Abstract
Objectives
The aim of this systematic reviews was to synthesize the current studies for the effectiveness of intradialytic resistance exercises with usual care on HD people.
Design
Meta‐analysis of randomized controlled studies.
Methods
A systematic search of seven electronic databases, including PubMed, EMBASE, the Cochrane Library, Web of Science, WanFang, China National Knowledge Infrastructure (CNKI) and SINOMED, was systematically searched up to May 2018. The reference lists of previously reported systematic review were also checked. Pooled analysis was used to determine effection of intradialytic resistance exercises for haemodialysis people. Physical performance, nutrient intake and quality of life were explored, by comparing the association between effect sizes.
Results
Fourteen studies of 594 people were included. Compared with control groups, intradialytic resistance exercises significantly improve physical performance included 6‐min walk test, sit‐to‐stand 30 and grip strength. However, no significant improvements were found in nutrient intakes such as dietary protein intake and quality of life.
Keywords: haemodialysis, intradialytic resistance exercise, meta‐analysis, nutrient intake, physical performance, quality of life
1. INTRODUCTION
End‐stage renal disease (ESRD), a life‐threatening illness, is the advanced stage of chronic kidney disease (CKD) resulting from various causes. In China, the incidence of ESRD is increasing year by year (Zheng et al., 2010). Haemodialysis (HD) is the major renal replacement therapy (RRT) for most people with ESRD. These people have significantly increased morbidity and mortality, with cardiovascular disease as the leading cause of death (Weiner et al., 2011). Frailty, a biologic syndrome, the state of increased vulnerability to physical stressors as a result of cumulative declines across multiple physiological systems, is common in HD people (Kim, Kalantar‐Zadeh, & Kopple, 2013). The most prevalent and potential change components of frailty in HD population are physical inactivity, together with “enforced” sedentary time during HD, contribute to poor physical performance, lower quality of life and increase healthcare resources (Matsuzawa & Roshanravan, 2018).
Exercise intervention has confirmed a potential benefit to target several of these issues. However, physical activity decreases with progression of kidney disease and reaches a nadir in HD people, particularly in the older population (Johansen et al., 2000). The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend that exercises should be one of the cornerstones of therapy for adults receiving dialysis (K/DOQI Workgroup, 2005). Intradialytic resistance exercises, namely various exercise programmes like upper extremity strengthening with free‐weight dumbbells implemented during dialysis, were considered as a strategy to prevent or even reverse people’ muscle wasting, are an important part of comprehensive management for HD people (Parker, 2016) and have been confirmed beneficial in the older population and some chronic diseases such as heart failure (Peterson, Rhea, Sen, & Gordon, 2010; Santos et al., 2018). The aim of this review was to provide an up‐to‐date synthesis of randomized controlled trials (RCT) comparing the effects of intradialytic resistance exercises versus usual care on physical performance, nutrient intake and quality of life in HD people.
2. METHODS
2.1. Inclusion criteria
We included studies that (a) were published in English and Chinese; (b) used a randomized controlled trial; (c) recruited participants with a diagnosis of ESRD requiring maintenance HD as RRT and who were >18 years, were on HD for >3 months and received intradialytic resistance exercises in a structured, supervised environment; and (d) included at least one primary or secondary outcome. Incomplete or duplicate data were excluded.
The primary outcomes were the mean difference in measures of physical performance included 6‐min walk test (6MWT), sit‐to‐stand 30 (STS 30) and grip strength (HG). The secondary outcomes including nutrient intake such as dietary protein intake (DPI) and dietary energy intake (DEI); quality of life (QoL) including mental (MCS) and physical (PCS) component dimensions of the short‐form 36 (SF‐36) health questionnaire.
2.2. Search strategy
We searched the English databases the Cochrane Library, EMBASE, PubMed, Web of Science and the Chinese databases CNKI, WanFang, SINOMED from inception to May 2018 to identify relevant studies using the following as MeSH search terms and free words: “resistance training”[Mesh], “resistance exercise*”, “strengthen train*”, “strengthen exercise*”, “hemodialysis, home”[Mesh], “hemodialysis unit, hospital”[Mesh], “renal dialysis”[Mesh], “renal replacement therapy”[Mesh], “end stage renal disease”[Mesh] etc. To confirm any articles missed by the initial search, we also evaluated the reference lists of previously reported systematic review. We limited our searches to studies published in English and Chinese involving stable adult human participants on HD. Reference sections of the retrieved articles were hand‐searched for citations missed by the electronic searches.
2.3. Data extraction
Two authors (ZF and ZYY) extracted data independently for study characteristics, including first authors, country, sample, mean age, mean duration of dialysis, exercise prescription (FITT: frequency; intensity; type; time), trial duration, and related outcomes. Whenever disagreements occurred, a third author (SQZ) resolved it.
2.4. Quality assessment
The quality of each included trials was assessed independently by both investigators (SH and WZC), using the Physiotherapy Evidence Database (PEDro) scale. Each item was scored with a maximum score of 10 (criterion 1 is not scored). Quality items for studies reviewed were as follows: (1) people were randomly allocated to groups; (2) allocation was concealed; (3) the groups were similar at baseline regarding the most important prognostic indicators; (4) there was blinding of all people; (5) there was blinding of all therapists who administered the therapy; (6) there was blinding of all assessors who measured at least one key outcome; (7) measures of at least one key outcome were obtained from more than 85% of the people initially allocated to groups; (8) all people for whom outcome measures were available received the treatment or control; and (9) the results of between‐group statistical comparisons are reported for at least one key outcome; and (10) the study provides both point measures and measures of variability for at least one key outcome.
2.5. Statistical methods
All analyses were computed in Stata software version 12.0 (Stata Corp LP, College Station, TX 77845, USA). Continuous data were analysed by using the standardized mean difference (SMD) and 95% confidence intervals (95% CI) at the end of treatment. When heterogeneity was significant (p < 0.05, I 2 > 50%), the random effects model was used to pool the sizes of the effect. However, when heterogeneity was insignificant (p ≥ 0.05, I 2 ≤ 50%), the fixed effects model was used. Potential publication bias was assessed by a funnel plot for all outcomes.
