Abstract
Objective:
Patients with underlying diseases are more vulnerable to coronavirus disease 2019 (COVID-19). The purpose of this study was to investigate cancer incidence in patients with COVID-19 and to determine whether cancer was associated with mortality among patients with COVID-19.
Methods:
Electronic searches of PubMed, Embase, Cochrane, Web of Science, and medRxiv were conducted to collect studies that provided data regarding the incidence and mortality of cancer patients with COVID-19. Meta-analyses were used to estimate pooled incidences, risk ratios (RRs), and 95% confidence intervals (CIs) using a random-effects model. Heterogeneity among studies was detected using I2 statistics.
Results:
A total of 19 retrospective studies involving 63,019 patients (2,682 patients with cancer) were included. Meta-analysis showed that the pooled incidence of cancer in COVID-19 patients was 6% (95% CI: 3%–9%). The mortality rate of COVID-19 patients with cancer was higher than that of those without cancer [risk ratio (RR): 1.8, 95% CI: 1.38–2.35, P < 0.01]. Studies on specific types of cancer showed that among COVID-19 patients, the mortality rate of lung cancer patients was higher than that of patients without lung cancer (RR: 1.8, 95% CI: 0.85–3.80, P = 0.02).
Conclusions:
Patients with cancer were more susceptible to COVID-19. As a risk factor, cancer increased mortality among COVID-19 patients. Among COVID-19 patients with cancer, those who had lung cancer had a higher mortality than those without lung cancer. Our findings suggested that clinicians should pay more attention to cancer patients diagnosed with COVID-19 and provide useful information for their clinical management.
Keywords: COVID-19, cancer, incidence, mortality, meta-analysis
Introduction
The novel coronavirus disease 2019 (COVID-19) outbreak has spread throughout the world1–8. Currently (September 16, 2020), the cumulative number of confirmed cases worldwide is 29,514,196, and the cumulative number of deaths is 933,8069. Most COVID-19 patients have mild to moderate respiratory symptoms10–16; however, 13.8% of COVID-19 patients become critically ill with diverse symptoms, leading to multiple organ failure or even death17–24. Recent studies have shown that COVID-19 patients with comorbidities, such as endocrinopathies, cardiac disease, chronic respiratory disease, renal disease, chronic neurological disease, and cancer, are more likely to have a relatively unfavorable prognosis25–35.
Cancer is a major public health problem that seriously threatens the health of the global population36. According to the Global Cancer Observatory, it was estimated that there will be 1.8 million novel cancer cases and 606,000 new cancer-associated deaths worldwide in 202037. Recent studies have demonstrated that cancer enhances susceptibility to COVID-19 and is a risk factor for worse clinical outcomes among patients with COVID-1938–46. Liang et al.47 reported a cancer prevalence of 1.13% [95% confidence interval (CI): 0.61%–1.65%] among 1,590 cases of COVID-19 in China, which was higher than the overall cancer incidence of 0.29% in the Chinese population. In addition, Giannakoulis et al.40 reported a meta-analysis of the outcomes of 46,499 COVID-19 patients with malignancies and showed that all-cause mortality was higher in patients with cancer vs. those without cancer [risk ratio (RR): 1.66, 95% CI: 1.33–2.07, P < 0.0001]. However, existing studies have been limited to a relatively small sample size, and the incidence of cancer in COVID-19 patients should be further investigated. It is therefore necessary to investigate the relationship between cancer and COVID-19 based on a larger sample size. In this study, we conducted a meta-analysis that included 63,019 participants in 19 clinical studies across 9 countries (China, USA, UK, Italy, Switzerland, Republic of Korea, Iran, Spain, and Portugal) to determine both the incidence and outcome of COVID-19 patients with malignancies.
Materials and methods
Search strategy
In this study, we systematically searched PubMed, Embase, Cochrane, Web of Science, and medRxiv databases on July 9, 2020. The search terms included: “COVID-19,” “2019-nCoV,” “SARS-CoV-2,” “clinical characteristics,” “cancer,” “comorbidities,” “malignancy,” “mortality,” “morbidity,” and “outcomes.” The retrieved studies were downloaded from the databases and imported into EndNote X9 for data management and analysis.
