline and also in comparison to the other outcome measures.
Patient’s Global Impression of Change
The mean score for the participants’ perceptions of treatment efficacy was 4.94 (SD = 2.08, scale of 0-7). This finding indicates that about 70% of participants rated their global improvement as “moderately better, and slight but noticeable changes”).
Satisfaction From APA
The majority (88%, n = 22) of the participants were satisfied with the treatment (32% [8] were very satisfied and 56% [n = 14] were somewhat satisfied). However, 12% (n = 3) of the participants were not satisfied with the treatment. These 3 participants stated that they did not feel significant changes in their pain intensity after the treatment.
Adherence to APA Practice
The average frequency of pressing the seeds per day was 2.93 times (SD = 2.27, range 0-10) and 1.60 minutes per time (SD = 2.64, range 0-10), indicating that the participants were able to adhere to the recommended pressing frequency per day (3 times per day), but they only pressed the ear points about 53% of suggested pressing time (3 minutes per time).
Side Effects of APA Treatment
Few participants indicated that the ear points exhibited soreness (n = 1), tenderness (n = 1), or redness due to tape sensitivity (n = 3). Four participants complained that the tape fell off easily. No adverse effects were reported; regardless, these participants continued their APA treatment for pain relief.
Qualitative Data Findings
Participants appreciated the instructional videos that provided background information about the concept of APA and the pain relief achieved when following the treatment protocol. The majority of the participants (80%, n = 20) expressed that they were able to learn how to self-administer APA using the smartphone app. However, five participants conveyed a need to re-watch the videos multiple times to accurately identify ear point locations. Furthermore, they were not aware of the need to use the probe to locate tender points on the ear and subsequently use those tender points as areas for seed placement. Five patients favored the possibility of doing an in-person training. Three participants indicated that the seeds/tapes did not stay on the ear well and that they possibly had sensitivity to the latex tape used. Four participants indicated that the training could be improved if they were able to review the videos on a computer or tablet since the screen on their smartphone was too small.
Discussion
APA is a relatively safe treatment to manage pain and can be used as an option to manage CMP. Minor concerns from the study participants were soreness or tenderness on their ears when self-administering the APA treatment, redness from the tapes used to secure the seeds, or tapes easily falling off. However, these participants stated that the soreness was bearable compared to their body pain and that the treatment was worth pursuing to achieve pain relief. To improve APA practice, hypoallergenic and latex-free tape and ear seeds can be used. Another option could be the development of a finger cot which only covers the finger with embedded pellets on the fingertip so that participants can stimulate the ear points anywhere and anytime as needed without using tape. Unlike body acupressure—which usually requires different degrees of pressure on body points for about 30–40 minutes per session and usually requires a therapist, especially when body acupoints are not accessible to the patients themselves—auricular points are at a superficial skin level, within easy reach, and take less time to implement. Patients simply press the seeds for 3 minutes, three times per day (i.e., 9 minutes total per day) to relieve pain. This is time-efficient, combines active participation with potential for immediate pain relief, and provides patients with a greater sense of control over their pain, thereby allowing them to resume daily tasks that CMP prevented them from performing. Our APA smartphone app protocol includes teaching patients regarding the treatment rationale (i.e., mechanisms by which APA can alleviate CMP), skill training (i.e., learning how to identify the ear points and press the seeds), and maintenance of learned skills (adherence to the treatment regimen) to help facilitate sustainability of the APA practice.
This is the first study to develop an APA smartphone app as a self-guided tool to self-administer APA for participants with CMP and to examine the feasibility and initial treatment efficacy of a 4-week APA protocol to manage CMP. Our smartphone app was found to be user friendly. Participants found it easy to follow and were able to use it to guide their APA practice. This app empowers participants to self-manage their pain. Our study findings indicate that it is feasible for participants to learn APA from the smartphone app and successfully self-administer APA to manage their pain, with sustained effects at 1-month follow-up. We reported the effect sizes of the study outcomes to inform sample size estimation for a future trial, although the interpretation needs to be taken with caution due to the following: (1) small sample size given the nature of the pilot study; (2) heterogeneity of pain conditions in CMP limit generalizability of study findings; (3) lack of a placebo-control group and randomization, so we were not able to differentiate the true effects of APA; (3) given that only 1-month follow-up data were collected, we were not able to assess the longer effects of APA on CMP; and (4) we excluded participants who did not have a smartphone. Future studies should include a larger sample size, robust study design (i.e., randomized controlled trials, comparative effectiveness trials), and a longer follow-up phase. Future directions in research should also include evaluating this app as part of a remotely delivered pain program for easy accessibility as well as for assessing its application among underserved and other pain populations.