3. RESULTS
3.1. Literature search results
As shown in Figure 1, according to the predefined search strategy, a total of 972 studies were initially obtained from databases and there were four additional records identified through other sources. After removing 381 duplicates, 595 studies remained. After browsing titles and abstracts, 542 studies were eliminated as irrelevant. Fifty‐three of full‐text articles assessed for eligibility and 39 were excluded (non‐resistance exercises or exercise not during dialysis: 7; only abstract: 7; not‐RCT: 9; no relevant outcomes: 12; data incomplete: 2; non‐English: 1; and repetitive study: 1), and 14 of studies were finally included in quantitative synthesis (Cheema et al., 2007; Chen et al., 2010; Kirkman et al., 2014; Kopple et al., 2007; Liu, 2017; Martin‐Alemañy et al., 2016; Olvera‐Soto, Valdez‐Ortiz, López Alvarenga, & Espinosa‐Cuevas, 2015; Pellizzaro, Thomé, & Veronese, 2013; Rosa et al., 2018; Seguraortí, Kouidi, & Lisón, 2009; Song & Sohng, 2012; Sun, 2016; Thompson et al., 2016; Wu et al., 2014).
Figure 1.
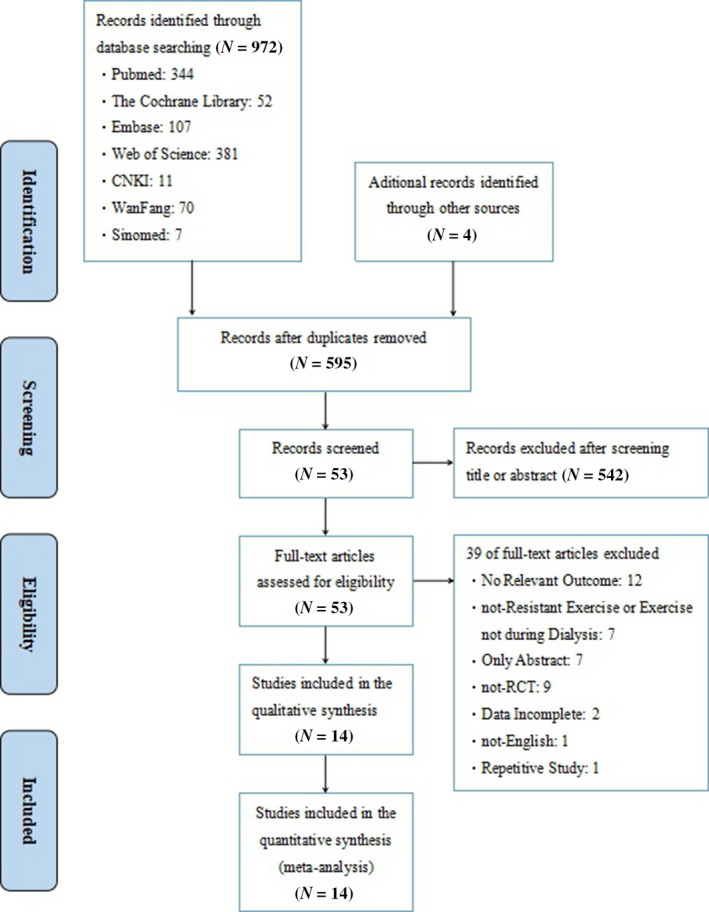
Flow diagram of study selection
3.2. Study characteristics
Studies took place in Europe, America and Asia and were published from 2007–2018. The number of participants in a trial ranged from 15–77, and mean age of study participants ranged from 28.5–81.4. The 14 eligible studies involved 594 HD people: 303 in the exercise group and 291 in the control group.
Trials in this meta‐analysis comprised kinds of regular exercise interventions, with ankle weights or elastic bands for knee extension–flexion and hip abduction flexion being the most common. Most trials had used a moderate to high‐intensity exercises. In terms of frequency of exercise training, all trials had an exercise frequency of 2–3 times per week. Single exercise sessions varied in duration from 30–50 min per session, although some studies did not report duration. Total follow‐up durations ranged from 12–24 weeks. The detailed characteristics of the included studies are listed in Table 1. We conducted a meta‐analysis of all the outcomes (6MWT, STS30, HG, DPI, DEI, the scores of PCS and MCS) at baseline between the exercise and control groups. No significant heterogeneity was found (Table 2).
Table 1.