Eligibility criteria
We included studies that met the following criteria: (1) patients studied were confirmed with COVID-19 through clinical and laboratory diagnoses; (2) the study contained information about the number of cases or deaths of cancer and noncancer patients in the population infected with COVID-19; and (3) the language was limited to English. We excluded studies that did not meet our criteria. The exclusion criteria were as follows: (1) articles categorized as reviews, case reports, conference abstracts, or basic experimental research literature; (2) articles that did not include an epidemiological analysis related to the observation indicators of this study; (3) articles that did not obtain complete data or were not a full-text study, and thus, the effective evaluation of the study quality could not be effectively evaluated; and (4) repetitive data published in the literature. To avoid duplication of the sample population, among the studies with overlapping data, we chose the study with the largest sample size among studies with overlapping data. Evaluation of the eligibility of the studies was performed by two authors (Ludi Yang and Peiwei Chai) independently of each other, and conflicts were resolved through consultation with a third review author (Jie Yu).
Data extraction and quality assessment
Two authors performed data extraction and quality assessment independently of one another. The extraction content included (1) study information: first author, type of study, study period, region of study, source of sample, and total population of the study; (2) population characteristics: age, sex, comorbidities, the number of cancer, and noncancer patients; and (3) outcomes: survival status of cancer and noncancer patients. The data were cross-checked by 2 authors using a standard electronic sheet to reach a consensus. We chose to use the Newcastle-Ottawa quality assessment scale (NOS) to evaluate the quality of the included articles48. Articles with ≥ 6 stars were defined as high quality articles, with a total score of 9 stars49. Throughout the entire process, if the 2 authors had conflicts or were uncertain, they would consult with the third author to resolve the issue.
Statistical analysis
We performed statistical analyses using the R META package in R Studio (version 3.6.2). Incidence data were first converted to conform to a normal distribution. Then, a meta-analysis of the conversion rate was conducted to calculate the pooled rate and its 95% CI. For binary variables, the overall effect of cancer on mortality was estimated by the pooled RR with a 95% CI using a random-effects model. I2 was calculated to assess heterogeneity, and the interpretations were as follows: unimportant, 0%–40%; moderate heterogeneity, 30%–60%; substantial heterogeneity, 50%–90%; and considerable heterogeneity, 75%–100%50. We conducted an assessment of publication bias to avoid excessively exaggerating the strength of the association between outcomes and risk factors. Significant heterogeneity was dissected via subgroup analysis and sensitivity analysis. A meta-regression was performed to illustrate the potential source of heterogeneity between studies.
Results
Data collection
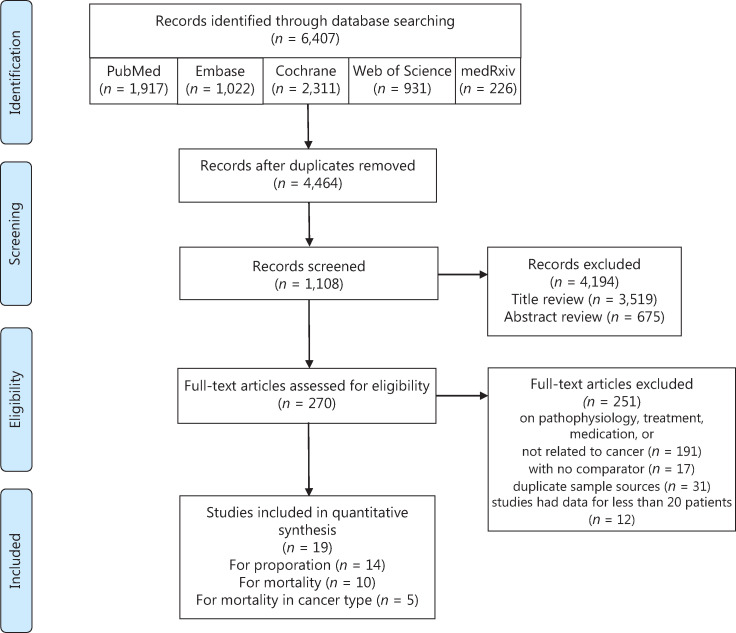
In this study, 6,407 articles were retrieved, and 19 articles met the eligibility criteria, with 6 in Asia, 8 in North America, and 5 in Europe. A total of 63,019 patients were included (Figure 1), of which 2,682 were cancer patients. For studies with repeated sample sources, we only selected the study with the largest number of samples to avoid duplication of the population. The major characteristics of the included studies are summarized in Table 1. The overall quality of these studies was high, with quality scores ranging between 5–9. Assessment of the risk of bias in the involved studies is shown in Supplementary Table S1.
Figure 1.
Flow chart of the search process.
Table 1.
