Abstract
Electronic nicotine delivery systems (ENDS) are a rapidly growing global market advertised as a safer alternative to combustible cigarettes. However, comprehensive investigations of END aerosol physicochemical and toxicological properties have not been fully explored across brands to assess relative safety. In this study, we evaluated aerosols collected from three ENDS – Juul Fruit Medley (5% nicotine), Logic Power (2.4% nicotine), and Mistic (1.8% nicotine). ENDS aerosols were generated using standard machine puffing regimen and collected with a novel fluoropolymer condensation trap. Triple quadrupole-inductively coupled plasma-mass determined the presence of heavy metals in collected aerosols. The toxicological effects of ENDS aerosols on normal human bronchial epithelial cells (NHBE) were investigated using cellular viability, reactive oxygen species, oxidative stress assays, along with DNA damage assessments using the CometChip©. Results indicated the total metal concentrations within collected ENDS aerosols were higher for Mistic and Logic compared to Juul. Logic Power aerosols elicited higher reactive oxygen species levels than Mistic and Juul in NHBE after 24-hr exposure. Similar dose-dependent reductions of cellular viability and total glutathione were found for each exposure. However, Logic and Juul aerosols caused greater single stranded DNA damage compared to Mistic. Our study indicates that regardless of brand, ENDS aerosols are toxic to upper airway epithelial cells and may pose a potential respiratory hazard to occasional and frequent users.
Keywords: Electronic nicotine delivery systems, airway epithelial cells, toxicity, aerosols, heavy metals
Introduction
In the United States, electronic nicotine delivery system (ENDS or electronic cigarettes) usage amongst adolescents has risen 78% despite warning signs of potential adverse impacts to public health (Cullen 2018). ENDS are available with different physical designs and chemistries. A basic design typically consists of three parts – the atomizer, refill liquid cartridge, and a battery. A newer design has a liquid cartridge, which contains the heating element (often referred to as a “pod”) that inserts into receptacle containing the battery and associated electronics. The liquid in each of these types of devices may have various formulations of glycerol, propylene glycol, tobacco alkaloids, pH modifiers, and a variety of flavoring agents (Kavvalakis et al. 2015; Lisko et al. 2015). The majority of the liquid constituents have been deemed safe as food additives but have not been confirmed safe for inhalation nor have comprehensive toxicological evaluations been performed related to pulmonary exposures (Mikheev et al. 2016). The rapid acceptance of ENDS is mainly due to their promotion and perception of being safer, less harmful alternatives to their tobacco-based predecessors. (Palazzolo 2013) While the number and variety of chemicals found within ENDS aerosols are significantly fewer than tobacco-based cigarette products,(Rodgman and Perfetti 2016) ENDS aerosols can contain a range of chemicals such as formaldehyde, diacetyl and acrolein, all of which have been implicated in the development and exacerbation of respiratory illnesses (Wang et al. 2017). The most challenging factors in determining the health risks associated with ENDS are the various design considerations and product materials, all of which may affect ENDS aerosol composition, toxicity and ultimately respiratory health effects.
While the relative safety of exposure to organic chemicals in ENDS aerosol is poorly understood, an additional concern is the presence of inorganic and toxic metals in ENDS aerosols. Recent evidence suggests that ENDS aerosols may contain high levels of toxic metals including copper, lead, and nickel that are generated in the aerosols of most first, second, and third generation ENDS (Palazzolo et al. 2016). The generation and release of toxic metal ions is due to the heating and subsequent deterioration of the ENDS metal components (Zucchet and Schmaltz 2017). The concentration of ENDS derived metals may be influenced by the puff topography, where puff duration, frequency, and type of ENDS may enhance nanoparticle inhalation exposure (Zhao et al. 2016). A recent puff topography study that compared ENDS to tobacco cigarette users revealed that ENDS users utilize longer puff durations than tobacco users (Hua et al. 2013). This longer duration could cause high lung burden of ENDS derived metal ions, eliciting cellular and molecular damage caused by either direct or indirect interactions (Geiss et al. 2015; Lee et al. 2017). Additionally, although puff frequency differ between users, studies have shown the mean number of puffs per day to range from 120–225 per day spread out over several smoking sessions (Etter and Bullen 2011; Robinson et al. 2015).
In this study, we characterized the physicochemical and toxicological profiles of three prominent ENDS - Juul Fruit Medley (5% nicotine), Logic Power (2.4% nicotine), and Mistic (1.8% nicotine). Metal concentrations in collected aerosols were determined by inductively coupled plasma mass spectrometry (ICP-MS). Normal human bronchial epithelial cells (NHBE) were used to determine the potential impact of ENDS aerosols on the upper respiratory tract via cellular viability, reactive oxygen species and oxidative stress assays including total glutathione analysis. Finally, genotoxicity assessments using a high throughput version of the comet assay called the CometChip was used to determine levels of single stranded DNA damage.
Methods
Selected ENDS Used in Study.
ENDS devices are trademarks of respective manufacturers and were obtained from vendors in the greater Atlanta, GA USA area or via the internet. Juul devices consist of a rechargeable battery and pre-filled replaceable pods. Logic Power devices consist of a single-use battery and pre-filled cartridge. Mistic devices consist of a rechargeable battery and pre-filled replaceable cartridges.
ENDS aerosol generation and sample preparation.
