Abstract
The World Health Organization (WHO) has declared the COVID-19 an international health emergency due to the severity of infection progression, which became more severe due to its continuous spread globally and the unavailability of appropriate therapy and diagnostics systems. Thus, there is a need for efficient devices to detect SARS-CoV-2 infection at an early stage. Nowadays, the reverse transcription polymerase chain reaction (RT-PCR) technique is being applied for detecting this virus around the globe; however, factors such as stringent expertise, long diagnostic times, invasive and painful screening, and high costs have restricted the use of RT-PCR methods for rapid diagnostics. Therefore, the development of cost-effective, portable, sensitive, prompt and selective sensing systems to detect SARS-CoV-2 in biofluids at fM/pM/nM concentrations would be a breakthrough in diagnostics. Immunosensors that show increased specificity and sensitivity are considerably fast and do not imply costly reagents or instruments, reducing the cost for COVID-19 detection. The current developments in immunosensors perhaps signify the most significant opportunity for a rapid assay to detect COVID-19, without the need of highly skilled professionals and specialized tools to interpret results. Artificial intelligence (AI) and the Internet of Medical Things (IoMT) can also be equipped with this immunosensing approach to investigate useful networking through database management, sharing, and analytics to prevent and manage COVID-19. Herein, we represent the collective concepts of biomarker-based immunosensors along with AI and IoMT as smart sensing strategies with bioinformatics approach to monitor non-invasive early stage SARS-CoV-2 development, with fast point-of-care (POC) diagnostics as the crucial goal. This approach should be implemented quickly and verified practicality for clinical samples before being set in the present times for mass-diagnostic research.
Keywords: Diagnostics, Electrochemical biosensors, Pandemic, Point-of-care, SARS-CoV-2
1. Introduction
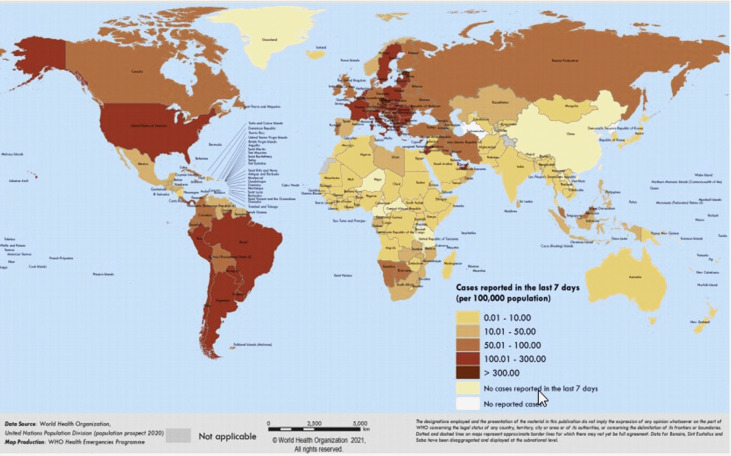
The emergency initiated via viral disease denotes a significant risk to community health globally. Various viral epidemics have arrived over the past several eras with growing intensity, like H1N1 influenza, SARS-CoV, Ebola virus, and, quite lately, the MERS-CoV as well [1]. COVID-19 is a current worldwide outbreak that has developed massive chaos of diverse activities across the globe. The WHO has announced a communal health calamity in the world on 30th January 2020 [2], because of the emergence of the latest CoV, known as the 2019 novel coronavirus (2019-nCoV), that arose in the Wuhan city in China's Hubei province [3,4]. On 11th February 2020, the WHO legitimately termed the outbreak as CoV 2019 disease (COVID-19) [5]. The US Center for Disease Control and Prevention (CDC) and WHO have also verified [6] human-to-human transmission with approval by three separate cases outside of China, particularly in the United States [7], Vietnam [8], and Germany [9]. COVID-19 is continuously expanding its roots in more than 213 countries [Fig. 1 ], with 113,472,187 confirmed infection cases and confirmed the death of 2,520,653 persons on 2nd March 2021 [10]. For controlling the spread of this disease, around 50 million people were blocked in China [11]. Italy also adopted the same measures on 8th March 2020, locking its northern portion, thereby impacting its 16 million citizens. The reproductive number represented by the symbol R0 (i.e. the approximate number of secondary complications produced by a normal infected person) and doubling time is expected to be 2.68 and 6.4 days, respectively [12].
Fig. 1.
This map shows the global distribution of confirmed cases of COVID-19. (This was reproduced from situation reports of the WHO coronavirus disease [4]. Copyright 2020 WHO.)
The struggles of monitoring and controlling COVID-19 infections worldwide are still unsatisfactory and restricted due to a deficiency of responsibility, organization, unavailability of diagnostic equipment, vaccines, and drugs to prevent the infection. The US government has asked the health organizations to spread awareness, understand and develop approaches to cope with the SARS-CoV-2 viral infection in the coming times. These health organizations encourage aiming at the fast-spreading of understanding of human-to-human transmission control programs, diagnostic development, and vaccine research. Many worldwide reports have suggested that sustained and committed public support, well-administrated infrastructure, global economic support, funding, and public–private partnerships are immediately needed for exploring economic medicines and diagnostic equipment. Particularly, studying the structure of SARS-CoV-2 has become crucial for developing specific human and animal models to understand the pathogenesis and symptoms of the virus, to design novel drugs, vaccines, and diagnostic kits to fight SARS-CoV-2 infection. Recently, a few animals and human models were proposed to study SARS-CoV-2 pathogenesis for facilitating the therapeutic and vaccine discovery. Until then, to prevent infection with SARS-CoV-2, the only options that a person can take are preventative measures such as stay at home, maintain hygiene, use a mask, keep social distance, perform yoga, maintain good immunity, etc.
The cheap cost, early diagnosis, and reduction of the chance of its transmission so that health workers do not get affected during the analysis are the primary concern linked with this virus to control and study SARS-coV-2 infection. Presently, the RT-PCR technique has been widely applied for testing of SARS-CoV-2. However, this method for the detection of SARS-CoV-2 is laborious, time-consuming, expensive, and requires a specialized facility and trained staff. Also, the SoA diagnostics time to approve SARS-CoV-2 infection fluctuates from 6 h to nearly 3 days, causing an interruption in therapeutic methods. Furthermore, some of the researchers have developed biosensors for the detection of COVID-19 in recent times through various ways, but most of them are invasive and include the exposure to particles of the virus. To overcome the issues as mentioned above of invasive diagnostic techniques and time-consuming PCR-based methods, researchers around the globe are making substantial efforts to fabricate different devices and approaches for an affordable, rapid, highly sensitive, easy, and selective detection of SARS-CoV-2 in low-resource settings (such as directly at home or doctors’ practices). For this purpose, a powerful and promising technique is the use of nano-enabled biomarker-based immunosensors that can play a pivotal role in diagnosing COVID-19. The qualities such as disposable, cheap cost, miniaturization, and multiplexing with the capability to construct flexible devices have made immunosensors very fascinating and suitable for various applications. As most electrical and electrochemical sensors are label-free sensing devices, their scope for detection of SARS-CoV-2 is large and extensively considerable as powerful and potential replacements over molecular SARS-CoV-2 detection methods. Thus, this report highlights and reiterates the need for SoA diagnostic techniques and also a call to fabricate sensitive, selective, and rapid POC detection system for SARS-CoV-2 at an early stage for global health care. We propose that adopting miniaturized non-invasive nano-enabled biomarker-based SARS-CoV-2 immunosensing technique can detect femto/pico/nanomolar (fM/pM/nM) level of virus concentration within a time frame of ~40 min in comparison to 3 days required for RT-PCR-based test or ELISA at nM levels. Smart health care focused on artificial intelligence (AI) and the Internet of Medical Things (IoMT) is gaining popularity in dealing with COVID-19 pandemic in the era of advanced digital technology. Therefore the innovative approach of AI and IoMT to handle and address COVID-19 in terms of assessment and benchmarking is also discussed in this study. This idea of AI and IoMT is ideally suited to this pandemic because it is important to link and track every individual across a vast network requiring effective spectrum management. Further, this review aims to provide a brief overview of SARS-CoV-2, outline the current and emerging detection methods, and reviewed immunosensors platforms used to detect other viruses along with the potential of immunosensors for the rapid detection and diagnosis of COVID-19. Finally, this pandemic has allowed all the health sector stakeholders and multiple government agencies to join and develop rapid diagnostic tests against infectious disease globally.
2. SARS-CoV-2: physical characteristics, structure, and genomics
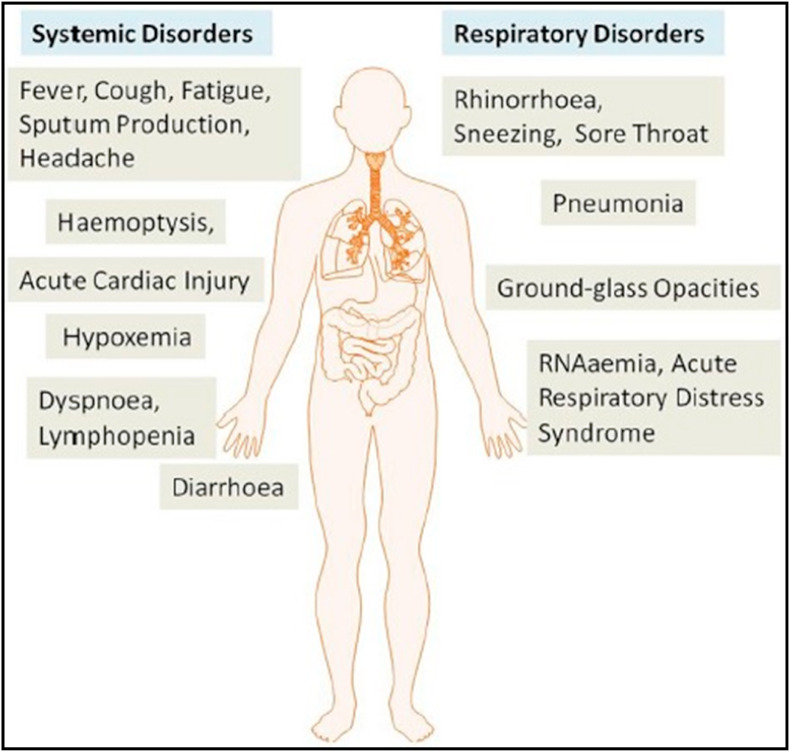
COVID-19 disease is highly infectious, as it spreads via air droplets and human-to-human touch [13]. The viruses accountable for SARS, MERS, and COVID-19 occurrence are, most of the time, spherical and pleomorphic sometimes [14]. These viruses have an average diameter of about 125 nM, as the glycoprotein spikes over the capsid's surface, and their capsid, which makes up these viruses, is around 20 nM and 85 nM, respectively [15]. CoV symptoms show similarity with influenza and appeared after 5.2 days of an incubation period [16]. The duration of the arrival of COVID-19 symptoms to death fluctuates from 6 to 41 days, with an average of 14 days [17]. The common symptoms found between previous β-CoVs and COVID-19 are fatigue, cough, and fever, along with headache, dyspnea, sputum generation, lymphopenia, diarrhea, and hemoptysis [17,18]. Though, COVID-19 exhibited distinct clinical traits that involve the lower airway infection, as evident from the symptoms from the upper respiratory tract such as sore throat, diarrhea, sneezing, and rhinorrhea, as shown in Fig. 2 . People infected with the SARS-CoV-2 virus indicate large leukocyte numbers, vast plasma pro-inflammatory cytokines, and irregular respiratory findings. One of the case reports of COVID-19 patient showed coarse breathing sounds of both lungs, cough, and body temperature of 39 °C on the 5th day of fever. The noteworthy pathogenesis as a respiratory system targeting virus of COVID-19 infection was RNAaemia, acute cardiac injury, severe pneumonia, together with the incidence of ground-glass opacities that lead to death [19]. Also, a considerable amount of high blood levels of chemokines and cytokines were found in patients with COVID-19 infection, including IL1RA, IL7, IL10, IL9, IL8, IL1-β, VEGFA GCSF, basic FGF2, IFNγ MIP1α, MCP1, TNFα, IP10, PDGFB, MIP1β, and GMCSF [20]. Hence, to recognize the numerous modes of transmission from urine and fecal samples, fabrication of suitable methods is instantly required to develop therapeutics to minimize and inhibit the spread of the disease.
Fig. 2.