Characteristics of the included studies
| Author | Country | Type of study | Total patients | Sex (male) | Median age | Cancer |
Non-cancer |
||
|---|---|---|---|---|---|---|---|---|---|
| Total | Dead | Total | Dead | ||||||
| Chinese CDC51 | China | Retrospective, multicenter cohort | 20,812 | NA | NA | 107 | 6 | 20,705 | 498 |
| Miyashita et al.52 | USA | Retrospective, multicenter cohort | 5,688 | NA | NA | 334 | 37 | 5,354 | 518 |
| Goyal et al.53 | USA | Retrospective, multicenter cohort | 393 | 238 | 62.2 | 23 | NA | 370 | NA |
| Baker et al.54 | UK | Retrospective, single-center cohort | 316 | 173 | 75 | 33 | 10 | 283 | 71 |
| Benelli et al.55 | Italy | Retrospective, single-center cohort | 411 | 359 | 70.5 | 33 | 9 | 378 | 63 |
| Rossi et al.56 | Italy | Retrospective, multicenter cohort | 2,653 | 1,328 | 63.2 | 301 | 44 | 2,352 | 173 |
| Nikpouraghdam et al.57 | Iran | Retrospective, single-center cohort | 2,964 | 1,955 | 56 | 17 | 1 | 2,947 | 238 |
| Borobia et al.58 | Spain | Retrospective, single-center cohort | 2,226 | 1,074 | 61 | 385 | 139 | 1,841 | 321 |
| Vasco et al.59 | Portugal | Retrospective, multicenter cohort | 20,270 | 8,370 | NA | 603 | 47 | 19,667 | 455 |
| Duanmu et al.60 | USA | Retrospective, single-center cohort | 100 | 56 | 45 | 3 | NA | 97 | NA |
| Gold et al.61 | USA | Retrospective, multicenter cohort | 305 | 151 | 60 | 12 | NA | 293 | NA |
| Sami et al.62 | Iran | Retrospective, single-center cohort | 490 | 299 | 56.6 | 15 | NA | 475 | NA |
| Regina et al.63 | Swiss | Retrospective, single-center cohort | 200 | 120 | 70 | 26 | NA | 174 | NA |
| Ji et al.64 | Korea | Retrospective, multicenter cohort | 5,172 | 2,289 | 42 | 364 | NA | 4,808 | NA |
| Joharatnam-Hogan et al.65 | UK | Retrospective, multicenter cohort | 52 | 31 | NA | 26 | 6 | 26 | 6 |
| Stroppa et al.66 | Italy | Retrospective, single-center cohort | 56 | NA | NA | 25 | 9 | 31 | 5 |
| Dai et al.41 | China | Retrospective, multicenter cohort | 641 | 302 | NA | 105 | 12 | 536 | NA |
| Mehta et al.67 | USA | Retrospective, single-center cohort | 218 | 127 | NA | 218 | 61 | 0 | 0 |
| Yang et al.68 | China | Retrospective, single-center cohort | 52 | 28 | 63 | 52 | 11 | 0 | 0 |
| Total | 63,019 | 16,900 | — | 2,682 | 392 | 60,337 | 2,348 | ||
NA, not available.
The incidence of cancer in COVID-19 patients
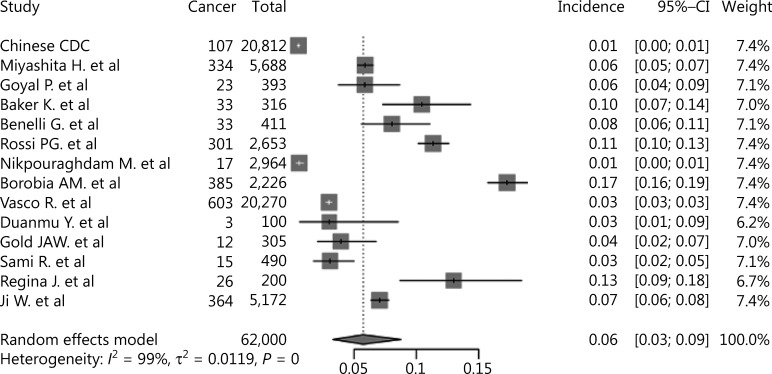
The data for cancer incidence in COVID-19 patients was provided by 14 studies (62,000 total patients, 2,256 with cancer). The incidence varied between different countries, with the highest incidence in Spain (17.296%, 385/2,226) and the lowest in China (0.514%, 107/20,812) (Supplementary Figure S1).
