Abstract
Background
Breast cancer (BC) is a leading cause of cancer-related death in females worldwide. Previous studies have demonstrated that matrix metalloproteinases (MMPs) play key roles in metastasis and are associated with survival in various cancers. The prognostic values of MMP2 and MMP9 expression in BC have been investigated, but the results remain controversial. Thus, we performed the present meta-analysis to investigate the associations between MMP2/9 expressions in tumor cells with clinicopathologic features and survival outcome in BC patients.
Methods
Eligible studies were searched in PubMed, Web of Science, EMBASE, CNKI and Wanfang databases. The associations of MMP2/9 overexpression in tumor cells with overall survival (OS), disease-free survival (DFS) and recurrence-free survival (RFS) were assessed by hazard ratio (HR) and 95% confidence interval (CI). The associations of MMP2/9 overexpression with clinicopathological features were investigated by calculating odds ratio (OR) and 95% CI. Subgroup analysis, sensitivity analysis, meta-regression, and analysis for publication bias were performed.
Results
A total of 41 studies comprising 6517 patients with primary BC were finally included. MMP2 overexpression was associated with an unfavorable OS (HR = 1.60, 95% CI 1.33 –1.94, P < 0.001) while MMP9 overexpression predicted a shorter OS (HR = 1.52, 95% CI 1.30 –1.77, P < 0.001). MMP2 overexpression conferred a higher risk to distant metastasis (OR = 2.69, 95% CI 1.35–5.39, P = 0.005) and MMP9 overexpression correlated with lymph node metastasis (OR = 2.90, 95% CI 1.86 – 4.53, P < 0.001). Moreover, MMP2 and MMP9 overexpression were both associated with higher clinical stage and histological grade in BC patients. MMP9 overexpression was more frequent in patients with larger tumor sizes.
Conclusions
Tumoral MMP2 and MMP9 are promising markers for predicting the prognosis in patients with BC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-07860-2.
Keywords: Breast cancer, MMP2, MMP9, Survival, Meta-analysis
Background
Breast cancer (BC) is the most prevalent malignancy and one of the leading causes of cancer-related death among females worldwide [1]. It accounts for 24.2% of newly diagnosed cancer cases and 15.0% of death from cancer in women [1]. Furthermore, the incidence and mortality rates of BC have been increasing in recent years [2]. Previous studies have identified metastasis, tumor stage, histological grade, expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) as prognostic factors for BC [3]. The significant findings of these biomarkers have promoted the development of molecular-targeted therapy of BC [4]. Therefore, identifying more and more molecular biomarkers would definitely improve the treatment and management of BC in the future.
Matrix metalloproteinases (MMPs) are a group of zinc endopeptidases critical for the decomposition of extracellular matrix (ECM) components and basement membrane (BM) [5]. MMPs thus play pivotal roles in various physiological and pathological processes, including morphogenesis, wound healing, inflammation, cancer invasion, and metastasis [6]. MMPs are structurally divided into several subtypes, among which MMP2 and MMP9 belong to the gelatinase family that mainly degrades gelatin, collagens IV and V in ECM and BM through their proteolytic function [7]. In cancer, the overproduction or increased activity of MMP2/9 leads to the degradation of ECM and BM, allowing for the invasion of tumor cells to other tissues and tumor cell metastasis to distant organs [8]. MMP2/9 have also been implicated in cancer development and progression through their functions in cell apoptosis, proliferation, and angiogenesis [9–11].
Previous studies demonstrated that MMP2/9 are important prognostic factors for various cancers. MMP2 and MMP9 overexpression was associated with poor prognosis in oral cancers [12], retinoblastoma [13], bladder cancer [14], and ovarian epithelial cancer [15]. The prognostic value of MMP2/9 in BC has also been investigated. Several studies reported that MMP2/9 overexpression was related to clinicopathological characteristics and associated with poor survival in patients with BC [16–19], indicating that MMP2/9 may function as good prognostic markers for BC. However, other studies showed no associations of MMP2/9 overexpression with survival [20–23]. Thus, the associations between MMP2/9 expression and clinicopathological features and survival in BC remain controversial.
To evaluate the prognostic values of MMP2/9 in BC, we performed a meta-analysis for the associations between MMP2/9 overexpression in tumor cells with the clinicopathologic features and survival outcomes in BC patients.
Methods
Literature search strategy
This was a Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement based meta-analysis [24]. A comprehensive literature search was performed in PubMed, Web of Science, EMBASE, CNKI and Wanfang databases from study inception to June 30, 2020. The following search terms were used: (breast cancer OR breast tumor OR breast neoplasm OR breast carcinoma) AND (matrix metalloproteinase OR MMP2 OR MMP9 OR gelatinase). There was no language restriction. References in relevant articles were furtherly scanned for more potentially eligible studies.
Inclusion and exclusion criteria
Studies that met the following criteria were included: 1) detecting the protein expression of MMP2 and/or MMP9 in tumor cells of breast cancer tissue by immunohistochemistry (IHC); 2) investigating the associations between MMP2/MMP9 overexpression and survival and/or clinicopathological features; 3) reporting hazard ratio (HR) with corresponding 95% confidence interval (CI) or survival curves for survival analysis, or providing sufficient data to calculate the odds ratio (OR) with 95% CI for clinicopathological features. Studies that measured mRNA expression or protein levels in serum or stromal cells were excluded. Reviews, meta-analyses and studies lacking sufficient data were excluded. If several studies had overlapping samples, only the largest one was included.
Data extraction and quality assessment
The following data were extracted by two independent researchers: first author, publication year, country, sample size, follow-up duration, percent of infiltrating ductal carcinoma (IDC), criteria for MMPs overexpression, survival outcomes, HR and 95% CI, clinicopathological features, tissue and antibody used for IHC staining, and the data for the calculation of OR and 95% CI. The quality of included studies were assessed by Newcastle-Ottawa Scale (NOS), which assigned a total of 9 stars to 8 items [25]. Studies awarded 6 or more stars were considered as high quality. The literature search, selection, data extraction and quality assessment were performed by two independent researchers (HJ and HL). Discrepancies were resolved by discussion.
Definition of MMP2/MMP9 overexpression
MMP2/MMP9 overexpression in tumor sections was assessed using specific cut-offs of percentage of stained cells or the stained index (SI) that combines both percentage and intensity of staining, or the other methods. The SI was calculated as either the sum or product of staining percentage and intensity scores or determined using other complex scoring methods.
Survival outcomes
The survival outcomes we investigated included overall survival (OS), disease-free survival (DFS), and recurrence-free survival (RFS). We obtained HR and 95% CI from univariate and/or multivariate analysis of associations between MMP2/MMP9 overexpression and survival. If no HR data were reported, we extracted survival data from the survival curves by using Engauge Digitizer software (https://github.com/markummitchell/engauge-digitizer) and estimated the HR and 95% CI by using the method by Tierney et al [26]. If a study reported HRs from both univariate and multivariate analysis, the latter was included in the overall analysis, and both were included in the subgroup analysis of univariate or multivariate analysis, respectively.
Clinicopathological features
The clinicopathological features investigated in our analysis included tumor size, lymph node metastasis, distant metastasis, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, TNM stage, and histological grade, as those are associated with the prognosis of BC.