The systemic and respiratory disorders caused by the SARS-CoV-2. The incubation period of COVID-19 infection is approximately 5.2 days. COVID-19 showed some unique clinical features that include the targeting of the lower airway, as evidenced by upper respiratory tract symptoms like rhinorrhea, sneezing, and sore throat. Additionally, patients infected with COVID-19 developed intestinal symptoms like diarrhea (adapted with permission from Ref. [19], Copyright 2020 Elsevier).
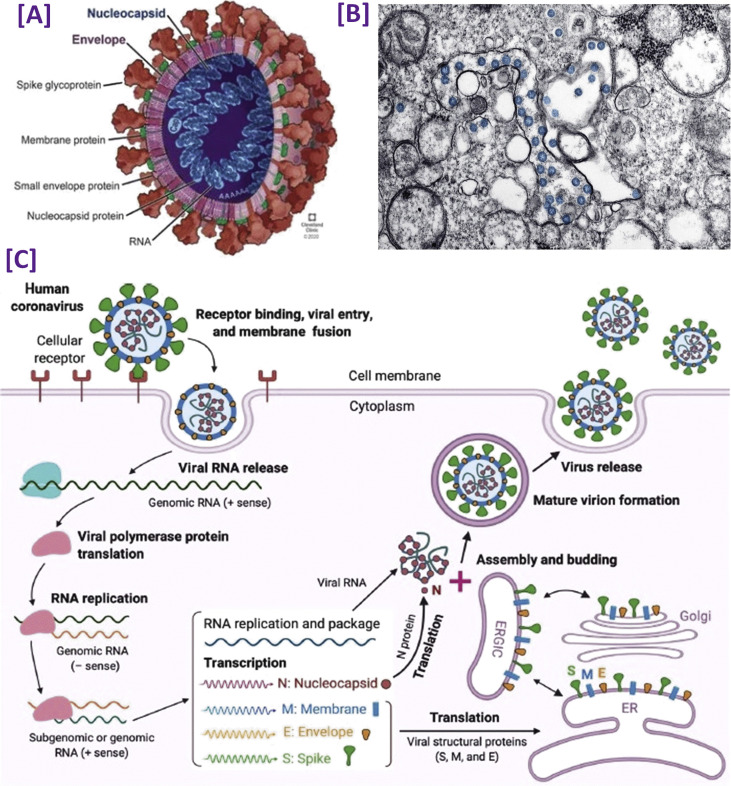
As the detection of COVID-19 by various methods is based on the structure and genomics of the novel SARS-CoV-2 in the present and upcoming times, it is relevant to review the new aspects of its progress. Due to the phylogenetic connection and similarity in the genome sequence, this novel species of CoV, SARS-CoV-2, has been placed under the β CoV genus of the CoV family. It has a positive sense of large 30 kb, single-stranded RNA (ssRNA) genome, which is spherical [18,21]. As the genetic material of this virus is RNA, its two-third part consists of non-structural polyproteins (ORF1a-ORF1b), i.e. RNA synthesis materials, and encodes viral polymerase RNA dependent RNA polymerase (RdRp). The rest part contains the four accessory and structural proteins, i.e. N (nucleocapsid protein), M (membrane protein), S (spike protein), and E (envelope protein) [22]. Fig. 3 (A) [23] represents a schematic diagram of the structure of the SARS-CoV-2 virus, and Fig. 3(B) [24] shows the TEM images of the SARS-CoV-2 virus. All four proteins are not only involved in virus structure, but they also play a crucial role during the replication cycle [25].
Fig. 3.
[A]. A schematic structural view of human SARS-CoV-2 with its surface protein (adapted with permission from Ref. [22], Copyright 2020 Cleveland Clinic [B]. Visualization of SARS-CoV-2 with transmission electron microscopy. The virus is shown in blue color (adapted with permission from Ref. [23], Copyright 2020 American chemical society) [C]. The life cycle of highly pathogenic human SARS-CoV-2. These CoVs enter host cells by first binding to their respective cellular receptors [angiotensin-converting enzyme 2(ACE2) for the severe acute respiratory syndrome (SARS)-CoV-2 or SARS-CoV and dipeptidyl peptidase 4 (DPP4) for the Middle East respiratory syndrome (MERS)-CoV] on the membranes of host cells expressing ACE2 (e.g., pneumocytes, enterocytes) or DPP4 (e.g. liver or lung cells including Huh-7, MRC-5, and Calu-3) via the surface spike (S) protein, which mediates virus–cell membrane fusion and viral entry. Viral genomic RNA is released and translated into viral polymerase proteins. The negative (−) sense genomic RNA is synthesized and used as a template to form subgenomic or genomic positive (+) sense RNA. Viral RNA and nucleocapsid (N) structural protein is replicated, transcribed, or synthesized in the cytoplasm. By contrast, other viral structural proteins, including S, membrane (M), and envelope (E), are transcribed, then translated in the endoplasmic reticulum (ER) and transported to the Golgi. The viral RNA–N complex and S, M, and E proteins are further assembled in the ER–Golgi intermediate compartment (ERGIC) to form a mature virion, then released from host cells (adapted with permission from Ref. [34], Copyright 2020 Cell Press).
E is the smallest and significant structural protein of CoV responsible for the composition and formation of the viral membrane. A small part of this protein is merged into the virion's envelope and is highly expressed during the host cell's replication cycle [26]. The primary purpose of the N protein is to attach the RNA molecule of CoV and to build the nucleo-capsid structure, which helps in protecting and enfolding the viral RNA [27]. In addition to its principal function in viral genome processes, it includes both the viral replication cycle and the host cell response to viral infection [28]. M is a 25–30 kDa structural protein that helps to define the shape of the viral envelope and has a role as a central organizer in the assembly of CoV by interacting with several other structural proteins [29]. The entrance of SARS-CoV-2 into host cells is made possible through the binding of S glycoprotein to the receptor of the host cell, as this protein helps in the merging of host cell and viral membranes to facilitate the easier entry of the virus into the host cell [26,30]. Also, this protein attaches with the transmembrane receptor ACE2 (the angiotensin-converting enzyme 2) specifically, which is highly expressed in the heart, gastrointestinal tissues, kidneys, and lungs [31]. The virus's binding with the host cell and further entrance into the cell is carried by a protease enzyme known as TMPRSS2. After SARS-CoV-2 entry into the host cells, the viral RNA converts into the proteins quickly together with RNA synthesis via the replication of the virus in the cytoplasm [14]. The variations in amino acid sequences are part of direct correlations between both the spike protein and the receptor of the host cell [32]. The complete life cycle of the SARS-CoV-2 virus is shown in Fig. 3(C) [[33], [34], [35]].
Furthermore, RdRp is considered to be an essential conserved sequence. As per the International Committee on Taxonomy of Viruses (ICTV) criteria, if a species displays >90% resemblance for a conserved RdRp sequence, it would be reflected as new species. As the RdRp sequence of virus collected from Wuhan reveals 86% similarity with the existing SL-CoVZC45 viral species, SARS-CoV-2 was declared a novel species. The genome sequence analysis shows 88% resemblance of SARS-CoV-2 with SARS-like bat-derived CoV, SL-CoVZXC21, and SL-CoVZC45. The entire sequencing of the virus genome taken from various spheres displays more than 99% of sequence homology with novel SARS-CoV-2 (from Wuhan, China) [36]. This virus differs from SARS-CoV only by some amino acid residues present in the receptor-binding domain, according to the homology modeling [37]. Therefore, knowledge of the structure and genomics of SARS-CoV-2 is essential to know the basics and for fabricating more sensitive, useful, and accurate detection devices.
2.1. Conventional techniques in diagnosis and detection of coronaviruses
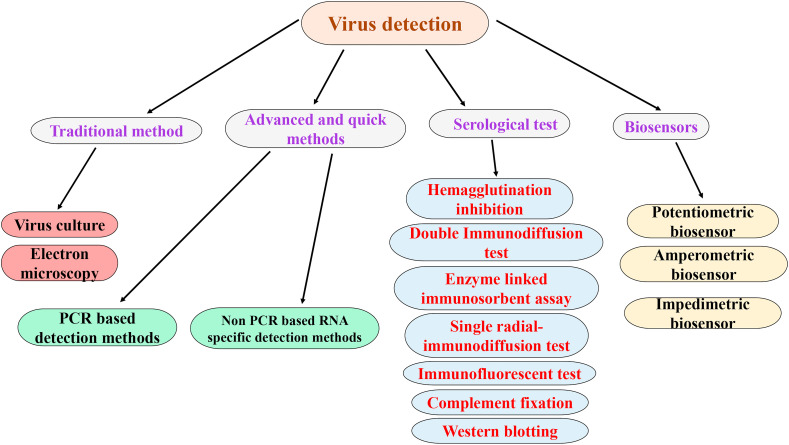
There lies a great challenge and threat for the diagnosis due to the rampant spread of SARS-CoV-2, as it shows similarity in the symptoms with that of previously occurred viruses such as adenoviruses, rhinoviruses, respiratory syncytial virus, parainfluenza, and influenza. The facilities of flights around the globe and other means of transportation, i.e. global civilization, have generated suitable circumstances for quick transmission of this pandemic. As the transmission of virus takes place within a time frame of 24 h, sample collection from the tracheal–throat and nasal swabs within 2–5 days are done and shifted in transport media for keeping them alive, so it reaches safely to particular laboratories for rapid detection [38]. Some of the conventional techniques that are already in use for detecting viruses are electron microscope (EM), serological methods, virus cultivation in cell cultures, and enzyme-linked immunosorbent assay (ELISA), as shown in Fig. 4 .
Fig. 4.
A generalized representation of all different areas of conventional detection methods for viruses.
2.1.1. Electron microscopy
EM in developed countries has been regarded as an efficient technique for determining the virus directly via visual counting of viral particles in stools, body fluids, or histopathologic samples. This technique needs a certain quantity of viral particles (~106 particles per mL) to identify the morphological characters specific to a particular family of viruses. However, there is a prior need to prepare the specimen [39], which makes analysis difficult, as it may decrease the virus's concentration. Moreover, it is time-consuming, expensive, challenging to locate the minute virus particles under a microscope and requires technical experts and assistance. Nevertheless, EM played an essential role in the initial discovery that CoV were the causative agents of SARS [40] and COVID-19 [41].
2.1.2. Virus cultivation in cell culture-based methods
Viral infection stimulates the body's immune system, which continues to produce antibodies, and the concentration of antibodies is found to increase between the 8th and 14th days of viral infection [42]. Virus cultivation in cell culture is another detection method for a virus, i.e. the standard gold method showing behavioral and morphological changes. This test is analyzed after 7–10 days of infection for the cytopathic effect to form cell lines and hem adsorption screening followed by immunofluorescent confirmation having 86–94% sensitivity and 100% specificity [43]. Successful plaque assays for HCOV-NL63 have been reported recently [44] and were also reported earlier for SARS-CoV [45] and MERS-CoV. These assays were considered the benchmark for identifying MERS-CoV during the MERS outbreak in mid-2010 [46]. Although these assays were challenging to conduct, long procedures, i.e. 5–7 days to obtain results and laborious, which limit its use [47].
On the other hand, some laboratories have substituted traditional culture with the shell-vial culture, which gave higher sensitivity and faster results than conventional culture methods [48]. But, this method is not valid for viruses that did not have cytopathic effect, as many days are invested in detecting the type of pathogen, thereby lacking timely bound detection. Culture-based approaches have revealed a significant impact in the field of infectious virus diagnosis that is still practiced in many hospital laboratories.
2.1.3. Immunological-based methods
In the history of clinical virology, complement fixation test (CFT) is one of the ancient methods, which was simple, convenient, and requires low-cost material [49]. This technique determines the complement-fixing antibodies found in the serum of a patient [50,51] and has the advantage of checking the number of pathogens at a particular time along with a description of a specific group. However, its demerit lies in recognizing different strains of the same kind of virus [52,53] and needs high cost, particular reagents, lacks sensitivity, time-consuming, and labor-intensive processes.
Another easy and simple method used to confirm infectious viruses such as parainfluenza virus and influenza arboviruses subtypes is the hemagglutination inhibition (HI) test. This test is also used to check the antigenic type of virus and give quantitative amounts of virus particles relatively [54,55]. Yet, this approach is demanding in terms of quality control, has very low reproducibility, and sometimes, cross-reacts with other viruses.
The single radial-immunodiffusion test is another approach for checking the hemagglutinin content in test antigens and the antigenicity of the virus hemagglutinin [56,57]. It also offers additional control for the antigenic structure of the vaccine [58]. It shows sensitivity toward antigenic composition and is useful for active antigen detection [59] along with simple and good reproducibility. In this process, specific antibody separation occurs from serum against viruses, and in agarose wells, antigens are poured. Determining the unknown concentration of virus titer or antigen is done through many known concentrations of different antigens in agarose solution. On the contrary, a double immunodiffusion (DID) test is another method for virus typing using a 1–1.5% agarose gel solution [60]. Although, this method is highly sensitive, low viral titer and prior virus cultivation for typing restrict its commercial use.
