Abstract
Background
Human alveolar echinococcosis (AE) caused by Echinococcus multilocularis is an underreported, often misdiagnosed and mistreated parasitic disease mainly due to its low incidence. The aim of this study was to describe the epidemiological and clinical characteristics of human AE patients in Hungary for the first time.
Method
Between 2003 and 2018, epidemiological and clinical data of suspected AE patients were collected retrospectively from health database management systems.
Results
This case series included a total of 16 AE patients. The mean age of patients was 53 years (range: 24–78 years). The sex ratio was 1:1. Four patients (25%) revealed no recurrence after radical surgery and adjuvant albendazole (ABZ) therapy. For five patients (31.3%) with unresectable lesions, a stabilization of lesions with ABZ treatment was achieved. In seven patients (43.8%), progression of AE was documented. The mean diagnostic delay was 33 months (range: 1–122 months). Three AE related deaths (fatality rate 18.8%) were recorded.
Conclusions
AE is an emerging infectious disease in Hungary with a high fatality rate since based on our results, almost every fifth AE patient died in the study period. Differential diagnosis and appropriate surgical and medical therapy for AE is an urging challenge for clinicians in Hungary, as well as in some other European countries where E. multilocularis is prevalent.
Keywords: Human alveolar echinococcosis, Echinococcus multilocularis, Clinical epidemiology, Case series, Hungary
Background
Human alveolar echinococcosis (AE) is one of the most dangerous and potentially lethal parasitic zoonosis in the temperate and arctic regions of Europe, which is caused by Echinococcus multilocularis (Em), a small tapeworm belonging to family Taeniidae [1]. Em develops into its adult form in the small intestine of canid definitive hosts, mainly the red fox (Vulpes vulpes) in Europe [2, 3]. To which extent dogs may play a central role in transmitting AE infection to humans in Europe, is still under debate [4, 5]. Infective Em eggs are mainly released into the environment with the feces of infected foxes and ingested by intermediate hosts, mainly small rodents belonging to family Arvicolinae. Oncospheres hatching from eggs penetrate the intestinal mucosa of Arvicolinae and migrate via portal venous and lymphatic circulation into the capillary bed of the main target organ, namely the liver. The oncospheres develop into a larval metacestode, which slowly grows into an infiltrative parasitic tissue. Humans are dead-end intermediate hosts and may become infected, similarly to small rodents, through the ingestion of Em eggs from contaminated environment or matrices. Human AE is a rare disease associated with nonspecific symptoms and a long incubation period of several years. Therefore, main drivers of Echinococcus spp. transmission pathways (food-borne, water-borne or hand-to-mouth) and the identification of potential risk factors are still under scientific debate [4–6].
Clinical management of human AE patients is challenging, even for experts. In humans, oncospheres established in the liver develop as aggregated vesicles (appearing as microcysts at imaging) and then evolving into a tumor-like parasitic mass with locally invasive growth pattern, mimicking malignancy. Histologically, AE-lesions are characterized by necrosis with intermingled fragments of the laminated layer surrounded by a granulomatous inflammation [3] (Fig. 1c; Fig. 2a). Initially, AE is often asymptomatic, and the time from infection to the development of a typical AE-liver lesion is 5 to 15 years in immunocompetent individuals. Diagnosis of AE is based on clinical and epidemiological data, imaging techniques, histopathology and/or nucleic acid detection and serology [7]. Imaging techniques has a central role in the differential diagnosis and clinical management of human AE. In particular, the importance of ultrasonography (US) in hepatic AE rely primarily in the opportunity for early diagnosis [8]. For further characterization of lesions and for investigate extension of AE lesion to adjacent structures and distant metastases, computed tomography (CT) and magnetic resonance imaging (MRI) are useful [7, 9, 10]. During the past decades, several advanced classification systems has been introduced to distinguish types of AE lesions based on different imaging characteristics adjusted for conventional imaging techniques (US, MRI, CT) [8, 11–15]. For interdisciplinary treatment planning, staging based on image findings according the WHO - Informal Working Group on Echinococcosis (WHO-IWGE) PNM classification is recommended [7]. Albendazole (ABZ) is the first drug of choice for medical treatment and is mandatory in all patients [16, 17]. Radical surgery, aiming to completely remove all lesions including satellite (metastatic) lesions followed by a two-year ABZ administration is the standard treatment aiming for cure. The majority of patients are inoperable and need long-term ABZ-treatment [18, 19]. Interventional procedures should be preferred to palliative surgery whenever possible, and radical surgery is the first choice in all cases suitable for total resection of the lesion(s). Rescue liver transplantation is a therapeutic option for AE patients with inoperable lesions and/or chronic liver failure [7, 20].
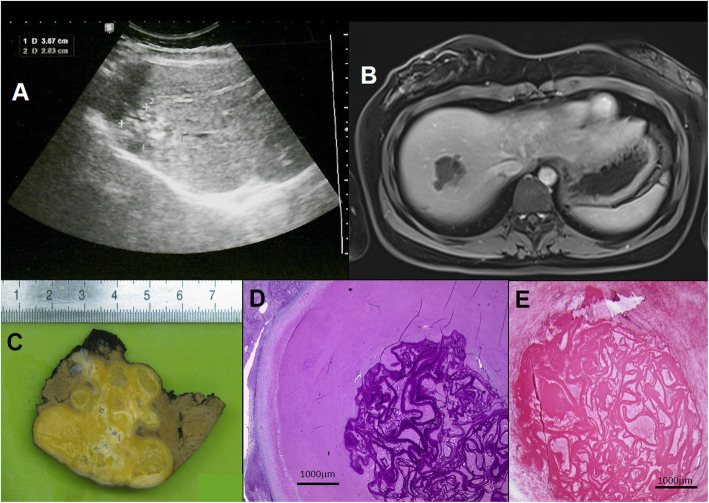
Fig. 1.
Patient No. 11. a Ultrasound image of alveolar echinococcosis (AE) lesion in the liver. b Axial T1 weighted MR image of the same AE lesion. c Gross picture of the lesion removed by segmentectomy. d Microscopic appearance of the lesion: note the multiplex, slender, PAS positive LL of the metacestode surrounded by abundant necrotic material (PAS). e Immunohistochemical staining using the monoclonal antibody Em2G11: the antibody marks the laminated layer of Em (LL – red colored band-like structures)
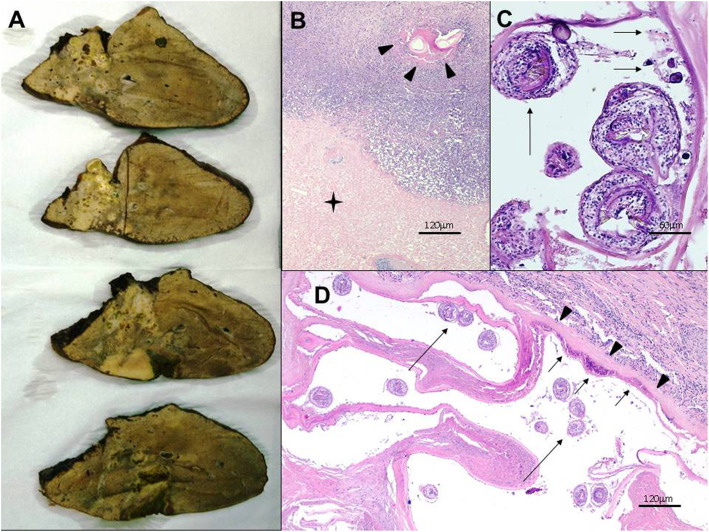
Fig. 2.
Patient No. 14. a Gross view of the resected right lobe of the liver shows heterogeneous solid mass lesion that contains externally budding vesicles and calcified foci with a maximum diameter of a single vesicle around 10 mm. b, c, d Histopathological characteristics of Echinococcus multilocularis lesion in the liver. Normal appearance of the liver is distorted. Severe inflammation is present around the necrotic liver tissue (asterisk in b). This inflammatory infiltrate consists of lymphocytes and histiocytes. In some areas fragment of cuticular membrane is observed within the necrotic area (arrowheads in b). This membrane displays the laminated layer as a tender band-like structure (arrowheads in d) with a germinal layer (short arrows in c and d). Note the invaginated protoscoleces found within the vesicles (long arrows in c and d)
In this context, our study aims to collect epidemiological and clinical data on all human AE patients diagnosed in Hungary so far by means of retrospective case-series analysis to present the consequences of parasite spreading in previously AE free European countries and provide guidance to clinicians for the management of this emerging helminthic infection in the future.
Methods
Case selection and inclusion criteria
A multicentre retrospective case-series analysis was conducted during the study period from 01/01/2003 to 31/12/2018, describing the epidemiological and clinical characteristics of all patients with probable or confirmed diagnosis of AE in Hungary.
