Highlights
-
•
Gastric neuroendocrine neoplasms (g-NENs) represent the most frequent digestive NENs and are increasingly recognized thanks to diffusion of upper gastrointestinal endoscopy.
-
•
g-NENs can be sporadic or associated with multiple endocrine neoplasia type 1 (MEN-1) and present with a functional Zollinger-Ellison syndrome.
-
•
We described a case of a 60 years old Caucasian male came to emergency room with diffuse abdominal pain and leukocytosis on blood tests.
-
•
At the level of the pyloric portion we found irregularly thickened walls associated with a small fluid collection and bubbles of free air. On exploratory laparoscopy we found a large perforation (about 5 cm of size) in the first duodenum portion.
-
•
Histological examination revealed a gastric NET perforation as a consequence of hypergastrinemia secondary to gastrinoma.
Keywords: Gastric NET, Gastric perforation, Emergency surgery, Exploratory laparoscopy
Abstract
Introduction
Neuroendocrine tumors (NETs) represent uncommon neoplasms with different characteristics. They can be asymptomatic and benign or they can also proliferate and manifest themselves with neoplastic mass symptoms such as intestinal occlusion or with carcinoid syndrome. Gastric neuroendocrine neoplasms (g-NENs) are the most frequent digestive NENs while duodenal neuroendocrine neoplasms (d-NENs) may be sporadic or associated with multiple endocrine neoplasia type 1 (MEN-1) and present a functional syndrome (e.g. gastrinoma with Zollinger-Ellison syndrome).
Presentation of case
We report a case of duodenal perforation due to a unknown gastrinoma responsible of Zollinger-Ellison Syndrome. He underwent an emergency contrast enhanced CT abdominal scan that showed a perforation. We performed a distal gastrectomy. The histopathological examination revealed a g-NET configuring a possible picture of Zollinger-Ellison Syndrome.
Discussion
The management of NETs is diffulcult and controversial because of their rarity. It is useful to know the pathologic assessment of tumor differentiation and/or grade, evaluate surgical resectability and control the carcinoid syndrome symptoms.
Conclusion
This case report shows that gastric NETs can be found in cases of duodenal perforation. Our future goal is to evaluate the possibilities to diagnose the Zollinger Ellison Syndrome as early as possible and to treat it with targeted therapy in order to prevent its related complications.
1. Introduction
Neuroendocrine tumors (NETs) can arise in the gastrointestinal tract, in the lungs and, occasionally, elsewhere. The term neuroendocrine tumor refers to well-differentiated neuroendocrine neoplasms, while neuroendocrine carcinoma (NEC) is attribute to poorly differentiated neuroendocrine cancers. Carcinoid syndrome is a clinical picture with a constellation of symptoms mediated by various humoral factors elaborated by well-differentiated NETs of the digestive tract and lungs which synthesize, store and release several polypeptides, biogenic amines, and prostaglandins. Most NETs are associated with carcinoid syndrome only when they metastasize to the liver. Gastric neuroendocrine neoplasms (g-NENs) represent the most frequent digestive NENs and are increasingly recognized thanks to diffusion of upper gastrointestinal endoscopy. g-NENs can be asymptomatic and benign or aggressive and can sometimes mimic a gastric adenocarcinoma. Duodenal neuroendocrine neoplasms (d-NENs) can be sporadic or associated with multiple endocrine neoplasia type 1 (MEN-1) and present with a functional Zollinger-Ellison syndrome. The fundamental principles of evaluation of NETs include radiological staging and tumor localization with CT scan or magnetic resonance imaging (MRI) [[1], [2], [3]]. Upper and lower endoscopy are used for the evaluation of metastatic NETs with an unknown primary site. For clinical management is useful to know tumor differentiation and/or grade (assessment of mitotic rate and/or Ki-67 index, presence/absence of necrosis and pleomorphism), surgical resectability, the control of carcinoid symptoms and an adequate use of antitumor therapy for unresectable metastatic disease [4]. In line with the SCARE criteria, in this case report we present a a patient with duodenal perforation due to a misunderstood gastrinoma with Zollinger-Ellison Syndrome [5,6].