Furthermore, since the 1990s, ELISA tests [61] are performed with high specificity and sensitivity. They are obtainable in two procedures: microtiter plates and paper strips. Traditionally, the influenza virus's detection was determined via specific interactions between antigen and antibody and immunocomplex–enzyme linkage, which caused a change in color [62]. Despite the wide acceptance of this test, low sensitivity limits its use compared to nucleic acid-based techniques. However, some researchers attempt to enhance the sensitivity by utilizing europium and gold nanoparticles showing progressive results. For instance, detection of 29 strains of virus influenza A and some B virus subtypes is achieved through the europium nanoparticle-based immunoassay (ENIA), with 16 times more sensitivity than ELISA assay, which is available commercially [63].
Western blotting is a related technique that relies on antibodies' specific interaction with target antigens present in a sample mixture. Upon separation of the sample proteins on an electrophoretic gel, they are then transferred to a membrane where primary and secondary antibodies bind and visualize the target protein [64]. Western blotting is generally slower than ELISA and hence not used as often in routine clinical testing. However, a Western blot method has been reported for the detection of SARS-COV [65].
The various conventional techniques for detecting viruses are more or less very time-consuming; for instance, the cell culture test takes about 4–7 days. Although ELISA is a rapid and straightforward technique utilized for serum antibody analysis, it cannot be used to detect virus practically or in diagnostics. On the other hand, immunoassays can be used directly to detect viruses from tissue specimens, but the requirement of labels, cross-reactions in some cases, and low sensitivity limit its use. Therefore, to overcome such economic and social costs, the need for the fabrication of rapid detection methods is essential, and biosensors have shown an abundant potential to fulfill such requirements. Herein, we propose a potential protocol based on biomarker-based electrochemical biosensors for COVID-19 detection, collected from recently published articles for other virus detection.
3. Current detection method for COVID-19 and the need for portable and rapid detection systems
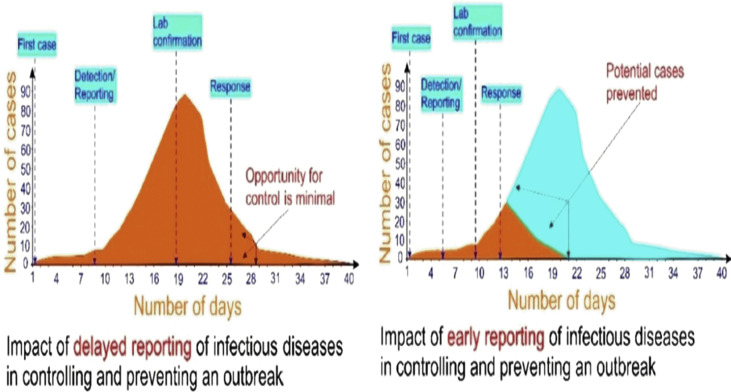
Several features of the influence of SARS-CoV-2 infection on communal health have been analyzed and studied worldwide [20,66], describing the details of its emergence, epidemiology, and discovery along with its diagnostics. The appearance of COVID-19 infection is not clearly defined, which includes fever, viral pneumonia, dyspnea, cough, and respiratory symptoms [19]. Thus, specific diagnostic tests for this disease are immediately required for confirming the infection in screened suspected patients and conduct virus surveillance. In this regard, a POC device (i.e. a robust, rapid, and economical system that can be utilized not only for the field but for on-site purposes without requiring the skilled technician for operating it) is vital and instantly necessary for detecting the COVID-19 at an early stage [67]. Fig. 5 shows the dramatic influence in the prior detection of COVID-19 disease to control an outbreak [[68], [69], [70], [71]].
Fig. 5.
The effect of rapid detection of infectious diseases in preventing and controlling an outbreak (adapted with permission from Ref. [70], Copyright 2020 Elsevier).
3.1. Molecular methods
PCR is a technique that utilizes DNA polymerase enzymes and a primer sequence for producing several duplicates (amplification) of series of genetic sequences or a gene to increase the quantity of required DNA exponentially. They are extensively employed for amplifying the trace amount of DNA in the samples to provide a sufficient number of viral DNA for laboratory diagnosis. Because of their high sequence specificity, sensitivity, and simplicity, these methods are reliably and routinely be applied to detect CoV infection in patients suffering from it [[72], [73], [74]]. In principle, such tests are employed to convert CoV RNA into complementary DNA via reverse transcription. Moreover, PCR is executed for amplifying the DNA, which is further subjected to analytical methods or specific detection such as sequencing or electrophoresis [72,75,76].
The most common and available PCR technique that is being utilized for detecting COVID-19 is the standard molecular method: real-time reverse transcription-polymerase chain reaction (rRT-PCR), which is based on detection of nucleic acid in biofluids comprising cerebrospinal fluid saliva, serum, and urine. rRT-PCR is considerably more sensitive than conventional approaches as it has been used for almost 10 years to detect various viruses. Additionally, it has better specificity (nearly 100%) and sensitivity compared to RAD techniques and viral culture [[77], [78], [79]]. Since 17th January 2020, the WHO has given the available and documented protocol for testing on its website, including [80].
-
(i)
Collection of the specimen;
-
(ii)
Packing, i.e. storage and shipping of the clinical samples;
-
(iii)
Excellent communication with the laboratory person providing valuable data;
-
(iv)
Testing in the laboratory;
-
(v)
Evaluation of reported results.
In the present times of challenges, this analysis method needs costly, sophisticated laboratory equipment, and various specialists along with highly expert analysts working at a central laboratory (Biosafety Level 2 or above) [7,80,81]. Transportation of samples is a cumbersome job; therefore, sample reports are obtained in 2–3 days. At this time of public health emergency where the COVID-19 outbreak has become pandemic all over the world, such delay in the testing procedure is problematic and dangerous for the person handling the sample as the virus is contagious.
Moreover, owing to the dependence on technical experts and costly reasons, commercial PCR-based techniques can only be applied to seriously ill patients and do not reflect acute disease every time due to the presence of viral DNA or RNA [[82], [83], [84]]. Also, its co-detection using PCR results in interference with other respiratory viruses of the CoVs family, and the results of positive CoV PCR causing disease severity are not revealed all the time. Besides, the recent PCR-based approach of investigation needs (predominantly while dealing with many infected patients) 4–8 h or more for processing. Along with the financial pressures, these requirements instigate one to send the samples hundreds of miles away for testing to provide necessary resources and facilities for executing the diagnostic analysis. This, in turn, can further increase the financial burden and the required time for performing the diagnostic analysis [85].
3.2. Computed tomography
Computed tomography, also known as CT scan, is used either in association or as a standalone treatment strategy to confirm inaccurate RT-PCR results for SARS-CoV-2 virus detection [86,87]. Throughout this process, a considerable number of chest X-ray measurements are typically gathered from various angles to generate a three-dimensional (3D) contrast image, which will then be evaluated by radiologists for aberrant functionalities to validate the existence of SARS-CoV-2 infection. Patterns of COVID-19 infection that occur in the CT scan usually involve areas of subpleural regions of ground glass opacification hampering the lower portion either of single or both lobes and has also shown consolidation of fluids in the lungs [88]. CT scan pictures of multiple patients on several days when different irregularities are fully evident, like focal ground-glass opacity along with peripheral and bilateral predominant consolidation after 20 days of onset of infection has been shown in Fig. 6 [1,89]. Although CT scans are willing to keep one of the most efficient strategies in COVID-19's early diagnosis, the critical challenge for radiologists is to differentiate symptoms from pneumonia or other disorders of the lung, which is not triggered by COVID-19 infections. Moreover, CT scanning is costly and requires specialized technical expertise for activity and evaluation, and it could only be suited for SARS-CoV-2 detection as a complementary technology with RT-PCR.
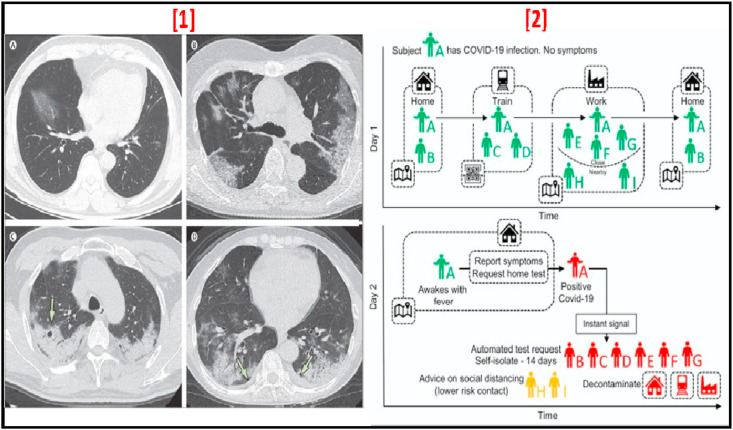
Fig. 6.
[1] Transverse thin-section CT scans in patients with COVID-19 disease (A) 56-year-old man, Day 3 after symptom onset, (B) 74-year-old woman, Day 10 after symptom onset, (C) 61-year-old woman, Day 20 after symptom onset (D) 63-year-old woman, Day 17 after symptom onset (adapted with permission from Ref. [88], Copyright 2020 The Lancet) [2]. Contact tracing application: using GPS contacts of Individual A and all individuals using the app, infections are traced. This is further supplemented by scanning QR codes displayed on high-traffic public amenities where GPS is too coarse. Using this application, Individual A requests a test for COVID-19 infection, and their positive test result is shared as an instant notification to individuals who have been in close contact (adapted with permission from Ref. [23], Copyright 2020 American chemical society).
3.3. Contact tracing
By using digital technologies, in particular, smartphone-based applications to assess the presence of both recovered and active infected cases have also been effectively applied to control the COVID-19 transmission [90]. A contact tracing application has been recently developed by Ferrite et al. [91], which is shown in Fig. 6 [2]. One of the benefits of such software-based systems is that this can be easily modified to be more accurate, such as quarantining areas if the infection spreads in that region gets unpredictable, quarantining individual families or flats, or tracing connections in the second/third degree if the number of cases rises along with the accessibility of technology with members of the community. One key drawback of contact tracing is that it is also vital to boost the number of diagnostic tests to unlock the maximum benefits of this technology. There is also another restriction, such as considering privacy, which is becoming more appropriate among the group members with changing times and circumstances [92]. Nevertheless, contact tracing usage is still beneficial for members of the community to monitor the movement of individuals, including asymptomatic ones, who came in contact with the infected person.
Therefore, through the evaluation of the above tests, it is necessary to develop smart diagnostic systems (i.e. POC test) for selective and sensitive detection of SARS-CoV-2 to identify infection at an early stage at low concentrations. The final objective for fabricating a POC test is to match with the ASSURED criteria that were set by the WHO: sensitive, robust, deliverable to those in need, user-friendly, affordable, equipment-free, and specific. Consequently, there is a need to develop new methodologies that can be sensitive along with practicability. In this regard, biomarker-based electrochemical biosensors could be outstanding substitute systems to overcome the disadvantages of the standard and conventional rapid techniques for COVID-19 detection. These approaches can provide the desired sensitive, cost-effective, fast, user-friendly, toward a portable, and selective response [93].
4. Diagnosis and biomarkers
Early and rapid diagnosis of diseases is continuously a significant concern for any nation. Now, the condition associated with COVID-19 is vast, as we do not have any method for fast and early virus detection. Numerous countries are trying hard to fabricate a quick kit based on antibodies or genes for detection. Some other techniques can solve this issue with a more adaptable approach and detect the virus quickly. In recent years, medical analysis has been significantly enhanced due to the advancements in new methods capable of quantifying specific components and molecules for performing the detection in the presence or absence of which gives data about the physiological state of a living being. One of these techniques is biomarker-based sensors, known as biosensors. Several reported biomarker-based biosensors can accurately detect diseases; for example, a non-invasive biosensor was developed by Kumar et al. for oral cancer detection using CYFRA-21, which is a specific biomarker for oral cancer and detection of antibiotics by Yadav et al., [94,95]. Moreover, immunosensors based on biomarkers was also fabricated for different virus's detection; such as Haji-Hashemi et al. and Freitas et al. prepared a label-free immunosensor for CTV (as viral antigen) detection [96,97]. Joshi et al. [98] show a thermally reduced graphene oxide (RGO) on the electrodes of indium tin oxide/glass for quantitative determination of influenza virus H1N1 as a tag-free electrochemical immunosensor. The electrochemical biosensor was shown by Navakul et al. [99] using graphene oxide-deposited gold electrodes to detect dengue virus particle. A sensitive and selective immunosensor was designed by Kaushik et al. for ZIKA virus detection using ZIKA virus protein as biomarker [100]; similar to these, particular biomarkers for COVID-19 can be used to develop biosensors for its detection.