ENDS aerosols were generated using a Cerulean (Richmond, VA, USA) CETI-8 e-cigarette aerosol machine using CORESTA Recommended Method 81 parameters: 55 mL puff volume, 3 second puff duration, rectangular puff profile, pressure drop ≤ 900 Pa with device in place, 30 second puff interval. Aerosols obtained from 75 puffs from each device and replicate were collected using a high metals purity fluoropolymer condensation trap previously described (Halstead 2019). For particle and toxicity studies, the traps were rinsed with ultrapure water rather than acids to prevent dissolution of metals, and diluted to 25.0 mL volume. Tube rinse blanks were collected daily using a cleaned trap with the same rinse and dilution procedure as samples.
ICP-MS analysis.
The concentrations of chromium, nickel, copper, zinc, cadmium, tin, and lead in ENDS aerosols were determined using an Agilent 8800 (Tokyo, Japan) triple quad-inductively coupled plasma-mass spectrometer with an Apex desolvating introduction system (Elemental Scientific, Omaha, NE, USA) and C400 concentric PFA nebulizer (Savillex, Minnetonka, MN, USA) as previously described (Halstead 2019).
Cell Culture and ENDS Exposure.
Primary normal human bronchial epithelial (NHBE) cells (ATCC® PCS-300–010™) were purchased mycoplasma free and source verified. NHBE were expanded for a period of 7 days in T-75 flasks until 70–80% confluency in Airway Epithelial Cell Basal Medium growth media (ATCC® PCS-300–030) supplemented with Bronchial/Tracheal Epithelial cell growth kit (ATCC® PCS-300–040™) and penicillin/streptomycin (100 units). Upon confluency, NHBE were seeded into 96 well plates at a density of 10,000 cells/well. After a complete monolayer was achieved, cells were exposed to ENDS aerosols that were diluted (1:3) in media from 75 puffs to 25 puffs, which was further diluted 1:1 in media to prepare the mid (12.5 puffs/ml) and low (6.25 puffs/ml) doses. Diluted aerosols were administered directly to cell monolayer for 24 hrs. Media controls and tube rinse blanks diluted in media were also evaluated.
Reactive oxygen species detection.
Using the CellROX® Orange ROS Detection Assay (Invitrogen), ENDS aerosol mediated reactive oxygen species in NHBE were determined. Briefly, following ENDS aerosol exposure, NHBE cell monolayers were washed with phosphate buffered saline (PBS). CellROX Orange diluted in PBS (5 µM) was added to cells for 30 minutes at 37°C. After the stain was removed, a nuclei post stain, NucBlue (Invitrogen) was added for 5 minutes, then removed, followed by two PBS washes. Fluorescent images were obtained using an EVOS FL Auto microscope (Thermo Fisher Scientific, Waltham, MA). These images were used for quantitative assessment of relative fluorescent intensity in the acquired images using Image J software.
Oxidative Stress Evaluations.
Total glutathione (GSH) assessments in ENDS aerosol exposed cells were performed to determine the impact of CellROX reactive oxygen species detected and described above. Following ENDS exposure and NHBE cell monolayer washing, a 1:100 dilution of Luciferin-NT and Glutathione S-Transferase in GSH-Glo™ Reagent was prepared and administered to the cells. The assay was conducted per manufacturer instructions and luminescence was measured using a microplate reader (EnSpire, PerkinElmer, Waltham, MA).
Metabolic activity assessments.
The metabolic activity of NHBE cells post-ENDS exposure was determined using the CellTiter 96 Aqueous One Solution (MTS) (Promega, Madison, WI) assay. ENDS exposed cells were washed twice with phosphate buffered saline (PBS). Fresh media containing MTS reagent (1:10) was then added to the exposed cells, which were then incubated for 1 hr. Plates were read at 490 nm using an EnSpire microplate reader.
CometChip - Single Cell Analysis for DNA Damage.
ENDS aerosol exposed NHBE cells (10,000 cells/ml) were trypsinized and then dispersed into individual macrowells within the Cometchip. The individual cells were subjected to alkaline lysis solution, electrophoresis for 30 minutes, and stained with SybrGold nucleic acid stain (Invitrogen). Fluorescent images of the CometChip gel were made using the fully automated EVOS FL Imaging system. Damaged cells or nuclei resemble a comet with a distinct head and tail. The head contains intact DNA, while the tail contains damaged (single-strand or double-strand breaks) or broken fragments of DNA. The length and intensity of the comet tail (% Tail DNA) are indicators of the level of DNA damage. OpenComet Image J, an open source free image analysis software developed by Schneider and coworkers, was used to score the images to provide quantitative data. At least 150 comets were analyzed per ENDS aerosols with comet scoring conducted blindly to eliminate any potential bias.
Statistical Analysis.
GraphPad Prism software was used to generate descriptive graphs and perform statistical analysis. Ordinary one way ANOVA was employed to assess differences among ENDS exposure groups followed by Dunnett’s test with α = 0.05. P-value <0.05 was deemed significant. All analyses were conducted in triplicate unless stated otherwise.
Results
Concentrations of metals in aerosols to which cells were exposed.
Concentrations of metals in the aerosols to which cells were exposed have been reported,(Halstead 2019) and are shown here in Table 1 for comparison with cytotoxicity studies. The aerosol cadmium concentrations were below the method 0.050 nanograms per 10 puff limit of detection for all devices, as expected, since cadmium is not a component of any device or pod (Halstead 2019).
Table 1.