Basic characteristics of the included RCTs
| Authors (Year) | Country | Sample (N) | Mean age (Years) | Mean duration of dialysis (Years) | Exercise prescription (FITT) | Trial duration | Outcomes |
|---|---|---|---|---|---|---|---|
| Kirkman (2014) | UK |
I:9 C:10 |
I:48 ± 18 C:58 ± 15 |
I:46 ± 54 C:66 ± 47 |
3/week; 80% of patient's predicted 1RM; leg press exercise; N.D. | 12 weeks | 6MWT, STS30 |
| Wu (2014) | China |
I:32 C:33 |
I:45 (37–48) C:44 (41–50) |
I:55.5 ± 37.3 C:39.8 ± 29.7 |
3/week; energy consumption of 70–100 calories, a Borg tiredness score of 12–16 and an increase in the heart rate of 20 beats/min; recumbent cycling; 30–40 min | 12 weeks | 6MWT, STS30, HG |
| Olvera (2015) | Mexico |
I:30 C:31 |
I:28.5 (23–46.5) C:29 (19–38) |
I:12(5.75–37.75) C:18(8–39) |
2/week; N.D.; arm extension, lower leg extension, etc; 50 min | 12 weeks | HG |
| Song (2012) | Korea |
I:20 C:20 |
I:52.1 ± 12.4 C:54.6 ± 10.1 |
I:38.9 ± 26.1 C:45.9 ± 56.2 |
3/week; warm up and cool down: 8–10 RPE; exercise: 11–15 RPE; two additional upper‐body stretches on top of eight movements; 30 min | 12 weeks | HG, STS30, PCS, MCS |
| Cheema (2007) | USA |
I:24 C:25 |
I:60.0 ± 15.3 C:65.0 ± 12.9 |
I:3.3(0.3, 16.7) C:1.6(0.6, 10.3) |
3/week; 15–17 out of 20 at the RPE scale; shoulder press, side shoulder raise, triceps extension, etc; N.D. | 12 weeks | 6MWT, PCS, MCS |
| Thompson (2016) | Canada |
I:7 C:8 |
I:59.7 (45.9, 81.4) C:49.3 (43.0, 62.3) |
I:2.8(2.0, 4.0) C:3.3(1.2, 6.2) |
3/week; 12–14 or “somewhat hard” on the RPE scale; ankle weights; N.D. | 12 weeks | 6MWT, STS30, PCS, MCS |
| Martin (2016) | Mexico |
I:22 C:22 |
I:35 (24–41.5) C:30 (24–47) |
N.D. | 3/week; 12–13 or “somewhat hard” on the RPE scale; lower leg extension, arm extension, etc; 40 min | 12 weeks | HG |
| Chen (2010) | USA |
I:22 C:22 |
I:71.1 ± 12.6 C:66.9 ± 13.4 |
I:2.6 ± 2.6 C:4.8 ± 5.2 |
2/week; 60% of 1RM; knee extension, leg curl, etc; N.D. | 24 weeks | PCS, MCS |
| Pellizzaro (2013) | Brazil |
I:14 C:14 |
I:48.9 ± 10.1 C:51.9 ± 11.6 |
I:54(10.7, 120) C:54(12, 78) |
3/week; 50% 1RM; knee extension; N.D. | 10 weeks | 6MWT |
| Liu (2017) | China |
I:39 C:38 |
60.75 ± 12.53 | 50.08 ± 41.25 | 3/week; elastic band is stretched and patient is well tolerated; ankle extension; 40 min | 12 weeks | PCS, MCS |
| Sun (2016) | China |
I:24 C:22 |
I:57.1 ± 2.9 C:58.1 ± 3.0 |
I:71.0 ± 13.2 C:81.4 ± 13.2 |
3/week; 13–16 on the RPE scale; shoulder press, side shoulder raise, triceps extension, etc; 30–60 min | 12 weeks | 6MWT, HG |
| Segura (2009) | Spain |
I:17 C:8 |
I:53.8 ± 18.0 C:60.1 ± 16.9 |
I:37.3 ± 34.9 C:53.7 ± 42.0 |
3/week; 12–14 on the RPE scale; unilateral triple extension; 35 min | 24 weeks |
6MWT, STS30, PCS, MCS |
| CSDC (2018) | Brazil |
I:28 C:24 |
I:54.49 ± 11.97 C:57.10 ± 16.20 |
I:1.54 ± 1.26 C:2.35 ± 1.66 |
3/week; N.D.; a repetition maximum training zone regime; 40–50 min | 12 weeks | 6MWT, HG, PCS, MCS |
| Kopple (2007) | USA |
I:15 C:14 |
I:46.0 ± 2.7 C:41.3 ± 3.33 |
I:51.9 ± 12.4 C:51.4 ± 21.0 |
3/week; 70% of the 5‐RM; leg extension/leg curl/calf extension; 45–50 min | 21 weeks | DPI, DEI |
6MWT: 6‐min walk test; C: control; DEI: dietary energy intake; DPI: dietary protein intake; FITT: frequency, intensity, type, time; HG: grip strength; I: intervention; MCS: Mental Component Dimensions of the SF‐36; N.D.: no data; PCS: Physical Component Dimensions of the SF‐36; RPE: rate of perceived exertion; STS 30: sit‐to‐stand 30.
Values are mean ± SD or median (first and third quartiles).
Mean ± SEM.
Table 2.
Comparison of baseline values of outcomes
| Outcomes | No. studies | No. people | p for heterogeneity | Pool estimate | 95% CI | pfor results |
|---|---|---|---|---|---|---|
| 6MWT | 7 | 284 | 0.303 | 0.289 | 0.052, 0.526 | 0.017 |
| STS 30 | 4 | 149 | 0.990 | 0.230 | −0.096, 0.556 | 0.166 |
| HG | 6 | 300 | 0.572 | 0.200 | −0.028, 0.428 | 0.086 |
| DPI | 5 | 215 | 0.293 | 0.216 | −0.055, 0.486 | 0.118 |
| DEI | 5 | 215 | 0.662 | 0.122 | −0.147, 0.391 | 0.373 |
| PCS | 6 | 282 | 0.640 | −0.212 | −0.448, 0.024 | 0.079 |
| MCS | 6 | 282 | 0.753 | −0.100 | −0.336, 0.135 | 0.405 |
6MWT: 6‐min walk test; DEI: dietary energy intake; DPI: dietary energy intake; HG: grip strength; MCS: Mental Component Dimensions of the SF‐36; PCS: Physical Component Dimensions of the SF‐36; STS30: sit‐to‐stand 30.
3.3. Publication bias
No publication bias was detected in all outcomes assessed.
3.4. Risk of bias
The methodological quality of included studies is presented in Table 3. All studies reported the “Random Allocation” and only five studies used the allocation concealed: two studies used the people and therapist blinding, three studies used assessor blinding. Only two studies did not report intention to treat. Moreover, all the studies reported that there were no statistically significant differences in age or duration of dialysis between the exercise and control groups at the baseline (p > 0.05).
Table 3.