Statistical analysis
Between-study heterogeneity was determined by using I2 and Q test. If I2 was 50% and P value for Q test was > 0.10, a fixed-effect model was used. Otherwise, a random-effect model was used. For survivals, pooled HR and 95% CI were calculated, and subgroup analyses regarding ethnicity (Caucasians, Asians), IHC analysis standard (percentage, SI, other cut-offs), HR data source (reported, estimated), analysis model (univariate, multivariate), sample size, cancer subtype, tissue (whole tissue, tissue microarray), IHC antibody (monoclonal, polyclonal) and antibody source (mouse, rabbit) were performed. For clinicopathological features, pooled OR and 95%CI were calculated, and subgroup analyses were also performed. Sensitivity analysis and meta-regression were performed to find the potential source of heterogeneity. Publication bias was assessed by funnel plot and Egger’s test. All the analyses were performed by using STATA 12.0 (Stata Corporation, TX, USA). P < 0.05 was considered statistically significant.
Results
Description of eligible studies
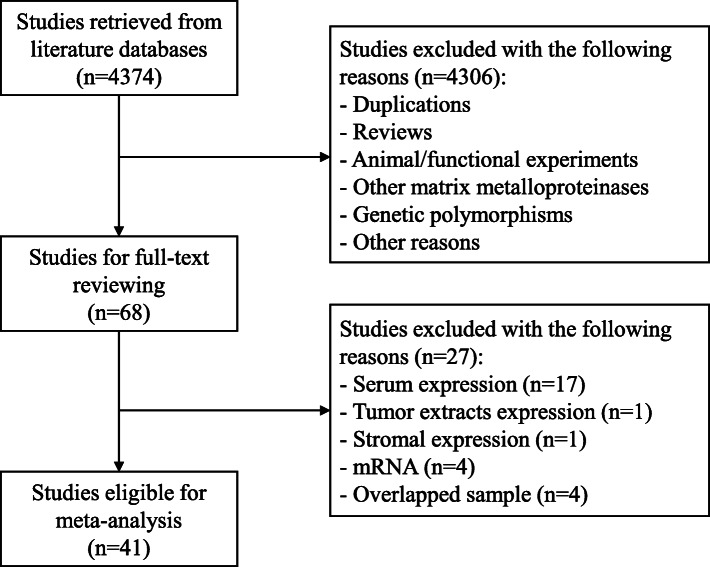
The literature search yielded a total of 68 studies for full-text reviewing. Then, 27 studies were excluded as they detected MMP2/MMP9 expression in serum (n = 17) or cytosol tumor extracts (n = 1) or in stromal cells (n = 1), investigated mRNA expression (n = 4), duplicated with others (n = 4). Finally, a total of 41 studies comprising 6517 patients with primary breast cancers [16–23, 27–59] were included in our meta-analysis (Fig. 1). Among them, 31 studies with 4895 patients were eligible for survival analysis and 30 studies with 4743 cases for the analysis of clinicopathological features. The sample sizes range from 41 to 675. Regarding ethnicity, 22 studies were conducted in Caucasian populations and 19 in Asian populations. Regarding the definition of overexpression, 19 studies used percentage criteria, 19 used SI criteria and 3 used the other criteria [57–59]. According to NOS, 12 studies had 6 stars, 20 had 7 stars and 9 had 8 stars, indicating that all studies were of high quality.
Fig. 1.
Flowchart of literature search and selection of eligible studies
For survival analysis, the HR and 95% CI were estimated from survival curves in 10 studies and were directly reported in 21 studies. Overexpression of MMP2 and MMP9 were investigated in 17 and 21 studies, respectively. The associations between MMP2 and MMP9 overexpression and clinicopathological features were reported in 14 and 20 studies, respectively. The characteristics of survival analysis and clinicopathological features were summarized in Table 1 and Table 2, respectively. The primary anti-huamn-MMP2/9 antibody used for IHC staining varied between studies and was summarized in Table S1.
Table 1.
Characteristics of eligible studies for survival analysis
| Author | Year | Country | Sample size | Percent of IDC (%) | Cut-offa | Follow-up | Protein | Survival outcome | HR data | Survival analysis | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Talvensaari-Mattila [51] | 1998 | Finland | 169 | 82 | P > 0% | 92 months | MMP2 | OS | Rep | U | 8 |
| Talvensaari-Mattila [50] | 1999 | Finland | 108 | 96 | P > 0% | 2 years | MMP2 | RFS | Est | U | 8 |
| Talvensaari-Mattila [49] | 2001 | Finland | 100 | 79 | P > 0% | 44 months | MMP2 | OS, RFS | Est | U | 7 |
| Scorilas | 2011 | Greece | 210 | 75 | HSCORE> 175 | 62 months | MMP9 | OS, RFS | Rep | U, M | 7 |
| Djonov [48] | 2002 | Switzerland | 75 | 61 | SI ≥ 1 | NR | MMP2 | OS, DFS | Rep | M | 6 |
| Hirvonen [47] | 2003 | Finland | 137 | NR | P > 0% | 10 years | MMP2 | RFS | Est | U | 6 |
| Fan [45] | 2003 | China | 66 | 86 | SI ≥ 1 | 30.5 months | MMP2, MMP9 | OS | Rep | M | 6 |
| Talvensaari-Mattila [44] | 2003 | Finland | 453 | 75 | P > 0% | 60–150 months | MMP2 | OS | Rep | M | 8 |
| Li [19] | 2004 | China | 270 | 90 | P > 0% | 61 months | MMP2, MMP9 | OS, RFS | Rep | U, M | 6 |
| Pellikainen [58] | 2004 | Finland | 415 | 64 | P > 85%b | 55 months | MMP9 | RFS | Rep | M | 7 |
| Rahko [23] | 2004 | Finland | 168 | 75 | P > 1% | 7–111 months | MMP9 | OS, DFS | Rep | U | 7 |
| Ban [52] | 2004 | China | 60 | 100 | SI ≥ 3 | > 5 years | MMP2 | OS | Est | U | 7 |
| Zhou [42] | 2005 | China | 112 | 84 | P > 5% | 48 months | MMP2 | OS | Rep | M | 7 |
| Zhang [38] | 2008 | China | 263 | 100 | SI ≥ 6 | 92.1 months | MMP2, MMP9 | OS | Rep | U | 6 |
| Zhao [53] | 2008 | China | 71 | 93 | P > 10% | 54.94 months | MMP9 | OS | Est | U | 6 |
| Sullu [22] | 2011 | Turkey | 140 | 100 | SI ≥ 5 | 63.2 months | MMP9 | OS, DFS | Est | U | 8 |
| Ranogajec [37] | 2012 | Croatia | 138 | 59 | SI ≥ 2 | 5 years | MMP2 | OS | Est | U | 7 |
| Fernandez-Guinea [21] | 2013 | Spain | 97 | 100 | SI | > 5 years | MMP2, MMP9 | RFS | Rep | U, M | 6 |
| Zhao [18] | 2013 | China | 127 | NR | SI ≥ 6 | NR | MMP9 | OS | Rep | M | 6 |
| Liu [17] | 2013 | China | 189 | 89 | P > 10% | NR | MMP9 | OS | Rep | U, M | 8 |
| Zeng [36] | 2013 | China | 253 | NR | P > 20% | 15 years | MMP9 | OS, DFS | Rep | U, M | 8 |
| Merdad [20] | 2014 | Saudi Arabia | 45 | 84 | SI ≥ 2 | 52.1 months | MMP9 | OS | Rep | U | 7 |
| Puzovic [35] | 2014 | Croatia | 121 | 100 | SI | 80.6 months | MMP2, MMP9 | OS, DFS | Rep | U | 7 |
| Min [32] | 2014 | Korea | 177 | 100 | SI ≥ 1, SI ≥ 5 | NR | MMP2, MMP9 | OS | Rep | M | 7 |
| Yousef [30] | 2014 | Canada | 300 | NR | SI ≥ 5 | NR | MMP9 | RFS | Est | U | 6 |
| Bottino [59] | 2014 | Brazil | 60 | 1 | MOD> 191 | NR | MMP9 | OS | Rep | U | 7 |
| Huang [55] | 2014 | China | 147 | 86 | P > 10% | 45.6 months | MMP9 | OS, DFS | Rep | U, M | 6 |
| Ramos [29] | 2016 | Brazil | 44 | 77 | P > 10% | NR | MMP2 | OS, DFS | Est | U | 7 |
| Li [16] | 2017 | China | 80 | NR | P > 25% | NR | MMP2, MMP9 | OS | Est | U | 7 |
| Yang [28] | 2018 | Korea | 173 | 100 | SI ≥ 2 | NR | MMP9 | OS, DFS | Rep | U, M | 8 |
| Zhang [56] | 2019 | China | 127 | NR | SI ≥ 6 | NR | MMP9 | OS | Rep | M | 7 |
aCut-off for overexpression of matrix metalloproteinases in tumor cells by immunohistochemistry
bMedian value
IDC infiltrating ductal carcinoma, P percentage of stained cells, SI staining index considering both percentage and intensity of staining, OS overall survival, DFS disease-free survival, RFS recurrence-free survival, Rep reported in the text, Est estimated from survival curve, U univariate analysis, M multivariate analysis, NR not reported, MOD mean optical density
Table 2.