Metal Concentrations in Aerosol from Respective Devices
| Chromium | Nickel | Copper | Zinc | Cadmium | Tin | Lead | |
|---|---|---|---|---|---|---|---|
| Mistic | 1.14 ± 0.62 | 4.97 ± 3.01 | 488* | 265 ± 111 | < 0.050 | 1.16 ± 0.78 | 3.28 ± 2.56 |
| Logic Power | 0.789 ± 0.422 | 2.28 ±0.96 | 360 ± 186 | 175 ± 101 | < 0.050 | 0.341 ± 0.067 | 1.06 ± 0.33 |
| Juul Mint | < 0.125 | < 0.250 | < 1.00 | < 10.0 | < 0.050 | < 0.100 | < 0.050 |
Units are ng per 10 puffs. *Insufficient inventory was available for triplicate analyses. This copper result is the mean of two analyses.
ENDS Aerosols Elicit Reactive Oxygen Species in NHBE Cells.
Figure 1 shows the qualitative and quantitative data for reactive oxygen species detected in NHBE cells exposed to 25 puffs of ENDS aerosols after 24 hrs. The CellROX dye is cell permeable and is non-fluorescent in its native state. Upon uptake by the NHBE cells, the stain becomes oxidized and fluorescent due to the ENDS aerosols and the reactive oxygen species present within the cell. Using fluorescent microscopy, a detectable signal and orange coloration can be observed in ENDS exposed cells. Figure 1A shows the presence of reactive oxygen species in Juul, Logic, and Mistic aerosol exposed cells in comparison to the media control as evidenced by the robust signal and orange fluorescence. In Figure 1B, a significant increase of relative fluorescence units (RFUs) is shown for each of the three ENDS evaluated in comparison to the media control, with Logic Power causing significantly higher levels of reactive oxygen species than Juul and Mistic at 25 puffs. These reactive oxygen species levels are significantly higher than the positive control, hydrogen peroxide, a known reactive oxygen species generator in biological systems.
Figure 1. Detection of ENDS mediated reactive oxygen species in NHBE cells after 24 hrs.
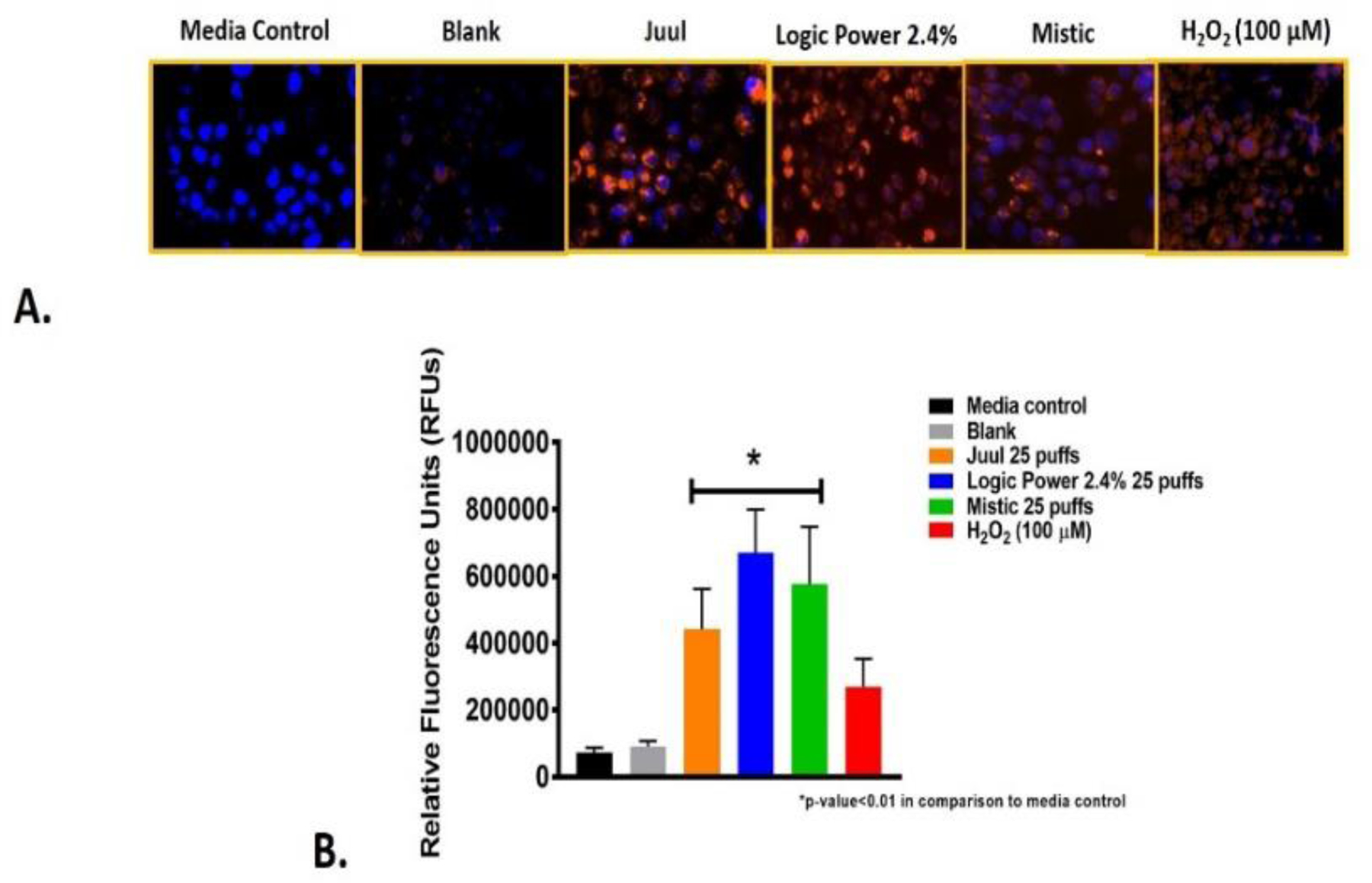
Figure A shows representative images of each exposure group. Figure B reveals the quantitative data derived from images obtained from three individual experiments. Each reveal a significant increase in ROS in comparison to the negative/media control and the blank.