While the APA smartphone app developed in this study showed preliminary efficacy to manage CMP, challenges remain that need to be addressed in order to maximize the usability and accessibility of APA. First, some participants preferred to watch the videos through a computer or tablet with bigger screens compared to smaller screens on smartphones. We suggest modifying the smartphone app to become compatible with web-based interfaces on computers or tablets.
Secondly, the APA treatment protocol, which includes auricular points corresponding to painful body parts, and the procedure for identifying auricular points are new concepts and techniques for the study participants and required multiple views of the videos to learn APA. The smartphone app videos developed in the current study included two sets of videos: one instructional video (approximately 5 minutes long) and one demonstration video (approximately 3 minutes). If the participants wanted to review a specific segment of the video, they needed to rewind the video many times to locate the exact video segment they needed. To facilitate learning outcomes, the “microlearning” technique can be used by breaking learning content into shorter videos, adding quality graphics, and incorporating visual aids to assist with participant retention of the knowledge they learn.92 We suggest that each piece of content be divided into approximately one-minute videos to facilitate easier learning and understanding. Microlearning can be self-paced, repeated, and re-emphasized based on the participants’ learning progress. Each video can be followed by a knowledge test to ensure APA retention before advancing to the next video. Additionally, the videos should accommodate closed captioning and have larger font sizes for the visually impaired.
Last, to facilitate self-monitoring and to motivate participant’s adherence to APA practice and effectively self-manage pain, we suggest that the APA smartphone app include an individualized dashboard with user-friendly visual summaries of study outcomes and APA practice. Another recommendation is to include pre-programmed motivational messages according to daily scores of pain outcomes to encourage APA practice based on tried quotes and postings given to patients with chronic disorders such as pain.93–95
We conclude that the APA smartphone app is an ideal tool for training individuals to self-administer APA. Up to 81% of Americans own a smartphone96 and online technology remains an effective tool to facilitate knowledge translation from research findings to end-users.24,97,98 In this study, we found that the participants were able to learn APA from the app and subsequently foster APA into a self-management plan to reduce their pain. APA is a non-invasive treatment and can be easily administered and used as an adjunct treatment to self-manage CMP, allowing patients to resume many daily tasks interrupted by their pain. More importantly, once learned, patients can incorporate APA into a self-management plan that best fits their specific needs given the nuances in various pain conditions to habitually manage their pain when present, especially since CMP oftentimes comes and goes. The availability of APA as an adjunct to standard care offers the potential to improve patients’ quality of life in a cost-effective manner. We believe that replicating this study in a randomized clinical trial with a larger sample size and a control group is the next step to confirm the efficacy of APA for treating CMP.
Footnotes
Authors' Note: Keenan Caswell is now affiliated with School of Medicine, Uniformed Services University, Maryland, US.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the grants to Dr. Yeh from the Sigma Theta Tau International, Under Armour Women’s Health & Breast Cancer Innovation Grant, Johns Hopkins Medicine, and National Institute On Aging of the National Institutes of Health under Award Number R01AG056587.
ORCID iDs: Chao Hsing Yeh https://orcid.org/0000-0001-5326-6660
Maurice Mazraani https://orcid.org/0000-0002-3621-4647
References
- 1.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morbid Mortal Wkly Rep. 2018; 67(36):1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Bone and Joint Initiative. United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States (BMUS). http://www.boneandjointburden.org. Published 2018.
- 3.Denkinger MD, Lukas A, Nikolaus T, Peter R, Franke S. Multisite pain, pain frequency and pain severity are associated with depression in older adults: results from the ActiFE Ulm study. Age Ageing. 2014; 43(4):510–514. [DOI] [PubMed] [Google Scholar]
- 4.Statista Dossier About Back Pain in the United States. https://www.statista.com/study/49118/back-pain-in-the-us/. Published 2019. Accessed October 1, 2020.