Risk of bias of included studies
| Studies (Author/Year) | Random allocation | Allocation concealed | Baseline similarity | Subject blinding | Therapist blinding | Assessor blinding | Adequate follow‐up | Intention to treat | Between‐group comparison | Point estimate and variability |
|---|---|---|---|---|---|---|---|---|---|---|
| Kirkman/2014 | √ | √ | √ | × | × | √ | √ | √ | √ | √ |
| Wu/2014 | √ | × | √ | × | × | × | √ | √ | √ | √ |
| Oivera/2015 | √ | × | √ | √ | √ | × | √ | √ | √ | √ |
| Song/2012 | √ | × | √ | × | × | × | √ | √ | √ | √ |
| Cheema/2007 | √ | √ | √ | × | × | √ | √ | √ | √ | √ |
| Stephaine/2016 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Martin/2016 | √ | × | √ | × | × | × | √ | √ | √ | √ |
| Chen/2010 | √ | √ | √ | × | × | × | √ | × | √ | √ |
| Pellizzaw/2013 | √ | × | √ | × | × | × | √ | √ | √ | √ |
| Liu/2017 | √ | × | √ | × | × | × | √ | √ | √ | √ |
| Sun/2016 | √ | × | √ | × | × | × | √ | √ | √ | √ |
| Segura/2009 | √ | × | √ | × | × | × | √ | √ | √ | √ |
| Rosa C/2018 | √ | √ | √ | × | × | × | √ | √ | √ | √ |
| Kopple/2007 | √ | × | √ | × | × | × | √ | √ | √ | √ |
“×” indicated high risk; “√” indicated low risk.
3.5. Pooled results
3.5.1. Effects on physical performance (6MWT, STS 30, HG)
There was a significant decrease in 6MWT with intradialytic resistance exercises versus control in HD people (8 studies; Cheema et al., 2007, Kirkman et al., 2014, Pellizzaro et al., 2013, Rosa et al., 2018, Seguraortí et al., 2009, Sun, 2016, Thompson et al., 2016, Wu et al., 2014), 299 people, SMD = 0.517; 95% CI: 0.283, 0.751; p = 0.000), without between‐study heterogeneity (χ 2 = 8.61; I 2 = 18.7%; p = 0.282; Figure 2).
Figure 2.
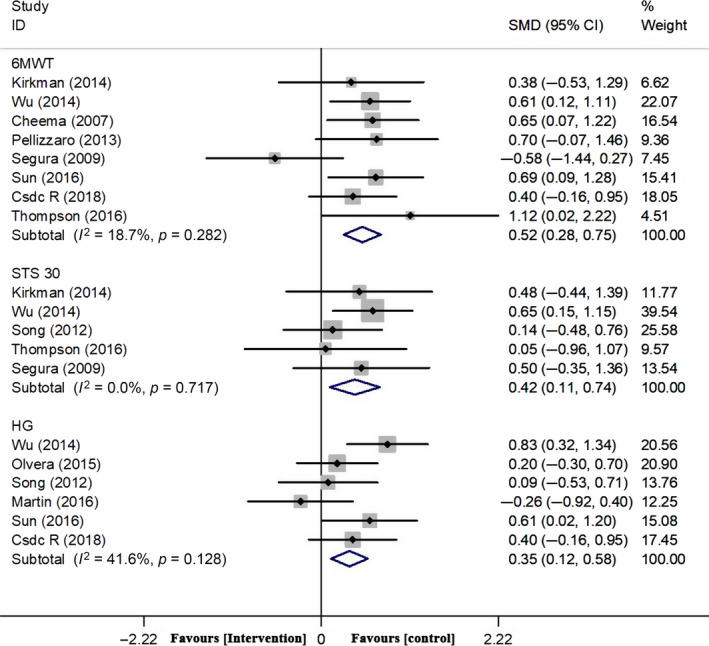
Forest plots of overall effect size of 6MWT, STS 30 and HG between the exercise and control groups
There was a significant decrease in STS 30 with intradialytic resistance exercises versus control in HD people (5 studies: Kirkman et al., 2014; Seguraortí et al., 2009; Song & Sohng, 2012; Thompson et al., 2016; Wu et al., 2014), 164 people, SMD = 0.422; 95% CI: 108, 0.736; p = 0.008), without between‐study heterogeneity (χ 2 = 2.10; I 2 = 0.0%; p = 0.717; Figure 2).
There was a significant increase in HG with intradialytic resistance exercises versus control in HD people (6 studies: Martin‐Alemañy et al., 2016; Olvera‐Soto et al., 2015; Rosa et al., 2018; Song & Sohng, 2012; Sun, 2016; Wu et al., 2014), 300 people, SMD = 0.353; 95% CI: 0.123, 0.583; p = 0.003), with between‐study no heterogeneity (χ 2 = 8.56; I 2 = 41.6%; p = 0.128; Figure 2).
3.5.2. Effects on nutrient intake
There were five studies (Abreu et al., 2017; Cheema et al., 2007; Dong et al., 2011; Kopple et al., 2007; Olvera‐Soto et al., 2015) reporting DPI, and because of no significant between‐study heterogeneity (χ 2 = 5.18; p = 0.269; I 2 = 22.8%), a fix‐effect model was used. The pooled analysis was 0.138 (95% CI: −0.139, 0.415; p = 0.328), which indicated that there was no significant change in exercise group (Figure 3).
Figure 3.