2. Presentation of case
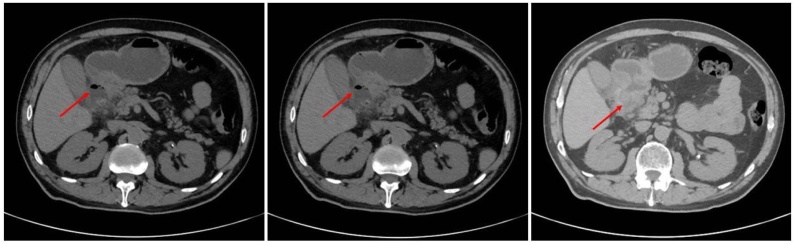
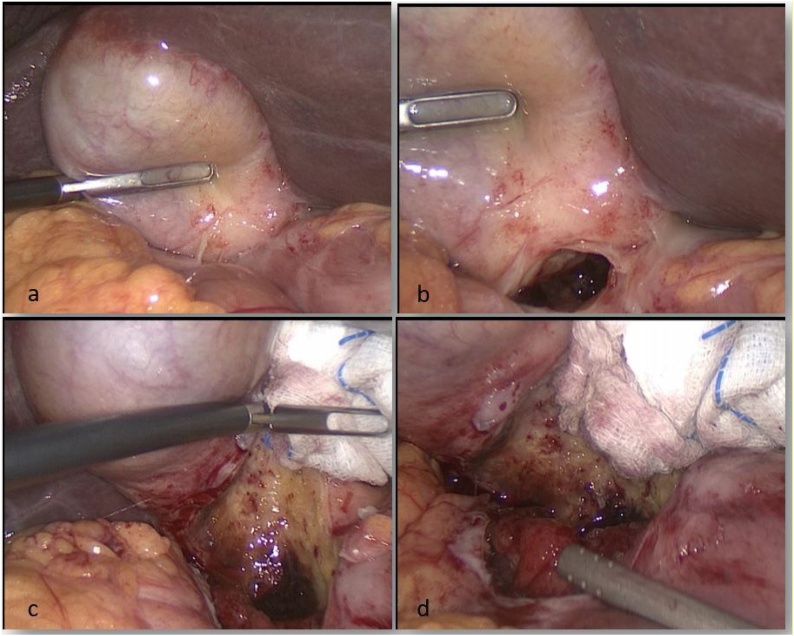
A 60 years-old Caucasian male came to emergency room with diffuse abdominal pain, leukocytosis on blood tests (WBC 19 × 103/mmc) and increased C-reactive protein (120 mg/L). The patient suffered from hypertension and he had a medical history of previous appendectomy and repair of umbilical hernia. Family history was negative for other diseases. He underwent an urgent contrast enhanced CT abdominal scan that showed a dilated stomach with hyperdense material of hematic nature in the lumen. At the level of the pyloric portion we found irregularly thickened walls associated with a small fluid collection and bubbles of free air (Fig. 1). These radiological and clinical findings appeared compatible with diagnosis of complicated peptic ulcer with covered perforation. Because of the worsening of the patient's clinical condition, we carried out an emergency surgery with distal gastrectomy for the large diameter and position of perforation. The procedure was performed by a young surgeon in urgent setting. We decided for a laparoscopic approach with pneumoperitoneum via trans-umbilical open Hasson technique [7]. We used a 12-mm trocar in the left hypochondrium and other two 5-mm trocars respectively in the right flank and in xiphoid region [[8], [9], [10]]. On exploratory laparoscopy we found a large perforation (about 5 cm of size) in the first duodenum portion (Fig. 2). We converted the procedure to open surgery in consideration of the extension and position of the lesion that did not allow us to continue safely in laparoscopy. We achieved a distal gastrectomy with Roux-en-Y side-to-side gastro-jejunostomy [11,12]. The patients were satisfied with the treatment received, the postoperative course was uneventful and the patient was discharged on POD 7. The intraoperative findings appeared to be not unequivocal, configuring on the one hand the hypothesis of perforation on a large peptic ulcer or on a chronic pancreatitic process but we could not exclude the presence of a neoplasm by a gastro-duodenal origin. The histopathological examination described, 2 cm from the distal pyloric margin and in the context of the pyloric type mucosa, a centimetric neoformation with histological and immunophenotypic characteristics of well-differentiated neuroendocrine tumor (NET G.1 s. WHO 2019 classification, Synaptofisina + and Chromogranina +) [13]. In the adjacent mucosa we saw multiple erosion/ulceration phenomena, with bleeding spillage and vascular congestion. The gastric wall was all affected by outbreaks of chronic inflammation, sometimes in follicular aggregation and with fibrosis also extended to the subserosa. These aspects, in relation to the presence of a NET G1 and multiple erosive/ulcerative areas near the pyloric margin, were suggestive of a clinical picture of Zollinger Ellison syndrome [14].
Fig. 1.