Biomarkers can be described as parameters that can be objectively evaluated or measured to get data of either a pathologic/physiologic state or as a response to therapeutic intervention by a non-regular activity or situation of a living individual [101]. Biomarkers, for instance, serum amyloid A (SAA), are secreted in a more ample amount in COVID-19 patients than healthy individuals. Simultaneously, serum ferritin is also an important biomarker that shows the potential for COVID-19 detection, primarily as there lies a difference between its secretion and the cut-off range. Apart from these, other biomarkers, which can also be considered and show changes in the blood qualitatively in the case of COVID-19 patients, listed in Table 1 [19,[102], [103], [104], [105], [106]] with the secretion range in healthy patients and COVID-19 infected patient. Lastly, few investigations reported some other biomarkers such as Interleukin (IL), IL-6, IL-10, and IL-1β [107]. Therefore, a biosensor based on biomarker approach for COVID-19 detection would be a non-invasive and user-friendly technique to overcome the limitation of highly qualified professionals. Furthermore, this biomarkers-based biosensor can be combined with a microfluidics system, which will reduce the amount of sample and the chances of spread of the virus, similar to the approach developed by Singh et al., in which they tried to fabricate a microfluidics-based biosensor for detecting the influenza virus [108].
Table 1.
List of considerable biomarkers for COVID-19.
| S.No. | Biomarker | Normal patient | Affected patient | Ref. |
|---|---|---|---|---|
| 1 | Serum ferritin, (ng/mL) | 15.0–150.0 | 800.4 (452.9–1,451.6) | [102] |
| 2 | Cholinesterase (CHE) (kU/L) | 8–18 | 1.5–8 | [106] |
| 3 | C-reactive protein (CRP) (mg/L) | 0.0–1.0 | 57.9 (20.9–103.2) | [102] |
| 4 | Creatinine (CREA) (μmol/L) | 60–120 | 30–80 | [106] |
| 5 | Interleukin-2R, (U/mL) | 223.0–710.0 | 757.0 (528.5–1,136.3) | [102] |
| 6 | Lactate dehydrogenase (LDH) (U/L) | 140–280 | 300–600 | [106] |
| 7 | IL-6 (pg/mL) | 0·0–7.0 | 7·9 | [103] |
| 8 | IL-10 (pg/mL) | 0–9.1 | >15 | [105] |
| 9 | Cystatin C (Cys C) mg/dl | 0.6–1.3 | >1.1 | [106] |
| 10 | D-dimer (μg/mL) | 0–0.243 | 0.4 | [19] |
| 11 | Urea (mmol/L) | 2.5–7.1 | 4–22 | [106] |
| 12 | Lymphocyte count (×109/L) | 0.8–4 | <0.6 | [104] |
| 13 | Serum amyloid A (SAA) (mg/L) | 0–10 | 108.4 | [19] |
| 14 | Procalcitonin (ng/mL) | 0–0.5 | <0.1 | [104] |
| 15 |
Serum direct bilirubin (DBIL) (μmol/L) |
5.1–17 |
>8-60 |
[106] |
|
Qualitative changes in the blood profile of COVID-19 patients. (The information is adapted from the clinical studies of [107]) | ||||
| Hematologic | WBC count Neutrophil count |
Increases | ||
| Lymphocyte count Platelet count Eosinophil count Hemoglobin |
Decreases | |||
| Biochemical | Alanine aminotransferase Aspartate aminotransferase Total bilirubin Blood urea nitrogen Creatinine Creatine kinase Lactate dehydrogenase Myoglobin Creatine kinase-MB Cardiac Troponin I |
Increases | ||
| Albumin | Decreases | |||
| Coagulation | Prothrombin time D-dimer |
Increases | ||
| Inflammatory biomarkers | Erythrocyte sedimentation rate CRP Serum-ferritin PCT IL-2R IL-6 IL-8 IL-10 |
Increases | ||
5. Electrochemical biosensors: toward point-of-care sensing
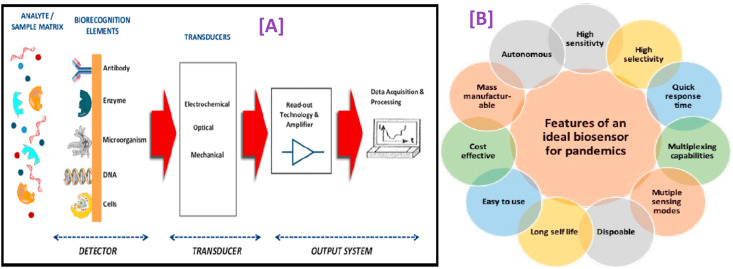
Till now, there is no adequate facility available for monitoring and detection of COVID-19. Therefore, considerable attention needs to be given to POC applications by developing a portable, selective, sensitive, and rapid tool for COVID-19 detection, resulting in early diagnosis required for determining the adequate and suitable therapeutic intervention at the right time. Currently, the available SARS-CoV-2 virus sensors are multi-component, which need sophisticated elements. An electrochemical biosensor is one of the groups of analytical devices that can be adapted to fulfill the characteristics requested for ASSURED criteria and POC testing, which majorly involves 11 features, as shown in Fig. 7 (B).
Fig. 7.
[A] A schematic presentation of the principle of a biosensor (reproduced from Ref. [105]); [B] Features of an ideal biosensor required to be developed for practical use in pandemics (adapted with permission from Ref. [23], Copyright 2020 American chemical society).
Because of their applicability and versatility in concrete situations, biosensors' field has gained incredible progress [109]. A biosensor is described as analytical devices combining bio-recognition element (which includes nucleic acids, tissue, cell receptors, microorganisms, organelles, antibodies, enzymes, proteins) with physio-chemical integrated with three-electrode systems [110] that are capable of detecting a chemical, physical, or biological property of a specific substance and an electronic transducer comprising of signal processing, amplifying, recording, and displaying the result in a readable format as shown in Fig. 7(A) [111,112]. Principally, on the interaction of target analyte with bioreceptor, the detecting element specifically identifies the target analyte via specific adsorption, reaction, or some other process such as chemical/physical interaction. Afterward, the transducers convert molecular interactions into detectable form, and for this, several materials are being employed for developing transducers that can be explored in the fabrication of biosensors. The following section explicitly explores the applicability of biosensors toward COVID-19 detection. Previous works have already explored the general aspects of these sensors, e.g. the various electrode materials in use for the detection of other viruses.
5.1. Recent developments in state of the art techniques for COVID-19 detection
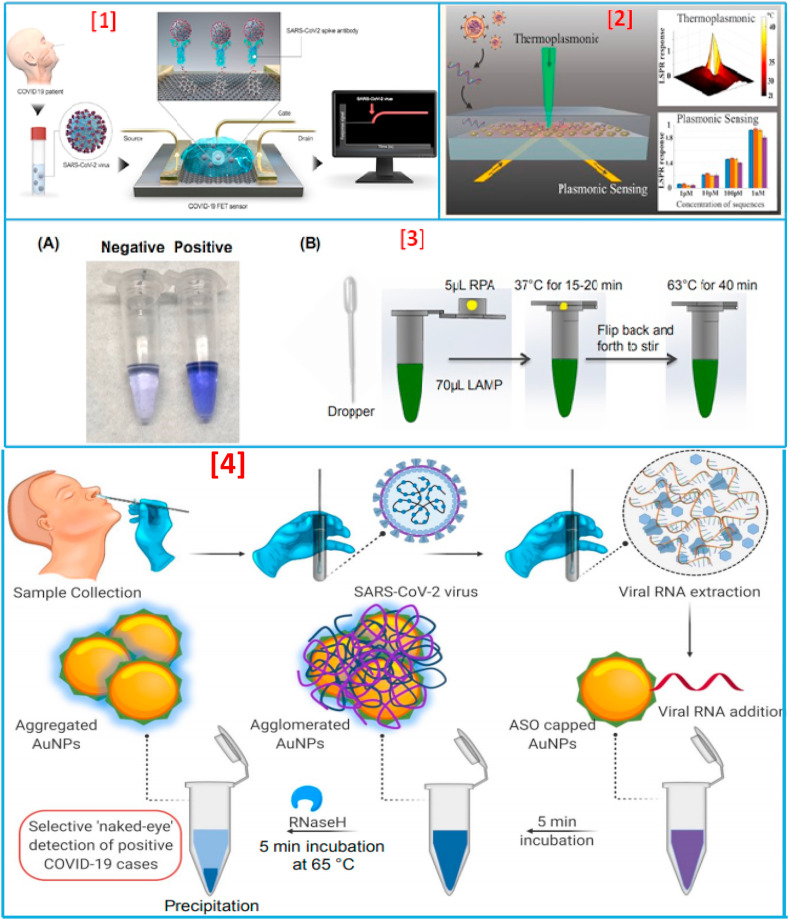
All around the globe, numerous researchers are working hard to develop a quick diagnostic approach for the detection of COVID-19, as the existing method of RT-PCR is costly and lengthy. To begin with the signs of progress, Qiu and his team developed a dual-functional plasmonic photothermal (PPT) biosensor, in which they used localized surface plasmon resonance (LSPR) and PPT effect with the aid of two-dimensional gold nanoislands (AuNIs), as shown in Fig. 8(2) . They utilized AuNIs for functionalizing the complementary DNA receptors to sensitively detect the selected sequences of SARS-CoV-2 [113]. Though this sensor is more accurate and fast compared to the results of RT-PCR, it was still not able to eliminate the problem of exposure to particles of virus. For improving the limit of detection (LOD) or detection limit, Seo et al. recently developed an immunosensor on SARS-CoV-2 spike protein using a graphene sheet on an FET-based system [Fig. 8(1)] [114]. This sensor has a better LOD but lacks the specificity in longer terms (such as the viral strain can get mutated and change its morphology). Recently, some other developments have also been done by several research groups for fast COVID-19 detection, whose parameters are listed in Table 2 [[113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133]]. However, none of these methods went toward the fabrication of non-invasive biosensors that can be user-friendly and cost-effective. Various studies have also revealed the successful application of LAMP assay to detect CoV RNA in multiple forms in patient samples [[134], [135], [136], [137]], showing 100-fold more sensitivity than conventional RT-PCR techniques [[136], [137], [138], [139], [140], [141]]. A POC COVID-19 sensing system based on reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay was well established, explained, and hypothesized for highly sensitive COVID-19 detection by Kashir et al. [85] and Trieu et al. [3]. Furthermore, Zhang et al. [142] have used colorimetric detection for identifying COVID-19 viral RNA from patient cell lysis or purified RNA via LAMP methodology. In a recent report, El-Tholoth et al. [143] modified the less sensitive LAMP assay of Zhang et al. to enhance sensitivity by Penn-RAMP strategy for COVID-19 detection. This is a two-step LAMP protocol, which is ~10–100-fold more sensitive than usual LAMP [144] and has a success rate of 100% at 7–10 copies of viral reaction/RNA as compared to 7,000 copies for COVID-19 qPCR and COVID-19 LAMP assay [142,144,145]. The principle for detecting COVID-19 is based on the variation of color formed in leuco crystal violet (LCV) dye. This dye is nearly colorless in the absence of dsDNA and deep violet in the presence of dsDNA, enabling the detection of amplicons, as shown in Fig. 8(3A). In this study, the authors examined the variation in color through the naked eye; therefore, the instrument-free POC detection device could be used at home. Moreover, it comprises a chemically heated cup for controlling the system's temperature without the requirement of electrical power [Fig. 8(3B)]. In another study, Moitra et al. [115] fabricated a gold NPs capped with thiol-modified antisense oligonucleotides colorimetric biosensing approach for the selective naked eye SARS-CoV-2 detection within 20 min, as shown in Fig. 8(4). This exhibits a linear range of 0.2–3 ng/μL, with a detection limit of 0.18 ng/μL for SARS-CoV-2 RNA. These advanced sensors facilitate detection in remote areas where clinical instruments and medical facilities are very confined, thus having potential as an efficient COVID-19 diagnostic tool [143].
Fig. 8.