The Hungarian patients included in this study were those who had positive serology for Em with one highly sensitive (ELISA or IHA) and one highly specific (Western blot) test and therefore fulfilled the clinical and laboratory diagnostic criteria for probable or confirmed AE patient as proposed by the WHO-IWGE [7]. A probable case was defined as any patient with clinical and epidemiological history, and imaging findings, and positive serology for AE with two positive tests. A confirmed case was defined as the above, plus i) histopathology compatible with AE and/or ii) detection of Em nucleic acid sequence(s) in a clinical specimen. Patient(s) who were serologically negative but Em infection was unequivocally confirmed by histopathological methods were also included in this case series.
Epidemiological and serological data
Baseline demographic and clinical data were collected from medical records from the database management systems of the following centres: National Public Health Centre, National Reference Laboratory for Human Parasitic Diseases, Budapest, Hungary; Central Hospital of Southern Pest National Institute of Hematology and Infectious Diseases, Budapest, Hungary (MedSol); Semmelweis University Department of Transplantation and Surgery, Budapest, Hungary (Medsol); National Institute of Oncology, Budapest, Hungary (MedWorks); Kaposi Mór Teaching Hospital, Kaposvár, Hungary (eMedsol); Borsod-Abaúj-Zemplén County Teaching Hospital, Miskolc, Hungary (Medworks); Petz Aladár County Teaching Hospital, Győr, Hungary (Hospitaly) and Jósa András County Teaching Hospital, Nyíregyháza, Hungary (Helise).
Data regarding potential risk factors were collected by means of questionnaires with dichotomous questions delivered to patients providing informed consent. The following potential risk factors on human infection with Em were investigated: dog ownership, playing with dogs, have a kitchen garden, farming occupation, did hay making in meadows not adjacent to water, went to forests for vocational reasons, ate unwashed strawberries, chewed grass, hunting, handling foxes, consumption of wild vegetables and fruit [4], and presence of foxes at the place of residence.
Serological data were obtained from the Hungarian Reference Laboratory for Human Parasitic Diseases (National Public Health Centre, Budapest, Hungary). Antibody titers were first determined by a high-sensitivity ELISA test (Hydatidosis ELISA, Vircell, Spain; Ridascreen Echinococcus IgG, R-Biopharm, Germany) or indirect hemagglutination test (Cellognost-Echinococcosis, Siemens, Germany). As a second high-specificity confirmatory test, Western blot (Echinococcus Western blot IgG, Ldbio, France) was applied [21].
Histopathology, immunohistochemistry, and molecular analysis
Tissue sections from samples included core-biopsies, surgical specimens and specimens from autopsies. Both haematoxylin-eosin (H&E) and Periodic Acid Schiff (PAS) staining were performed. Histological criteria for diagnosis included the identification of multiple cysts, a PAS positive slender (< 1 mm) laminated layer within abundant necrotic areas, a tubular growth pattern accompanied by a granulomatous cell-reaction without any definite fibrous capsule around the lesion [22] (Fig. 1d-e, Fig. 2b-d). Further confirmation derived from an immunohistochemical staining with the monoclonal antibody Em2G11 (Ulm, Germany) [23] (Fig. 1e) or by the detection of Em specific DNA by PCR method (Wien, Austria). Cytological analysis of samples gained by fine-needle aspiration biopsy was also carried out. Cytology alone without further confirmatory tests such as immunocytochemistry was regarded as inappropriate method in diagnosing AE in this case series.
Clinical data and case follow-up
For detailed clinical analysis, we registered the date (month, year) of the first symptoms and/or findings; the initial symptoms; the first physical and laboratory findings; the initial and final radiomorphological characteristics of AE lesions investigated by conventional imaging methods (US, CT or MRI) with the largest diameter of the lesions in millimeters; the preliminary diagnosis; extrahepatic localization of the parasite at first diagnosis; the date (month, year) of diagnosis of probable or confirmed AE (Table 1). The diagnostic delay in each identified patient as the time period between the first symptoms/findings and the recognition of AE was calculated. Treatment modalities were also analyzed. ABZ was given at a dose of 800 mg per day in two divided doses continuously or intermittently. Duration of ABZ administration were registered in months. In AE patients with resectable parasitic lesion, curative surgical treatment followed the rules of tumor surgery aiming to perform R0 resection without any parasitic residue. Endoscopic and percutaneous interventions (Table 2) and potentially immunosuppressive co-morbidities were also recorded.
Table 1.
Diagnostic features of human alveolar echinococcosis cohort patients in Hungary (2003–2018)
| case no. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| onset of symptoms or first findings | 09.2001 | 09.2003 focal hepatic lesions during imaging studies | 10.2004 | 08.2008 |
| initial symptoms and physical findings | epigastric pain, vomitus | asymptomatic, hepatomegaly | epigastric and right hypochondriac pain | jaundice, pruritus, right hypochondriac pain |
|
liver function tests: liver enzymes (U/l); sebi (μmol/l) |
normal | – | elevated GGT (104) | elevated ALP (1254), GGT (570) and sebi (202) |
|
initial US/CT/MRI (date) radiomorphology largest diameter of AE lesion(s) in mm |
US (09.2001) – 15 mm hyperreflective area in SIV CT (03.2003) –echinococcal cysts inboth lobes, number, size, localization unknown |
US (04.2005) and CT (08.2009) – 10 typical AE lesions in SIV, SV, SVI, SVIII, 10–30 mm, largest lesion 50 mm |
US (10.2004), CT (11.2004) and MRI (06.2005) – one typical AE lesion – 100 mm –in SV, SVI, SVII, SVIII |
US (08.2008) – typical central AE lesion −110 mm – in the dichotomy of hepatic common duct, SIV, SV |
| preliminary diagnosis | echinococcosis |
liver tumor, echinococcosis |
liver tumor, HCC, liver metastasis |
liver tumor, adenocarcinoma |
| serology Westernblot (Ldbio) P3 Em | positive | positive | positive | positive |
| core biopsy/surgical sample/autopsy | – | – |
core biopsy (2x) surgical sample (1x) |
core biopsy during PTC, surgical sample |
| histopathology/IH/PCR | – | – |
histopathology and PCR |
histopathology and PCR |
| type of diagnosis | probable | probable | confirmed | confirmed |
| month.year of diagnosis | 04.2003 | 04.2004 | 07.2005 | 09.2008 |
| latency of diagnosis (in months) | 20 | 8 | 10 | 2 |
| extrahepatic localizationat the time of diagnosis | no pulmonary lesion | no pulmonary lesion |
peritoneal dissemination no pulmonary lesion |
no pulmonary lesion |
| PNM at diagnosis | PxNxMx | P1N0Mx | P2N1Mx | P3N0Mx |
| case no. | 5 | 6 | 7 | 8 |
| onset of symptoms orfirst findings |
2002 asymptomatic hepatic cyst; patient denied investigations |
11.2011 | 12.2012 | 11.2012 |
| initial symptoms andphysical findings |
right hypochondriac pain, vomitus, anasarca, palpable liver tumor (12.2010) |
right hypochondriac pain, weightloss, hepatomegaly |
asymptomatic, mild hepatomegaly |
asymptomatic |
|
liver function tests: liver enzymes (U/l); sebi (μmol/l) |
AST (177), ALT (177), GGT (920), ALP (1152), sebi (16) |
GGT (105), ALP (543), sebi (7,1) |
GGT (335), ALP (999), sebi (7,3) |
normal |
|
initial US/CT/MRI (date) radiomorphology largest diameter of AE lesion(s) in mm |
US (12.2010) and CT (01.2011) two interconnected pseudocystic AE lesionsin both lobes – 130 mm and 120 mm – dilatated intrahepatic bileducts |
US (11.2011) and CT (12.2011) typical AE lesion in SV, SVI −83 mm – and some smaller lesions |
CT (04.2013) typical AE lesion in right lobe, 135 mm, periportal biliary and vascular involvement (right v. portae, v. hepatica intermedia) |
CT (11.2012) and MRI (08.2014) multiplying small calcified lesions in SV, SVI, SVII, SVIII |
| preliminary diagnosis | metastasis, tumor, CE | hemangioma, tumor, CE |
cholangiocellular carcinoma |
liver metastasis |
| serology Westernblot (Ldbio) P3 Em | positive | equivocal | positive | negative (postoperatively 2x) |
| core biopsy/surgical sample/autopsy | parasitology and cytology from lesion fluid (FNAB) negative | corebiopsy | corebiopsy | corebiopsy and surgical sample |
| histopathology/IH/PCR | – | histopathology | histopathology | histopathology andIH |