Characteristics of eligible studies for clinicopathological features
| Author | Year | Country | Sample size | Cut-offa | Protein | T | N | M | ER | PR | HER2 | S | G | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Talvensaari-Mattila [51] | 1998 | Finland | 169 | P > 1% | MMP2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |
| Talvensaari-Mattila [50] | 1999 | Finland | 108 | P > 1% | MMP2 | ✓ | ✓ | ✓ | ✓ | 8 | ||||
| Talvensaari-Mattila [49] | 2001 | Finland | 100 | P > 1% | MMP2 | ✓ | ✓ | 7 | ||||||
| Scorilas | 2011 | Greece | 210 | HSCORE> 175 | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||
| Hirvonen [47] | 2003 | Finland | 137 | P > 1% | MMP2 | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Nakopoulou | 2003 | Greece | 135 | P > 10% | MMP2 | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Fan [45] | 2003 | China | 66 | SI ≥ 1 | MMP2, MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | |||
| Talvensaari-Mattila [44] | 2003 | Finland | 453 | P > 1% | MMP2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Li [19] | 2004 | China | 270 | P > 1% | MMP2, MMP9 | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Rahko [23] | 2004 | Finland | 168 | P > 1% | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Ban [52] | 2004 | China | 60 | SI ≥ 3 | MMP2 | ✓ | ✓ | 7 | ||||||
| Sivula | 2005 | Finland | 194 | P > 20% | MMP2 | ✓ | ✓ | ✓ | ✓ | 7 | ||||
| Zhou [42] | 2005 | China | 112 | P > 5% | MMP2 | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Mylona | 2007 | Greece | 175 | P > 20% | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| Hao | 2007 | China | 76 | SI ≥ 5 | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||
| Wu | 2008 | China | 60 | P > 50% | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||
| Sullu [22] | 2011 | Turkey | 140 | SI ≥ 5 | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |
| Zhao [18] | 2013 | China | 127 | SI ≥ 6 | MMP9 | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Zeng [36] | 2013 | China | 253 | P > 20% | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Wu | 2014 | China | 41 | SI ≥ 1 | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||
| Tang | 2014 | China | 156 | SI ≥ 6 | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |
| Min [32] | 2014 | Korea | 177 | SI ≥ 1, SI ≥ 5 | MMP2, MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |
| Youssef | 2014 | Egypt | 67 | P > 10% | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |
| Huang [55] | 2014 | China | 147 | P > 10% | MMP9 | ✓ | ✓ | 6 | ||||||
| Ramos [29] | 2016 | Brazil | 44 | P > 10% | MMP2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |
| Li [16] | 2017 | China | 80 | P > 25% | MMP2, MMP9 | ✓ | ✓ | ✓ | 7 | |||||
| Yang [28] | 2018 | Korea | 173 | SI ≥ 2 | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |
| Zhang [56] | 2019 | China | 127 | SI ≥ 6 | MMP9 | ✓ | ✓ | ✓ | ✓ | 7 | ||||
| Zhou [54] | 2009 | China | 43 | SI ≥ 3 | MMP9 | ✓ | ✓ | ✓ | ✓ | 7 | ||||
| Joseph [27] | 2020 | British | 675 | SI | MMP9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
aCut-off for overexpression of matrix metalloproteinases in tumor cells by immunohistochemistry
P percentage of stained cells, SI staining index considering both percentage and intensity of staining, T tumor size, N lymph node status, M distant metastasis, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, S TNM stage, G histological grade
MMP2 overexpression and survival
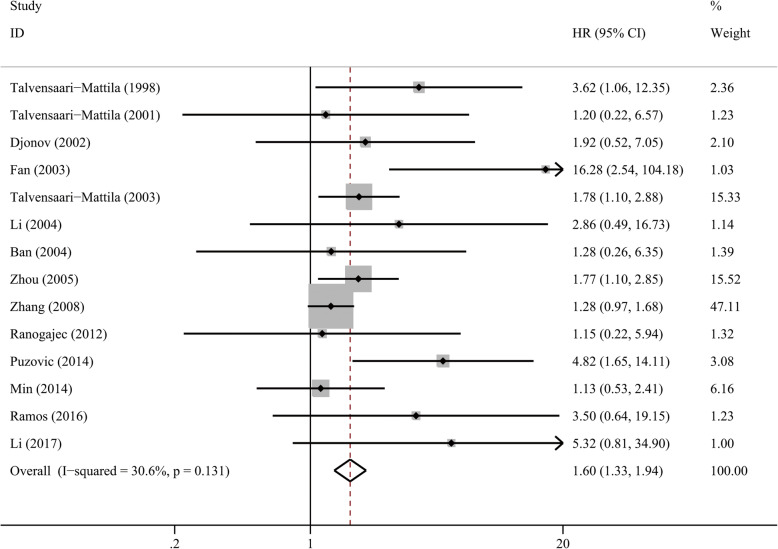
As shown in Table 3, there were no significant associations between MMP2 overexpression with DFS (HR = 1.79, P = 0.096) or RFS (HR = 1.21, P = 0.338) in BC. However, after pooling 14 studies, patients with MMP2 overexpression showed an unfavorable OS (HR = 1.60, 95% CI 1.33–1.94, P < 0.001, Fig. 2). The association was significant regarding ethnicity, IHC analysis standard, sample size and the anti-human-MMP2 antibody used for IHC staining (Table 3, Table S2). Subgroup of multivariate analysis adjusting HR for the other confounders (ER, PR, HER2, clinicopathological features) revealed that MMP2 overexpression was associated with unfavorable OS (HR = 1.78, 95%CI 1.32–2.39, P < 0.001) and may be an independent prognostic factor.
Table 3.