ENDS Mediated Reactive Oxygen Species Cause Oxidative Stress in exposed NHBE cells.
To assess the impact of the ENDS mediated reactive oxygen species in NHBE cells exposed for 24 hrs, we evaluated cellular glutathione levels at doses equivalent to 6.25, 12.5, 25 puffs to assess dose response. In Figure 2, a reduction in glutathione indicates elevated levels of reactive oxygen species within exposed cells, which implies cellular oxidative stress. In comparison to the media control, only the high dose (25 puffs) of each of the three ENDS aerosol was found to significantly alter glutathione levels indicating that ENDS aerosols cause oxidative stress in NHBE cells.
Figure 2. Alteration of glutathione levels in ENDS exposed NHBE cells after 24 hrs.
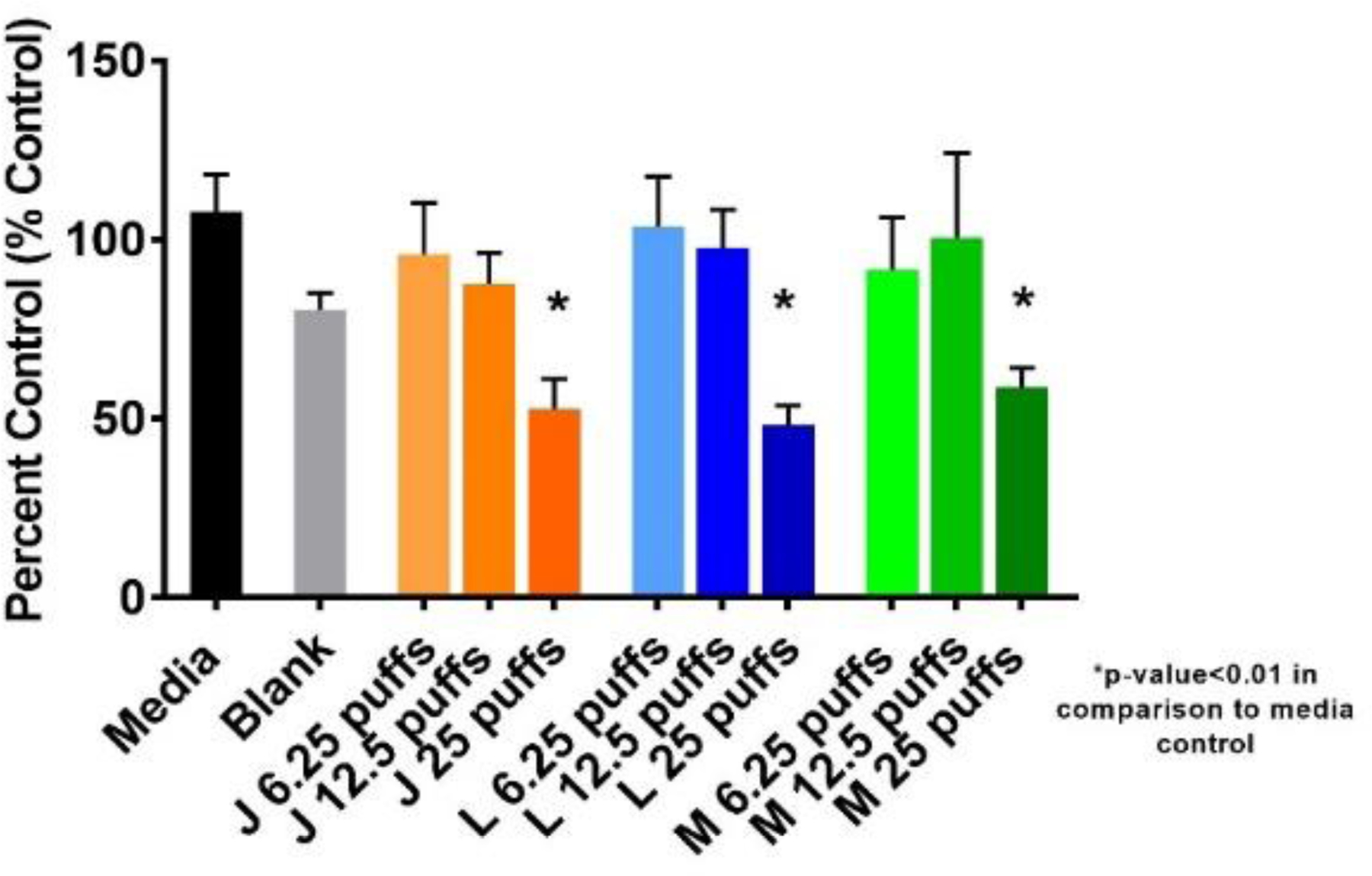
Significant reductions in total glutathione levels are observed in each ENDS exposure at the highest dose of 25 puffs, which correlates with reactive oxygen species data shown above. J: Juul, L: Logic, M: Mistic
ENDS aerosols reduce NHBE metabolic capacity.