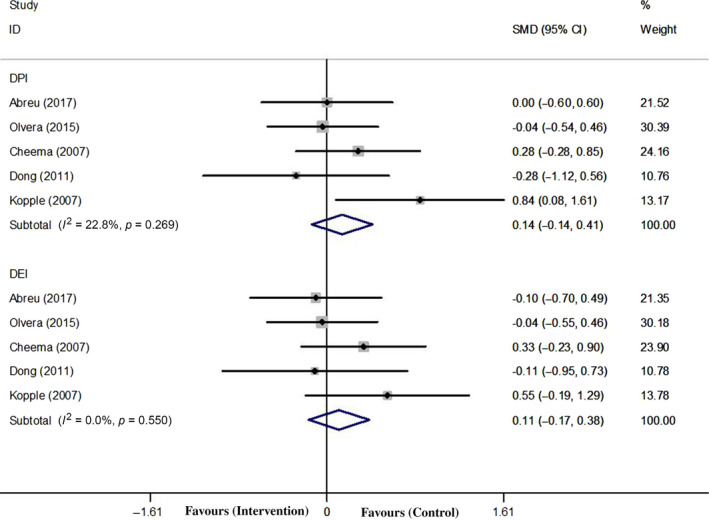
Forest plots of overall effect size of DPI and DEI between the exercise and control groups
There were five studies (Abreu et al., 2017; Cheema et al., 2007; Dong et al., 2011; Kopple et al., 2007; Olvera‐Soto et al., 2015) reporting DEI, and because of no significant between‐study heterogeneity (χ 2 = 3.04; p = 0.550; I 2 = 0.0%), a fix‐effect model was used. The pooled analysis was 0.108 (95% CI: −0.167, 0.384; p = 0.441), which indicated that there was no significant change in exercise group (Figure 3).
3.5.3. Effects on quality of life
Seven studies (Cheema et al., 2007; Chen et al., 2010; Liu, 2017; Rosa et al., 2018; Seguraortí et al., 2009; Song & Sohng, 2012; Thompson et al., 2016) assessed the change in the PCS, with a total of 297 people, 155 people in the exercise group while 142 people in the control group. The heterogeneity was not significant (χ 2 = 5.27; I 2 = 0.0%; p = 0.509). The pooled analysis showed that there was no significant change in the PCS after resistance exercises (SMD = 0.226; 95% CI: −0.005, 0.457; p = 0.055; Figure 4).
Figure 4.
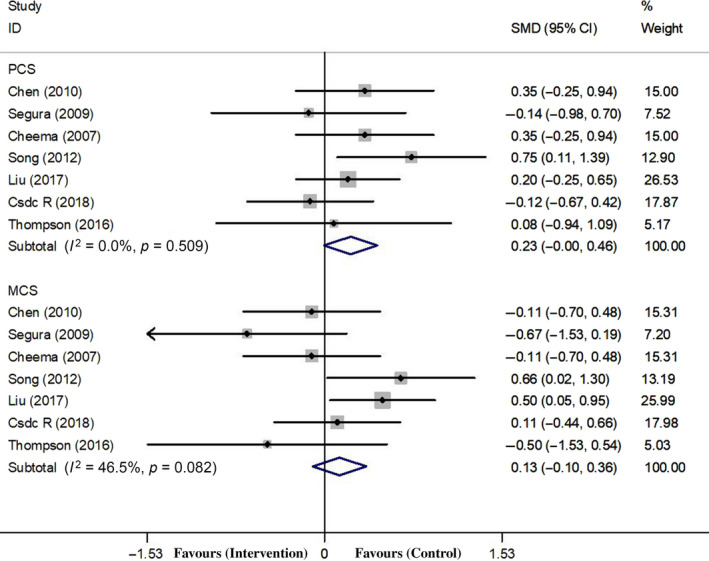
Forest plots of overall effect size of PCS and MCS between the exercise and control groups
Seven studies (Cheema et al., 2007; Chen et al., 2010; Liu, 2017; Rosa et al., 2018; Seguraortí et al., 2009; Song & Sohng, 2012; Thompson et al., 2016) assessed the change in the MCS, with a total of 297 people. The heterogeneity was not significant (χ 2 = 11.21; I 2 = 46.5%; p = 0.082). The pooled analysis showed that there was no significant change in the MCS after resistance exercises (SMD = 0.129; 95% CI: −0.102, 0.361; p = 0.082; Figure 4).
4. DISCUSSION
To date, the precise optimal dose of resistance exercises during HD has not been established yet. Various international health organization and the KDOQI recommended that regular exercise training should be a component for HD people to reduce the risk of cardiovascular complications and the exercise programmes should be designed and delivered by appropriately trained and qualified personnel and take into account individual patient's needed (K/DOQI Workgroup, 2005; Smart et al., 2013). However, there was still insufficient evidence regarding the effects of intradialytic resistance exercises for people undergoing HD. Results of the present study indicated that physical performance is improved by resistance exercises regardless of exercise frequency, intensity or time. Of course, the people must adhere to the exercise intervention to preserve this dominant effect. This finding is in agreement with the recent study in older adults (Liu et al., 2017). Type of exercises that improved physical performance was those that used large muscle group continuously including ankle weights, knee extension and leg curl. It could be helpful for nephrologists, nurse staffs and HD people to understand the effects of intradialytic resistance exercises comprehensively.
Frailty was a common complication in people with ESRD undergoing HD (Brown & Johansson, 2010; Johansen, Chertow, Jin, & Kutner, 2007). A previous study found there were 67.7% of people undergoing HD existed frailty, which was strongly associates with all‐cause morbidity, mortality and lower quality of life (Johansen et al., 2007). Physical performance, a “gold standard” to reflect frailty status (Fried et al., 2001), is defined as the capability to conduct normal daily physical activities, which is often measured by such activities as the time required to climb a defined time of sit‐to‐stand stairs (like STS30) or the distance walked (like 6MWT) (Storer, Casaburi, Sawelson, & Kopple, 2005). It is reported that people on HD with physical performance levels 20%~50% lower than age‐ and sex‐matched sedentary population controls (O'Hare, Tawney, Bacchetti, & Johansen, 2003). In our pooled analysis, intradialytic resistance exercises appeared to have a beneficial influence on physical performance. The possible underlying mechanism of resistance exercises to it may be its ability to improve oxidative metabolism (Stray‐Gundersen, Howden, Parsons, & Thompson, 2016), muscle mitochondrial biogenesis (Vaidyanatha & Balakrishnan, 2010) and reduced systemic inflammation (Castaneda et al., 2004; Viana et al., 2014). Positive effects could be observed in as soon as 12 weeks of regular exercises. In a recent multicenter RCT, Manfredini revealed that an exercise programme improved functional status compared with usual care in people with ESRD (Manfredini et al., 2017).