Association between MMP2 overexpression and survival in patients with breast cancer
| Survival | Subgroup | No. of studies | No. of patients | I2 (%) | P for heterogeneity | Pooled HR (95%CI) | P for effect size |
|---|---|---|---|---|---|---|---|
| DFS | Overall | 3 | 240 | 0 | 0.724 | 1.79 (0.90–3.54) | 0.096 |
| OS | Overall | 14 | 2128 | 30.6 | 0.131 | 1.60 (1.33–1.94) | < 0.001 |
| Ethnicity | |||||||
| Caucasians | 7 | 1100 | 0 | 0.569 | 2.21 (1.47–3.06) | < 0.001 | |
| Asians | 7 | 1028 | 47.5 | 0.075 | 1.75 (1.14–2.70) | 0.011 | |
| IHC analysis standard | |||||||
| Percentage | 7 | 1228 | 0 | 0.729 | 1.97 (1.46–2.68) | < 0.001 | |
| SI | 7 | 900 | 53.2 | 0.046 | 1.87 (1.08–3.26) | 0.026 | |
| HR data | |||||||
| Reported | 9 | 1706 | 51.5 | 0.036 | 1.95 (1.37–2.79) | < 0.001 | |
| Estimated | 5 | 422 | 0 | 0.641 | 1.90 (0.89–4.07) | 0.097 | |
| Analysis model | |||||||
| Univariate | 9 | 1245 | 32.2 | 0.160 | 1.52 (1.19–1.94) | 0.001 | |
| Multivariate | 6 | 1153 | 29.9 | 0.211 | 1.78 (1.33–2.39) | < 0.001 | |
| Sample size | |||||||
| > 150 | 5 | 796 | 23.3 | 0.266 | 1.43 (1.15–1.78) | 0.002 | |
| ≤150 | 9 | 1332 | 20.2 | 0.263 | 2.19 (1.53–3.13) | < 0.001 | |
| Cancer subtype | |||||||
| IDC | 4 | 621 | 48.0 | 0.123 | 1.35 (1.06–1.73) | 0.017 | |
| RFS | Overall | 5 | 712 | 0 | 0.892 | 1.21 (0.82–1.81) | 0.338 |
OS overall survival, DFS disease-free survival, RFS recurrence-free survival, IHC immunohistochemistry, IDC infiltrating ductal carcinoma, SI staining index, HR hazard ratio
Fig. 2.
Forest plot of MMP2 overexpression with overall survival in patients with breast cancer
MMP9 overexpression and survival
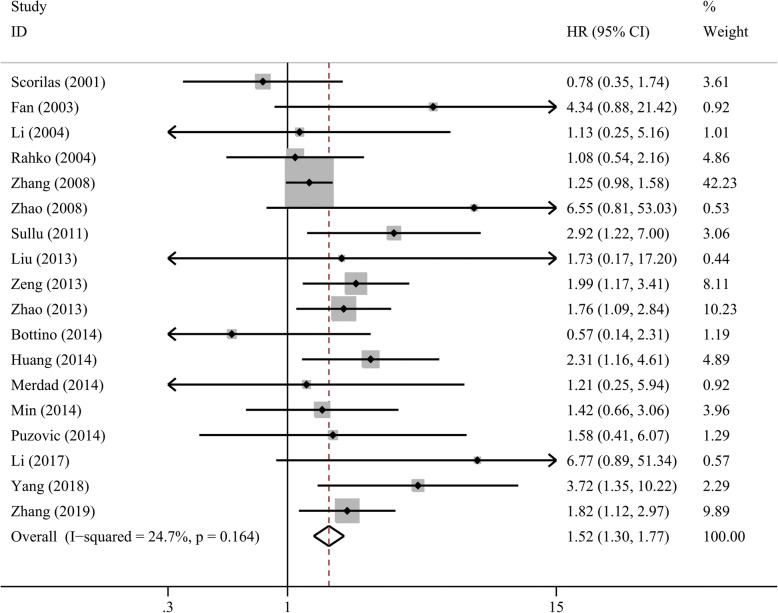
We found an association between MMP9 overexpression and DFS that almost reached significance (HR = 1.73, 95%CI 0.99–3.01, P = 0.052), whereas subgroup of univariate and multivariate analysis both suggested a shorter DFS (P = 0.034 and 0.006, respectively). MMP9 overexpression was not associated with RFS (HR = 1.53, 95%CI 0.73–3.18, P = 0.259, I2 = 79.7%) when we used a random-effect model. For OS, we pooled 18 studies with 2687 patients together by a fixed-effect model. The pooled HR was 1.52 (95% CI 1.30–1.77, P < 0.001, Fig. 3), suggesting an unfavorable OS in patients with overexpressed MMP9. A significantly shorter OS with MMP9 overexpression was found in Asian patients (HR = 1.58, 95% CI 1.34–1.86, P < 0.001) but not in Caucasian patients (P = 0.344) which may be due to small sample size (n = 744). The association was also significant in all of the other subgroup analyses regarding IHC analysis standard, analysis model, HR data source, sample size, tissue and antibody used for IHC analysis (Table 4, Table S2). In addition, MMP9 overexpression was correlated with unfavorable OS in patients with IDC (HR = 1.37, 95% CI 1.11–1.68, P = 0.003) and triple-negative breast cancer (TNBC) (HR = 1.88, 95% CI 1.39–2.55, P < 0.001).
Fig. 3.
Forest plot of MMP9 overexpression with overall survival in patients with breast cancer
Table 4.
Association between MMP9 overexpression and survival in patients with breast cancer
| Survival | Subgroup | No. of studies | No. of patients | I2 (%) | P for heterogeneity | Pooled HR (95%CI) | P for effect size |
|---|---|---|---|---|---|---|---|
| DFS | Overall | 6 | 1002 | 70.5 | 0.005 | 1.73 (0.99–3.01) | 0.052 |
| Analysis model | |||||||
| Univariate | 6 | 1002 | 74.4 | 0.002 | 1.86 (1.05–3.31) | 0.034 | |
| Multivariate | 3 | 573 | 56.7 | 0.100 | 2.73 (1.33–5.61) | 0.006 | |
| OS | Overall | 18 | 2687 | 24.7 | 0.164 | 1.52 (1.30–1.77) | < 0.001 |
| Ethnicity | |||||||
| Caucasians | 6 | 744 | 22.0 | 0.268 | 1.21 (0.81–1.80) | 0.344 | |
| Asians | 12 | 1943 | 25.0 | 0.198 | 1.58 (1.34–1.86) | < 0.001 | |
| IHC analysis standard | |||||||
| Percentage | 7 | 1178 | 2.8 | 0.404 | 1.85 (1.32–2.59) | < 0.001 | |
| SI | 9 | 1239 | 24.4 | 0.226 | 1.51 (1.26–1.80) | < 0.001 | |
| Other cut-offs | 2 | 270 | 0 | 0.703 | 0.72 (0.36–1.45) | 0.359 | |
| HR data | |||||||
| Reported | 14 | 2249 | 9.9 | 0.344 | 1.43 (1.21–1.67) | < 0.001 | |
| Estimated | 4 | 438 | 0 | 0.644 | 2.84 (1.71–4.73) | < 0.001 | |
| Analysis model | |||||||
| Univariate | 14 | 2190 | 63.8 | 0.001 | 1.79 (1.24–2.59) | 0.002 | |
| Multivariate | 9 | 1592 | 1.4 | 0.422 | 1.75 (1.37–2.22) | < 0.001 | |
| Sample size | |||||||
| > 150 | 8 | 1703 | 18.8 | 0.281 | 1.33 (1.10–1.61) | 0.003 | |
| ≤150 | 10 | 984 | 0 | 0.494 | 1.97 (1.51–2.56) | < 0.001 | |
| Cancer subtype | |||||||
| IDC | 6 | 934 | 43.3 | 0.116 | 1.37 (1.11–1.68) | 0.003 | |