The MTS assay is a gold standard test in toxicological studies evaluating various environmental toxicants. In Figure 3, the percent control as a function of each ENDS exposure and negative controls are shown. A dose dependent reduction in metabolic capacity can be seen for Juul exposures due to 6.25, 12.5, 25 puffs, which caused 27%, 44%, and 43% reduction in metabolic capacity, respectively. Logic exposure also reduced NHBE cell metabolic capacity but not in a dose-dependent manner. Mistic exposures significantly reduced metabolic capacity by 29% at the high dose of 25 puffs.
Figure 3. Metabolic capacity of ENDS exposed NHBE cells as determined by MTS assay.
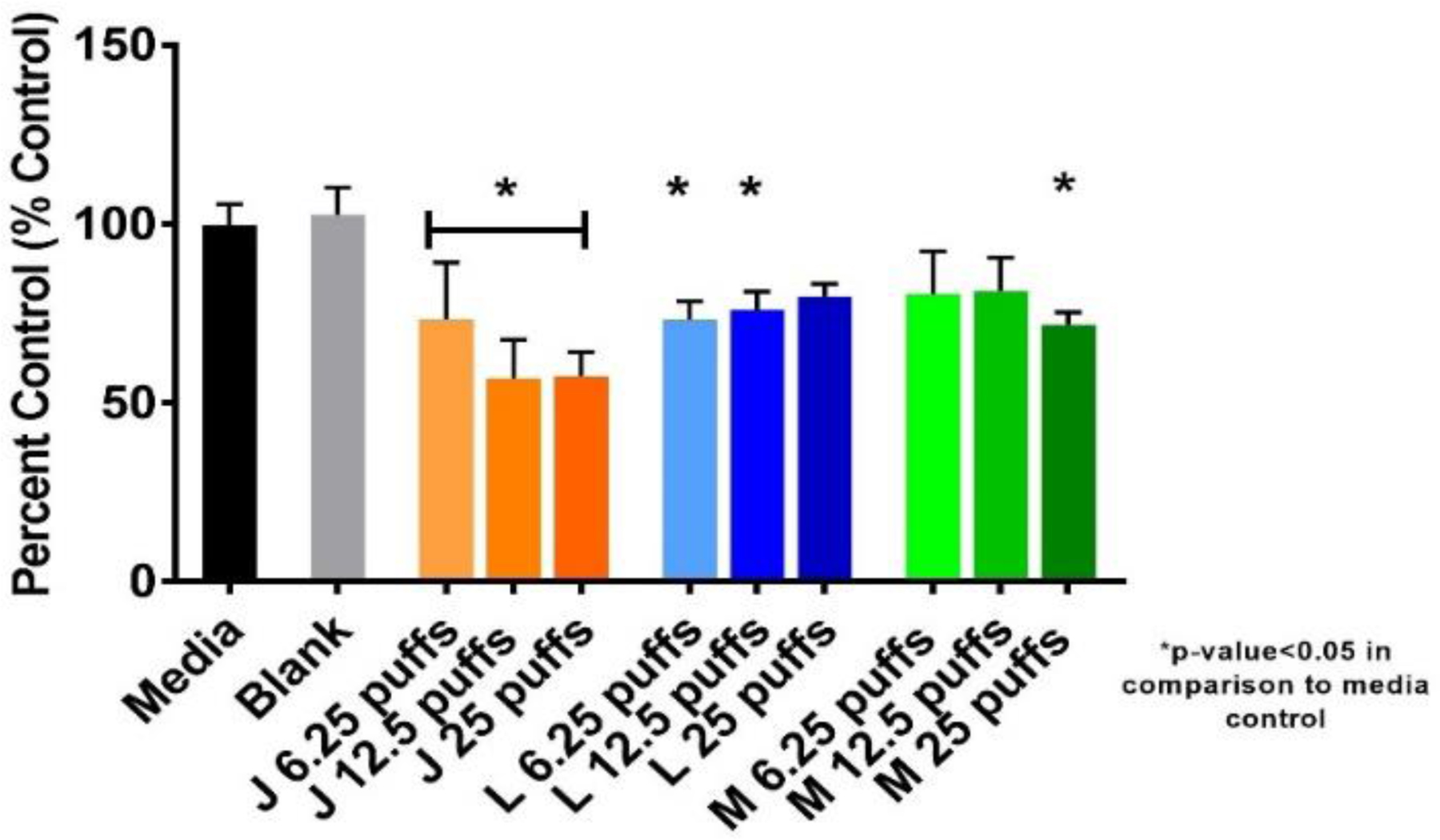
Dose dependent decreases in cellular viability were found due to exposures of Juul and Mistic ENDS aerosols at 24 hrs. J: Juul, L: Logic, M: Mistic
Induction of DNA damage by ENDS aerosols exposures in NHBE cells.
To determine the genotoxicity of aerosols derived from Juul, Logic Power, and Mistic ENDS, we employed the CometChip assay. The CometChip assay is a high-throughput version of the comet assay that allows users to assess multiple environmental toxicants, concentrations and cell types concurrently due to the 96 well array format. Using the CometChip, we determined the genotoxic potential of ENDS aerosols at a dose of 25 puffs. Figure 4A shows images taken from CometChip assessment of Juul, Logic, and Mistic exposed NHBE cell/nuclei. In Figure 4B, the percent Tail DNA, a metric determined by CometChip assessment and used to quantify the level of DNA damage is shown. All three ENDS aerosols elicited single stranded DNA breaks in NHBE cells after 24 hrs that were significantly higher than for cells exposed to procedural blanks. However, ENDS aerosols derived from Logic and Juul induced significantly higher levels of DNA damage than aerosol from Mistic.