Remarkably, prevention and treatment of frailty require adequate intake of nutrients. Most guidelines recommended similar amounts of DPI for HD people, ranging from 1.0–1.3 g kg−1 day−1 with at least 50% of high biologic value (Dukkipati, Noori, Feroze, & Kopple, 2010). The KDOQI recommended for energy intake in HD people is 35 kcal kg−1 day−1 for people <60 years and 30 kcal kg−1 day−1 among people <65 years (Kopple, 2001). However, most people undergoing HD will not be able to ingest these quantities of protein and energy and their nutrient intake may need to be augmented to meet these goals. Some scholar reported that it may be that an increase in protein and energy intake in HD people which is offset by exercises (Castaneda et al., 2004). However, the present study showed no significant improvement in DPI and DEI following intradialytic resistance exercises. Fortunately, Martin (Martin‐Alemany et al., 2016) considered that resistance exercises and oral nutritional supplementation during HD may be an effective strategy to increase nutrient intake in HD people.
The lack of significant difference for QoL in our analysis should also be interpreted with caution. The QoL in HD people is universally low and is influenced by factors like age, sex, functional status, work status and some others (Fukuhara et al., 2003). Although our meta‐analysis showed that intradialytic resistance exercises have a predominant benefit in physical performance, there is no difference in the SF‐36 score for PCS and MCS between the exercise and control groups. A possible explanation for this might be due to the proportion of sex, adult and other possible underlying factors.
The adverse events of intradialytic resistance exercises should not be ignored, as HD people have a greater risk of cardiovascular and musculoskeletal complications than normal individuals. Several trials in our meta‐analysis showed that exercises during HD with few serious adverse reactions even without occurred (Cheema et al., 2007; Chen et al., 2010; Kirkman et al., 2014; Olvera‐Soto et al., 2015; Thompson et al., 2016; Wu et al., 2014). It seemed that exercises appeared to be safely in HD people if begun at low intensity and increase gradually. For minimum risk considered, one should first take a full medical history and clinical examination including cardiovascular assessment including blood pressure.
Even so, why has resistance exercises not been broadly applied in HD people? There are several reasons need to be taken into consideration. Firstly, patient‐related barriers: specific symptoms related to comorbid conditions include chronic pain, fatigue, poor physical function and dyspnoea, and comorbid diseases such as cardiovascular disease, diabetes; secondly, staff‐related barriers: few nephrologists assess physical activity or counsel people to increase activity; thirdly, exercise‐related barriers: whether there is a prescription (frequency, intensity, type, time) of intradialytic resistance exercises that can be suitable for most HD people is still a controversial question (Bossola et al., 2014; Delgado & Johansen, 2012; Johansen, 2007; Roshanravan, Gamboa, & Wilund, 2017).
5. LIMITATION
There is some limitation should be noted. First, there were deficiencies in the reporting of methodological information, such as method of randomization, blinding and allocation conceal, and most of the included studies were small‐scale. Second, different exercise prescription including intensity, duration and modality may be a factor that potentially influences the effectiveness of exercise regime; these complicate comparisons between trials and the meta‐analysis of the trials’ results. Finally, our review was restricted to studies published in English; some literature might have been overlooked.
6. CONCLUSIONS
In conclusion, our meta‐analysis confirmed the positive effects of intradialytic resistance exercises on physical performance, but the nutrient intake and quality of life in the general HD population. However, the development of well‐designed studies that have a low risk of bias and multicenter, multidisciplinary studies is needed. Anyhow, intradialytic resistance exercises are an inexpensive, safe and feasible intervention and could be as beneficial auxiliary therapeutic strategy for HD people.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTION
Fan Zhang: Study design. Fan Zhang, Ying Zhang, Zhai Yingying, Zichun Wang and Hui Su: Data collection and analysis. In addition, all authors read and approved the final manuscript.
ETHICAL APPROVAL
There is no ethical statement for this trial.
ACKNOWLEDGEMENTS
No funding sources were provided for this study. The authors deeply thank Professor Cui Xuejun, PhD, Adjunct Research Fellow of Shanghai University of Traditional Chinese Medicine, who read the manuscript and made many valuable comments and suggestions.