| TNBC | 4 | 590 | 0 | 0.933 | 1.88 (1.39–2.55) | < 0.001 | |
| RFS | Overall | 5 | 1292 | 79.7 | 0.001 | 1.53 (0.73–3.18) | 0.259 |
OS overall survival, DFS disease-free survival, RFS recurrence-free survival, IHC immunohistochemistry, IDC infiltrating ductal carcinoma, TNBC triple-negative breast cancer, SI staining index, HR hazard ratio
MMPs overexpression and clinicopathological features
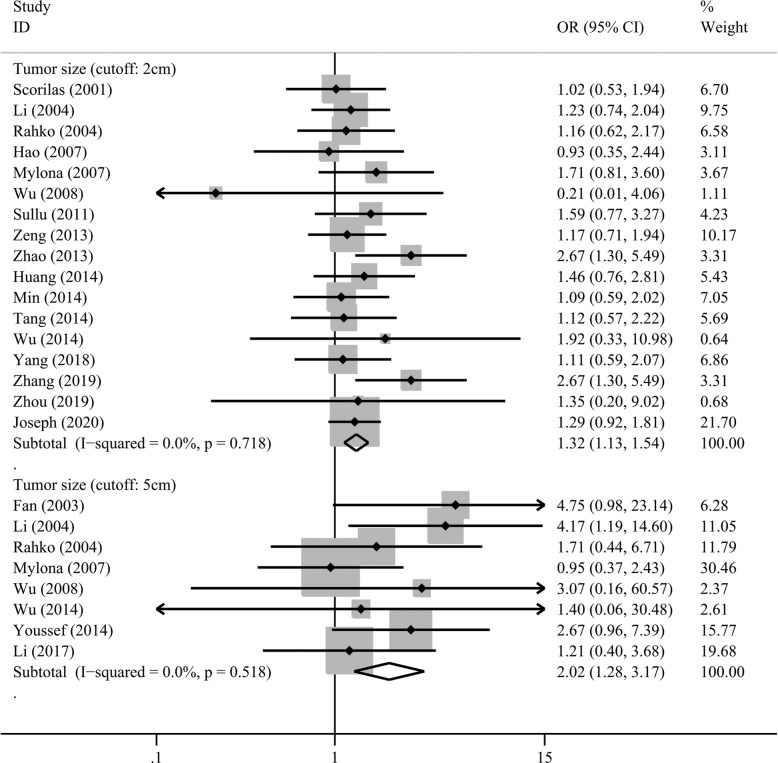
We investigated the associations between MMP2/MMP9 overexpression and clinicopathological features (Table 5, Table S3, Table S4). MMP2 overexpression was significantly associated with higher histological grades (for grade 2–3 vs 1, OR = 2.11, P < 0.001; for grade 3 vs. 1–2, OR = 1.53, P = 0.005; Fig. 4), higher tumor stages (OR = 2.09, P = 0.001) and distant metastasis (OR = 2.69, P = 0.005), but not with the other clinicopathological features. Meanwhile, MMP9 overexpression was found to be associated with higher histological grades (grade 3 vs. 1–2, OR = 1.77, P < 0.001), larger tumor size (for > 2 cm vs. ≤2 cm, OR = 1.32, 95% CI 1.13–1.54, P < 0.001; for > 5 cm vs. ≤5 cm, OR = 2.02, 95%CI 1.28–3.17, P = 0.002; Fig. 5), lymph node metastasis (OR = 2.90, P < 0.001), and positive HER2 (OR = 1.41, P = 0.021).
Table 5.
Association between MMP2/9 overexpression and clinicopathological features in breast cancer patients
| Clinicopathological feature | MMP2 | MMP9 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | I2 (%) | Model | Pooled OR (95%CI) | P | No. of patients | I2 (%) | Model | Pooled OR (95%CI) | P | |
| Tumor size (> 2 cm vs ≤2 cm) | 1254 | 48.4 | R | 1.17 (0.78–1.75) | 0.448 | 3005 | 0 | F | 1.32 (1.13–1.54) | < 0.001 |
| Tumor size (> 5 cm vs ≤5 cm) | 1286 | 15.9 | F | 1.12 (0.76–1.64) | 0.568 | 924 | 0 | F | 2.02 (1.28–3.17) | 0.002 |
| Lymph node status (+ vs -) | 1606 | 40.3 | R | 1.22 (0.88–1.70) | 0.225 | 1945 | 77.1 | R | 2.90 (1.86–4.53) | < 0.001 |
| Distant metastasis (+ vs -) | 219 | 22.0 | F | 2.69 (1.35–5.39) | 0.005 | – | – | – | – | – |
| ER (+ vs -) | 1784 | 47.1 | R | 0.82 (0.57–1.18) | 0.290 | 1975 | 58.2 | R | 1.00 (0.71–1.39) | 0.990 |
| PR (+ vs -) | 1660 | 5.7 | F | 1.07 (0.85–1.35) | 0.545 | 1876 | 55.2 | R | 1.00 (0.73–1.38) | 0.991 |
| HER2 (+ vs -) | 361 | 64.8 | R | 1.28 (0.49–3.37) | 0.612 | 1007 | 0 | F | 1.41 (1.05–1.90) | 0.021 |
| TNM stage (III-IV vs I-II) | 666 | 28.0 | F | 2.09 (1.36–3.21) | 0.001 | 2419 | 70.7 | R | 2.00 (1.26–3.19) | 0.004 |
| Grade (2–3 vs 1) | 1437 | 0 | F | 2.11 (1.55–2.88) | < 0.001 | 2051 | 61.9 | R | 1.55 (0.91–2.62) | 0.107 |
| Grade (3 vs 1–2) | 1089 | 0 | F | 1.53 (1.14–2.06) | 0.005 | 2609 | 48.4 | R | 1.77 (1.32–2.36) | < 0.001 |
ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor 2, F fixed effect model, R random effect model, OR odds ratio
+: positive; −: negative
Fig. 4.
Forest plot of MMP2 overexpression with histological grade in patients with breast cancer
Fig. 5.
Forest plot of MMP9 overexpression with tumor size in patients with breast cancer
Sensitivity analysis and meta-regression
Sensitivity analysis revealed that excluding a single study did not obviously change the pooled effect size. Meta-regression showed that sample size had a significant impact on the association of PR status with MMP9 overexpression (P = 0.025) and ER status with MMP2 overexpression (P = 0.004).
Publication bias
We observed significant publication bias in the analysis of the association of MMP2 overexpression with survival (P < 0.05), ER status (P = 0.003), and lymph node metastasis (P = 0.003), as well as MMP9 overexpression with lymph node status (P = 0.045) and TNM stage (P = 0.042). In the other analyses, the funnel plots were symmetric and P values of Egger’s test were > 0.05, indicating there was no obvious publication bias.
Discussion
MMP2 and MMP9, also known as Gelatinase A and B, play key roles in the carcinogenesis of BC, with functions in cell proliferation, inflammation, angiogenesis, tumor invasion and metastasis [60]. However, studies on the potential associations between MMP2/9 expression and clinicopathological features and survival in BC have yielded conflicting results. Here, we performed a meta-analysis including 41 studies with 6517 BC patients and evaluated the prognostic values of MMP2/9 expression in tumor cells for BC. We found that MMP2 overexpression was significantly associated with shorter OS while MMP9 overexpression was related to shorter DFS and OS, indicating that MMP2 and MMP9 may serve as promising prognostic biomarkers for the treatment and management of BC patients.