- 5.By The Numbers: Musculoskeletal Conditions Is Sourced From The Burden of Musculoskeletal Diseases in the United States (BMUS). http://www.boneandjointburden.org/docs/By%20The%20Numbers%20-%20Back%20Pain.pdf. Accessed September 11, 2016.
- 6.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. 2010; 11(11):1230–1239. [DOI] [PubMed] [Google Scholar]
- 7.Knauer SR, Freburger JK, Carey TS. Chronic low back pain among older adults: a population-based perspective. J Aging Health. 2010; 22(8):1213–1234. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine of the National Academies Report. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 9.Skelly AC, Chou R, Dettori JR, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. Comparative Effectiveness Review No. 227. (Prepared by the Pacific Northwest Evidence-based Practice Center Under Contract No. 290-2015-00009-I.) AHRQ Publication No. 20-EHC009. Rockville, MD: Agency for Healthcare Research and Quality; 2020. [PubMed] [Google Scholar]
- 10.Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Int Med. 2016; 176(7):958–968. [DOI] [PubMed] [Google Scholar]
- 11.Frieden TR, Houry D. Reducing the risks of relief—the CDC opioid-prescribing guideline. N Engl J Med. 2016; 374(16):1501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manchikanti L, Ailinani H, Koyyalagunta D, et al. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011; 14(2):91–121. [PubMed] [Google Scholar]
- 13.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I–evidence assessment. Pain Physician. 2012; 15(3 Suppl):S1–S65. [PubMed] [Google Scholar]
- 14.American Geriatrics Society 2015. Updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatrics Soc. 2015; 63(11):2227–2246. [DOI] [PubMed] [Google Scholar]
- 15.Malanga G, Wolff E. Evidence-informed management of chronic low back pain with nonsteroidal anti-inflammatory drugs, muscle relaxants, and simple analgesics. Spine J. 2008; 8(1):173–184. [DOI] [PubMed] [Google Scholar]
- 16.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008; 11(2 Suppl):S105–S120. [PubMed] [Google Scholar]
- 17.Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013; 173(17):1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015; 156(4):569–576. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention NCfIPaC. Prescription Opioids. https://www.cdc.gov/drugoverdose/opioids/prescribed.html. Published 2017. Accessed June 1, 2020.
- 20.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ (Clinical Research Ed). 2015; 350:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petzke F, Klose P, Welsch P, Sommer C, Hauser W. Opioids for chronic low back pain: an updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks of double-blind duration. Eur J Pain (London, England). 2020; 24(3):497–517. [DOI] [PubMed] [Google Scholar]
- 22.Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017; 166(7):514–530. [DOI] [PubMed] [Google Scholar]
- 23.Kawi J. Predictors of self-management for chronic low back pain. Appl Nurs Res: ANR. 2014; 27(4):206–212. [DOI] [PubMed] [Google Scholar]
- 24.Sparks T, Kawi J, Menzel NN, Hartley K. Implementation of health information technology in routine care for fibromyalgia: pilot study. Pain Manag Nurs. 2016; 17(1):54–62. [DOI] [PubMed] [Google Scholar]
- 25.Talusan R, Kawi J, Candela L, Filler J. Implementation and evaluation of an APRN-led opioid monitoring clinic. Feder Practition. 2016; 33(11):22–27. [PMC free article] [PubMed] [Google Scholar]
- 26.Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007; 147(7):492–504. [DOI] [PubMed] [Google Scholar]
- 27.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews. Cochrane Database Syst Rev. 2017; 4:CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krein SL, Metreger T, Kadri R, et al. Veterans walk to beat back pain: study rationale, design and protocol of a randomized trial of a pedometer-based internet mediated intervention for patients with chronic low back pain. BMC Musculoskelet Disord. 2010; 11:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eleswarapu AS, Divi SN, Dirschl DR, Mok JM, Stout C, Lee MJ. How effective is physical therapy for common low back pain diagnoses? A multivariate analysis of 4597 patients. Spine. 2016; 41(16):1325–1329. [DOI] [PubMed] [Google Scholar]