Zhang F, Zhou W, Sun Q, et al. Effects of intradialytic resistance exercises on physical performance, nutrient intake and quality of life among haemodialysis people: A systematic review and meta‐analysis. Nursing Open.2021;8:529–538. 10.1002/nop2.274
REFERENCES
- Abreu, C. C. , Cardozo, L. F. M. F. , Stockler‐Pinto, M. B. , Esgalhado, M. , Barboza, J. E. , Frauches, R. , & Mafra, D. (2017). Does resistance exercise performed during dialysis modulate Nrf2 and NF‐βB in patients with chronic kidney disease? Life Sciences, 188, 192–197. [DOI] [PubMed] [Google Scholar]
- Bossola, M. , Pellu, V. , Di, S. E. , Tazza, L. , Giungi, S. , & Nebiolo, P. E. (2014). Self‐reported physical activity in patients on chronic hemodialysis: Correlates and barriers. Blood Purification, 38(1), 24–29. [DOI] [PubMed] [Google Scholar]
- Brown, E. A. , & Johansson, L. (2010). Old age and frailty in the dialysis population. Journal of Nephrology, 23(5), 502. [PubMed] [Google Scholar]
- Castaneda, C. , Gordon, P. L. , Parker, R. C. , Uhlin, K. L. , Roubenoff, R. , & Levey, A. S. (2004). Resistance training to reduce the malnutrition‐inflammation complex syndrome of chronic kidney disease. American Journal of Kidney Diseases the Official Journal of the National Kidney Foundation, 43(4), 607. [DOI] [PubMed] [Google Scholar]
- Cheema, B. , Abas, H. , Smith, B. , O'Sullivan, A. , Chan, M. , Patwardhan, A. , … Lloyd, B. (2007). Progressive exercise for anabolism in kidney disease (PEAK): A randomized, controlled trial of resistance training during hemodialysis. Journal of the American Society of Nephrology: JASN, 18(5), 1594–1601. [DOI] [PubMed] [Google Scholar]
- Chen, J. L. T. , Godfrey, S. , Tan, T. N. , Moorthi, R. , Liangos, O. , Ruthazer, R. , … Castanedasceppa, C. (2010). Effect of intra‐dialytic, low‐intensity strength training on functional capacity in adult haemodialysis patients: A randomized pilot trial. Nephrology Dialysis, Transplantation, 25(6), 1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado, C. , & Johansen, K. L. (2012). Barriers to exercise participation among dialysis patients. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association – European Renal Association, 27(3), 1152–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J. , Sundell, M. B. , Pupim, L. B. , Wu, P. , Shintani, A. , & Ikizler, T. A. (2011). The effect of resistance exercise to augment long‐term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. Journal of Renal Nutrition the Official Journal of the Council on Renal Nutrition of the National Kidney Foundation, 21(2), 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukkipati, R. , Noori, N. , Feroze, U. , & Kopple, J. D. (2010). Dietary protein intake in patients with advanced chronic kidney disease and on dialysis. Seminars in Dialysis, 23(4), 365–372. [DOI] [PubMed] [Google Scholar]
- Fried, L. P. , Tangen, C. M. , Walston, J. , Newman, A. B. , Hirsch, C. , Gottdiener, J. … Cardiovascular Health Study Collaborative Research Group . (2001). Frailty in older adults: Evidence for a phenotype. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56(3), M146–M156. [DOI] [PubMed] [Google Scholar]
- Fukuhara, S. , Lopes, A. A. , Bragggresham, J. L. , Kurokawa, K. , Mapes, D. L. , Akizawa, T. , … Held, P. J. (2003). Health‐related quality of life among dialysis patients on three continents: The Dialysis Outcomes and Practice Patterns Study. Kidney International, 64(5), 1903–1910. [DOI] [PubMed] [Google Scholar]
- Johansen, K. L. (2007). Exercise in the end‐stage renal disease population. Journal of the American Society of Nephrology: JASN, 18(6), 1845–1854. [DOI] [PubMed] [Google Scholar]
- Johansen, K. L. , Chertow, G. M. , Jin, C. , & Kutner, N. G. (2007). Significance of frailty among dialysis patients. Journal of the American Society of Nephrology, 18(11), 2960–2967. [DOI] [PubMed] [Google Scholar]
- Johansen, K. L. , Chertow, G. M. , Ng, A. V. , Mulligan, K. , Carey, S. , Schoenfeld, P. Y. , & Kentbraun, J. A. (2000). Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney International, 57(6), 2564–2570. [DOI] [PubMed] [Google Scholar]
- K/DOQI Workgroup . (2005). K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. American Journal of Kidney Diseases the Official Journal of the National Kidney Foundation, 45(3), 1–153. [PubMed] [Google Scholar]
- Kim, J. C. , Kalantar‐Zadeh, K. , & Kopple, J. D. (2013). Frailty and protein‐energy wasting in elderly patients with end stage kidney disease. Journal of the American Society of Nephrology: JASN, 24(3), 337–351. [DOI] [PubMed] [Google Scholar]
- Kirkman, D. L. , Paul, M. , Junglee, N. A. , Mick, K. , Jibani, M. M. , & Macdonald, J. H. (2014). Anabolic exercise in haemodialysis patients: A randomised controlled pilot study. Journal of Cachexia Sarcopenia & Muscle, 5(3), 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopple, J. D. (2001). National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. American Journal of Kidney Diseases the Official Journal of the National Kidney Foundation, 37(1 Suppl 2), S66. [DOI] [PubMed] [Google Scholar]
- Kopple, J. D. , Wang, H. , Casaburi, R. , Fournier, M. , Lewis, M. I. , Taylor, W. , & Storer, T. W. (2007). Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. Journal of the American Society of Nephrology, 18(11), 2975–2986. [DOI] [PubMed] [Google Scholar]
- Liu, C. J. , Chang, W. P. , Araujo, I. D. C. , Savage, K. , Radford, L. W. , & Amuthavalli, J. T. (2017). Effects of physical exercise in older adults with reduced physical capacity: Meta‐analysis of resistance exercise and multimodal exercise. International Journal of Rehabilitation Research, 40(4), 303–314. [DOI] [PubMed] [Google Scholar]
- Liu, Y. (2017). The effects of resistant exercise on maintenance hemodialysis patients with intradialytic hypotension. Dalian Medical University.