Contrast enhanced CT abdominal scan that showed a dilated stomach with hyperdense material of hematic nature in the lumen. At the level of the pyloric portion we found irregularly thickened walls associated with a small fluid collection and bubbles of free air (arrows).
Fig. 2.
Intraoperative findings during laparoscopic exploration. a, b) we can see a covered perforation with no free fluid in the abdominal cavity. c, d) only blunt dissection of gallbladder detects the large duodenal perforation requiring conversion to open surgery.
3. Discussion
Zollinger-Ellison syndrome (ZES) is caused by a secretion of gastrin from duodenal or pancreatic neuroendocrine tumors named gastrinomas. The annual incidence of gastrinomas is 0.5–2 per million. Most patients are between 20 and 50 years-old with a higher incidence in men as compared with women. Approximately 80% of gastrinomas are sporadic, but 20–30% occurs in association with multiple endocrine neoplasia type 1 (MEN 1) and hypersecretion of other hormones [15,16]. The hyperproduction of gastrin stimulates gastric acid secretion and the proliferation of parietal and enterochromaffin cells of the gastric wall. Acid hypersecretion causes diarrhea and a consequent hypokalaemia, gastro-oesophageal reflux disease, recurrent and/or conventional treatment-resistant gastric and duodenal ulcers (over in 90% of affected patients). These peptic lesions are the main reason of abdominal pain felt by subjects suffering from gastrinoma. In 25% of cases it can occur in the context of a Multiple Endocrine Neoplasia (MEN 1) which must always be excluded in the case of hypergastrinemia. MEN is a rare syndrome characterized by the presence of several malignant and benign tumors affecting the endocrine system. It is generally a hereditary disease, linked to mutations in the homonymous gene (MEN1). There are more than 20 types of endocrine and non-endocrine tumors, which occur in different combinations in the case of MEN1. In general, the most frequent tumors concern parathyroid glands (80–100%), pancreas (pancreatic NETs: 30–80%), pituitary glands (15–50%) and, in the 25% of the case, as we sayed, can occur duodenal NETs [17]. Many people find mutations in the MEN1 gene during specific tests after that another family member has been diagnosed with type 1 multiple endocrine neoplasia. We can suspect the presence of the MEN 1 syndrome even in the absence of other family cases, based on typical signs and symptoms such as in presence of hyperparathyroidism or in treatment-resistant peptic ulcers. Blood tests can assess the levels of potentially altered typical hormones and radiological features allow us to see if there are suspicious formations. MEN1 is due by mutations in the MEN1 gene and DNA examination is useful to obtain diagnosis and to study the other family members. For this reason, in our patients in addition to CT scan of chest and abdomen we performed genetic test which were negative. Therefore we concluded that the cause of its perforation is attributable to a sporadic ZES related to gastrinoma and with no association with MEN1 [18,19].