Tumor metastasis is a crucial event of BC that severely affects the survival of patients, and may influence the determination of appropriate therapeutic strategies [61]. Overproduction of MMP2//9 induces the degradation of the major components of ECM and BM, allowing the escape of tumor cells and promoting subsequent metastasis [62]. Higher expression of MMP2/9 was found in tumor tissues compared with adjacent normal tissues [16]. In present study, MMP2 overexpression was associated with higher risk of distant metastasis and MMP9 overexpression correlated with lymph node metastasis, suggesting MMP2/9 may be indicators for BC metastasis. Moreover, both markers were associated with advanced clinical stages and poor tumor differentiation of BC. Therefore, MMP2/9 may be markers for poor prognosis and the detection of MMP2/9 protein expression may help determine strategies for treatment and follow-ups.
As described above, MMP2/9 overexpression has been associated with tumor size, metastasis, clinical stages and histological grades, all of which were well-known clinicopathological features influencing the survivals of BC patients. Therefore, the hazards ratio may be biased in univariate analysis and should be adjusted for these confounders using multivariate methods to investigate the independent roles of MMP2/9. In present analysis, subgroup of multivariate analysis demonstrated that both MMP2 and MMP9 overexpression predicted a significantly shorter OS after adjustment for known prognostic markers including ER, PR, HER2, and other clinicopathological features, indicating that MMP2 and MMP9 were independent predictors for survival of BC patients. Thus, MMP2/MMP9 overexpression with independent prognostic values may help make strategies for treatment and management of BC alone or together with known markers.
While the predictive roles of MMP2 and MMP9 were separately investigated in our meta-analysis, the role of MMP2 and MMP9 co-expression were not studied because of the small number of eligible studies. Li et al [19] reported that MMP2/9 co-expression was associated with shorter RFS in univariate and multivariate analyses but not with OS. Whereas, Puzovic et al [35] did not find associations of MMP2/9 co-expression with DFS and OS in BC patients. Since MMP2 and MMP9 belong to the same subtype of matrix metalloproteinases and share similar mechanism in promoting carcinogenesis, it is necessary to explore the prognostic value of the co-expression of both proteins in BC patients.
The present study mainly focused on the protein expression in tumor cells and has excluded MMP2/9 mRNA and protein expression in serum and stromal cells. Several studies detected serum MMP2/9 expressions by ELISA and correlated them with survival outcomes in BC patients [63–66]. However, the optimal cutoffs for high- and low-expression were mostly established using the median values, which varied among studies and were largely dependent on the enrolled samples. Thus, it was not suitable to pool these studies together or with the studies investigating protein expression derived from tumor cells. More efforts are needed to establish the optimal cutoff for serum expression of MMP2/9.
Some studies detected MMP2/9 expression in stromal cells by semi-quantitative analysis with IHC [21, 32, 41]. MMP2/9 are mainly expressed by neoplastic cells but also are derived from non-neoplastic stromal and inflammatory cells [67, 68]. Stromal MMP2/9 may also participate in tumor tissue remodeling and contribute to cancer progression [69, 70]. Min et al [32] found that stromal but not tumoral MMP2 was an independent predictive factor of OS, implying different prognostic roles of tumor- and stroma-derived MMP2 in BC. Mylona et al [41] reported significant associations of stromal MMP9 with poor OS and DFS. However, the prognostic value of stromal MMP2/9 in BC requires further investigation. Because the percentages of MMP2/9 overexpression were much higher in tumor cells compared with in stromal cells under the same IHC criteria [32, 41], we focused on tumoral MMP2/9 and excluded stromal MMP2/9 to keep the homogeneity of eligible studies in present study.
IDC is the most common subtype of BC [71]. Subgroup analysis demonstrated that MMP2/9 overexpression predicted significantly shorter OS in patients with IDC. TNBC is featured by the lack of ER, PR, and HER2 expression and comprises almost one-fifth of BC cases [72] and new prognostic indicators and treatment approaches for TNBC are urgently needed. Our analysis demonstrated that MMP9 overexpression was associated with poorer OS, larger tumor size, and higher TNM stage in TNBC, suggesting the promising role of MMP9 in the prognosis of TNBC.
There are some limitations in our study. Firstly, the HR and corresponding 95% CI in some studies were estimated from survival curves, which may deviate from the true values and affect the pooled effect sizes. For example, a significant association between MMP2 and OS was found in the subgroup with reported data but not in the subgroup with estimated data. To minimize the inaccuracy, two researchers independently extracted the data from survival curves. Secondly, there is currently no consensus on the threshold for MMPs overexpression by IHC. The cut-off values for percentage or staining index differ between studies, resulting in inconsistent positivity rates and predictive values of MMPs overexpression. This may be an important source of heterogeneity and limit the clinical use of MMP expression for the prediction of BC prognosis. Thirdly, we found obvious publication bias in the analysis of MMP overexpression associated with survival. The bias may potentially come from studies with univariate analysis (Egger’s test, P < 0.05) but not multivariate analysis (Egger’s test, P > 0.05), since studies with negative results of univariate analysis may tend to be unpublished.
Conclusions
Our meta-analysis demonstrated that MMP2 and MMP9 overexpression in tumor cells was associated with poor survival, larger tumor size, lymph node metastasis, distant metastasis, higher clinical stage, and histological grade in patients with BC. These results suggest that MMP2 and MMP9 are potential markers for the prediction of BC prognosis.
Supplementary Information
Additional file 1: Table S1. Tissues and antibodies for immunohistochemistry. Table S2. Subgroup analysis of overall survival stratified by immunohistochemistry antibody. Table S3. Subgroup analysis of association between MMP2 overexpression and clinicopathological features in breast cancer patients. Table S4. Subgroup analysis of association between MMP9 overexpression and clinicopathological features in breast cancer patients.
Acknowledgements
Not applicable.