Figure 4. Single stranded DNA damage elicited by ENDS aerosol exposures on NHBE cells after 24 hrs.
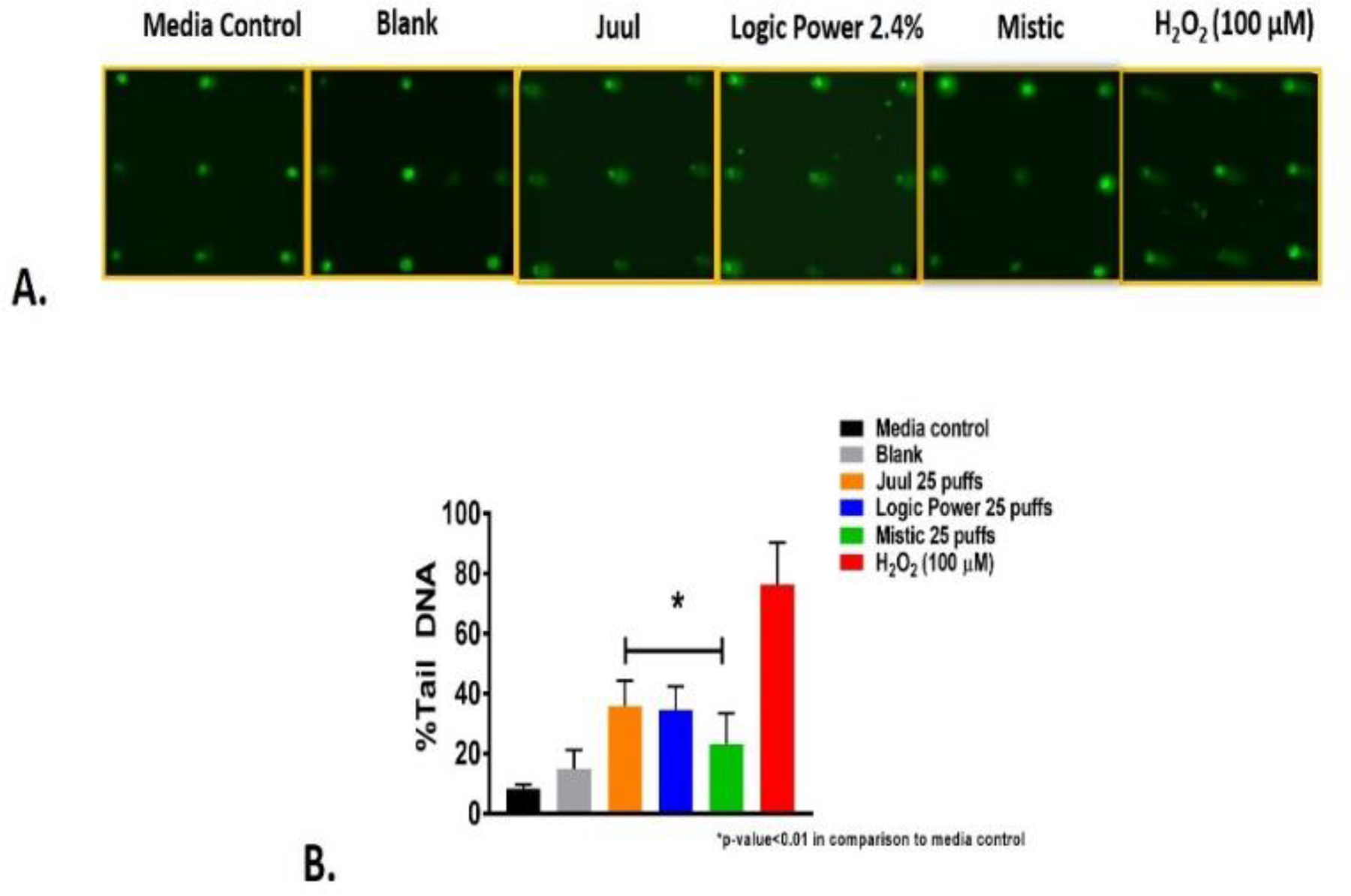
A.) shows images of CometChip assessment of NHBE cells exposed to ENDS aerosols. B.) Quantitative assessment of CometChip images in Panel A.
Discussion
Numerous studies have reported that e-liquids and aerosols vary across different manufacturers, devices, cartridges, and even puff to puff, which when combined with stratification in ENDS user behavior may contribute to differences in ENDS aerosol exposures (DeVito and Krishnan-Sarin 2018). Reports on the toxicological properties of ENDS aerosols have also been somewhat inconsistent due to differences in experimental design and biological parameters (Ferrari et al. 2015; Flouris et al. 2013). The majority of these studies focused on older, less-popular, off-brand or research based ENDS with no data on the potential market for the products. This leaves a major knowledge gap regarding potential health effects from products with significant market presence and sales among current ENDS users. At the end of 2018, the Juul brand held the largest market share of 29% and a 15 billion dollar valuation in comparison to all other ENDS devices (King 2018). To date, only a few studies have examined the toxicological effects of Juul aerosols and none have compared these effects to other aerosols produced by comparably less popular ENDS. This is needed to assess human health risks associated with each brand. Within this study, we compared the toxicological profiles of three popular ENDS devices (with varying levels of nicotine and one popular flavoring) consisting of Juul Fruit Medley (5% nicotine), Logic Power (2.4% nicotine), and Mistic (1.8% nicotine) to determine relative toxicity of each.