| type of diagnosis | probable | confirmed | confirmed | confirmed |
| month.year of diagnosis | 03.2011 | 01.2012 | 04.2013 | 10.2014 |
| latency of diagnosis (in months) | 111 | 1 | 5 | 24 |
| extrahepatic localizationat the time of diagnosis | no pulmonary lesion | no pulmonary lesion |
undignified pulmonary lesions |
no |
| PNM at diagnosis | P4N0Mx | P2N0Mx | P4N0Mx | P1N0M0 |
| case no. | 9 | 10 | 11 | 12 |
| onset of symptoms or first findings | 10.2013 | 04.2012 | 02.2017 | 03.2017 |
| initial symptoms and physical findings | right hypochondriac pain, urticaria | right hypochondriac pain, hepatomegaly | epigastric pain, vomitus | right hypochondriac pain |
|
liver function tests: liver enzymes (U/l); sebi (μmol/l) |
ALP (125), GGT (86) | normal | normal | elevated ALP |
|
initial US/CT/MRI (date) radiomorphology largest diameter of AE lesion(s) in mm |
MRI (12.2015) and CT (01.2016) 2 typical AE lesions in the dichotomy of hepatic veins; in SV/SIVB 55 mm; in SVIII/IVA 53 mm |
CT (04.2012) and MRI (10.2012) typical AE lesion in SIV 42 mm |
US (02.2017), CT (02.2017) and MRI (03.2017) two AE lesions in SVIII 44 mm and in SVII 12 mm |
US (05.2017), CT (05.2017) multiplex AE lesions in both lobes, 40 mm |
| preliminary diagnosis | atypical rare malignancy liver metastasis | hemangioma, adenoma, liver tumor |
hemangioma cholangiocellular carcinoma, fibrolamellar carcinoma |
liver metastasis, sarcoidosis, granulomatous hepatitis |
| serology Westernblot (Ldbio) P3 Em | positive | positive | Echinococcus genus P5 | positive |
| core biopsy/surgical sample/autopsy | – | – | (FNAB) and surgical sample | corebiopsy (2x) |
| histopathology/IH/PCR | – | – | IH | histopathology |
| type of diagnosis | probable | probable | confirmed | confirmed |
| month.year of diagnosis | 01.2016 | 06.2016 | 05.2017 | 07.2017 |
| latency of diagnosis (in months) | 28 | 50 | 4 | 5 |
| extrahepatic localizationat the time of diagnosis | no | no | no | no |
| PNM at diagnosis | P3N0M0 | P1N0M0 | P1N0M0 | P2N0M0 |
| case no. | 13 | 14 | 15 | 16 |
| onset of symptoms orfirst findings | 09.2017 | 09.2016 | 04.2008 | 2008 |
| initial symptoms and physical findings |
right hypochondriac pain, hepatomegaly |
asymptomatic | asymptomatic |
right hypochondriac pain |
|
liver function tests: liver enzymes (U/l); sebi (μmol/l) |
elevated liver enzymes | GGT (115) | – | – |
|
initial US/CT/MRI (date) radiomorphology largest diameter of AE lesion(s) in mm |
US (10.2017), CT (10.2017) typical AE lesion in SV 80 mm |
MRI (09.2016) 15 mm wide hypodens area in right lobe, CT (09.2017) and MRI (11.2017) 75 mm typical AE lesion in SV and SVIII, dilatation of intrahepatic bileducts |
US (04.2008), CT (07.2008) typical AE lesion in SV – 54 mm –and three small calcified lesions |
US (2008) 20 mm hyperechoic liver lesion, CT (10.2016) and MRI (06.2017) 120 mm typical AE lesion in right lobe (SV-VI-VIII) |
| preliminary diagnosis | liver tumor | cholangiocellular carcinoma, Klatskin tumor | atypical hepatic cyst | hemangioma, cystadenocarcinoma |
| serology Westernblot (Ldbio) P3 Em | positive | positive | positive | positive |
| core biopsy/surgical sample/autopsy | -(FNAB 2x) | surgical sample | surgical sample autopsy | surgical sample |
| histopathology/IH/PCR | – | histopathology | histopathology | histopathology |
| type of diagnosis | probable | confirmed | confirmed | confirmed |
| month.year of diagnosis | 12.2017 | 01.2018 | 05.2018 | 08.2018 |
| latency of diagnosis (in months) | 4 | 17 | 122 | 115 (+ 12) |
| extrahepatic localizationat the time of diagnosis | no pulmonary lesion | subphrenic abscess, peribiliar vascular invasion, no pulmonary lesion | falciform ligament, no pulmonary lesion | no pulmonary lesion |
| PNM at diagnosis | P4N0M0 | P4N1Mx | P4N1Mx | P4N0Mx |
E.m Echinococcus multilocularis, AE alveolar echinococcosis, CE cystic echinococcosis, v vena, d ductus, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT gamma-glutamyltransferase, ALP alkaline phosphatase, sebi serum bilirubin, US ultrasound, CT computer tomography, MRI magnetic resonance imaging, IH immunohistochemistry using monoclonal antibody mAbEm2G11, PCR polymerase chain reaction, tx treatment, EPI endoscopic and percutaneous interventions, ERCP endoscopic retrograde cholangiopancreatography, PTC percutaneous transhepatic cholangiography, PTD percutaneous transhepatic drainage, FNAB fine needle aspiration biopsy, S liver segment, ABZ albendazole, HCC hepatocellular carcinoma
Table 2.
Therapeutic features of human alveolar echinococcosis cohort patients in Hungary(2003–2018)
| case no. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| antiparasitic drug tx (duration in months) | – | ABZ (3) 10.2004–12.2004 |
ABZ (162) continuously since 07.2005 |
ABZ (12) 11.2008 – 01.2009 and 06.2016–03.2017 |
| surgery | – | – | exploration –unresectable |
exploration, fenestration, marsupialisation |
| EPI | – | – | – | PTD (2x), ERCP |
| follow-up period in months | 27 | 177 | 162 | 124 |
| radiomorphology on final control, largest diameter of AE lesion(s) in mm (date) | CT (04.2005) – pseudocystic AE lesion in left lobe 49 mm, two more AE lesions in right lobe, 35 mm and 24 mm | US (09.2014) stabilization | MRI (12.2018) stabilization | US (12.2018) residual cavity in SIV 70 mm, giant biloma in porta hepatis |
| PNM at final imaging | P1N0Mx | P1NxMx | P2N1Mx | P3N0Mx |
| complications | elevated GGT (136), ALP (621), sebi (36,3) | – | – | central biliary obstruction, cholangitis, biloma, bile-leaking |
| outcome | progression, AE unrelated death | stabilization | stabilization | progression |
| case no. | 5 | 6 | 7 | 8 |
| antiparasitic drug tx (duration in months) | – | ABZ (5) 02.2012–07.2012 | ABZ (67) continuously since 06.2013 | ABZ (24) postoperatively |
| surgery | marsupialization, drainage | extended right hemihepatectomy | – | segmentectomy |
| EPI | ERCP – biliary stent implantation, nasobiliary stent | – | – | – |
| follow-up period in months | 9 | 84 | 69 | 51 |
| radiomorphology on final control, largest diameter of AE lesion(s) in mm (date) | US (11.2011) residual cavity 45 mm, atrophy of right lobe | US (02.2018) no recurrence | MRI (10.2018) and US (10.2018), 109 mm | US (07.2018) no recurrence |
| PNM at final imaging | P4N0Mx | P0N0Mx | P4N0Mx | P0N0M0 |
| complications | central biliary obstruction, bile-leaking, bilio-peritoneal fistula, injury of bileducts during surgical manipulation, cachexia | postoperative peritonitis, haematoma, bile-leaking, Kehr-drainage | v. cava inferior compression | – |
| outcome | progression, AE related death | no recurrence | stabilization | no recurrence |
| case no. | 9 | 10 | 11 | 12 |
| antiparasitic drug tx (duration in months) | ABZ (3) lowered dose intermittently in 2016, finally ceased | ABZ (30) continuously since 07.2016 | ABZ (21) postoperatively | ABZ (3) 09.2017–11.2017 |
| surgery | – | – | segmentectomy | – |
| EPI | – | – | – | – |
| follow-up period in months | 36 | 30 | 20 | 18 |
| radiomorphology on final control, largest diameter of AE lesion(s) in mm (date) | MRI (10.2018), CT (10.2018) no progression in liver, new pulmonary micronodules (09.2017) | MRI (11.2018) SIV 70 mm, progression | MRI (06.2018), US (10.2018) no recurrence | US (07.2018) AE lesion in left lobe 65 mm, AE lesion in right lobe 44 mm |
| PNM at final imaging | P3N0Mx | P1N0Mx | P0N0Mx | P2N0Mx |
| complications | ABZ hepatotoxicity and allergic reactions, undignified pulmonary microlesions | – | – | – |
| outcome | stabilization | progression | no recurrence | progression |
| case no. | 13 | 14 | 15 | 16 |
| antiparasitic drug tx (duration in months) | ABZ (12) continuously since 01.2018 | ABZ (12) continuously since 01.2018 | ABZ (3) 06.2018–09.2018 | – |
| surgery | – | right hemihepatectomy, exstirpation of d. choledochus and cholecystectomy, hepaticojejunostomia | explorative laparotomy | right hemihepatectomy, exstirpation of d. choledochus and cholecystectomy, hepaticojejunostomia |
| EPI | – | ERCP | ERCP (2x), stent implantation (2x) | ERCP, stent implantation, PTD (2x) |
| follow-up period in months | 13 | 12 | 5 | 1 |
| radiomorphology on final control, largest diameter of AE lesion(s) in mm (date) | MRI (10.2018) AE lesion in SV 79 mm | CT (10.2018) no recurrence | US (05.2018) 120 mm AE lesion occupying left lobe, ascites, dilatated intrahepatic bileducts | – |
| PNM at final imaging | P4N0Mx | P0N1Mx | P4N1Mx | P0N0Mx |