4. Conclusion
This case report shows that gastric NET can be found in cases of duodenal perforation as a consequence of hypergastrinemia secondary to gastrinoma. The literature data about management of these clinical conditions in emergency setting are poor because of the rarity of that pathologies. However, surgical treatment remains the best therapeutic option. Elective surgery is based on an early endoscopic diagnosis of Zollinger Ellison Syndrome and targeted therapy with proton-pump inhibitors (PPIs) and antiacid in order to prevent any complications
Conflicts of interest
Di Buono Giuseppe and other co-authors have no conflict of interest.
Funding
Di Buono Giuseppe and other co-authors have no study sponsor.
Ethical approval
Ethical Approval was not necessary for this study.
We obtained written patient consent to publication.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Di Buono Giuseppe: study design, data collections, data analysis and writing.
Bonventre Giulia: study design, data collections, data analysis and writing.
Badalamenti Giuseppe: data collections.
Buscemi Salvatore: data collections.
Romano Giorgio: study design, data collections, data analysis and writing.
Agrusa Antonino: study design, data collections, data analysis and writing.
Registration of research studies
Not applicable.
Guarantor
Di Buono Giuseppe
Agrusa Antonino
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
This article is part of a supplement entitled Case reports from Italian young surgeons, published with support from the Department of Surgical, Oncological and Oral Sciences – University of Palermo.
Contributor Information
Giuseppe Di Buono, Email: giuseppe.dibuono@unipa.it.
Giulia Bonventre, Email: giulia.bonv@gmail.com.
Giuseppe Badalamenti, Email: giuseppe.badalamenti@unipa.it.
Salvatore Buscemi, Email: buscemi.salvatore@gmail.com.
Giorgio Romano, Email: giorgio.romano@unipa.it.
Antonino Agrusa, Email: antonino.agrusa@unipa.it.
References
- 1.Galia M., Albano D., Picone D., Terranova M.C., Agrusa A., Di Buono G., Licata A., Lo Re G., La Grutta L., Midiri M. Imaging features of pancreatic metastases: a comparison with pancreatic ductal adenocarcinoma. Clin. Imaging. 2018;51(September-October):76–82. doi: 10.1016/j.clinimag.2018.01.016. Epub 2018 Feb 6. [DOI] [PubMed] [Google Scholar]
- 2.Galia M., Albano D., Bruno A., Agrusa A., Romano G., Di Buono G., Agnello F., Salvaggio G., La Grutta L., Midiri M., Lagalla R. Imaging features of solid renal masses. Br. J. Radiol. 2017;90(August (1077)):20170077. doi: 10.1259/bjr.20170077. Epub 2017 Jul 13. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albano D., Agnello F., Midiri F., Pecoraro G., Bruno A., Alongi P., Toia P., Di Buono G., Agrusa A., Sconfienza L.M., Pardo S., La Grutta L., Midiri M., Galia M. Imaging features of adrenal masses. Insights Imaging. 2019;10(January (1)):1. doi: 10.1186/s13244-019-0688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oronsky B., Ma P.C., Morgensztern D., Carter C.A. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(December (12)):991–1002. doi: 10.1016/j.neo.2017.09.002. Epub 2017 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., For the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Thomaschewski M., Neeff H., Keck T., Neumann H.P.H., Strate T., von Dobschuetz E. Is there any role for minimally invasive surgery in NET? Rev. Endocr. Metab. Disord. 2017;18(December (4)):443–457. doi: 10.1007/s11154-017-9436-x. [DOI] [PubMed] [Google Scholar]