Although ENDS are promoted as a smoking cessation tool due to their presumed safer composition, ENDS aerosols are not devoid of toxic substances (Gaur and Agnihotri 2019). Trace metal ions including nickel, chromium, tin, aluminum, and lead have been detected in END aerosols (Halstead 2019). Likewise, our ICP-MS analysis indicated that Mistic and Logic aerosols had significantly higher total metal ions than the Juul (Halstead 2019). Specifically, the Mistic and Logic had 488 nanograms per 10 puffs and 360 nanograms per 10 puffs, respectively, of copper in comparison to the Juul, which had less than 10 nanograms per 10 puffs. Zinc, nickel, tin, and lead were also found in higher amounts in the Mistic in comparison to the Logic Power and Juul. The presence of any of these metals has been shown to offset and even inhibit crucial biological processes in cellular systems (Halstead 2019). For example, inhaling nickel, chromium, and lead have been found to cause adverse nervous and respiratory system effects (Williams et al. 2013). Additionally, many of these ENDS aerosols have seemingly infinite combinations of metals and other chemicals, which may cause variations in toxicity (Williams et al. 2017; Williams et al. 2019).
The production of reactive oxygen species and subsequent overwhelming cellular antioxidant defenses is a well-recognized paradigm of toxicity (Bhattacharya 2015). We observed the Logic and Mistic aerosols, which had higher total metal concentrations than Juul, caused greater increases in reactive oxygen species production. Similar findings were found due to ENDS aerosol exposure in vascular endothelial cells where induction of reactive oxygen species were shown to contribute to observed cytotoxicity (Anderson et al. 2016). Cellular oxidative stress as measured by depletion of glutathione in ENDS aerosol exposed NHBE cells to highest concentrations of aerosols was similar for all three devices. These data are in line with previous in vitro and human outcomes in which ENDS aerosols were found to decrease antioxidant capacity and increase oxidative stress (Chatterjee et al. 2019). Oxidative stress over time can result in damage to biomolecules such as DNA (Pizzino et al. 2017). Interestingly, exposures of the cells to aerosol from Logic and Juul devices resulted in similar high levels of single strand DNA breakage as measured by the CometChip assay, although metal concentrations were lower in aerosol from Juul. Juul and Logic had the highest amounts of nicotine, however, other studies have shown that ENDS aerosols are genotoxic independent of nicotine levels (Yu et al. 2016).
In summary, exposure of human bronchial epithelial cells to higher metal concentrations in ENDS aerosol may contribute to greater increases in production of reactive oxygen species, but other factors may be involved in causing glutathione depletion and single strand DNA breakage (Gaur and Agnihotri 2019). Possible additional contributors to the toxicities induced by exposure to aerosols could be the pH of the ENDS liquids from which the aerosols are produced, nicotine levels, volatile organic compounds and flavor additives. Most recently, Omaiye and coworkers reported the toxicological effects of Juul fluids and aerosols in Cool Mint and Crème Brulee flavorings (Omaiye et al. 2019). The authors reported moderate to weak correlations between total flavor chemical concentration and menthol concentrations. It was concluded that certain flavor chemicals such as ethyl maltol may have contributed to the observed adverse cellular effects found in e-liquid and ENDS aerosol exposed BEAS-2B lung epithelial cells. Since Juul had the highest nicotine levels, it is unlikely that nicotine was a principal contributor to glutathione depletion or DNA single strand breakage. The pH of liquids used in the devices were 8.20 ± 0.05 (Logic), 4.93 ± 0.05 (Mistic), and 5.67 ± 0.05 (Juul). Since the pH from the liquids used in the Logic and Mistic devices bracket the pH of the liquid in Juul pods, there is no solid evidence that pH alone was a major contributor to DNA single strand breakage or depletion of glutathione. This study has not ruled out flavor additives as contributors to depletion of glutathione or genotoxicity. It is likely that a combination of interacting factors contribute to cellular responses following ENDS aerosol exposures, thereby increasing the complexity of studies examining toxicity.
The data demonstrates proof-of-concept for method validity. Toxicity data for three ENDS aerosols, Juul, Logic Power, and Mistic are presented and statistical analysis was conducted. Aerosols from each of the three ENDS evaluated possessed significant biological activity and toxicity. Exposure of cells to aerosol from Juul resulted in the greatest cytotoxicity and genotoxicity of the three ENDS devices evaluated with these assays, conveying a 43% reduction in metabolic activity and 35.8% tail DNA or DNA damage at the highest dose of 25 puffs. Exposure of the cells to aerosol from Mistic resulted in reduction of cellular viability of 29%. However, the amount of single stranded DNA breaks elicited by Mistic aerosol exposures were not significantly different than background levels found in control cells. The Logic Power did not cause overt dose-dependent toxicity (20% reduction for low and mid doses) but did elicit high levels of reactive oxygen species and DNA damage (34.5 percent tail DNA) along with significantly altered glutathione levels. The dose-dependent responses of cells exposed to aerosol from Juul and Mistic suggests that lower doses are toxic as well and that ENDS users who may not frequently use their devices may also be at risk for potential adverse respiratory effects. While 25 puffs may be less than the typical amount per day for users, 25 puffs may be considered a high dose when considering the surface area of a 96-well plate compared to the human lung. Additionally, every puff in a human would be distributed across the respiratory system but the bronchi would be exposed to a higher concentration. More work is needed to evaluate which constituents and their respective concentrations are driving the damage, especially since this is a snapshot at a single time point and there may be toxicity present at various time points.