| complications | thrombosis and parasitic infiltration of right v. portae, compression of d. hepaticus dexter | leukopenia, hairloss, haematoma in residual left lobe (32 mm) and undignified pulmonary microlesions | compression of d. hepaticus communis, peritonitis, cholangiogen sepsis | compression of d. hepaticus communis, abscessus hepatis, liver insufficiency, septic shock |
| outcome | stabilization | no recurrence | progression, AE related death | progression, AE related death |
E.m Echinococcus multilocularis, AE alveolar echinococcosis, CE cystic echinococcosis, v vena, d ductus, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT gamma-glutamyltransferase, ALP alkaline phosphatase, sebi serum bilirubin, US ultrasound, CT computer tomography, MRI magnetic resonance imaging, IH immunohistochemistry using monoclonal antibody mAbEm2G11, PCR polymerase chain reaction, tx treatment, EPI endoscopic and percutaneous interventions, ERCP endoscopic retrograde cholangiopancreatography, PTC percutaneous transhepatic cholangiography, PTD percutaneous transhepatic drainage, FNAB fine needle aspiration biopsy, S liver segment, ABZ albendazole, HCC hepatocellular carcinoma
Clinical follow-up period was determined as the time interval between the date of AE diagnosis and the date of AE related or unrelated death or the endpoint of this study (31/12/2018). In given cases, the WHO-IWGE PNM classification [7] for AE was carried out based on the initial and final imaging during the study period. We registered AE associated complications including both the sequelae of the natural course of the infection and the consequences of pharmacological, surgical and/or endoscopic interventions. With regards to the outcome, course of AE lesions on conventional imaging was assessed as follows: “recurrence/no recurrence” after radical resection regardless of the duration of adjuvant ABZ treatment, “regression” (decreased size or stable size and declining symptoms or disappearance of former complications such as cholestasis, cholangitis), “progression” (increase in size and/or extension to neighboring organs and/or metastases or new clinical complications due to the AE as cholestasis, need for ERCP-intervention, cholangitis), “stabilization” (no change in size of the lesions and no new complications such as cholestasis and cholangitis) [19]. AE related death or AE unrelated death was also used as clinical outcome categories. At the end of the study, assessment of AE lesions was done by conventional imaging and is given as PNM re-staging (Table 1; Table 2).
Results
Epidemiological features
Between 2003 and 2018, a total of 16 patients of AE were reported to the National Public Health Centre in Hungary. Based on clinical and laboratory diagnostic criteria (WHO-IWGE expert consensus), 10 were diagnosed as confirmed (62.5%) and six as probable (37.5%) AE patients. The sex ratio was 1:1. The mean age of patients at the time of first symptoms or findings related to AE was 53 years (range: 24–78 years). Patients originated from rural areas (n = 7; 43.8%), suburban areas (n = 4; 25%), and urban areas (n = 5; 31.3%). Data regarding potential risk factors were collected from 12 out of 16 AE patients. The following potential risk factors were recorded in our series: have a kitchen garden (91.7%), went to forests for vocational reasons (83.3%), dog ownership (66.7%), playing with dogs (66.7%), consumption of wild vegetables and fruits (66.7%), recognizing foxes at the place of residence (58.3%), farming occupation (50%), eat unwashed strawberries (50%), did haymaking in meadows not adjacent to water (16.7%), and chewed grass (16.7%).
Diagnostic features
Five patients (31.3%) were asymptomatic at first examination, and AE lesions were incidental imaging findings. In symptomatic cases, the most frequent clinical signs were epigastric and/or right hypochondriac pain. Hepatomegaly, vomitus, weight loss and pruritus were observed in six patients (37.5%), while palpable liver mass was detected in one patient (6.3%). Further physical signs were urticaria and anasarca. One patient (stage P3N0Mx) had jaundice (sebi 202 μmol/l) at first presentation (6.3%). Nine patients (56.3%) had elevated liver enzymes (ALP and GGT). Imaging studies (US, CT, MRI) revealed typical hepatic AE lesions in 14 patients (87.5%). In one probable patient, two interconnected AE pseudocystic lesions were detected in both lobes with 130 mm and 120 mm in diameter. In one confirmed patient, multiple small calcified lesions were identified [22]. Hepatic localization of the parasite and environmental parasitic infiltration at the time of diagnosis was observed in 15 patients (93.8%). The disease stage was P1 in four (25%), P2 in three (18.8%), P3 in two (12.5%) and P4 in six patients (37.5%). Extrahepatic involvement of neighboring organs (N1) was detected in three patients (18.8%). Subphrenic abscess (n = 1; 6.3%), dissemination along falciform ligament (n = 1; 6.3%) and dissemination along omental peritoneum (n = 1; 6.3%) were present. The absence or presence of distant metastasis was completely evaluated only in six patients (37.5%). Distant metastasis was not found either of these patients M0 (n = 6; 37.5%). Pulmonary metastasis at the time of diagnosis was excluded in 15 patients (93.8%). Based on radiological findings, the following preliminary diagnoses were made: echinococcosis, cystic echinococcosis, liver metastasis, sarcoidosis, granulomatous hepatitis, haemangioma, hepatocellular adenoma, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, cystic neoplasm of the liver and fibrolamellar carcinoma. Based on histopathological findings, the following preliminary diagnoses were made: granulomatous hepatitis, chronic hepatitis with fibrosis, helminthosis and echinococcosis. In 10 patients confirmed by histology, protoscoleces or hooklets were evident in two patients (20%) (Fig. 2c-d). In four patients (25%), immunohistochemistry (n = 2; 12.5%) and polymerase chain reaction (n = 2; 12.5%) were used to confirm diagnosis. Em antibodies were detected in 13 out of 16 patients (81.3%). Diagnostic delay ranged from 1 to 122 months (mean diagnostic delay: 33 months) (Table 1).
Therapeutic features
Thirteen out of 16 AE patients (81.3%) received ABZ. Three patients (18.8%) received no ABZ treatment due to misdiagnosis (Table 2). During the whole study period, five AE patients (31.25%) received a daily dose of 800 mg uninterrupted ABZ treatment from the time of diagnosis because of multiple and/or extended unresectable AE lesion(s). In one patient (6.3%), AE was recognized in an advanced stage (P4N1Mx), and the patient died after 3 months of treatment. One unresectable patient (6.3%) was treated with ABZ for a total of 12 months with long interruptions during his 124 months follow-up period. Three patients (18.8%) with unresectable AE lesion(s) received ABZ treatment for less than 4 months from the time of diagnosis. Causes of ceasing therapy were drug-related hepatotoxicity, allergic reactions, virtual stabilization of AE lesions and propagation of liver lesions (with supposed uneffectivity of ABZ treatment). Four patients (25%) received adjuvant ABZ therapy following removal of AE lesion(s) by radical (R0) liver surgery. Four patients (25%) received an incomplete concomitant ABZ treatment with a total of 5 months duration (Table 2).
Surgery was performed in nine out of 16 AE patients (56.3%). The timely diagnosis of AE, as a major impact on choosing the proper method of surgical intervention, was only confirmed in one out of the nine patients (6.3%). In three patients (18.8%), explorative laparotomy was carried out for diagnostic purposes to assess resectability and gain tissue-sample for histopathological analysis. Unresectability was detected in three patients (18.8%) due to extrahepatic peritoneal dissemination (n = 1; 6.3%), central localization compressing ductus hepaticus communis (n = 1; 6.3%) and peritoneal dissemination and also the compression of ductus hepaticus communis (n = 1; 6.3%). Radical resection aiming to excise the entire parasitic lesion with safety margin (R0) were done in five patients (31.3%) as follows: extended right hemihepatectomy with feeding catheter jejunostomy (n = 1; 6.3%), right hemihepatectomy with hepaticojejunostomy (n = 2; 12.5%) and segmentectomy (n = 2; 12.5%). In two patients (12.5%) fenestration and marsupialisation of AE lesions, as palliative methods were performed.