Abbreviations
- MMP
Matrix metalloproteinases
- OS
Overall survival
- DFS
Disease-free survival
- RFS
Recurrence-free survival
- HR
Hazard ratio
- OR
Odds ratio
- BC
Breast cancer
- IDC
Infiltrating ductal cancer
- TNBC
Triple-negative breast cancer
- IHC
Immunohistochemistry
Authors’ contributions
HJ and HL conceived and designed the study, collected, analyzed and interpreted the data. HJ drafted the manuscript. HJ and HL critically revised the manuscript and approved the submission.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15(6):593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 4.Marti JLG, Hyder T, Nasrazadani A, Brufsky AM. The evolving landscape of HER2-directed breast Cancer therapy. Curr Treat Options in Oncol. 2020;21(10):82. doi: 10.1007/s11864-020-00780-6. [DOI] [PubMed] [Google Scholar]
- 5.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells. 2020;9(5):1313. [DOI] [PMC free article] [PubMed]
- 7.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48(3):222–272. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 8.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18(5):1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 9.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 10.Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer. 2003;89(10):1817–1821. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41(2):271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng W, Peng W, Wang T, Chen J, Zhu S. Overexpression of MMPs functions as a prognostic biomarker for Oral Cancer patients: a systematic review and meta-analysis. Oral Health Prev Dent. 2019;17(6):505–514. doi: 10.3290/j.ohpd.a43636. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Zhang X, Ai L, Yuan R, Ye J. Clinicohistopathological implications of MMP/VEGF expression in retinoblastoma: a combined meta-analysis and bioinformatics analysis. J Transl Med. 2019;17(1):226. doi: 10.1186/s12967-019-1975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao C, Liang C, Zhu J, Xu A, Zhao K, Hua Y, Zhang J, Chen W, Suo C, Zhang C, et al. Prognostic role of matrix metalloproteinases in bladder carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8(19):32309–32321. doi: 10.18632/oncotarget.15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia H, Zhang Q, Liu F, Zhou D. Prognostic value of MMP-2 for patients with ovarian epithelial carcinoma: a systematic review and meta-analysis. Arch Gynecol Obstet. 2017;295(3):689–696. doi: 10.1007/s00404-016-4257-9. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Qiu Z, Li F, Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol Lett. 2017;14(5):5865–5870. doi: 10.3892/ol.2017.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Xin T, Jiang QY, Huang DY, Shen WX, Li L, Lv YJ, Jin YH, Song XW, Teng C. CD147, MMP9 expression and clinical significance of basal-like breast cancer. Med Oncol. 2013;30(1):366. doi: 10.1007/s12032-012-0366-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhao S, Ma W, Zhang M, Tang D, Shi Q, Xu S, Zhang X, Liu Y, Song Y, Liu L, et al. High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Med Oncol. 2013;30(1):335. doi: 10.1007/s12032-012-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS, Di GH, Liu G, Li FM, Ou ZL, et al. Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Res Treat. 2004;88(1):75–85. doi: 10.1007/s10549-004-1200-8. [DOI] [PubMed] [Google Scholar]
- 20.Merdad A, Karim S, Schulten HJ, Dallol A, Buhmeida A, Al-Thubaity F, Gari MA, Chaudhary AG, Abuzenadah AM, Al-Qahtani MH. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res. 2014;34(3):1355–1366. [PubMed] [Google Scholar]
- 21.Fernandez-Guinea O, Alvarez-Cofino A, Eiro N, Gonzalez LO, del Casar JM, Fernandez-Garcia B, Lamelas ML, Andicoechea A, Vizoso FJ. Low microvascular density at the tumor center is related to the expression of metalloproteases and their inhibitors and with the occurrence of distant metastasis in breast carcinomas. Int J Clin Oncol. 2013;18(4):629–640. doi: 10.1007/s10147-012-0428-2. [DOI] [PubMed] [Google Scholar]
- 22.Sullu Y, Demirag GG, Yildirim A, Karagoz F, Kandemir B. Matrix metalloproteinase-2 (MMP-2) and MMP-9 expression in invasive ductal carcinoma of the breast. Pathol Res Pract. 2011;207(12):747–753. doi: 10.1016/j.prp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Rahko E, Jukkola A, Melkko J, Paavo P, Bloigu R, Talvensaari-Mattila A, Turpeenniemi-Hujanen T. Matrix metalloproteinase-9 (MMP-9) immunoreactive protein has modest prognostic value in locally advanced breast carcinoma patients treated with an adjuvant antiestrogen therapy. Anticancer Res. 2004;24(6):4247–4253. [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 26.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph C, Alsaleem M, Orah N, Narasimha PL, Miligy IM, Kurozumi S, Ellis IO, Mongan NP, Green AR, Rakha EA. Elevated MMP9 expression in breast cancer is a predictor of shorter patient survival. Breast Cancer Res Treat. 2020;182(2):267–282. doi: 10.1007/s10549-020-05670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Min KW, Kim DH, Son BK, Moon KM, Wi YC, Bang SS, Oh YH, Do SI, Chae SW, et al. High TNFRSF12A level associated with MMP-9 overexpression is linked to poor prognosis in breast cancer: gene set enrichment analysis and validation in large-scale cohorts. PLoS One. 2018;13(8):e0202113. doi: 10.1371/journal.pone.0202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos EA, Silva CT, Manica GC, Pereira IT, Klassen LM, Ribeiro EM, Cavalli IJ, Braun-Prado K, Lima RS, Urban CA, et al. Worse prognosis in breast cancer patients can be predicted by immunohistochemical analysis of positive MMP-2 and negative estrogen and progesterone receptors. Rev Assoc Med Bras (1992) 2016;62(8):774–781. doi: 10.1590/1806-9282.62.08.774. [DOI] [PubMed] [Google Scholar]
- 30.Yousef EM, Tahir MR, St-Pierre Y, Gaboury LA. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer. 2014;14:609. doi: 10.1186/1471-2407-14-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youssef NS, Hakim SA. Association of Fascin and matrix metalloproteinase-9 expression with poor prognostic parameters in breast carcinoma of Egyptian women. Diagn Pathol. 2014;9:136. doi: 10.1186/1746-1596-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min KW, Kim DH, Do SI, Kim K, Lee HJ, Chae SW, Sohn JH, Pyo JS, Oh YH, Kim WS, et al. Expression patterns of stromal MMP-2 and tumoural MMP-2 and -9 are significant prognostic factors in invasive ductal carcinoma of the breast. APMIS. 2014;122(12):1196–1206. doi: 10.1111/apm.12285. [DOI] [PubMed] [Google Scholar]
- 33.Tang D, Piao Y, Zhao S, Mu X, Li S, Ma W, Song Y, Wang J, Zhao W, Zhang Q. Expression and correlation of matrix metalloproteinase-9 and heparanase in patients with breast cancer. Med Oncol. 2014;31(7):26. doi: 10.1007/s12032-014-0026-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu QW, Yang QM, Huang YF, She HQ, Liang J, Yang QL, Zhang ZM. Expression and clinical significance of matrix metalloproteinase-9 in lymphatic invasiveness and metastasis of breast cancer. PLoS One. 2014;9(5):e97804. doi: 10.1371/journal.pone.0097804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puzovic V, Brcic I, Ranogajec I, Jakic-Razumovic J. Prognostic values of ETS-1, MMP-2 and MMP-9 expression and co-expression in breast cancer patients. Neoplasma. 2014;61(4):439–446. doi: 10.4149/neo_2014_054. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Y, Liu C, Dong B, Li Y, Jiang B, Xu Y, Meng L, Wu J, Qu L, Shou C. Inverse correlation between Naa10p and MMP-9 expression and the combined prognostic value in breast cancer patients. Med Oncol. 2013;30(2):562. doi: 10.1007/s12032-013-0562-3. [DOI] [PubMed] [Google Scholar]
- 37.Ranogajec I, Jakic-Razumovic J, Puzovic V, Gabrilovac J. Prognostic value of matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9) and aminopeptidase N/CD13 in breast cancer patients. Med Oncol. 2012;29(2):561–569. doi: 10.1007/s12032-011-9984-y. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Cao X, Liu Y, Cao W, Zhang F, Zhang S, Li H, Ning L, Fu L, Niu Y, et al. Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer. 2008;8:83. doi: 10.1186/1471-2407-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, Xu XC. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008;122(9):2050–2056. doi: 10.1002/ijc.23337. [DOI] [PubMed] [Google Scholar]