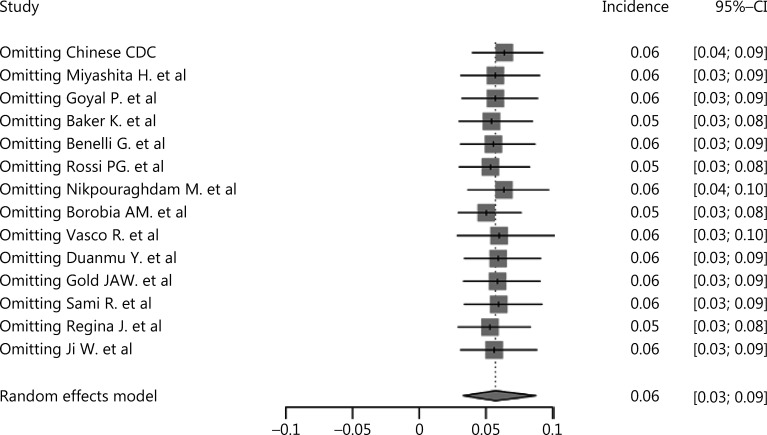
As shown in Figure 2, the pooled incidence of cancer in COVID-19 patients was 6% (95% CI: 3%–9%), which was much higher than the global cancer incidence (approximately 0.2%)69. There was no significant publication bias in our study (P = 0.09). However, our analysis showed that heterogeneity was considerable among the studies (I2 = 99%). Sensitivity analysis revealed that our results were robust, and the results did not vary significantly after separately omitting each study (Figure 3). Subgroup analyses based on the region and sample size were conducted. However, neither could address the source of heterogeneity (Supplementary Figures S2 and S3). Using meta-regression, we detected that sex was not the source of heterogeneity (P = 0.50, R2 = 0.00%). Due to the limitation of information, we could not further determine the potential source of heterogeneity.
Figure 2.
Forest plot showing the incidence of cancer in COVID-19 patients.
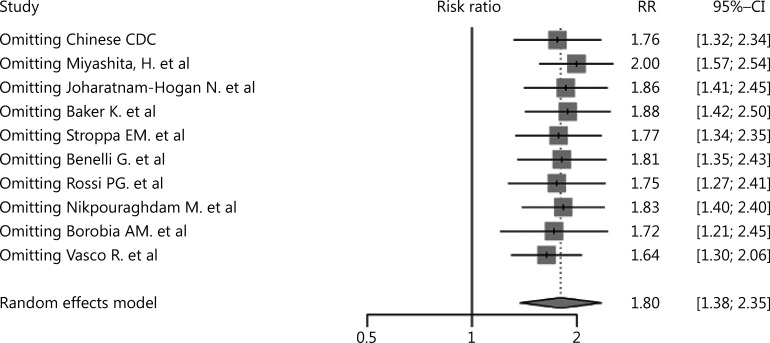
Figure 3.
Sensitivity analysis of cancer incidence in COVID-19 patients.
Cancer-associated mortality in COVID-19 patients
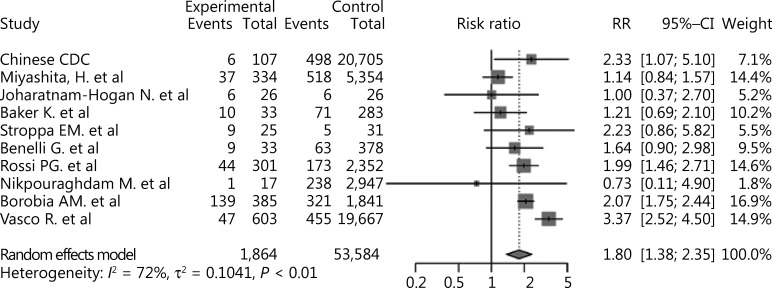
For analysis of the mortality of the cancer patients infected with COVID-19, 10 studies were included. The total population was 55,448, of which 1,864 were cancer patients. As shown in Supplementary Figure S4, the mortality of cancer patients with COVID-19 varied between different countries, with the highest in Spain (36.1%, 139/385) and the lowest in China (5.6%, 6/107).