Endoscopic-retrograde-cholangio-pancreatography was performed in five patients (31.3%) because of AE associated biliary obstruction. Endoscopic biliary stents were placed five times in three patients. In one patient, nasobiliary stent placement was also necessary to facilitate bile passage. A total of four percutaneous transhepatic drainage of AE lesions in two patients were performed. Among the five patients (31.3%) who needed endoscopic and/or percutaneous interventions, the following stages were determined at diagnosis: P4N1Mx (n = 2; 12.5%), P4N0Mx (n = 2; 12.5%); P3N0Mx (n = 1; 6.3%) (Table 1; Table 2).
Outcome
Clinical follow up period ranged from 1 to 177 months (mean 52.4 months). Because of misdiagnosis, the probable first documented Hungarian AE patient was left untreated. Based on the laboratory findings, AE presumably progressed but lack of pathological investigations did not allow to draw any conclusion on this very first patient. In four patients (25%), no recurrence of AE was detected after radical surgery and concomitant ABZ treatment. In these patients, disease free period from curative surgery to the date of final imaging ranged from 10 to 74 months. Stabilization of AE lesions with continuous ABZ treatment was achieved in three (60%) out of five patients with unresectable lesions. One probable AE patient with multiple hepatic AE lesions received ABZ treatment for only 3 months. Ten years later, no progression was detected despite the lack of continuous ABZ therapy. In one probable and unresectable patient intermittent low dose (2 × 100 mg per day) of ABZ therapy had to be ceased because of drug-related hepatotoxicity and allergic reactions. After 26 months without treatment, hepatic AE lesions stabilized, but pulmonary microlesions emerged. Unfortunately, the patient was permanently supported with hydrocortisone for hypadrenia, which may have influenced the course of AE. Progression of AE during the study period was proved in seven patients (43.8%). In our series, progression was not generally accompanied with PNM upstaging of cases but increasing size of AE lesion(s) and/or worsening clinical condition directly related to AE. In two (33.3%) out of six patients, short-term or interrupted ABZ treatment is a plausible explanation for progression. In one case, the size of AE lesion increased beside adequate continuous ABZ treatment. In this patient, disseminating malignant neuroendocrine tumor with liver metastases was diagnosed and simultaneously treated with a probable liver AE lesion. Malignancy, administration of somatostatin analog sandostatin, targeted radionuclide therapy and classical radiotherapy, may play a role in the course of AE as immunocompromising factors. We registered three AE related deaths (18.8%) in our study. In patient No. 5 (P4N0Mx), giant pseudocystic AE was presumably misdiagnosed as abscessing CE. Endoscopic Retrograde Cholangiopancreatography (ERCP), stent implantation, surgical marsupialisation and drainage were performed. Central biliary obstruction, bilioperitoneal fistula, injury of bile ducts with subsequent bile leaking, complete lack of ABZ treatment, cachexia and advanced age were possible factors contributing to death. In patient No. 15 (P4N1Mx), imaging studies and explorative laparotomy revealed a central unresectable AE lesion (120 mm) occupying the left lobe and compressing the common hepatic duct with ascites and parasitic invasion along the falciform ligament. AE was confirmed by histopathology from the surgical sample. Peritonitis and cholangiogen sepsis were the causes of death in this advanced case. In patient No. 16 (P4N0Mx), imaging studies revealed a 120 mm AE lesion misdiagnosed as cystic echinococcosis, abscess or tumor of the liver occupying the right lobe and compressing the common hepatic duct. ERCP, biliary stent implantation, repeated percutaneous transhepatic drainage and finally right hemihepatectomy were performed with hepaticojejunostomy. Postoperative bleeding, liver failure and septic shock led to the death of the patient. AE was confirmed by histopathology postoperatively from surgical sample. Mean diagnostic delay in the three lethal AE patients was around 10 years (116 months) (Table 1; Table 2).
Discussion
By the end of the 1980s, the “historic endemic area” where Em was known to occur in foxes was composed by four countries (Austria, France, Germany and Switzerland) from Central and Western Europe [24, 25]. In these countries nowadays, the majority of AE patients have a nearly-normal life expectancy and a good quality of life while treated according to WHO-IWGE guidelines [26–28]. The subsequent increased emergence of Em in European red foxes has been traced back to the increase in fox population size due to antirabies vaccination, change of human attitudes towards foxes and other ecological factors [25]. Examination of foxes performed since 1989 revealed that the European endemic area of Em is much larger than previously assumed, and cases of human AE have been described in countries previously not recognized as endemic. Out of the European “historic endemic area”, confirmed and presumably autochthonous human AE cases were reported in Belarus, Belgium, Croatia, Czech Republic, Lithuania, Latvia, Poland, Romania, Slovenia, Slovakia, The Netherlands and Hungary [29–31]. Regarding countries adjacent to Hungary, the first human AE cases were detected in Romania in 1999 [32, 33], Slovakia in 2004 [34], Slovenia between 2001 and 2005 [35] and Croatia in 2014 [36].
In Hungary, Em was first recorded in red foxes near to the Hungarian-Slovak border in the Northern Mountain Range in 2002 [37]. After the first confirmation of this parasite in Hungary, further studies were carried out to determine the burden of this parasitic infection in the major wild definitive host, the red fox. Between 2008 and 2019, Em was detected in 18 out of the 19 Hungarian counties, comprising Budapest, with an average prevalence of 7.6% in 3265 analyzed red foxes ([38, 39],unpublished). Highest prevalence was detected in the North-Western half of the country (Fig. 3) [38, 39]. As reported in Fig. 3, the spatial distribution of Em in foxes was highly clustered. This spatial distribution pattern of the parasite can be explained by environmental factors. In fact, the mean annual temperature and the annual precipitations resulted as major statistically significant determinants for the spatial distribution of Em in foxes in Hungary [39]. These results can be attributed to the sensitivity of Em eggs to high temperature and desiccation [40]. Similar relationships with temperature or precipitation and Em infection of foxes or water voles were also observed in France, Germany and Switzerland [41–44]. As a consequence of the fox population increase and change of human behavior, red foxes inhabited urban and suburban areas in Hungary [45] as in other Central European countries [46], increasing the risk of human AE infections. Moreover, golden jackals (Canis aureus) have also been identified as definitive hosts in Hungary [47, 48]. Data regarding occurrence of the parasite in intermediate hosts in Hungary are scarce [31]. During a veterinary surveillance study on echinococcosis in livestock conducted between 2015 and 2018, three swine were found to be infected with Em in Hungary, suggesting that swine cases may be regarded as indicators of the environmental contamination by Em eggs [49].
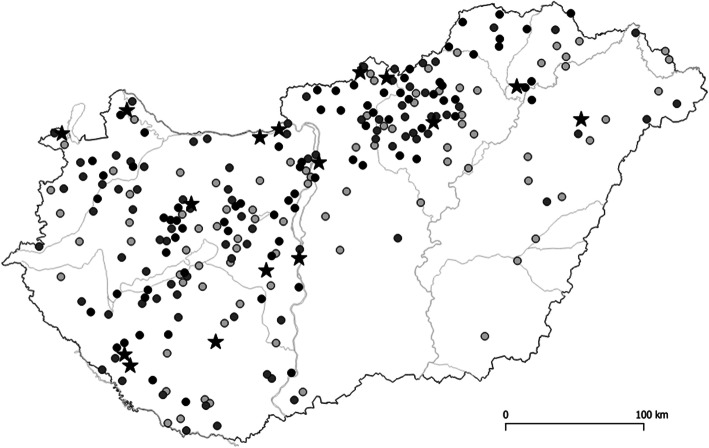
Fig. 3.
Spatial distribution of 16 AE human cases (stars) and that of 247 red foxes (Vulpes vulpes) (dots) infected with Echinococcus multilocularis out of 3265 foxes examined between 2008 and 2019. The darkness of the dots reflects the intensity of infection in foxes (light grey: < 10 worms; grey: 1–100 worms; black: > 100 worms) Aapproximately 4% of the fox population of each county was sampled
Regarding human echinococcosis in Hungary, cystic echinococcosis (CE) caused by E. granulosus sensu lato is a well-known parasitic disease since the nineteenth century. In fact, CE is the most prevalent reportable zoonotic helminthosis in Hungary, which is endemic in several regions with considerable disease burden [50]. In contrast, despite the prevalence of Em in foxes, the first confirmed human AE patient in Hungary was reported only in 2008 [51], and the first confirmed autochthonous human AE case was described in 2016 [22]. Herein we reported further 14 AE cases. The limitation of our study may rely on sampling bias, possibly underestimating AE cases. In fact, vast majority of our case series (94%) were collected from the official registry of National Public Health Centre, which is only based on seropositivity for Em. Inadequacies in the reporting system [50] have resulted in the lack of reporting of seronegative AE cases, which might had been diagnosed exclusively by histopathology. Therefore, we cannot exclude that the true number of AE cases might have been higher in Hungary in the past decade. A matter of debate whether the emergence of human AE in Europe is a consequence of the expansion of the classical endemic area through increasing number of fox populations and consequently infected animals or the result of improved awareness and better availability of diagnostic tools. In Hungary, the spread and emergence of Em could be observed in both animals and humans in the past two decades [37–39, 47–49]. Our results showed that the residence of human AE patients overlaps the geographical distribution of Em infected red foxes in Hungary (Fig. 3), suggesting that the majority of human cases may be autochthonous. The majority of AE patients (n = 11; 68,8%) originated from rural or suburban areas, which is in line with the observations by other researchers [46] and supporting the hypothesis that living in rural areas may be a proxy for environmental contamination and a driver for AE infection [4]. Regarding other main potential risk factors, almost three quarters of the patients had kitchen garden or visited forests for vocational reasons.