Funding
Funding in part was obtained as an award from the U.S. Centers for Disease Control and Prevention Innovation Fund.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The use of product or brand names in this manuscript do not represent an endorsement of any product by the authors or by the Centers for Disease Control and Prevention.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Anderson C, Majeste A, Hanus J, Wang S. 2016. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicological sciences : an official journal of the Society of Toxicology. 154(2):332–340. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S 2015. Reactive oxygen species and cellular defense system In: Yadav V, editor. Free radicals in human health and disease. Springer, New Delhi: p. 17–29. [Google Scholar]
- Chatterjee S, Tao J-Q, Johncola A, Guo W, Caporale A, Langham MC, Wehrli FW. 2019. Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. American Journal of Physiology-Lung Cellular and Molecular Physiology. 317(2):L155–L166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, & King BA 2018. Notes from the field: Use of electronic cigarettes and any tobacco product among middle and high school students — united states, 2011–2018. . MMWR Morb Mortal Wkly Rep. (67):1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Krishnan-Sarin S. 2018. E-cigarettes: Impact of e-liquid components and device characteristics on nicotine exposure. Current neuropharmacology. 16(4):438–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F, Bullen C. 2011. Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction. 106(11):2017–2028. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Zanasi A, Nardi E, Labate AMM, Ceriana P, Balestrino A, Pisani L, Corcione N, Nava S. 2015. Short-term effects of a nicotine-free e-cigarette compared to a traditional cigarette in smokers and non-smokers. BMC pulmonary medicine. 15(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsakis AM, Koutedakis Y. 2013. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation toxicology. 25(2):91–101. [DOI] [PubMed] [Google Scholar]
- Gaur S, Agnihotri R. 2019. Health effects of trace metals in electronic cigarette aerosols—a systematic review. Biological Trace Element Research. 188(2):295–315. [DOI] [PubMed] [Google Scholar]
- Geiss O, Bianchi I, Barahona F, Barrero-Moreno J. 2015. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. International Journal of Hygiene and Environmental Health. 218(1):169–180. [DOI] [PubMed] [Google Scholar]
- Halstead M, Gray N, Gonzalez-Jimenez N, Fresquez M, Valentin-Blasini L, Watson C, & Pappas RS 2019. Analysis of toxic metals in electronic cigarette aerosols using a novel trap design. . J Anal Toxicol. (44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Yip H, Talbot P. 2013. Mining data on usage of electronic nicotine delivery systems (ends) from youtube videos. Tobacco control. 22(2):103–106. [DOI] [PubMed] [Google Scholar]
- Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, Kouretas D, Liesivuori J, Alegakis AK, Vynias D, Tsatsakis AM. 2015. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. Journal of Analytical Toxicology. 39(4):262–269. [DOI] [PubMed] [Google Scholar]
- King BA, Gammon DG, Marynak KL, & Rogers T 2018. Electronic cigarette sales in the united states, 2013–2017. . Journal of the American Medical Association. (320):1379–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-S, LeBouf RF, Son Y-S, Koutrakis P, Christiani DC. 2017. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environmental Health. 16(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. 2015. Chemical composition and evaluation of nicotine, tobacco alkaloids, ph and selected flavors in e-cigarette cartridges and refill solutions. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 17(10):1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. 2016. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res. 18(9):1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye EE, McWhirter KJ, Luo W, Pankow JF, Talbot P. 2019. High-nicotine electronic cigarette products: Toxicity of juul fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chemical research in toxicology. 32(6):1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo DL. 2013. Electronic cigarettes and vaping: A new challenge in clinical medicine and public health. A literature review. Frontiers in public health. 1:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo DL, Crow AP, Nelson JM, Johnson RA. 2016. Trace metals derived from electronic cigarette (ecig) generated aerosol: Potential problem of ecig devices that contain nickel. Frontiers in physiology. 7:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. 2017. Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev. 2017:8416763–8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. 2015. Electronic cigarette topography in the natural environment. PLOS ONE. 10(6):e0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgman A, Perfetti TA. 2016. The chemical components of tobacco and tobacco smoke. CRC press. [Google Scholar]
- Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, Shusterman D, Mendell M, Kumagai K. 2017. A device-independent evaluation of carbonyl emissions from heated electronic cigarette solvents. PLOS ONE. 12(1):e0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Bozhilov K, Ghai S, Talbot P. 2017. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLOS ONE. 12(4):e0175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Li J, Talbot P. 2019. Effects of model, method of collection, and topography on chemical elements and metals in the aerosol of tank-style electronic cigarettes. Scientific Reports. 9(1):13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. 2013. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLOS ONE. 8(3):e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Crotty Alexander LE et al. 2016. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncology. 52:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pyrgiotakis G, Demokritou P. 2016. Development and characterization of electronic-cigarette exposure generation system (ecig-egs) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhalation toxicology. 28(14):658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchet A, Schmaltz G. 2017. Electronic cigarettes—a review of the physiological health effects. FACETS. 2(1):575–609. [Google Scholar]