The results showed that cancer was a risk factor for mortality among COVID-19 patients (RR: = 1.80, 95% CI: 1.38–2.35, P < 0.01, Figure 4). Significant publication bias was not detected among the studies included (P = 0.36). There was substantial heterogeneity (I2 = 72%) in this study, which was studied using subgroup analyses based upon the region and sample sizes. However, inspection of the forest plots of the subgroup analyses built on region and sample size did not reveal the source of the heterogeneity (Supplementary Figures S5 and S6). Using sensitivity analysis, we did not observe any obvious change in the results after omission of each of these studies (Figure 5). We also performed a meta-regression analysis on sex and found that it was the source of the heterogeneity (P < 0.01, R2 = 96.93%, Supplementary Figure S7).
Figure 4.
Forest plot showing the mortality of cancer patients with COVID-19.
Figure 5.
Sensitivity analysis of cancer mortality in COVID-19 patients.
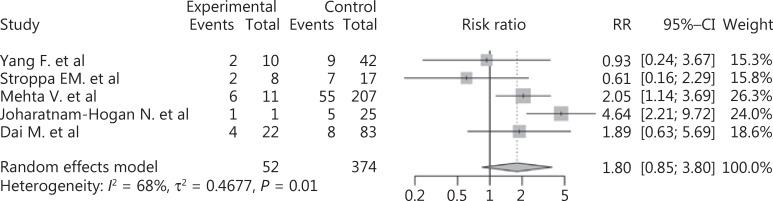
We also included 4 studies to compare the mortalities of lung cancer and non-lung cancer patients among cancer patients with COVID-19. The results showed that lung cancer patients were at higher risk of death than non-lung cancer patients (RR: 1.80, 95% CI: 0.85–3.80, P = 0.02, Figure 6). We further analyzed the effects of COVID-19 on non-lung cancer patients, which indicated that non-lung cancer also increased the mortality of COVID-19 patients (RR: 1.96, 95% CI: 1.57–2.45, P < 0.01, Supplementary Figure S8). Due to the small number of included studies, no subgroup or sensitivity analysis was performed.
Figure 6.
Forest plot showing the mortality of lung cancer patients vs. non-lung cancer patients among the COVID-19 population.
Discussion
With changes in the living environment and lifestyle, the incidence of cancer is increasing worldwide. According to the Global Cancer Observatory, it is estimated that there will be 1.8 million novel cancer cases and 606,000 new cancer-associated deaths worldwide in 202037. With the outbreak of COVID-19, cancer patients have been affected. A recent study reported that COVID-19 infection of host cells is facilitated by transmembrane protease serine 2 (TMPRSS2), angiotensin-converting enzyme 2, and other host cell proteases, such as cathepsin L, which is highly expressed in cancer patients15,70–74. Compared with the general population, the immunosuppressive states of cancer patients make them more vulnerable to severe complications, which may affect the prognosis of the disease41,52,75. Apart from the immunosuppressive state, the mean age of cancer patients is greater than the general population, which could be another risk factor for severe COVID-1947,75. Several studies have reported that cancer is a risk factor for COVID-19 patients, which could lead to unfavorable clinical outcomes43,76. However, a COVID-19 case report from Switzerland detailed a breast cancer patient with immunosuppression who recovered faster and had a better prognosis than her husband, who was in good health77. This report indicates that immunosuppression may not always cause severe complications and could even provide advantages in preventing cytokine storms. In addition, Barlesi et al. reported that the death rates of COVID-19 did not differ significantly between the population with and without cancer because of the low percentage of treatment-related adverse events (5.5%)75,78. Another study reported that the percentages of severe events in breast cancer patients with COVID-19 were the same as the general population, which might be related to the implementation of much stricter social distancing procedures by cancer patients79. Therefore, it is necessary to conduct a comprehensive meta-analysis to identify the relationship between cancer and COVID-19.
This study included 19 high quality articles and we systematically analyzed 63,019 COVID-19 patients worldwide using a meta-analysis. We estimated the incidence of cancer among the COVID-19 population (6%, 95% CI: 3%–9%) and found that it was much higher than that in the general population (approximately 0.2%69). Cancer also appeared to be a risk factor for mortality in COVID-19 patients (RR: 1.80, 95% CI: 1.38–2.35, P < 0.01), and the mortality was higher in patients with lung cancer vs. those without lung cancer (RR: 1.80, 95% CI: 0.85–3.80, P = 0.01). A meta-regression was also performed on mortality and found that sex was the source of heterogeneity, which could be related to different sex compositions among different countries80. In terms of the susceptibility of cancer patients, the risk of infection was related to the physique of each individual. For example, differentially expressed genes in cancer patients could facilitate the entry of viruses into cells, and dysfunction of the immune system of cancer patients could lead to weaker resistance to viruses. In addition, cancer patients need to go to the hospital for treatment or follow-up regularly, which increases the risk of COVID-19 infection.