The incidence of human AE is low in Hungary and it is often misdiagnosed as CE, liver abscess or malignancy of the hepatobiliary tract, and most of the patients are only recognized in an advanced and unresectable stage. Six patients (37.5%) were diagnosed with hilar extension of the parasite (P4) and lethality reached 50% in this subgroup. PNM system is useful for AE treatment according WHO-IWGE and assessing prognosis. Retrospective application of PNM has made our results partially comparable on an international level [19]. Unfortunately, 18F-FDG-PET/CT, which has found to be useful in the follow-up of AE [52–54], is currently not covered by the health insurance system for patients with non-malignant conditions in Hungary. Furthermore, the lack of complete and detailed CT imaging data did not allow an advanced typing of AE lesions, like EMUC-CT classification [13, 55].
Diagnosis of AE was often delayed (mean diagnostic delay was 33 months in this series) because of misdiagnosis. Hepatobiliary malignancies, especially intrahepatic cholangiocarcinoma (ICC) were frequent misdiagnoses in our AE series. Enhancement pattern and matrix calcifications are the only imaging criteria with a high discriminating power between AE and ICC. During imaging evaluation, the combined presence of no or septal enhancement and calcification ensures 100% specificity for AE [56]. In our series, no or septal enhancement and/or calcification were detectable during preliminary imaging evaluation (CT and MRI) in 11 out of 16 patients (68.8%), which would have allowed the radiologists to distinguish AE from ICC. Non-malignancies such as CE are also pitfalls during differential diagnosis, not allowing further investigations and contributing to diagnostic delay [57]. We emphasize that the awareness of specific imaging characteristics of AE could help to reduce diagnostic delay and accelerate the introduction of antiparasitic drug treatment. In most patients, diagnostic uncertainty or missing histological confirmation led to short-term/interrupted or complete lack of ABZ treatment and to inappropriate surgical interventions, including radical liver surgery with severe complications, which is in line with previous observations made by other researchers [57]. Endoscopic and percutaneous interventions were generally used in defined advanced AE patients. We registered three lethal cases of human AE in our study (fatality rate 18.8%). Since 2005, only three lethal cases caused by autochthonous parasitic infections have been recognized in Hungary, all of them were involved in this series. Misdiagnosis or diagnostic delay may have brought to an inadequate treatment of these three AE cases, resulting in an increased death rate due to palliative surgery or lack of proper medical treatment [58, 59]. In fact, before the advent of medical treatment with benzimidazoles, fatality rate exceeded 90% of AE cases within 10–15 years from diagnosis [60]. The major current causes of death due to AE are either septic shock, liver failure, complications after major liver surgery and complications due to secondary biliary cirrhosis [9, 61–63].
Conclusions
We conclude that AE is currently the most dangerous human parasitic infection in Hungary. Our results highlight the need of early differential diagnosis supported by accurate imaging evaluation and if serology is not conclusive, additional core-biopsies from progressive liver lesions for histopathological analysis are needed. Histology is a corner stone to distinguish AE from malignancies, bacterial liver abscess and CE, which is also endemic and much more prevalent in Hungary than AE [64]. Early diagnosis and staging of AE allow applying evidence-based treatment methods according to WHO-IWGE and may increase cure rates [28]. ABZ lifelong treatment prevents disease progression and leads to a favorable outcome in most patients. Stage-specific treatment avoids inadequate/palliativeinterventions and potential complications associated with these procedures [61]. The course of AE in immunosuppressed patients needs further observations and precise evaluation [65]. As the results of this study indicate, there is an urgent need for the education of physicians in the diagnosis and clinical management of AE in Hungary and in other newly endemic European countries. This is very important since in historic endemic European countries it has been shown a favorable outcome of AE-patients with adequate treatment.
Acknowledgements
We are grateful to István Kucsera (Head of the National Reference Laboratory for Human Parasitic Diseases, National Public Health Centre, Budapest, Hungary) for supporting this research. We are also thankful to György Somorácz (Saint Pantaleon Teaching Hospital, Dunaújváros, Hungary), Andrea Horváth, Szilvia Tóth and Katalin Fried (Central Hospital of Southern Pest National Institute of Hematology and Infectious Diseases, Budapest, Hungary), Balázs Nemes and Attila Doros (Department of Transplantation and Surgery, Semmelweis University, Budapest, Hungary), Tünde Nagy and Tamás Mersich (National Institute of Oncology, Budapest, Hungary), László Nehéz (1st Department of Surgery, Semmelweis University, Budapest, Hungary), Mónika Francz (Jósa András County Teaching Hospital, Nyíregyháza, Hungary), Judit Kovács (Borsod-Abaúj-Zemplén County Teaching Hospital, Miskolc, Hungary), Prof. Ákos Pap and Prof. Béla Hunyady (Somogy County Teaching Hospital, Kaposvár, Hungary), Albert Szabó (Petz Aladár County Teaching Hospital, Győr, Hungary) for their contribution to the diagnosis and treatment of AE patients involved in this study. We also thank the colleagues who volunteered to help with retrieving official human data from Hungary: Dóra Somorácz-Kiss, Levente Kővágó, Tibor Martyin and Piroska Lakatos.
Authors’ contributions
BD conceived and designed the study. BD, ZK, TS, BG, ACa and TFEB drafted the work. BD, ZD, TS, EC, VC, ZK, MF, AS, ACs, AO, KA, AP, DG, ZS, ZT, TS, JD and HA acquired the data. BD, ZD, TS, EC, VC, ZK, MF, AS, ACs, AO, KA, AP, DG, ZS, ZT, TS, JD, HA, BG, TFEB and ACa critically revised the work. All authors have approved the submitted version. All authors have agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
The research that will lead to these results was partially supported by ERANet-LAC 2nd Joint Call (http://www.eranet-lac.eu) and the Italian Ministry of Health - NDTND project. The funding sources had no involvement in the preparation, ideas, writing, interpretation, or the decision to submit this article.
Availability of data and materials
The data that support the findings of this study are available at the database management systems of participating study centres but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the participating institutional ethical boards.
Ethics approval
This study was approved by all of the participating institutional ethical boards and by the Scientific and Research Ethics Committee of the Hungarian Medical Research Council (48967–5/2019/EKU). Data regarding potential risk factors were collected by means of questionnaires with dichotomous questions delivered to patients providing informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declared no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Balázs Dezsényi, Email: dezsenyibalazs13@gmail.com.
Adriano Casulli, Email: adriano.casulli@iss.it.