[1] Detection of SARS-CoV-2 using field-effect transistors: The schematic shows collection of biological samples from the patient and its application on the graphene-based sensing area of the FET biosensor. The sensor in real time can capture binding events associated with the SAR-CoV-2 (adapted with permission from Ref. [23], Copyright 2020 American chemical society) [2]. LSPR detection of nucleic acid sequences from the SARS-CoV2. The schematic shows the architecture of the LSPR substrate consisting of gold nanoparticles in which light is illuminated on the substrate to generate local heat and detect nucleic acid-binding events. The graph also shows the LSPR response to their plasmonic effect and the detection of nucleic acid sequences at low concentrations (adapted with permission from Ref. [23], Copyright 2020 American chemical society) [3]. Home Test for COVID-19. (A) Visual detection of COVID-19 with one-tube Penn-RAMP with LCV dye. Negative: 0 copies of COVID-19 synthesized DNA; positive: 100 copies of synthesized DNA. (B) The sequence of operations of the home test. The reactions can be incubated either in a block heater or in a domestic oven with temperature control (adapted with permission from Ref. [84], Copyright 2020 Elsevier) [4]. Schematic representation for the selective naked-eye detection of SARS-CoV-2 RNA-mediated by the suitably designed ASO-capped AuNPs (adapted with permission from Ref. [109], Copyright 2020 American chemical society).
Table 2.
A recently developed SoA techniques for COVID-19 detection and their parameters.
| S.No | Techniques used | Materials used | Limit of detection | Analytes | Ref. |
|---|---|---|---|---|---|
| 1 | Surface plasmon resonance | Gold nanoislands | 0.22 pM | DNA | [113] |
| 2 | Field-effect transistor | Graphene sheet | 1.6 × 101 pfu/mL | Protein | [114] |
| 3 | Colorimetric | ASOs-capped plasmonic nanoparticles | 0.18 ng/μL | SARS-CoV-2 N-protein | [115] |
| 4 | LAMP | Polymer nanoparticles | 12 copies/reaction | Spike protein | [116] |
| 5 | RT-PCR | Iron oxide nanoparticles | 10 copies/reaction | RNA | [117] |
| 6 | Colorimetric | Gold nanoparticles | 0.5 ng | RNA | [118] |
| 7 | Lateral flow immunoassay (LFA) | Gold nanoparticles | – | IgM and IgG antibodies | [119] |
| 8 | Lateral flow immunoassay (LFA) | Lanthanide-doped nanoparticles | – | Anti-SARS-CoV-2 IgG | [120] |
| 9 | Optomagnetic | Iron oxide nanoparticles | 0.4 fM | RdRp sequence | [121] |
| 10 | Amperometric | Screen-printed carbon electrode (SPCE) | 10 fM | Protein | [122] |
| 11 | Bioelectric recognition assay (BERA) | Mammalian Vero cells | 1 fg/mL | S1 spike protein | [123] |
| 12 | Plasmonic metasensor | Functionalized gold nanoparticles (NPs) | 4.2 fmol | spike protein | [124] |
| 13 | Electrochemical | Cobalt-functionalized TiO2 nanotubes | 0.7 nM | SARS-CoV-2 S-RBD protein | [125] |
| 14 | P-FABU-bent optical fiber | Gold nanoparticles | 10–18 M | N- protein | [126] |
| 15 | Optical Fiber | Gold nanoparticles | 100 units/ml | IgG-type antibodies | [127] |
| 16 | RT-LAMP | – | 100 copies/reaction | genomic RNA | [128] |
| 17 | Surface plasmon resonance | Monolayer of 3-mercapto propionic-Leu-His-Asp-Leu-His-Asp-COOH | 1 μg/mL | Nucleocapsid antibodies | [129] |
| 18 | CRISPR–Cas12-based lateral flow assay | – | 10 copies per μl input | E gene and N gene | [130] |
| 19 | LFIA | Gold nanoparticles | – | IgM and IgG | [131] |
| 20 | Chemiluminescence immunoassay | – | – | IgM and IgG (recombinant nucleocapsid) | [132] |
| 21 | Colloidal gold-based immune-chromatographic (ICG) strip | – | – | IgM or IgG | [133] |
Furthermore, biosensor based on combined LSPR sensing and PPT effect [113], modified LAMP assay [142], the instrument-free LAMP [143], and graphene-based FET sensor [114] can be utilized as a platform for the development of miniaturized POC systems for COVID-19 estimation and detection. However, while RT-LAMP can be used to confirm infection cases, it cannot exclude them, thus limiting the usefulness of this technique. Yet, a somewhat portable form of the method has been developed in recent times, but the analysis takes around 1 h and must usually be performed in a controlled laboratory setting. Also, these approaches are multi-component colorimetric methods that require validation for clinical use. Therefore, there remains scope for developing smart sensing systems capable of performing under the ASSURED paradigm [[146], [147], [148]].
6. Biomarker-based electrochemical immunosensors for rapid detection of SARS-CoV-2
To overcome the current laborious detection and time-taking technique using RT-qPCR and to create an ASSURED environment for detection of SARS-CoV-2 infection, we recommend nano-enabled biomarker-based electrochemical immunosensing methods. These nano-enabled biomarker-based electrochemical immunosensors are an essential class of successful and widespread commercial devices for biomolecular electronics. They have several advantages because of their ability of miniaturization, the short response time (unlike bioluminescent ones), to perform quantitative analysis with fewer background noises to operate in turbid media (unlike optical ones), economical, and reach low detection limit, in comparison to other forms of biosensors. Because of the unique and exceptionally high selectivity, sensitivity, and specificity an antibody manifests for its specific antigen, immunosensors have now become common in clinical analytics. Therefore, attempts have been made to develop nano-enabled antibody-based immunosensing techniques (immunosensor) to take advantage of the numerous physicochemical properties of nano-dimensions, specifically electrical, optical, magnetic, and optomagnetic, toward virus detection responsible for respiratory infections like SARS-CoV-2. Unsurprisingly, at the time of writing, the literature is relatively sparse in immunosensors, specifically targeting SARSCoV-2.
Electrochemical immunosensors are defined as one of the analytical devices to measure and detect antigen or antibody concentration of the analytes. They are based on the antigen–antibody interactions, which provide signals to determine the analyte in the sample. This method is highly sensitive, selective, and low cost than the other conventional detection techniques for virus detection [149]. The sensor accompanied by bioreceptors is used to determine the specific analyte concentration in the biological sample in various fields of clinical, biological, biotechnology, and research center [150]. It produces an electrical signal proportional to the concentration of a group of analytes or a single analyte. The biomarker-based electrochemical biosensor methodology has been proposed to determine target analytes from the patient's sample in the physiological range [[151], [152], [153]]. For the desirable performance of electrochemical biosensor toward a target disease, various SoA technologies like molecular recognition (comprising antibody [154], aptamers [155], DNA [156], enzymes [157], and others), different nanostructured materials for immobilization, i.e. one-dimensional (1D) or 2D or 3D nanostructure of inorganic-organic, and composites [158], transduction methods [159], advanced flexible substrates [160], novel sensing arrays [161] and fluidic systems to develop BioMEMS [162] should be included. Numerous nano-enabled biosensors have been fabricated to determine metabolites like targeted biomarkers, antigens, nucleic acids, and pathogens for disease analysis [[163], [164], [165]]. Over the years, remarkable developments have been made in electrochemical immunosensing technology to detect the concentrations of viruses for the diagnosis of infectious diseases [[166], [167], [168]]. Based on the targets, a lot of research has been done for the development of biomarker-based electrochemical immunosensor toward the analysis of several types of viruses, including MERS-CoV and SERS-CoV [Table 3 ] to prevent considerable death and for controlling the spread of the virus from one country to the other [[169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187]].
Table 3.
Comprehensive list of recent virus biosensing methodologies based on immunosensors and comparison of the analytical performances in terms of limits of detection (LOD) and linear working ranges.
| Viral | Affinity reagent | Approach | Transducer (s) | LOD | Linear range | Ref. |
|---|---|---|---|---|---|---|
| DENV, NS1 | mAb, IgG | Fuel cell Prussian blue nanotube membrane | Amperometry | 0.04 pfu mL−1 | 3–45 pfu mL−1 | [169] |
| DENV, NS1 | mAb, IgG | Nanoporus alumina membrane electrode | Voltammetry | 1 pfumL−1 | 1–103 pfumL−1 | [170] |
| DENV, NS1 | mAb | Protein A-coated gold CD electrode | Voltammetry | 0.33 ng mL−1 | 1–100 ng mL−1 | [171] |
| DENV, NS1 | mAb, IgG | Nanoporus alumina membrane electrode | EIS | 1 pfumL−1 | 1–900 pfumL−1 | [172] |
| HV, antigens | mAb | Protein A/nanogold-modified GE | Voltammetry | <1.0 ng mL−1 | ~1–350 ng mL−1 | [173] |
| HBV, IgG | E protein | AuNP based Magnetosandwich immunoassay | Chronoamperometry | 3 mIUmL−1 | 5–69.2 mIUmL−1 | [174] |
| HBV, surface antigen | Single chain Ab | GBP/single-chain Ab fusion protein – GE | EIS | 0.14 ng mL−1 | 1 ngmL−1-50 mgmL−1 | [175] |
| HCV, core antigen | Ab | Nanocomposite-modified GCE | Voltammetry | 0.17 ng mL−1 | 2–512 ng mL−1 | [176] |
| HCV, cDNA | PNA | SAM-thiolated PNA probe modified GE | Voltammetry | 0.57 pM | 1–50 nM | [177] |
| HCV, cDNA | PNA | SAM-thiolated PNA probe-modified GE | Voltammetry | 1.8 pM | 10–100 pM | [178] |
| HCV, Protease | Peptide | Ferrocene-conjugated peptide-modified GE | EIS | 5 pM | 10–100 pM | [179] |
| HCV, RNA | PNA | TADC SAM PNA probe-modified GE | EIS | 23 pM | <10–500 nM | [180] |
| HIV, C protein | pAb | AuNP/MWCNT/AEP film-modified GE | Voltammetry | 6.4 pgmL−1 | 0.01–60 ng mL−1 | [181] |
| HIV, C protein | mAb | AuNP-electroplated GCE | Voltammetry | 8 pgmL−1 | 0.01–100 ng mL−1 | [182] |
| HIV, RT | Peptide | Ferrocene-labeled lipoic acid – AuNP/SPCE | Voltammetry, EIS | 0.8 pgmL−1 | 1–500 pgmL−1 | [183] |
| IAV, whole virus | pAb | Neutravidin/biotinylated Ab/thiol-modified GE | EIS | 8 ng mL−1 | 0–64 ng mL−1 | [184] |
| JEV, E protein | mAb/IgG | MWCNT/magnetic beads/HRP and magnetic GE | Voltammetry | 2 × 103pfumL−1 | 2 × 103 to 5 × 105pfumL−1 | [185] |
| JEV, antigen | Serum Ab | Protein A/GA-silanised interdigited electrode | EIS | 0.75 mg mL−1 | 1–10 mg mL−1 | [186] |
| MERS-CoV spike protein S1 | mAb | Gold NPs/carbon electrode | Voltammetry | 1.0 pg mL−1 | 0.001–100 ng mL−1 | [187] |
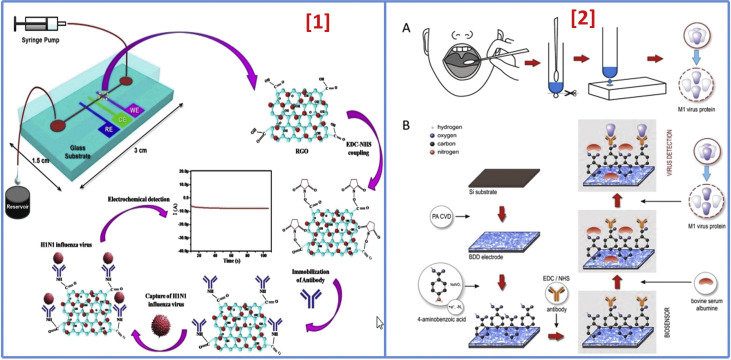
According to a report, an RGO-based electrochemical immunosensor was fabricated using EDC-NHS coupling chemistry between –COOH groups of graphene, i.e. attached to the gold surface of working electrode, and –NH2 of antibody specific for H1 of H1N1 influenza. RGO was made for assembling on the surface of the working electrode, and virus-specific antibodies were used to analyze electrochemical spectra via various concentrations for direct determination of the whole virus, as shown in Fig. 9(1) . The detection limit of 0.5 PFU/mL was reported [108]. In 2017, a boron-doped diamond (BDD) sensor comprising a three-electrode system was fabricated where diazonium salt of 4-aminobenzoic acid was utilized to form binding sites that would attach to anti-M1 on the electrode surface [Fig. 9(2)]. For this work, extraction of M1 protein from the virus was done, followed by an experiment using K3Fe(CN)6 via electrochemical impedance technique with a detection limit of 1 fg/mL in response time of 5 min [188]. SiO2-inverse opal (SiO2-IO) based biosensor (2018) was developed where α HA-specific antibodies were made to immobilize on the SiO2-IO surface utilizing 3-aminopropyl trimethoxysilane (APTMS) that reveals the amine group and an NHS-PEG4-maleimide linker to bind APTMS with thiolated protein G (Cys-ProG) that exhibited high sensitivity of 103–105 plaque-forming unit (PFU) [189]. A gold NPs/carbon electrode-based electrochemical immunosensor was fabricated by Layqah et al. for simultaneous detection of various CoVs, including human CoVs (HCoVs) and MERS CoV. This immunosensor had linear range and LOD of 0.001–100 ngmL−1 and 1.0 pgmL−1, 0.01–10,000 ngmL−1 and 0.4 pgmL−1 for MERS-CoV and HCoVs, respectively, with an assay time of 20 min [187]. Such research indicates that nanostructure-enabled immunosensing could be a potential approach to early and rapid detection of virus-induced respiratory infections. Therefore, various researches based on immunosensor methodology are continuously going to obtain excellent specificity and sensitivity via a different variation on the electrode's surface.