Unlike previous studies, our study included a large number of samples, covering wide geographic regions. In terms of data extraction, we expanded the sample size as much as possible. However, for articles with duplicate samples, we only included studies with the largest sample size to avoid duplication of sample sources. The quality of the included studies was assessed using the Newcastle-Ottawa quality assessment scale. Studies with Newcastle-Ottawa scores ≥ 6 were considered to be of high quality. We used the method of sequentially eliminating each study for sensitivity analysis, with the results not changing significantly, indicating that the results of this study were stable and highly representative.
There were also some limitations to our study. First, due to the exclusion of the studies with duplicate samples, the number of included studies was relatively limited. Second, the data used in meta-analysis were from hospital-based studies, which may have deviated from data in the real world. Therefore, some COVID-19 patients with cancer who have not been admitted to the hospital may be ignored. Third, there was a certain degree of heterogeneity in our research. There was also substantial heterogeneity in the incidence study analysis. However, we performed subgroup analysis, sensitivity analysis, and meta-regression, but these analyses could not explain the source of the heterogeneity. We assumed that there were other potential sources of heterogeneity which were not reported. For example, the sample sources involved different regions and different countries where health concepts and lifestyles could have influenced the susceptibility to cancer or COVID-19. Furthermore, the management of COVID-19 and the strategies used to control and prevent COVID-19 differed among countries. These could all be potential sources of the heterogeneity that affected the results.
Conclusions
In conclusion, cancer was a risk factor for COVID-19 patients, especially lung cancer. The main advantage of our research was that the sample size was large and representative, covering a wide range of regions. This study emphasized the importance of management of patients with comorbidities during the epidemic. Our results could provide useful information as references to protect people at risk of COVID-19. For example, considering the susceptibility of cancer patients, the follow-up interval and the frequency of radiotherapy and chemotherapy during the epidemic could be appropriately delayed to reduce the risk of nosocomial infection. Medical staff must be vigilant for cancer patients in the COVID-19 population, and personalized treatment plans should be developed to prevent the deterioration of the disease.
Supporting Information
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
Grant support
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81772875, 81770961, and U1932135) and the Science and Technology Commission of Shanghai (Grant Nos. 20DZ2270800 and 19JC1410200).
References
- 1.Contini C, Di Nuzzo M, Barp N, Bonazza A, De Giorgio R, Tognon M, et al. The novel zoonotic COVID-19 pandemic: an expected global health concern. J Infect Dev Ctries. 2020;14:254–64. doi: 10.3855/jidc.12671. [DOI] [PubMed] [Google Scholar]
- 2.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–6. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan KW, Wong VT, Tang SCW. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 Novel Coronavirus Disease. Am J Chin Med. 2020;48:737–62. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 4.Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39:1011–9. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13:667–73. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palacios Cruz M, Santos E, Velazquez Cervantes MA, Leon Juarez M. COVID-19, a worldwide public health emergency. Rev Clin Esp. 2020 doi: 10.1016/j.rceng.2020.03.001. S0014-2565(20)30092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Int Health. 2020;25:278–80. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Cai K, Xiong L. Coronavirus disease 2019: a new severe acute respiratory syndrome from Wuhan in China. Acta Virol. 2020;64:245–50. doi: 10.4149/av_2020_201. [DOI] [PubMed] [Google Scholar]
- 9.Organization WH. Coronavirus disease (COVID-2019) situation reports. 2020 [Google Scholar]
- 10.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–6. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288:192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–83. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond) 2020;20:124–7. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Chai P, Ge S, Fan X. Recent understandings toward coronavirus disease 2019 (COVID-19): from bench to bedside. Front Cell Dev Biol. 2020;8:476. doi: 10.3389/fcell.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili SM, Bahreini E. A comprehensive review of covid-19 characteristics. Biol Proceed Online. 2020;22:19. doi: 10.1186/s12575-020-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020;33:e00028. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–23. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajema KL, Oster AM, McGovern OL, Lindstrom S, Stenger MR, Anderson TC, et al. Persons evaluated for 2019 novel coronavirus – United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:166–70. doi: 10.15585/mmwr.mm6906e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo IM, Halepoto DM, Iqbal M, et al. Novel coronavirus 2019-nCOV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012–9. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 24.Singh A, Shaikh A, Singh R, Singh AK. COVID-19: from bench to bed side. Diabetes Metab Syndr. 2020;14:277–81. doi: 10.1016/j.dsx.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinosa OA, Zanetti ADS, Antunes EF, Longhi FG, Matos TA, Battaglini PF. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020;62:e43. doi: 10.1590/S1678-9946202062043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow N, Fleming-Dutra K, Gierke R, Hall A, Hughes M, Pilishvili T, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–6. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang WH, Guan WJ, Li CC, Li YM, Liang HR, Zhao Y, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur Respir J. 2020;55:2000562. doi: 10.1183/13993003.00562-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 29.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lucena TMC, da Silva Santos AF, de Lima BR, de Albuquerque Borborema ME, de Azevedo Silva J. Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab Syndr. 2020;14:597–600. doi: 10.1016/j.dsx.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dayal D. We urgently need guidelines for managing COVID-19 in children with comorbidities. Acta Paediatr. 2020;109:1497–8. doi: 10.1111/apa.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, et al. Covid-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13:1833–9. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajgain KT, Badal S, Bajgain BB, Santana MJ. Prevalence of comorbidities among individuals with COVID-19: a rapid review of current literature. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.06.213. S0196-6553(20)30637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aghagoli G, Gallo Marin B, Soliman LB, Sellke FW. Cardiac involvement in COVID-19 patients: risk factors, predictors, and complications: a review. J Card Surg. 2020;35:1302–5. doi: 10.1111/jocs.14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: a review. Scand J Public Health. 2018;46:27–36. doi: 10.1177/1403494817715400. [DOI] [PubMed] [Google Scholar]
- 37.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 38. Addeo A, Friedlaender A. Cancer and COVID-19: unmasking their ties. Cancer Treat Rev. 2020;88:102041. doi: 10.1016/j.ctrv.2020.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y, Liu M, Shi S, Chen Y, Sun Y, Chen J, et al. Cancer is associated with the severity and mortality of patients with COVID-19: a systematic review and meta-analysis. MedRxiv. 2020 doi: 10.1101/2020.05.01.20087031. [DOI] [Google Scholar]
- 40.Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Global Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–91. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center’s retrospective study. J Infect. 2020;81:318–56. doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai A, Sachdeva S, Parekh T, Desai R. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557–9. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cook G, John Ashcroft A, Pratt G, Popat R, Ramasamy K, Kaiser M, et al. Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with multiple myeloma receiving systemic anti-cancer therapy. Br J Haematol. 2020;190:e83. doi: 10.1111/bjh.16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharpless NE. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 46.Extance A. COVID-19 and long term conditions: what if you have cancer, diabetes, or chronic kidney disease. BMJ. 2020;368:m1174. doi: 10.1136/bmj.m1174. [DOI] [PubMed] [Google Scholar]
- 47.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 49.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2011 [Google Scholar]
- 50.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019) Cochrane 2019. 2019 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epidemiology Working Group for NCIP Epidemic Response CCDC, Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, et al. Do patients with cancer have a poorer prognosis of COVID-19. An experience in New York city. Ann Oncol. 2020;31:1088–9. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of COVID-19 in New York city. N Engl J Med. 2020;382:2372–4. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker KF, Hanrath AT, Schim van der Loeff I, Tee SA, Capstick R, Marchitelli G, et al. COVID-19 management in a UK NHS Foundation Trust with a high consequence infectious diseases centre: a detailed descriptive analysis. medRxiv. 2020 doi: 10.3390/medsci9010006. 2020.05.14.20100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benelli G, Buscarini E, Canetta C, La Piana G, Merli G, Scartabellati A, et al. SARS-CoV-2 comorbidity network and outcome in hospitalized patients in Crema, Italy. medRxiv. 2020 doi: 10.1371/journal.pone.0248498. 2020.04.14.20053090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R. Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the province of Reggio Emilia, Italy. medRxiv. 2020 doi: 10.1371/journal.pone.0238281. 2020.04.13.20063545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikpouraghdam M, Jalali Farahani A, Alishiri G, Heydari S, Ebrahimnia M, Samadinia H, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in Iran: a single center study. J Clin Virol. 