References
- 1.Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, et al. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982-2000. Emerg Infect Dis. 2003;9:343–349. doi: 10.3201/eid0903.020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oksanen A, Siles-Lucas M, Karamon J, Possenti A, Conraths FJ, Romig T, et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta-analysis. Parasit Vectors. 2016;9:519. doi: 10.1186/s13071-016-1746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casulli A, Barth TFE, Tamarozzi F. Echinococcus multilocularis. Trends Parasitol. 2019;35:738–739. doi: 10.1016/j.pt.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, Busani L, Casulli A. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11(7):e0005801. doi: 10.1371/journal.pntd.0005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torgerson PR, Robertson LJ, Enemark HL, Foehr J, van der Giessen JWB, Kapel CMO, Klun I, Trevisan C. Source attribution of human echinococcosis: a systematic review and meta-analysis. Plos Negl Trop Dis. 2020;14(6):e0008382. doi: 10.1371/journal.pntd.0008382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamarozzi F, Deplazes P, Casulli A. Reinventing the wheel of Echinococcus granulosus sensu lato transmission to humans. Trends Parasitol. 2020;36(5):427–434. doi: 10.1016/j.pt.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti E, Kern P, Vuitton DA. Writing panel for the WHO IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2009;114:10–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Kratzer W, Gruener B, Kaltenbach TE, Ansari-Bitzenberger S, Kern P, Fuchs M, Mason RA, Barth TF, Haenle MM, Hillenbrand A, Oeztuerk S, Graeter T. Proposal of an ultrasonographic classification for hepatic alveolar echinococcosis: Echinococcosis multilocularis Ulm classification-ultrasound. World J Gastroenterol. 2015;21(43):12392–12402. doi: 10.3748/wjg.v21.i43.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bresson-Hadni S, Vuitton DA, Bartholomot B, Heyd B, Godart D, Meyer JP, Hrusovsky S, Becker MC, Mantion G, Lenys D, Miguet JP. A twenty year history of alveolar echinococcosis: analysis of a series of 117 patients from eastern France. Eur J Gastroenterol Hepatol. 2000;12:327–336. doi: 10.1097/00042737-200012030-00011. [DOI] [PubMed] [Google Scholar]
- 10.Bresson-Hadni S, Delabrousse E, Blagosklonov O, Bartholomot B, Koch S, Miguet JP, Mantion GA, Vuitton DA. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Int. 2006;55(Suppl):S267–S272. doi: 10.1016/j.parint.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 11.Reuter S, Nüssle K, Kolokythas O, Haug U, Rieber A, Kern P, Kratzer W. Alveolar liver echinococcosis: a comparative study of three imaging techniques. Infection. 2001;29(3):119–125. doi: 10.1007/s15010-001-1081-2. [DOI] [PubMed] [Google Scholar]
- 12.Kodama Y, Fujita N, Shimizu T, Endo H, Nambu T, Sato N, Todo S, Miyasaka K. Alveolar echinococcosis: MR findings in the liver. Radiology. 2003;228(1):172–177. doi: 10.1148/radiol.2281020323. [DOI] [PubMed] [Google Scholar]
- 13.Graeter T, Kratzer W, Oeztuerk S, Haenle MM, Mason RA, Hillenbrand A, Kull T, Barth TF, Kern P, Gruener B. Proposal of a computed tomography classification for hepatic alveolar echinococcosis. World J Gastroenterol. 2016;22(13):3621–3631. doi: 10.3748/wjg.v22.i13.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantarci M, Bayraktutan U, Karabulut N, Aydinli B, Ogul H, Yuce I, Calik M, Eren S, Atamanalp SS, Oto A. Alveolar echinococcosis: spectrum of findings at cross-sectional imaging. Radiographics. 2012;32(7):2053–2070. doi: 10.1148/rg.327125708. [DOI] [PubMed] [Google Scholar]
- 15.Bulakçı M, Kartal MG, Yılmaz S, Yılmaz E, Yılmaz R, Şahin D, Aşık M, Erol OB. Multimodality imaging in diagnosis and management of alveolar echinococcosis: an update. Diagn Interv Radiol. 2016;22(3):247–256. doi: 10.5152/dir.2015.15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamarozzi F, Horton J, Muhtarov M, Ramharter M, Siles-Lucas M, Gruener B, Vuitton DA, Bresson-Hadni S, Manciulli T, Brunetti E. A case for adoption of continuous albendazole treatment regimen for human echinococcal infections. Plos Negl Trop Dis. 2020;14(9):e0008566. doi: 10.1371/journal.pntd.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siles-Lucas M, Casulli A, Cirilli R, Carmena D. Progress in the pharmacological treatment of human cystic and alveolar echinococcosis: Compounds and therapeutic targets. Plos Negl Trop Dis. 2018;12(4):e0006422. doi: 10.1371/journal.pntd.0006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern P. Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis. 2010;23(5):505–512. doi: 10.1097/QCO.0b013e32833d7516. [DOI] [PubMed] [Google Scholar]
- 19.Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, et al. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. 2006;55(S):283–287. doi: 10.1016/j.parint.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Koch S, Bresson-Hadni S, Miguet JP, Crumbach JP, Gillet M, Mantion GA, Heyd B, Vuitton DA, Minello A, Kurtz S. European collaborating clinicians. Experience of liver transplantation for incurable alveolar echinococcosis: a 45-case European collaborative report. Transplantation. 2003;75(6):856–863. doi: 10.1097/01.TP.0000054230.63568.79. [DOI] [PubMed] [Google Scholar]
- 21.Liance M, Janin V, Bresson-Hadni S, Vuitton DA, Houin R, Piarroux R. Immunodiagnosis of Echinococcus infections: confirmatory testing and species differentiation by a new commercial Western blot. J Clin Microbiol. 2000;38(10):3718–3721. doi: 10.1128/JCM.38.10.3718-3721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dezsényi B, Strausz T, Makrai Z, Csomor J, Danka J, Kern P, et al. Autochthonous human alveolar echinococcosis in a Hungarian patient. Infection. 2017;45:107–110. doi: 10.1007/s15010-016-0918-7. [DOI] [PubMed] [Google Scholar]
- 23.Barth TFE, Herrmann TS, Tappe D, Stark L, Grüner B, Buttenschoen K. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. Plos Negl Trop Dis. 2012;6:e1877. doi: 10.1371/journal.pntd.0001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert J, Deplazes P. Alveolar echinococcosis in humans: the current situation in Central Europe and the need for countermeasures. Parasitol Today. 1999;15(8):315–319. doi: 10.1016/s0169-4758(99)01476-3. [DOI] [PubMed] [Google Scholar]
- 25.Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, et al. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torgerson PR, Schweiger A, Deplazes P, Pohar M, Reichen J, Ammann RW, Tarr PE, Halkic N, Müllhaupt B. Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J Hepatol. 2008;49(1):72–77. doi: 10.1016/j.jhep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, Watelet J, Dumortier J, Gérard A, Beytout J, Abergel A, Mantion G, Vuitton DA, Bresson-Hadni S. Clinical features and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a survey in 387 patients. J Hepatol. 2011;55(5):1025–1033. doi: 10.1016/j.jhep.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Grüner B, Kern P, Mayer B, Gräter T, Hillenbrand A, TEF B, Muche R, Henne-Bruns D, Kratzer W, Kern P. Comprehensive diagnosis and treatment of alveolar echinococcosis: A single-center, long-term observational study of 312 patients in Germany. GMS Infect Dis. 2017;5:Doc01. doi: 10.3205/id000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, Li W, the Interdisciplinary Echinococcosis Working Group Ulm Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. 2019;47:703–727. doi: 10.1007/s15010-019-01325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuitton DA, Demonmerot F, Knapp J, Richou C, Grenouillet F, Chauchet A, Vuitton L, Bresson-Hadni S, Millon L. Clinical epidemiology of human AE in Europe. Vet Parasitol. 2015;213(3–4):110–120. doi: 10.1016/j.vetpar.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RC, Jenkins EJ. Global distribution of alveolar and cystic Echinococcosis. Adv Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Panaitescu D, Pop M. Alveococoza la om. [Alveococcosis in man] Rev Rom Parazitol. 1999;9(2):55. [Google Scholar]
- 33.Sikó SB, Deplazes P, Ceica C, Tivadar CS, Bogolin I, Popescu S, Cozma V. Echinococcus multilocularis in South-Eastern Europe (Romania) Parasitol Res. 2011;108(5):1093–1097. doi: 10.1007/s00436-010-2150-1. [DOI] [PubMed] [Google Scholar]
- 34.Antolova D, Miterpakova M, Radoňak J, Hudačkova D, Szilagyiova M, Začek M. Alveolar echinococcosis in a highly endemic area of northern Slovakia between 2000 and 2013. Euro Surveill. 2014;19(34):20882. doi: 10.2807/1560-7917.ES2014.19.34.20882. [DOI] [PubMed] [Google Scholar]
- 35.Logar J, Soba B, Lejko-Zupanc T, Kotar T. Human alveolar echinococcosis in Slovenia. Clin Microbiol Infect. 2007;13(5):544–546. doi: 10.1111/j.1469-0691.2007.01701.x. [DOI] [PubMed] [Google Scholar]