- 30.Morone NE, Greco CM, Moore CG, et al. A mind-body program for older adults with chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2016; 176(3):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morone NE, Rollman BL, Moore CG, Li Q, Weiner DK. A mind–body program for older adults with chronic low back pain: Results of a pilot study. Pain Med. 2009; 10(8):1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. Jama. 2016; 315(12):1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall AM, Maher CG, Lam P, Ferreira M, Latimer J. Tai chi exercise for treatment of pain and disability in people with persistent low back pain: a randomized controlled trial. Arthritis Care Res. 2011; 63(11):1576–1583. [DOI] [PubMed] [Google Scholar]
- 34.Weifen W, Muheremu A, Chaohui C, Md LW, Lei S. Effectiveness of Tai Chi practice for non-specific chronic low back pain on retired athletes: a randomized controlled study. J Musculoskelet Pain. 2013; 21(1):37–45. [Google Scholar]
- 35.Nambi GS, Inbasekaran D, Khuman R, Devi S, Shanmugananth, Jagannathan K. Changes in pain intensity and health related quality of life with Iyengar yoga in nonspecific chronic low back pain: a randomized controlled study. Int J Yoga. 2014; 7(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cramer H, Lauche R, Haller H, Dobos G. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013; 29(5):450–460. [DOI] [PubMed] [Google Scholar]
- 37.Henschke N, Ostelo R, van Tulder MW, et al. Behavioural treatment for chronic low‐back pain. Cochrane Database Syst Rev. 2010; 2010(7):CD002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubinstein SM, de Zoetes A, van Middelkoop M, Assendelft WJJ, de Boer MR, van Tulder MW. Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: systematic review and meta-analysis of randomised controlled trials. BMJ (Clin Res Ed). 2019; 13(364):1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou R, Deyo R, Devine B, et al. The Effectiveness and Risks of Long-Term Opioid Treatment of Chronic Pain. Evidence Report/Technology Assessment No. 218. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2012-00014-I.) AHRQ Publication No. 14-E005-EF. www.effectivehealthcare. ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141007.pdf. Published 2014. Accessed December 28, 2015. [DOI] [PubMed]
- 40.Deyo RA, Smith DH, Johnson ES, et al. Prescription opioids for back pain and use of medications for erectile dysfunction. Spine. 2013; 38(11):909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010; 152(11):712–720. [DOI] [PubMed] [Google Scholar]
- 42.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. Jama. 2011; 305(13):1315–1321. [DOI] [PubMed] [Google Scholar]
- 43.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015; 175(4):608–615. [DOI] [PubMed] [Google Scholar]
- 44.Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morbid Mortal Wkly Rep 2011; 60(43):1487–1492. [PubMed] [Google Scholar]
- 45.Fields HL. The doctor’s dilemma: opiate analgesics and chronic pain. Neuron. 2011; 69(4):591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nogier P. Handbook to Auriculotherapy. 1st ed Moulins-les-Metz: Maisonneuve; 1981. [Google Scholar]
- 47.Nogier R. How did Paul Nogier establish the map of the ear? Med Acupunct. 2014; 26(2):76–83. [Google Scholar]
- 48.Huang LC. Auricular Medicine: A Complete Manual of Auricular Diagnosis and Treatment. 1st ed Orlando, FL: Auricular International Research & Training; 2005. [Google Scholar]
- 49.Oleson T. Auriculotherapy manual: Chinese and western systems of ear acupuncture. 3rd ed London: Churchill Livingstone; 2003. [Google Scholar]
- 50.Oleson T. Auriculotherapy Manual: Chinese and Western Systems of Ear Acupuncture. 4th ed Edinburgh: Churchill Livingstone, Elsevier; 2014. [Google Scholar]