- 8.Agrusa A., Di Buono G., Buscemi S., Cucinella G., Romano G., Gulotta G. 3D laparoscopic surgery: a prospective clinical trial. Oncotarget. 2018;9(April (25)):17325–17333. doi: 10.18632/oncotarget.24669. eCollection 2018 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrusa A., Romano G., Navarra G., Conzo G., Pantuso G., Buono G.D., Citarrella R., Galia M., Monte A.L., Cucinella G., Gulotta G. Innovation in endocrine surgery: robotic versus laparoscopic adrenalectomy. Meta-analysis and systematic literature review. Oncotarget. 2017;8(October (60)):102392–102400. doi: 10.18632/oncotarget.22059. eCollection 2017 Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrusa A., Frazzetta G., Chianetta D., Di Giovanni S., Gulotta L., Di Buno G., Sorce V., Romano G., Gulotta G. “Relaparoscopic” management of surgical complications: the experience of an Emergency Center. Surg. Endosc. 2016;30:2804–2810. doi: 10.1007/s00464-015-4558-2. [DOI] [PubMed] [Google Scholar]
- 11.Falconi M., Eriksson B., Kaltsas G., Bartsch D.K., Capdevila J., Caplin M., Kos-Kudla B., Kwekkeboom D., Rindi G., Klöppel G., Reed N., Kianmanesh R., Jensen R.T. Vienna consensus conference participants. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103(2):153–171. doi: 10.1159/000443171. Epub 2016 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romano G., Agrusa A., Galia M., Di Buono G., Chianetta D., Sorce V., Gulotta L., Brancatelli G., Gulotta G. Whipple’s pancreaticoduodenectomy: surgical technique and perioperative clinical outcomes in a single center. Int. J. Surg. 2015;21(September (Suppl 1)):S68–71. doi: 10.1016/j.ijsu.2015.06.062. Epub 2015 Jun 26. [DOI] [PubMed] [Google Scholar]
- 13.Klimstra D.S., Kloppell G., La Rosa S., Rindi G. Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours Editorial Board, editor. WHO Classification of Tumours: Digestive System Tumours. 5th ed. International Agency for Research on Cancer; Lyon: 2019. p. 16. [Google Scholar]
- 14.Gibril F., Jensen R.T. Advances in evaluation and management of gastrinoma in patients with Zollinger-Ellison syndrome. Curr. Gastroenterol. Rep. 2005;7(May (2)):114–121. doi: 10.1007/s11894-005-0049-2. [DOI] [PubMed] [Google Scholar]
- 15.Anderson B., Sweetser S. Gastrointestinal: Zollinger-Ellison Syndrome: a rare cause of chronic diarrhea and abdominal pain. J. Gastroenterol. Hepatol. 2017;32(July (7)):1281. doi: 10.1111/jgh.13630. [DOI] [PubMed] [Google Scholar]
- 16.Strosberg J.R., Halfdanarson T.R., Bellizzi A.M., Chan J.A., Dillon J.S., Heaney A.P., Kunz P.L., O’Dorisio T.M., Salem R., Segelov E., Howe J.R., Pommier R.F., Brendtro K., Bashir M.A., Singh S., Soulen M.C., Tang L., Zacks J.S., Yao J.C., Bergsland E.K. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46(July (6)):707–714. doi: 10.1097/MPA.0000000000000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F., Venzon D.J., Serrano J., Goebel S.U., Doppman J.L., Gibril F., Jensen R.T. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J. Clin. Oncol. 1999;17(February (2)):615–630. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 18.Scherübl H., Cadiot G., Jensen R.T., Rösch T., Stölzel U., Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42(August (8)):664–671. doi: 10.1055/s-0030-1255564. Epub 2010 Jul 28. [DOI] [PubMed] [Google Scholar]
- 19.Norton J.A., Fraker D.L., Alexander H.R., Jensen R.T. Value of surgery in patients with negative imaging and sporadic Zollinger-Ellison syndrome. Ann. Surg. 2012;256(September (3)):509–517. doi: 10.1097/SLA.0b013e318265f08d. [DOI] [PMC free article] [PubMed] [Google Scholar]