- 40.Hao L, Zhang C, Qiu Y, Wang L, Luo Y, Jin M, Zhang Y, Guo TB, Matsushima K, Zhang Y. Recombination of CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human breast cancer. Cancer Lett. 2007;253(1):34–42. doi: 10.1016/j.canlet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Mylona E, Nomikos A, Magkou C, Kamberou M, Papassideri I, Keramopoulos A, Nakopoulou L. The clinicopathological and prognostic significance of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-9 according to their localization in invasive breast carcinoma. Histopathology. 2007;50(3):338–347. doi: 10.1111/j.1365-2559.2007.02615.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou S, Liu C, Wu SM, Wu RL. Expressions of CD147 and matrix metalloproteinase-2 in breast cancer and their correlations to prognosis. Ai Zheng. 2005;24(7):874–879. [PubMed] [Google Scholar]
- 43.Sivula A, Talvensaari-Mattila A, Lundin J, Joensuu H, Haglund C, Ristimaki A, Turpeenniemi-Hujanen T. Association of cyclooxygenase-2 and matrix metalloproteinase-2 expression in human breast cancer. Breast Cancer Res Treat. 2005;89(3):215–220. doi: 10.1007/s10549-004-0714-4. [DOI] [PubMed] [Google Scholar]
- 44.Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br J Cancer. 2003;89(7):1270–1275. doi: 10.1038/sj.bjc.6601238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan SQ, Wei QY, Li MR, Zhang LQ, Liang QC. Expression and clinical significance of MMP-2, MMP-9,TIMP-1, and TIMP-2 in breast carcinoma. Ai Zheng. 2003;22(9):968–973. [PubMed] [Google Scholar]
- 46.Nakopoulou L, Tsirmpa I, Alexandrou P, Louvrou A, Ampela C, Markaki S, Davaris PS. MMP-2 protein in invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Res Treat. 2003;77(2):145–155. doi: 10.1023/A:1021371028777. [DOI] [PubMed] [Google Scholar]
- 47.Hirvonen R, Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) in T(1-2)N0 breast carcinoma. Breast Cancer Res Treat. 2003;77(1):85–91. doi: 10.1023/A:1021152910976. [DOI] [PubMed] [Google Scholar]
- 48.Djonov V, Cresto N, Aebersold DM, Burri PH, Altermatt HJ, Hristic M, Berclaz G, Ziemiecki A, Andres AC. Tumor cell specific expression of MMP-2 correlates with tumor vascularisation in breast cancer. Int J Oncol. 2002;21(1):25–30. [PubMed] [Google Scholar]
- 49.Talvensaari-Mattila A, Paakko P, Blanco-Sequeiros G, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) is associated with the risk for a relapse in postmenopausal patients with node-positive breast carcinoma treated with antiestrogen adjuvant therapy. Breast Cancer Res Treat. 2001;65(1):55–61. doi: 10.1023/A:1006458601568. [DOI] [PubMed] [Google Scholar]
- 50.Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. MMP-2 positivity and age less than 40 years increases the risk for recurrence in premenopausal patients with node-positive breast carcinoma. Breast Cancer Res Treat. 1999;58(3):287–293. doi: 10.1023/A:1006326513176. [DOI] [PubMed] [Google Scholar]
- 51.Talvensaari-Mattila A, Paakko P, Hoyhtya M, Blanco-Sequeiros G, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer. 1998;83(6):1153–1162. doi: 10.1002/(SICI)1097-0142(19980915)83:6<1153::AID-CNCR14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 52.Ban SY, Tan XX, Qiao JJ. The relationship between MMP-2 and TIMP2 expression and metastasisi and prognosis of breat infiltrative ductal cancer. J Postgrad Med. 2004;27(6):27–29. [Google Scholar]
- 53.Zhao JX, Lin MZ, Yao HW, Wan YL. The expression of transferrin and MMP-9 in breast cancer and its significance in prognosis. Chin J Clin Oncol. 2008;35(1):22–25. [Google Scholar]
- 54.Zhou N, Tan XS, Xu TT, Wang FR, Hu SY. Expression of RECK and MMP9 proteins in triple negative breast cancer and the relationship with clinical characteristics and prognosis. Oncol Progress. 2019;17(16):1942–1945. [Google Scholar]
- 55.Huang S, Chen J, Jia YJ, Wang YK, Lu YS, Wu KJ. Research on the correlation of MMP9 and P53 expression with the prognosis of triple negative breast cancer. Progress Modern Biomed. 2014;14(5):881–884. [Google Scholar]
- 56.Zhang J, Yao SH, Wang XH. Expression of CD147 and matrix metalloproteinases 9 in triple-begative creast cancer before neoadjuvant chemotherapy and its guiding significance. Eval Anal Drug-Use Hospit China. 2019;19(11):1321–1323. [Google Scholar]
- 57.Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, Talieri M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001;84(11):1488–1496. doi: 10.1054/bjoc.2001.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10(22):7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- 59.Bottino J, Gelaleti GB, Maschio LB, Jardim-Perassi BV, Zuccari DA. Immunoexpression of ROCK-1 and MMP-9 as prognostic markers in breast cancer. Acta Histochem. 2014;116(8):1367–1373. doi: 10.1016/j.acthis.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Dofara SG, Chang SL, Diorio C. Gene polymorphisms and circulating levels of MMP-2 and MMP-9: a review of their role in breast Cancer risk. Anticancer Res. 2020;40(7):3619–3631. doi: 10.21873/anticanres.14351. [DOI] [PubMed] [Google Scholar]
- 61.Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 2009;15(2):RA32–RA40. [PubMed] [Google Scholar]
- 62.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237(3):273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 63.Darlix A, Lamy PJ, Lopez-Crapez E, Braccini AL, Firmin N, Romieu G, Thezenas S, Jacot W. Serum NSE, MMP-9 and HER2 extracellular domain are associated with brain metastases in metastatic breast cancer patients: predictive biomarkers for brain metastases? Int J Cancer. 2016;139(10):2299–2311. doi: 10.1002/ijc.30290. [DOI] [PubMed] [Google Scholar]
- 64.Kuvaja P, Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. Low serum level of pro-matrix metalloproteinase 2 correlates with aggressive behavior in breast carcinoma. Hum Pathol. 2006;37(10):1316–1323. doi: 10.1016/j.humpath.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 65.Talvensaari-Mattila A, Turpeenniemi-Hujanen T. Preoperative serum MMP-9 immunoreactive protein is a prognostic indicator for relapse-free survival in breast carcinoma. Cancer Lett. 2005;217(2):237–242. doi: 10.1016/j.canlet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 66.Leppa S, Saarto T, Vehmanen L, Blomqvist C, Elomaa I. A high serum matrix metalloproteinase-2 level is associated with an adverse prognosis in node-positive breast carcinoma. Clin Cancer Res. 2004;10(3):1057–1063. doi: 10.1158/1078-0432.CCR-03-0047. [DOI] [PubMed] [Google Scholar]
- 67.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200(4):448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 68.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19(56):6642–6650. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 69.Stuelten CH, DaCosta BS, Arany PR, Karpova TS, Stetler-Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-alpha and TGF-beta. J Cell Sci. 2005;118(Pt 10):2143–2153. doi: 10.1242/jcs.02334. [DOI] [PubMed] [Google Scholar]
- 70.Singer CF, Kronsteiner N, Marton E, Kubista M, Cullen KJ, Hirtenlehner K, Seifert M, Kubista E. MMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal-epithelial interactions. Breast Cancer Res Treat. 2002;72(1):69–77. doi: 10.1023/A:1014918512569. [DOI] [PubMed] [Google Scholar]
- 71.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93(9):1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9(5):R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Tissues and antibodies for immunohistochemistry. Table S2. Subgroup analysis of overall survival stratified by immunohistochemistry antibody. Table S3. Subgroup analysis of association between MMP2 overexpression and clinicopathological features in breast cancer patients. Table S4. Subgroup analysis of association between MMP9 overexpression and clinicopathological features in breast cancer patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.