- Manfredini, F. , Mallamaci, F. , D'Arrigo, G. , Baggetta, R. , Bolignano, D. , Torino, C. , … Zoccali, C. (2017). Exercise in patients on dialysis: A multicenter, randomized clinical trial. Journal of the American Society of Nephrology: JASN, 28(4), 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Alemany, G. , Valdez‐Ortiz, R. , Olvera‐Soto, G. , Gomez‐Guerrero, I. , Aguire‐Esquivel, G. , Cantu‐Quintanilla, G. , … Espinosa‐Cuevas, A. (2016). The effects of resistance exercise and oral nutritional supplementation during hemodialysis on indicators of nutritional status and quality of life. Nephrology Dialysis Transplantation, 31(10), 1712–1720. [DOI] [PubMed] [Google Scholar]
- Martin‐Alemañy, G. , Valdez‐Ortiz, R. , Olvera‐Soto, G. , Gomez‐Guerrero, I. , Aguire‐Esquivel, G. , Cantu‐Quintanilla, G. , … Espinosa‐Cuevas, A. (2016). The effects of resistance exercise and oral nutritional supplementation during hemodialysis on indicators of nutritional status and quality of life. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association – European Renal Association, 31(10), 1712. [DOI] [PubMed] [Google Scholar]
- Matsuzawa, R. , & Roshanravan, B. (2018). Management of physical frailty in patients requiring hemodialysis therapy. Contributions to Nephrology, 196, 101–109. [DOI] [PubMed] [Google Scholar]
- O'Hare, A. M. , Tawney, K. , Bacchetti, P. , & Johansen, K. L. (2003). Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. American Journal of Kidney Diseases, 41(2), 447–454. [DOI] [PubMed] [Google Scholar]
- Olvera‐Soto, M. G. , Valdez‐Ortiz, R. , López Alvarenga, J. C. , & Espinosa‐Cuevas, M. L. (2015). Effect of resistance exercises on the indicators of muscle reserves and handgrip strength in adult patients on hemodialysis. Journal of Renal Nutrition, 26(1), 53–60. [DOI] [PubMed] [Google Scholar]
- Parker, K. (2016). Intradialytic exercise is medicine for hemodialysis patients. Current Sports Medicine Reports, 15(4), 269–275. [DOI] [PubMed] [Google Scholar]
- Pellizzaro, C. O. , Thomé, F. S. , & Veronese, F. V. (2013). Effect of peripheral and respiratory muscle training on the functional capacity of hemodialysis patients. Renal Failure, 35(2), 189–197. [DOI] [PubMed] [Google Scholar]
- Peterson, M. D. , Rhea, M. R. , Sen, A. , & Gordon, P. M. (2010). Resistance exercise for muscular strength in older adults: A meta‐analysis. Ageing Research Reviews, 9(3), 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, C. , Nishimoto, D. Y. , Souza, G. , Ramirez, A. P. , Carletti, C. O. , Daibem, C. , … Monteiro, H. L. (2018). Effect of continuous progressive resistance training during hemodialysis on body composition, physical function and quality of life in end‐stage renal disease patients: A randomized controlled trial. Clinical Rehabilitation, 32(7), 899–908. [DOI] [PubMed] [Google Scholar]
- Roshanravan, B. , Gamboa, J. , & Wilund, K. (2017). Exercise and CKD: Skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. American Journal of Kidney Diseases, 69(6), 837–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, F. V. , Chiappa, G. R. , Ramalho, S. , De, A. L. , De, F. S. , Cahalin, L. P. , … Cipriano, J. G. (2018). Resistance exercise enhances oxygen uptake without worsening cardiac function in patients with systolic heart failure: A systematic review and meta‐analysis. Heart Failure Reviews, 23(1), 73–89. [DOI] [PubMed] [Google Scholar]
- Seguraortí, E. , Kouidi, E. , & Lisón, J. F. (2009). Effect of resistance exercise during hemodialysis on physical function and quality of life: Randomized controlled trial. Clinical Nephrology, 71(5), 527–537. [DOI] [PubMed] [Google Scholar]
- Smart, N. A. , Williams, A. D. , Levinger, I. , Selig, S. , Howden, E. , Coombes, J. S. , & Fassett, R. G. (2013). Exercise & Sports Science Australia (ESSA) position statement on exercise and chronic kidney disease. Journal of Science & Medicine in Sport, 16(5), 406–411. [DOI] [PubMed] [Google Scholar]
- Song, W. J. , & Sohng, K. Y. (2012). Effects of progressive resistance training on body composition, physical fitness and quality of life of patients on hemodialysis. Journal of Korean Academy of Nursing, 42(7), 947–956. [DOI] [PubMed] [Google Scholar]
- Storer, T. W. , Casaburi, R. , Sawelson, S. , & Kopple, J. D. (2005). Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrology Dialysis Transplantation, 20(7), 1429–1437. [DOI] [PubMed] [Google Scholar]
- Stray‐Gundersen, J. , Howden, E. J. , Parsons, D. B. , & Thompson, J. R. (2016). Neither hematocrit normalization nor exercise training restores oxygen consumption to normal levels in hemodialysis patients. Journal of the American Society of Nephrology: JASN, 27, 3769–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. J. (2016). Effects of the exercise related interventions during hemodialysis on physical motility and health condition of MHD patients. SuZhou University.
- Thompson, S. , Klarenbach, S. , Molzahn, A. , Lloyd, A. , Gabrys, I. , Haykowsky, M. , & Tonelli, M. (2016). Randomised factorial mixed method pilot study of aerobic and resistance exercise in haemodialysis patients: DIALY‐SIZE! BMJ Open, 6(9), e12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanatha, S. , & Balakrishnan, M. R. V. M. (2010). Resistance training increases muscle mitochondrial biogenesis in patients with chronic kidney disease. Clinical Journal of the American Society of Nephrology, 5(6), 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana, J. L. , Kosmadakis, G. C. , Watson, E. L. , Bevington, A. , Feehally, J. , Bishop, N. C. , & Smith, A. C. (2014). Evidence for anti‐inflammatory effects of exercise in CKD. Journal of the American Society of Nephrology: JASN, 25(9), 2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, D. E. , Scott, T. M. , Giang, L. M. , Agganis, B. T. , Sorensen, E. P. , Tighiouart, H. , & Sarnak, M. J. (2011). Cardiovascular disease and cognitive function in maintenance hemodialysis patients. American Journal of Kidney Diseases the Official Journal of the National Kidney Foundation, 58(5), 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , He, Q. , Yin, X. , He, Q. , Cao, S. , & Ying, G. (2014). Effect of individualized exercise during maintenance haemodialysis on exercise capacity and health‐related quality of life in patients with uraemia. Journal of International Medical Research, 42(3), 718–727. [DOI] [PubMed] [Google Scholar]
- Zheng, J. , You, L. M. , Lou, T. Q. , Chen, N. C. , Lai, D. Y. , Liang, Y. Y. , … Zhai, C. Q. (2010). Development and psychometric evaluation of the Dialysis patient‐perceived Exercise Benefits and Barriers Scale. International Journal of Nursing Studies, 47(2), 166–180. [DOI] [PubMed] [Google Scholar]