Fig. 9.
[1]. Schematic illustration of the microfluidics-integrated electrochemical immunosensing chip coated with RGO, followed by antibody immobilization using EDC/NHS coupling to detect influenza virus H1N1 (adapted with permission from Ref. [102], Copyright 2017 Nature) [2]. Schematic illustration of the biosensing system. (A) Throat swab culture acquisition. (B) Boron-doped diamond electrode surface modification with polyclonal anti-M1 antibodies that can identify the universal biomarker for influenza virus, the M1 protein (adapted with permission from Ref. [162], Copyright 2017 Nature).
We advise that sincere efforts should be taken toward (a) functional and genetic modification of bioreceptors molecules, e.g. CRISPR-Cas9, DNA, aptamers for sequence-specific detection; (b) exploring novel enzymes and antibodies to determine the concentrations of the virus selectively; (c) fabricating sensing array of electro-active materials for building highly sensitive sensing systems; (d) integrating advanced sensing components for developing a miniaturized system for application in clinics; (e) wireless communication to store data that can provide benefit bioinformatics for management of the disease; and (f) designing fluidic structures for automated sampling for precise real-time detection [190]. These tactics should be adopted to develop a diagnostic tool in relevant physiological ranges for detecting the SARS-CoV-2 virus.
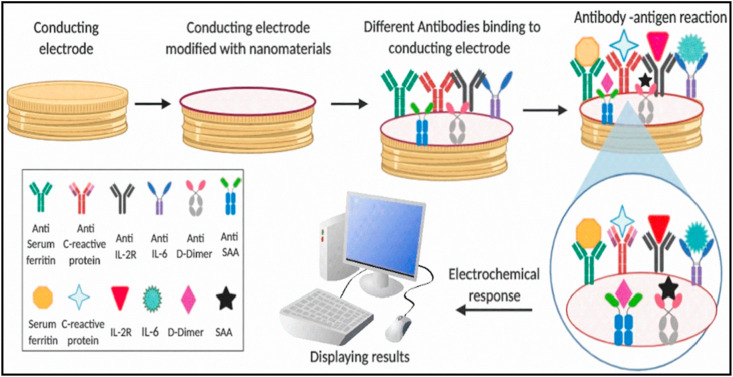
In our proposed approach, SARS-CoV-2 proteins, involving the SARS-CoV-2 envelope protein, biomarkers, and nonstructural proteins, as mentioned above, via the usage of respective specific antibodies, as shown in Scheme 1 (key scheme), could be able to detect at fM/pM/nM concentrations. By the usage of micro/nano-electrodes [191], nano-structured sensing materials [192], miniaturized sensing transducers, and microelectronics [193,194] to develop an electrochemical sensor for COVID-19 may improve the selective monitoring, screening, and SARS-CoV-2 detection. But, these studies on various sensing systems are still in the exploratory stage for detection of COVID-19. Hence, we encourage the proposal of nano-enabled biomarker-based electrochemical immunosensing technology for SARS-CoV-2 detection in biofluids of infected patients. We also believe that miniaturized nano-enabled biomarker-based electrochemical immunosensor systems could be a potential candidate for the detection of COVID-19 for the POC device that would provide rapid, personalized health-care supply to infected patients for better therapeutics and disease management.
Scheme 1.
A schematic illustration of the hypothetical workflow of nano-enabled biomarker-based immunosensor for COVID-19 detection. A conducting microelectrode can be modified with various nanostructures for high loading of SARS-CoV-2-specific antibodies to detect SARS-CoV-2 proteins or multiple biomarkers at picomolar/nanomolar concentrations using an appropriate electrochemical transduction technique.
6.1. Challenges with antibody-based immunosensors
A lot of challenges are related to antibody-based biosensors, which are majorly divided between target and reagent. The first challenge lies in generating the consistency of high-affinity antibodies that can selectively direct the analyte of interest. Also, antibodies show extreme variability, i.e. antibodies-based sensors can work for a particular batch but not for the other one. Generally, a better response in the case of reproducibility has been shown by the monoclonal antibodies, but due to a single binding epitope, potential problems arise owing to the number of binding sites. Immobilization of antibodies on the electrode's surface is another critical issue. If proper care is not taken during the experiment, the binding of antigen becomes impossible, as the substantial amount, i.e. 75% of antibodies, is not immobilized or denatured. So, considerable attention should be given to techniques of immobilization and hindering the non-specific adsorption.
Furthermore, antibodies are less stable than RNA/DNA reagents and often need low-temperature conditions for maintaining their shelf-life. As for reducing agents, ionic concentrations and increased temperatures can deactivate the antibodies and decrease the sensor's efficiency dramatically; therefore, suitable temperature and buffer compositions are required concerns while using antibody-based sensors. In addition to this, the significant challenges to target arise from the biology itself. As most of the viruses are known for mutating, i.e. changing their protein coats and having many similarities in a protein coat, this poses a big problem in fabricating the antibodies against the mutating species. Similarly, the likeness in the proteins coat of various viruses creates a unique challenge in the selectivity of antibody-based sensors. For instance, dengue viruses are of four types, and fabricating antibodies against these types of viruses is non-trivial. Hence, antibodies need to be carefully selected and treated for their use in biosensors to detect the target analyte successfully.
Another one of the significant difficulties in the design of immunosensors is to capture the signals of very low magnitude between biological species (antibodies and analytes). Nanomaterials (NMs) may be used as labels to solve this problem to achieve substantial signal enhancement, strong enough to be easily detectable. The properties of NMs, such as a high surface-to-volume ratio, quantum size effects, high adsorption, and reactive ability compared to their pure form, are critical during the fabrication of an immunosensor. Furthermore, the size and morphology of NMs can be easily modified and, thus, surface modification/immobilization with a wide range of antibodies by covalent or non-covalent bonding can boost immunosensing capabilities in terms of low detection limit, high sensitivity, selectivity, and rapid response to the sample analyte [195].
7. Artificial intelligence (AI) and Internet of Medical Things (IoMT) for COVID-19 management
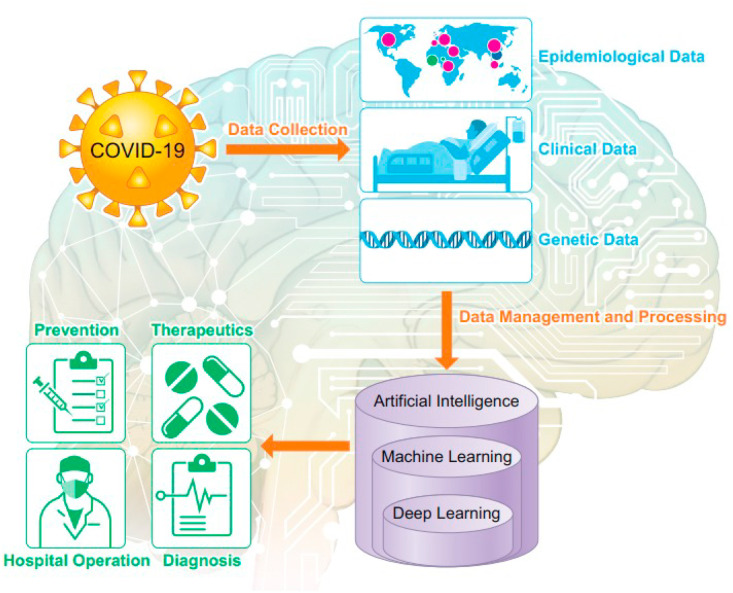
The current section emphasizes on the overview of some applicable AI- and IoMT-based approaches that could help the existing standard methods to deal with COVID-19 in health centers worldwide. At present, the COVID-19 pandemic has turned out to be a hotspot for medical research that provides new developing strategies, a remedy for this global emergency [196]. The need for essential medical equipment and supplies has been rising and is expected to outpace the ability for quick, but extremely essential replenishment. Potential patients and citizens have to leave their houses for getting medical aid that creates a gap in quarantine and isolation efforts, posing a threat in disease control. In addition to this, the lack of proper medical devices and isolation wards has encouraged the medical groups to inspire people with suspected or mild symptoms to stay at their residence [197]. Also, it is not possible that health-care workers and physicians will be able to give treatment to all with the exponential rise in number of patients suffering with COVID-19. Undoubtedly, another strategy, i.e. home-based diagnostic test, could offer a valuable way out to fulfill this unmet and urgent need. Therefore, computer scientists can provide contribution by presenting more intelligent solutions for fighting against COVID-19 to attain fast control over SARS-CoV-2 virus [198].
In this context, AI and IoMT technologies have a potential to maximize safety throughout the pandemic and avert the fast transmission of COVID-19 [[199], [200], [201]]. AI implies the computer usage without the inference of humans for modeling intelligent behavior [202]. The AI usage has increased in various fields particularly in medical detection [203] that has led to benefits such as decrease decision time, lesser burden and more precise detection results as compared to the detection procedure of conventional methods [204,205]. Patients suffering from COVID-19 can give novel information that are significant for attaining medical aims. Social media, mobile health apps, and self-tracking devices can delivers data that can be made available to clinicians and patients regarding COVID-19, thereby helping to check their health status [[206], [207], [208]]. Many AI methods such as machine learning, convolutional neural networks, deep learning, and cognitive computing can play a crucial part in estimating likely interactions with the newly explored therapies for this virus, reduction of caretaker workload, monitoring, large-scale screening, and detection [209,210]. The advancement of AI techniques in recognizing the threats of pandemic diseases is measured as an important aspect in the progression of the detection, prevention, and prediction of upcoming health risks globally [211,212]. Various kinds of AI classifiers have been described by some researchers having actual COVID-19 records with diverse targets and case studies [213]. For instance, a smartphone application has been developed that gathers updated regions of the outbreak, travel history, earlier locations of the patient, symptoms, and signs, and later this information gets processed, filtered using algorithms so that physicians could examined the suspected cases only (Fig. 10 ) [214,215]. The Center for Systems Science and Engineering located at Johns Hopkins University on January 22, 2020, initiated a publicly shared Web-based interactive dashboard [URL: https://coronavirus.jhu.edu/map.html], which precisely tracks and visualizes COVID-19 reported cases in real time, thereby helping in fast detection of new cases caused by the disease. This has led to a new spectrum of AI to recommend and predict quarantine in regions where a large number of cases have reported and also helps in timely diagnosis of patients if they are traveling to these regions [216]. An AI-augmented method was used by Hurt et al. [217] for plain chest radiographs for predicting and tracking the pulmonary progression of hospitalized patients suffering from COVID-19, which aided to recognize the patients requiring critical care. Moreover, the infrared thermal cameras utilized for screening the fever of public have been combined with AI-powered facial recognition techniques for determining whether persons wears the masks or not [218]. The efficiency of AI for predicting the outbreaks of COVID-19 in various geographical areas has been revealed by Effenberger et al. [219]. Blockchain technology in conjunction with AI has been developed for COVID-19 surveillance that is utilized for storing the health care-associated data [220]. One more significant use of AI is the remote monitoring of home-quarantined families and patients through smart bracelets or smartphones that provide an automatic alarm or warning message sound on breaking of quarantine [221]. Therefore, fast progression of automated diagnostic techniques based on AI not only serves enhanced diagnostic speed and accuracy, but rather decreases the burden and protect the health-care staffs by reducing their contacts with patients suffering from COVID-19 and work more effectively in a harmless environment. Though AI systems could be advantageous for classification and diagnosis of COVID-19, but choosing suitable AI methods that provides precise results is still a challenging problem [222,223].