- 51.Lu W, Rosenthal DS. Recent advances in oncology acupuncture and safety considerations in practice. Curr Treatment Options Oncol. 2010; 11(3–4):141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gottschling S, Reindl TK, Meyer S, et al. Acupuncture to alleviate chemotherapy-induced nausea and vomiting in pediatric oncology—a randomized multicenter crossover pilot trial. Klinische Padiatrie. 2008; 220(6):365–370. [DOI] [PubMed] [Google Scholar]
- 53.Yeh CH, Chien LC, Balaban D, et al. A randomized clinical trial of auricular point acupressure for chronic low back pain: a feasibility study. Evid-Based Complement Alternat Med. 2013; 2013:196978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeh CH, Morone NE, Chien LC, et al. Auricular point acupressure to manage chronic low back pain in older adults: a randomized controlled pilot study. Evid Based Complement Alternat Med. 2014; 2014:375173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeh CH, Suen LKP, Chien LC, et al. Day-to-day changes of auricular point acupressure to manage chronic low back pain: a 29-day randomized control study. Pain Med. 2015; 16(10):1857–1869. [DOI] [PubMed] [Google Scholar]
- 56.Yeh CH, Chien LC, Lin WC, Bovbjerg DH, van Londen G. Pilot randomized controlled trial of auricular point acupressure to manage symptom clusters of pain, fatigue, and disturbed sleep in breast cancer patients. Cancer Nurs. 2016; 39(5):402–410. [DOI] [PubMed] [Google Scholar]
- 57.Yeh CH, Chien LC, van Londen G, Bovbjerg DH. Auricular point acupressure (APA) to manage a symptom cluster of pain, fatigue, and disturbed sleep in breast cancer patients: a pilot study. J Pain Relief. 2015; 4:199. [DOI] [PubMed] [Google Scholar]
- 58.Yeh CH, Lukkahatai N, Campbell C, et al. Preliminary effectiveness of auricular point acupressure on chemotherapy-induced neuropathy: part 1 self-reported outcomes. Pain Manag Nurs. 2019; 20(6):614–622. [DOI] [PubMed] [Google Scholar]
- 59.Yeh CH, Chien LC, Huang LC, Suen KPL. Auricular point acupressure for chronic pain: a feasibility study of a four-week treatment protocol. Holist Nurs Pract. 2014; 28(3):184–194. [DOI] [PubMed] [Google Scholar]
- 60.Yeh CH, Chien LC, Albers KM, et al. Function of auricular point acupressure in inducing changes in inflammatory cytokines during chronic low back pain: a pilot study. Med Acupunct. 2014; 26(1):31–39. [Google Scholar]
- 61.Lin WC, Yeh CH, Chien LC, Morone NE, Glick RM, Albers KM. The anti-inflammatory actions of auricular point acupressure for chronic low back pain. Evid Based Complement Alternat Med. 2015; 2015:103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeh CH, Lukkahatai N, Campbell C, et al. Preliminary effectiveness of auricular point acupressure on chemotherapy-induced neuropathy: part 2 laboratory-assessed and objective outcomes. Pain Manag Nurs. 2019; 20(6):623–632. [DOI] [PubMed] [Google Scholar]
- 63.Yeh CH, Chien LC, Chiang YC, Suen L, Ren D. Analgesic effect of auricular point acupressure as an adjunct treatment for cancer patients with pain. Pain Manag Nur. 2015; 16(3):285–293. [DOI] [PubMed] [Google Scholar]
- 64.Yeh CH, Caswell K, Pandiri S, et al. Dynamic brain activity change after auricular point acupressure on patients with chemotherapy-induced peripheral neuropathy: a pilot longitudinal functional magnetic resonance imaging study. Glob Adv Health Med. 2020; 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeh CH, Van De Castle B. Integrating auricular point acupressure into real-world nursing practice to manage cancer-related pain. Baltimore, MD: Brager Award; 2019. [Google Scholar]
- 66.Yeh CH C, P, Lukahatai N., Morone N.E. Management of Chronic Low Back Pain in Older Adults Using Auricular Point Acupressure (R01): Preliminary Data Analysis on 133 Study Participants. 2020.
- 67.Yeh CH VDCB. Integrating auricular point acupressure into real-world nursing practice to manage cancer-related pain: Preliminary report. Brager Award Presentation; Janurary 30, 2020; Baltimore, MD.