2020;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borobia AM, Carcas AJ, Arnalich F, Alvarez-Sala R, Montserrat J, Quintana M, et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. medRxiv. 2020 doi: 10.3390/jcm9061733. 2020.04.29.20080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricoca Peixoto V, Vieira A, Aguiar P, Sousa P, Carvalho C, Thomas DR, et al. COVID-19: Determinants of hospitalization, ICU and death among 20,293 reported cases in Portugal. medRxiv. 2020 doi: 10.2807/1560-7917.ES.2021.26.33.2001059. 2020.05.29.20115824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duanmu YY, Brown IP, Gibb WR, Singh J, Matheson LW, Blomkalns AL, et al. Characteristics of emergency department patients with COVID-19 at a single site in Northern California: clinical observations and public health implications. Acad Emerg Med. 2020;27:505–9. doi: 10.1111/acem.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gold JAW, Wong KK, Szablewski CM, Patel PR, Rossow J, da Silva J, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 – Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:545–50. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sami R, Soltaninejad F, Amra B, Naderi Z, Haghjooy Javanmard S, Iraj B, et al. A one-year hospital-based prospective COVID-19 open-cohort in the Eastern Mediterranean region: the Khorshid COVID Cohort (KCC) study. medRxiv. 2020 doi: 10.1371/journal.pone.0241537. 2020.05.11.20096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Regina J, Papadimitriou-Olivgeris M, Burger R, Filippidis P, Tschopp J, Desgranges F, et al. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss University Hospital: an observational retrospective study. medRxiv. 2020 doi: 10.1371/journal.pone.0240781. 2020.05.11.20097741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in South Korea. medRxiv. 2020 doi: 10.3346/jkms.2020.35.e237. 2020.05.08.20095174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joharatnam-Hogan N, Hochhauser D, Shiu K-K, Rush H, Crolley V, Butcher E, et al. Outcomes of the 2019 novel coronavirus in patients with or without a history of cancer – a multi-centre North London experience. medRxiv. 2020 doi: 10.1177/1758835920956803. 2020.04.16.20061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stroppa EM, Toscani I, Citterio C, Anselmi E, Zaffignani E, Codeluppi M, et al. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy) Future Oncol. 2020;16:1425–32. doi: 10.2217/fon-2020-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10:935–41. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020;92:2067–73. doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 69.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 70.Chai P, Yu J, Ge S, Jia R, Fan X. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: a pan-cancer analysis. J Hematol Oncol. 2020;13:43. doi: 10.1186/s13045-020-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katopodis P, Anikin V, Randeva HS, Spandidos DA, Chatha K, Kyrou I, et al. Pancancer analysis of transmembrane protease serine 2 and cathepsin L that mediate cellular SARSCoV2 infection leading to COVID-19. Int J Oncol. 2020;57:533–9. doi: 10.3892/ijo.2020.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hou Y, Zhao J, Martin W, Kallianpur A, Chung MK, Jehi L, et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020;18:216. doi: 10.1186/s12916-020-01673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J, Li H, Hu S, Zhou Y. ACE2 correlated with immune infiltration serves as a prognostic biomarker in endometrial carcinoma and renal papillary cell carcinoma: implication for COVID-19. Aging (Albany NY) 2020;12:6518–35. doi: 10.18632/aging.103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bao R, Hernandez K, Huang L, Luke JJ. ACE2 and TMPRSS2 expression by clinical, HLA, immune, and microbial correlates across 34 human cancers and matched normal tissues: implications for SARS-CoV-2 COVID-19. J Immunother Cancer. 2020;8:e001020. doi: 10.1136/jitc-2020-001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu C, Zhao Y, Okwan-Duodu D, Basho R, Cui X. COVID-19 in cancer patients: risk, clinical features, and management. Cancer Biol Med. 2020;17:519–27. doi: 10.20892/j.issn.2095-3941.2020.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Serraino D. COVID-19 and cancer: looking for evidence. Eur J Surg Oncol. 2020;46:929–30. doi: 10.1016/j.ejso.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spezzani V, Piunno A, Iselin HU. Benign COVID-19 in an immunocompromised cancer patient – the case of a married couple. Swiss Med Wkly. 2020;150:w20246. doi: 10.4414/smw.2020.20246. [DOI] [PubMed] [Google Scholar]
- 78.Barlesi F, Foulon S, Bayle A, Gachot B, Pommeret F, Willekens C, et al. Outcome of cancer patients infected with COVID-19, including toxicity of cancer treatments. AACR Annual Meeting. 2020 April 28;:abstr.CT403. 2020 Online. [Google Scholar]
- 79.Vuagnat P, Frelaut M, Ramtohul T, Basse C, Diakite S, Noret A, et al. COVID-19 in breast cancer patients: a cohort at the Institut Curie Hospitals in the Paris area. Breast Cancer Res. 2020;22:55. doi: 10.1186/s13058-020-01293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.