Fig. 10.
Use of machine learning and artificial intelligence in battle against COVID-19 (adapted with permission from Ref. [207], Copyright©2020 the American Physiological Society).
7.1. Internet of Medical Things (IoMT)
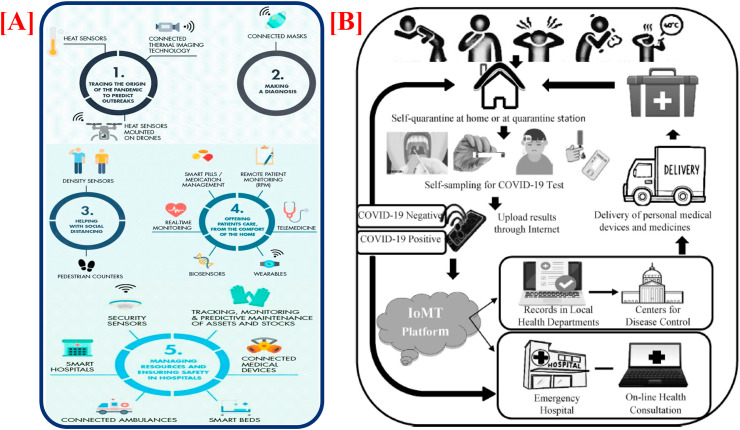
IoMT is the particular class of IoT for health industry that plays a significant role in smart health-care monitoring, which is used throughout the COVID-19 pandemic. IoMT is described as the application of the concepts, techniques, tools, principles, and fundamentals of the well-known internet techniques predominantly for the health-care and medical domains [224]. It is an inventive mean of combining health-care devices and their applications for connecting with the health-care data technology organizations through the usage of networking equipment. Leaving behind the conventional treatment modalities, IoMT can provide a solution for making remarkable progress to regulate the challenges of control, contact tracing, monitoring, and detection of real-time COVID-19, including fresh new cases of the disease [22,197]. As the whole population gets affected from the disease, it is not possible for some individuals to tackle the situation until live updates of numbers are accessible. So, IoMT can be applied in different major zones to handle COVID-19, as depicted in Fig. 11 (A).
Fig. 11.
[A] Role of IoMT in various major areas to tackle COVID-19. [B] Schematic for combating COVID-19 with combined implementation of POC diagnostics and the IoMT (adapted with permission from Ref. [190], Copyright©2020 Elsevier).
The critical roles of developed IoMT concepts arise when the health facilities are needed in some distant regions [224]. The hygiene medical care, information analysis and gathering, patient tracking, report monitoring, data sharing, etc. are the different cloud and connected network-based amenities of IoMT that have totally transformed the medical services, operations, and health-care systems [[224], [225], [226]]. Also, the real-time psychological information of the patient such as expression, speech, etc., as well as physiological information such as the oxygen level, glucose level, heart rate, ECG, blood pressure, EEG, body temperature, etc. are accessible to health workers distantly via IoMT [197]. With regard to COVID-19 crisis management, IoMT offers a chance to aid health-care experts to distantly test COVID-19 at quarantine/home center, monitor their health in real time, deliver online health facilities for patients, increase the accuracy and speed of treatment and diagnosis, and access correct personalized information about their patients that completely change the working design of the health-care centers with more satisfaction and superior level of care. With such techniques like telemedicine and connected assistance, remote patient monitoring (RPM), tracking sensors and devices, individuals can manage their health more efficiently, obtain online diagnoses and record their behaviors, without leaving their houses. This could be effective way to improve patient prognosis in both the directions. Further, it could also provide a medical platform for the databases management beneficial for health-care services and government, as shown in Fig. 11(B) [196].
8. Future prospects
As deliberated above, lacking proper safety, workstations, and rapid and efficient selective SARS-CoV-2 diagnostic approaches are the critical challenges for fighting a battle against COVID-19 in the future time. The WHO has alerted globally to fulfill the demands of treating and monitoring the COVID-19 for setting up BSL-4 level laboratories worldwide. This must be done to explore more applied and fundamental research in COVID-19 to develop novel drugs, vaccines, and effective diagnostic coordination. SARS-CoV-2 infection's symptoms are not seen at the initial stages but progress later to a severe infection when a patient is diagnosed with the disease. Therefore, developing an early-stage smart sensing system to detect SARS-CoV-2 selectively at very low levels would be useful for better diagnostics. Many techniques are being explored to prevent SARS-CoV-2 pathogenesis, transmission, health effects, and replication. Unfortunately, despite so much of advancements, these methods and assays have not been applied in case of a confirmed patient suffering from COVID-19, as these studies depend on the ‘simulated’ patient samples where blood samples and swabs were artificially ‘spiked’ with the RNA of COVID-19, which in turn gave false results, randomly arising uncertain issues. However, POC and smart miniaturized SARS-CoV-2 diagnostic kits or sensing devices have not been used for rapid and selective SARS-CoV-2 detection yet, i.e. necessary for filling the gap between making quick therapeutic decisions and estimating the infection level.
Thus, developing an affordable, sensitive, portable, rapid, selective, and reliable sensor is in high demand for controlling this virus would be commendable. This can be accomplished via a biomarker-based immunosensor approach, as nano-platforms and nano-electronics-based miniaturized devices and immunosensors can detect various (at fM/pM/nM concentration) disease at clinics location. The enhancement of such miniaturized sensing devices with MEMS/NEMS is done for the automation of sample and precise and accurate measurement. We assume that the fabrication of specific SARS-CoV-2 biomarker's monoclonal antibody-based nano-enabled immunosensor would be a possible way out for non-invasive rapid screening and estimation of SARS-CoV-2 virus at fM/pM/nM level within ~40 min, particularly at an initial stage. The analysis of miniaturized potentiostat for selective, sensitive, and rapid SARS-CoV-2 detection at POC would be a great relief in taking therapeutic decisions for infected patients of COVID-19 quickly. Furthermore, the information obtained by a quick assessment of SARS-CoV-2 using biomarker-based miniaturized electrochemical sensing systems would improve the SARS-CoV-2 progression for personalized health care of patients [Fig. 12 ] [227]. In the upcoming time, a fully optimized POC CoV sensing device for the onsite purpose could be utilized to diagnose infected patients that would be of great significance for selecting therapeutics. Moreover, due to the extensive techniques and algorithms that can tackle the virulent spread of the SARS-CoV-2 virus worldwide, AI and IoMT can possibly deliver novel and effective paradigms for health-care services. To eradicate COVID-19, effective deployment of AI and IoMT through the use of immunosensing and novel machine learning approaches may prove crucial. The purpose of this study is to assess the applicability of immunosensing approach using AI and IoMT to prevent and monitor COVID-19 and to create a holistic view of how current systems can be helpful in specific areas that would benefit many health-care managers, computer scientists, and policy makers around the world.
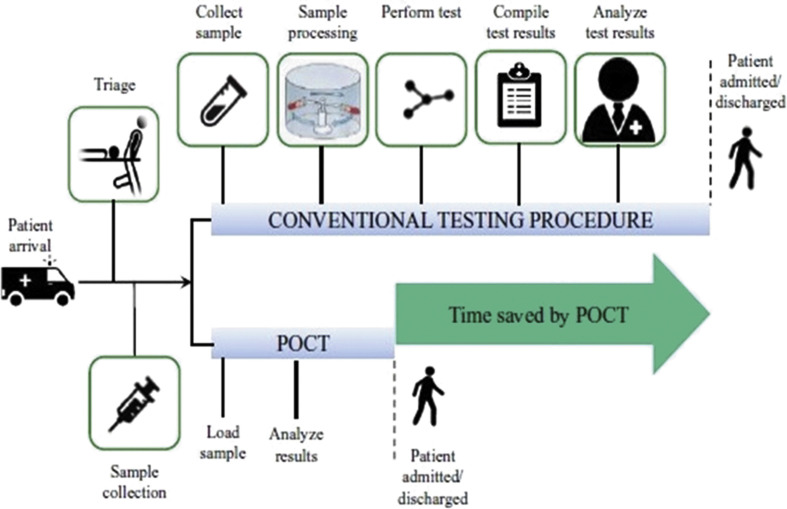
Fig. 12.
Rapid turnaround of results with POCT compared to the current testing procedure for COVID-19 detection (adapted with permission from Ref. [169], Copyright 2015 Scimago).
9. Conclusions
This review highlights the COVID-19 outbreak, SARS-CoV-2-related health effects, AI, IoMT and SoA of COVID-19 diagnostics for the health care point of view to decide therapeutics. Impactful research reports have been carried out for exploring tactics to control the spread and pandemic. For achieving selective and rapid detection of COVID-19 at the initial stage to decide therapeutics, efforts are being made to improve the detection performance of diagnostic techniques. The problems arising from the discrimination of COVID-19 symptoms and other deadly viruses such as Ebola and influenza remain unsolved. It raises the demand for developing highly sensitive non-invasive diagnostic tools at an initial stage combined with bioinformatics based analysis for timely decisions. The existing diagnostic techniques can detect the concentration of the SARS-CoV-2 virus, but portability and lengthy-time for analysis limit their use on the field. Through clinical studies on COVID-19, there has been found considerable variation in the secretion range of biomarkers like IL-2R, serum ferritin in the person suffering from COVID-19 and healthy persons. According to the SoA of miniaturized sensing technology, POC COVID-19 detection could be obtained by adopting a biomarker-based SARS-CoV-2 immunosensing approach that will decrease the detection time and reduce the chances of transmission of the virus while the diagnosis is going on. We also look forward to integrate immunosensor with AI and IoMT that could be successfully applied for expedite diagnostics for timely decisions. Therefore, significant efforts, assisted by public–private collaboration, are required to encourage cutting-edge research in the context of designing an electrochemical SARS-CoV-2 immunosensing device using AI and IoMT to conduct intelligent COVID-19 management in a personalized manner. We hypothesize that this assay would be rapid, sensitive, selective, and non-invasive, giving results within an hour of testing compared to RT-PCR, which takes 3–4 days. This approach could also be explored in the field in less time duration and used by the needy with minimal teaching. The objective is not a quantitative measure of infection, but somewhat an easy negative/positive assay for quick confirmation of detection.
At last, this review is an appeal to start high-level multidisciplinary research with the association of biologists, engineers, and nanotechnologists for developing the smart compartments to integrate POC COVID-19 sensing devices in the BSL-4 environment. The active proposal of COVID-19 sensing devices for detection of SARS-CoV-2 at disease site at fM/pM/nM level within a time duration of ~40 min for deciding therapeutics should be the objective of the upcoming research methodology. In our view, this strategy should be explored quickly and confirmed for practicality before being moved out for mass-diagnostic testing in the current times.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work done here is supported by the Government of India through DBT; Indo-Russia project (DBT/IC-2/Indo-Russia/2017-19/02), and Department of Science and Technology (DST); BDTD, Government of India.
Abbreviations
- SARS
Severe acute respiratory syndrome
- MERS
Middle East respiratory syndrome
- CoV
Coronavirus
- CoV-2
Coronavirus 2
- WHO
World health organization
- COVID-19
Coronavirus disease 2019
- CDC
Center for Disease Control and Prevention
- SoA
State of the art
- PCR
Polymerase chain reaction
- POC
point of care
- rRT-PCR
real-time reverse transcription-polymerase chain reaction
- ELISA
Enzyme-linked immunosorbent assay
- β-CoVs
Beta coronaviruses
- SAA
serum amyloid A
- IL
Interleukin
- RdRp
RNA-dependent RNA polymerase
- ACE2
Angiotensin-converting enzyme 2
- TMPRSS2
Transmembrane serine protease 2
- EM
Electron microscope
- CFT
Complement fixation test
- HI
Hemagglutination inhibition
- DID
Double immunodiffusion
- ENIA
Europium nanoparticle-based immunoassay
- CT
computer tomography
- RT-LAMP
Reverse transcription-loop-mediated isothermal amplification
- PPT
Plasmonic Photothermal
- LSPR
Localized surface plasmon resonance
- BSL-4
Biosafety level 4
References
- 1.Khan H. Letter to editor-impact of age factor in COVID-19 infectivity in population of nowshera KP, Pakistan. Middle East. J. Fam. Med. 2020;7(10):75. [Google Scholar]
- 2.W. Coronavirus. WHO; 2020. https://covid19 int. Accessed. [Google Scholar]
- 3.Nguyen T., Duong Bang D., Wolff A. 2019 novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines. 2020;11:306. doi: 10.3390/mi11030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paliwal P., Sargolzaei S., Bhardwaj S.K., Bhardwaj V., Dixit C., Kaushik A. Grand challenges in bio-nanotechnology to manage COVID-19 pandemic. Front. Nanotechnol. 2020;2:3. [Google Scholar]
- 5.W. H. Organisation:. Novel coronavirus (2019-nCoV) situation report-48.