- 68.You E, Kim D, Harris R, D’Alonzo K. Effects of auricular acupressure on pain management: a systematic review. Pain Manag Nurs. 2019; 20(1):17–24. [DOI] [PubMed] [Google Scholar]
- 69.Nielsen A, Gereau S, Tick H. Risks and safety of extended auricular therapy: a review of reviews and case reports of adverse events. Pain Med. 2020; 21(6):1276–1293. [DOI] [PubMed] [Google Scholar]
- 70.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977; 84 2:191–215. [DOI] [PubMed] [Google Scholar]
- 71.Grady PA, Gough LL. Self-management: a comprehensive approach to management of chronic conditions. Am J Public Health. 2014; 104(8):e25–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003; 26(1):1–7. [DOI] [PubMed] [Google Scholar]
- 73.Yeh CH, Huang LC, Suen KPL. The application of a comprehensive and systematic auricular diagnosis for musculoskeletal system disorder: a treatment protocol for chronic low back pain. Med Acupunct. 2014; 26(3):148–153. [Google Scholar]
- 74.Suen LKP, Yeh CH, Yeung SKW. Using auriculotherapy for osteoarthritic knee among elders: a double-blinded randomised feasibility study. BMC Complement Alternat Med. 2016; 16:257–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeh CH, Lin WC, Suen LKP, et al. Auricular point acupressure to manage arthralgia related to aromatase inhibitors in breast cancer survivors. Oncol Nurs Forum. 2017; 44(4):476–487. [DOI] [PubMed] [Google Scholar]
- 76.Yeh CH, Huang LC. Comprehensive and systematic auricular diagnosis protocol. Med Acupunct. 2013; 25(6):423–436. [Google Scholar]
- 77.Suen KPL, Yeh CH. Auricular diagnosis in chronic illnesses. Med Acupunct. 2014; 26(2):125–129. [Google Scholar]
- 78.MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 2010; 7(6):e1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med, Singapore. 1994; 23(2):129–138. [PubMed] [Google Scholar]
- 80.Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009; 146(3):238–244. [DOI] [PubMed] [Google Scholar]
- 81.Roland M, Fairbank J. The Roland-Morris disability questionnaire and the Oswestry disability questionnaire. Spine. 2000; 25(24):3115–3124. [DOI] [PubMed] [Google Scholar]
- 82.Roland M, Morris R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983; 8(2):141–144. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995; 7(4):524–532. [Google Scholar]
- 84.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993; 52(2):157–168. [DOI] [PubMed] [Google Scholar]
- 85.Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014; 17(8):846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS((R))-29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018; 27(7):1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hays RD, Revicki DA, Feeny D, Fayers P, Spritzer KL, Cella D. Using Linear Equating to Map PROMIS((R)) Global Health Items and the PROMIS-29 V2.0 Profile Measure to the Health Utilities Index Mark 3. PharmacoEconomics. 2016; 34(10):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulat Physiol Ther. 2004; 27(1):26–35. [DOI] [PubMed] [Google Scholar]
- 89.Yeh CH, Chien LC, Chiang YC, Huang LC. Auricular point acupressure for chronic low back pain: a feasibility study for 1-week treatment. Evid Based Complement Alternat. 2012; ▪: 383257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin WC, Burke LE, Schlenk EA, Yeh CH. Use of an ecological momentary assessment application to assess the effects of auricular point acupressure for chronic low back pain. Comput Informat Nurs. 2019; 37(5):276–282. [DOI] [PubMed] [Google Scholar]
- 91.Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed Thousand Oaks, CA: SAGE Publications; 1994. [Google Scholar]
- 92.Shail MS. Using micro-learning on mobile applications to increase knowledge retention and work performance: a review of literature. Cureus. 2019; 11(8):e5307–e5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bedrov A, Bulaj G. Improving self-esteem with motivational quotes: opportunities for digital health technologies for people with chronic disorders. Front Psychol. 2018; 9:2126–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fader J. The Science Behind Why Motivational Quotes Motivate You. https://jonathanfader.com/the-science-behind-why-motivational-quotes-motivate-you/. Published 2020. Accessed May 18, 2020.
- 95.Las Vegas Recovery Center. 28 Inspirational Chronic Pain Quotes That Can Help You Cope When You’re in Pain. https://lasvegasrecovery.com/chronic-pain-quotes/. Published 2017. Accessed May 18, 2020.
- 96.Pew Research Center. Mobile Fact Sheet 2020. Published 2020. Accessed April 14, 2020.
- 97.Devan H, Farmery D, Peebles L, Grainger R. Evaluation of self-management support functions in apps for people with persistent pain: systematic review. JMIR mHealth and uHealth. 2019; 7(2):e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mirkovic J, Jessen S, Kristjansdottir OB, Krogseth T, Koricho AT, Ruland CM. Developing technology to mobilize personal strengths in people with chronic illness: positive Codesign approach. JMIR Format Res. 2018; 2(1):e10774. [DOI] [PMC free article] [PubMed] [Google Scholar]