- 36.Dušek D, Vince A, Kurelac I, Papić N, Višković K, Deplazes P, Beck R. Human Alveolar Echinococcosis. Croatia Emerg Infect Dis. 2020;26(2):364–366. doi: 10.3201/eid2602.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sréter T, Széll Z, Zs E, Varga I. Echinococcus multilocularis: an emerging pathogen in Hungary and Central Eastern Europe? Emerg Infect Dis. 2003;9:384–386. doi: 10.3201/eid0903.020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casulli A, Széll Z, Pozio E, Sréter T. Spatial distribution and genetic diversity of Echinococcus multilocularis in Hungary. Vet Parasitol. 2010;174:241–246. doi: 10.1016/j.vetpar.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Tolnai Z, Széll Z, Sréter T. Environmental determinants of the spatial distribution of Echinococcus multilocularis in Hungary. Vet Parasitol. 2013;198:292–297. doi: 10.1016/j.vetpar.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Viel JF, Giraudoux P, Abrial V, Bresson-Hadni S. Water vole (Arvicola terrestris scherman) density as risk factor for human alveolar echinococcosis. Am J Trop Med Hyg. 1999;61(4):559–565. doi: 10.4269/ajtmh.1999.61.559. [DOI] [PubMed] [Google Scholar]
- 41.Denzin N, Schliephake A, Fröhlich A, Ziller M, Conraths FJ. On the move? Echinococcus multilocularis in red foxes of Saxony-Anhalt (Germany) Transbound Emerg Dis. 2014;61(3):239–246. doi: 10.1111/tbed.12026. [DOI] [PubMed] [Google Scholar]
- 42.Fischer I, Graeter T, Kratzer W, Stark K, Schlingeloff P, Schmidberger J. Echinococcosis working group Ulm. Distribution of alveolar echinococcosis according to environmental and geographical factors in Germany, 1992-2018. Acta Trop. 2020;212:105654. doi: 10.1016/j.actatropica.2020.105654. [DOI] [PubMed] [Google Scholar]
- 43.Chauchet A, Grenouillet F, Knapp J, Richou C, Delabrousse E, Dentan C, Millon L, Di Martino V, Contreras R, Deconinck E, Blagosklonov O, Vuitton DA, Bresson-Hadni S. FrancEchino network. Increased incidence and characteristics of alveolar echinococcosis in patients with immunosuppression-associated conditions. Clin Infect Dis. 2014;59(8):1095–1104. doi: 10.1093/cid/ciu520. [DOI] [PubMed] [Google Scholar]
- 44.Burlet P, Deplazes P, Hegglin D. Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformis in Arvicola terrestris. Parasit Vectors. 2011;4:6. doi: 10.1186/1756-3305-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szemethy L, Heltai M, Zs B. Effect of oral immunization against rabies on the red fox population in Hungary. Rabies Bull Eur. 1999;23:12. [Google Scholar]
- 46.Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Széll Z, Marucci G, Pozio E, Sréter T. Echinococcus multilocularis and Trichinella spiralis in golden jackals (Canis aureus) of Hungary. Vet Parasitol. 2013;197:393–396. doi: 10.1016/j.vetpar.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 48.Balog T, Nagy G, Halász T, Csányi E, Zomborszky Z, Csivincsik Á. The occurrence of Echinococcus spp. in golden jackal (Canis aureus) in southwestern Hungary: Should we need to rethink its expansion? Parasitol Int. 2020;80:102214. doi: 10.1016/j.parint.2020.102214. [DOI] [PubMed] [Google Scholar]
- 49.Dán Á, Rónai Z, Széll Z, Sréter T. Prevalence and genetic characterization of Echinococcus spp. in cattle, sheep and swine in Hungary. Parasitol Res. 2018;117:3019–3022. doi: 10.1007/s00436-018-5977-5. [DOI] [PubMed] [Google Scholar]
- 50.Dezsényi B, Somorácz Á, Danka J, Kucsera I, Barth TFE, Casulli A. Human cystic echinococcosis in Hungary (2000-2014): a retrospective case series analysis from a single-center study. Infection. 2018;46:477–486. doi: 10.1007/s15010-018-1146-0. [DOI] [PubMed] [Google Scholar]
- 51.Horváth A, Patonai A, Bánhegyi D, Szlávik J, Balázs GY, Görög D, et al. A humán Echinococcus multilocularis infectio első hazai esete. Orv Hetil. 2008;149:795–799. doi: 10.1556/OH.2008.28281. [DOI] [PubMed] [Google Scholar]
- 52.Reuter S, Schirrmeister H, Kratzer W, Dreweck C, Reske SN, Kern P. Pericystic metabolic activity in alveolar echinococcosis: assessment and follow-up by positron emission tomography. Clin Infect Dis. 1999;29(5):1157–1163. doi: 10.1086/313438. [DOI] [PubMed] [Google Scholar]
- 53.Reuter S, Buck A, Manfras B, Kratzer W, Seitz HM, Darge K, Reske SN, Kern P. Structured treatment interruption in patients with alveolar echinococcosis. Hepatology. 2004;39(2):509–517. doi: 10.1002/hep.20078. [DOI] [PubMed] [Google Scholar]
- 54.Ammann RW, Stumpe KD, Grimm F, Deplazes P, Huber S, Bertogg K, Fischer DR, Müllhaupt B. Outcome after discontinuing long-term Benzimidazole treatment in 11 patients with non-resectable alveolar Echinococcosis with negative FDG-PET/CT and anti-EmII/3-10 serology. Plos Negl Trop Dis. 2015;9(9):e0003964. doi: 10.1371/journal.pntd.0003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graeter T, Bao H, Delabrousse E, Brumpt E, Shi R, Li W, Jiang Y, Schmidberger J, Kratzer W, Liu W, XUUB consortium Hepatic alveolar echinococcosis: Comparative computed tomography study between two Chinese and two European centres. Food Waterborne Parasitol. 2020;19:e00082. doi: 10.1016/j.fawpar.2020.e00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller J, Stojkovic M, Berger AK, Rosenberger KD, Schlett CL, Kauczor HU, Junghanss T, Weber TF. How to not miss alveolar echinococcosis in hepatic lesions suspicious for cholangiocellular carcinoma. Abdom Radiol (NY) 2016;41(2):221–230. doi: 10.1007/s00261-015-0561-2. [DOI] [PubMed] [Google Scholar]
- 57.Stojkovic M, Mickan C, Weber TF, Junghanss T. Pitfalls in diagnosis and treatment of alveolar echinococcosis: a sentinel case series. BMJ Open Gastroenterol. 2015;2(1):e000036. doi: 10.1136/bmjgast-2015-000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buttenschoen K, Gruener B, Carli Buttenschoen D, Reuter S, Henne-Bruns D, Kern P. Palliative operation for the treatment of alveolar echinococcosis. Langenbeck's Arch Surg. 2009;394(1):199–204. doi: 10.1007/s00423-008-0367-6. [DOI] [PubMed] [Google Scholar]
- 59.Hillenbrand A, Gruener B, Kratzer W, Kern P, Graeter T, Barth TF, Buttenschoen K, Henne-Bruns D. Impact of safe distance on long-term outcome after surgical therapy of alveolar Echinococcosis. World J Surg. 2017;41(4):1012–1018. doi: 10.1007/s00268-016-3813-6. [DOI] [PubMed] [Google Scholar]
- 60.Wilson JF, Rausch RL, McMahon BJ, Schantz PM. Parasiticidal effect of chemotherapy in alveolar hydatid disease: review of experience with mebendazole and albendazole in Alaskan Eskimos. Clin Infect Dis. 1992;15(2):234–249. doi: 10.1093/clinids/15.2.234. [DOI] [PubMed] [Google Scholar]
- 61.Frei P, Misselwitz B, Prakash MK, Schoepfer AM, Prinz Vavricka BM, Müllhaupt B, Fried M, Lehmann K, Ammann RW, Vavricka SR. Late biliary complications in human alveolar echinococcosis are associated with high mortality. World J Gastroenterol. 2014;20(19):5881–5888. doi: 10.3748/wjg.v20.i19.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graeter T, Ehing F, Oeztuerk S, Mason RA, Haenle MM, Kratzer W, Seufferlein T, Gruener B. Hepatobiliary complications of alveolar echinococcosis: a long-term follow-up study. World J Gastroenterol. 2015;21(16):4925–4932. doi: 10.3748/wjg.v21.i16.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambregna S, Koch S, Sulz MC, Grüner B, Öztürk S, Chevaux JB, Sulima M, de Gottardi A, Napoléon B, Abergel A, Bichard P, Boytchev I, Deprez P, Dumortier J, Frossard JL, Kull E, Meny B, Moradpour D, Prat F, Vanbiervliet G, Thevenot T, Vuitton DA, Bresson-Hadni S, Vuitton L. A European survey of perendoscopic treatment of biliary complications in patients with alveolar echinococcosis. Expert Rev Anti-Infect Ther. 2017;15(1):79–88. doi: 10.1080/14787210.2017.1252260. [DOI] [PubMed] [Google Scholar]
- 64.Reinehr M, Micheloud C, Grimm F, Kronenberg PA, Grimm J, Beck A, Nell J, Meyer Zu Schwabedissen C, Furrer E, Müllhaupt B, Barth TFE, Deplazes P, Weber A. Pathology of Echinococcosis: a morphologic and Immunohistochemical study on 138 specimens with focus on the differential diagnosis between cystic and alveolar Echinococcosis. Am J Surg Pathol. 2020;44(1):43–54. doi: 10.1097/PAS.0000000000001374. [DOI] [PubMed] [Google Scholar]
- 65.Piarroux M, Gaudart J, Bresson-Hadni S, Bardonnet K, Faucher B, Grenouillet F, Knapp J, Dumortier J, Watelet J, Gerard A, Beytout J, Abergel A, Wallon M, Vuitton DA, Piarroux R. FrancEchino network. Landscape and climatic characteristics associated with human alveolar echinococcosis in France, 1982 to 2007. Euro Surveill. 2015;20(18):21118. doi: 10.2807/1560-7917.es2015.20.18.21118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available at the database management systems of participating study centres but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the participating institutional ethical boards.