- 6.Zeri F., Naroo S.A. Contact lens practice in the time of COVID-19. Contact Lens Anterior Eye. 2020;43:193–195. doi: 10.1016/j.clae.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q., et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N. Engl. J. Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.W. H. Organization . 2020. Novel Coronavirus ( 2019-nCoV) Situation Report, 3. [Google Scholar]
- 11.Cohen J., Kupferschmidt K. American Association for the Advancement of Science; 2020. Strategies shift as coronavirus pandemic looms; pp. 962–963. [DOI] [PubMed] [Google Scholar]
- 12.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar D., Manuel O., Natori Y., Egawa H., Grossi P., Han S.H., et al. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am. J. Transplant. 2020;20(7):1173–1779. doi: 10.1111/ajt.15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehr A.R., Perlman S. Springer; 2015. Coronaviruses: an Overview of Their Replication and Pathogenesis. Coronaviruses; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuman B.W., Adair B.D., Yoshioka C., Quispe J.D., Orca G., Kuhn P., et al. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J. Virol. 2006;80:7918–7928. doi: 10.1128/JVI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T., et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalandra R., Yadav A.K., Verma D., Dalal N., Sharma M., Singh R., et al. Strategies and perspectives to develop SARS-CoV-2 detection methods and diagnostics. Biomed. Pharmacother. 2020:110446. doi: 10.1016/j.biopha.2020.110446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai L., Yang D., Wang X., Tong L., Zhu X., Zhong N., et al. Chinese experts' consensus on the Internet of Things-aided diagnosis and treatment of coronavirus disease 2019 (COVID-19) Clin. eHealth. 2020;3:7–15. [Google Scholar]
- 23.Bergmann C.C., Silverman R.H. COVID-19: coronavirus replication, pathogenesis, and therapeutic strategies. Cleve. Clin. J. Med. 2020;87(6):321. doi: 10.3949/ccjm.87a.20047. [DOI] [PubMed] [Google Scholar]
- 24.Bhalla N., Pan Y., Yang Z., Payam A.F. Opportunities and challenges for biosensors and nanoscale Analytical tools for pandemics: COVID-19. ACS Nano. 2020;14(7):7783–7807. doi: 10.1021/acsnano.0c04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:1–22. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siu Y., Teoh K., Lo J., Chan C., Kien F., Escriou N., et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulswit R., De Haan C., Bosch B.J. Elsevier; 2016. Coronavirus Spike Protein and Tropism Changes. Advances in Virus Research; pp. 29–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride R., Van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., et al. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han Q., Lin Q., Jin S., You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J. Infect. 2020;80:373–377. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du L., Yang Y., Zhou Y., Lu L., Li F., Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin. Ther. Targets. 2017;21:131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J.M., Chung Y.S., Jo H.J., Lee N.J., Kim M.S., Woo S.H., et al. Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res. Perspect. 2020;11:3. doi: 10.24171/j.phrp.2020.11.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalal A., Mohan H., Prasad M., Pundir C. Detection methods for influenza A H1N1 virus with special reference to biosensors: a review. Biosci. Rep. 2020;40 doi: 10.1042/BSR20193852. BSR20193852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldsmith C.S., Miller S.E. Modern uses of electron microscopy for detection of viruses. Clin. Microbiol. Rev. 2009;22:552–563. doi: 10.1128/CMR.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 41.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller E., Hoschler K., Hardelid P., Stanford E., Andrews N., Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 43.Roa P.L., Catalán P., Giannella M., de Viedma D.G., Sandonis V., Bouza E. Comparison of real-time RT-PCR, shell vial culture, and conventional cell culture for the detection of the pandemic influenza A (H1N1) in hospitalized patients. Diagn. Microbiol. Infect. Dis. 2011;69:428–431. doi: 10.1016/j.diagmicrobio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Wilson H.L., Tran T., Druce J., Dupont-Rouzeyrol M., Catton M. Neutralization assay for Zika and dengue viruses by use of real-time-PCR-based endpoint assessment. J. Clin. Microbiol. 2017;55:3104–3112. doi: 10.1128/JCM.00673-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bermingham A., Heinen P., Iturriza-Gómara M., Gray J., Appleton H., Zambon M.C. Laboratory diagnosis of SARS. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004;359:1083–1089. doi: 10.1098/rstb.2004.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reusken C., Mou H., Godeke G.J., van der Hoek L., Meyer B., Müller M., et al. Specific serology for emerging human coronaviruses by protein microarray. Euro Surveill. 2013;18:20441. doi: 10.2807/1560-7917.es2013.18.14.20441. [DOI] [PubMed] [Google Scholar]
- 47.Gueudin M., Vabret A., Petitjean J., Gouarin S., Brouard J., Freymuth F. Quantitation of respiratory syncytial virus RNA in nasal aspirates of children by real-time RT-PCR assay. J. Virol Methods. 2003;109:39–45. doi: 10.1016/S0166-0934(03)00042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gavin P.J., Thomson R.B., Jr. Review of rapid diagnostic tests for influenza. Clin. Appl. Immunol. Rev. 2004;4:151–172. [Google Scholar]
- 49.Casals J., Palacios R. The complement fixation test in the diagnosis of virus infections of the central nervous system. J. Exp. Med. 1941;74:409–426. doi: 10.1084/jem.74.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.W. H. Organization . 1959. Expert Committee on Respiratory Virus Diseases. First report, Stockholm, 11-15 August 1958. Expert Committee on Respiratory Virus Diseases First Report, Stockholm, 11-15 August 1958. [PubMed] [Google Scholar]
- 51.Hoyle L., Fairbrother R. Isolation of the influenza virus and the relation of antibodies to infection and immunity. Brit. Med. J. 1937;1:655. doi: 10.1136/bmj.1.3977.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pyhälä R., Kleemola M. The value of complement fixation and haemagglutination inhibition tests in the diagnosis of influenza A. Acta Virol. 1976;20:66–69. [PubMed] [Google Scholar]
- 53.Fairbrother R.W., Hoyle L. Observations on the aetiology of influenza. J. Pathol. Bacteriol. 1937;44:213–223. [Google Scholar]
- 54.Julkunen I., Pyhälä R., Hovi T. Enzyme immunoassay, complement fixation and hemagglutination inhibition tests in the diagnosis of influenza A and B virus infections. Purified hemagglutinin in subtype-specific diagnosis. J. Virol Methods. 1985;10:75–84. doi: 10.1016/0166-0934(85)90091-6. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen J.C. Springer; 2014. Hemagglutination-inhibition Assay for Influenza Virus Subtype Identification and the Detection and Quantitation of Serum Antibodies to Influenza Virus. Animal Influenza Virus; pp. 11–25. [DOI] [PubMed] [Google Scholar]
- 56.Dowdle W., Galphin J., Coleman M., Schild G. A simple double immunodiffusion test for typing influenza viruses. Bull. World Health Organ. 1974;51:213. [PMC free article] [PubMed] [Google Scholar]
- 57.Schild G., Wood J., Newman R. A single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen: proposals for an assay method for the haemagglutinin content of influenza vaccines. Bull. World Health Organ. 1975;52:223. [PMC free article] [PubMed] [Google Scholar]
- 58.Williams M. Single-radial-immunodiffusion as an in vitro potency assay for human inactivated viral vaccines. Vet. Microbiol. 1993;37:253–262. doi: 10.1016/0378-1135(93)90027-5. [DOI] [PubMed] [Google Scholar]
- 59.Choi Y., Lee S., Kwon S.Y., Lee Y., Park Y.K., Ban S.J. Analysis of the proficiency of single radial immunodiffusion assays for quality control of influenza vaccines in Korea. Biologicals. 2017;50:137–140. doi: 10.1016/j.biologicals.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Tulloch W. Observations on the virus of influenza, with a view to elaborating a simple diagnostic test whereby its presence in the respiratory tract of man may Be revealed—Part I. Edinb. Med. J. 1939;46:117. [PMC free article] [PubMed] [Google Scholar]
- 61.Leirs K., Tewari Kumar P., Decrop D., Pérez-Ruiz E., Leblebici P., Van Kelst B., et al. Bioassay development for ultrasensitive detection of influenza a nucleoprotein using digital ELISA. Anal. Chem. 2016;88:8450–8458. doi: 10.1021/acs.analchem.6b00502. [DOI] [PubMed] [Google Scholar]
- 62.Lin C., Guo Y., Zhao M., Sun M., Luo F., Guo L., et al. Highly sensitive colorimetric immunosensor for influenza virus H5N1 based on enzyme-encapsulated liposome. Anal. Chim. Acta. 2017;963:112–118. doi: 10.1016/j.aca.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 63.Zhang P., Vemula S.V., Zhao J., Du B., Mohan H., Liu J., et al. A highly sensitive europium nanoparticle-based immunoassay for detection of influenza A/B virus antigen in clinical specimens. J. Clin. Microbiol. 2014;52:4385–4387. doi: 10.1128/JCM.02635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pillai-Kastoori L., Schutz-Geschwender A.R., Harford J.A. A systematic approach to quantitative Western blot analysis. Anal. Biochem. 2020;593:113608. doi: 10.1016/j.ab.2020.113608. [DOI] [PubMed] [Google Scholar]
- 65.He Q., Chong K.H., Chng H.H., Leung B., Ling A.E., Wei T., et al. Development of a Western blot assay for detection of antibodies against coronavirus causing severe acute respiratory syndrome. Clin. Diagn. Lab. Immunol. 2004;11:417–422. doi: 10.1128/CDLI.11.2.417-422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., et al. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen T., Zoëga Andreasen S., Wolff A., Duong Bang D. From lab on a chip to point of care devices: the role of open source microcontrollers. Micromachines. 2018;9:403. doi: 10.3390/mi9080403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.James A.S., Alwneh J.I. COVID-19 infection diagnosis: potential impact of isothermal amplification technology to reduce community transmission of SARS-CoV-2. Diagnostics. 2020;10:399. doi: 10.3390/diagnostics10060399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Isere E.E., Fatiregun A.A., Ajayi I.O. An overview of disease surveillance and notification system in Nigeria and the roles of clinicians in disease outbreak prevention and control. Niger. Med. J. 2015;56:161. doi: 10.4103/0300-1652.160347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones G., Le Hello S., Jourdan-da Silva N., Vaillant V., De Valk H., Weill F., et al. The French human Salmonella surveillance system: evaluation of timeliness of laboratory reporting and factors associated with delays, 2007 to 2011. Euro Surveill. 2014;19:20664. doi: 10.2807/1560-7917.es2014.19.1.20664. [DOI] [PubMed] [Google Scholar]
- 71.Mahapatra S., Chandra P. Clinically practiced and commercially viable nanobio engineered analytical methods for COVID-19 diagnosis. Biosens. Bioelectron. 2020:112361. doi: 10.1016/j.bios.2020.112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen M., Zhou Y., Ye J., Al-Maskri A.A.A., Kang Y., Zeng S., et al. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020;10(2):97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balboni A., Gallina L., Palladini A., Prosperi S., Battilani M. A real-time PCR assay for bat SARS-like coronavirus detection and its application to Italian greater horseshoe bat faecal sample surveys. Sci. World J. 2012:2012. doi: 10.1100/2012/989514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uhlenhaut C., Cohen J.I., Pavletic S., Illei G., Gea-Banacloche J.C., Abu-Asab M., et al. Use of a novel virus detection assay to identify coronavirus HKU1 in the lungs of a hematopoietic stem cell transplant recipient with fatal pneumonia. Transpl. Infect. Dis. 2012;14:79–85. doi: 10.1111/j.1399-3062.2011.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adachi D., Johnson G., Draker R., Ayers M., Mazzulli T., Talbot P., et al. Comprehensive detection and identification of human coronaviruses, including the SARS-associated coronavirus, with a single RT-PCR assay. J. Virol Methods. 2004;122:29–36. doi: 10.1016/j.jviromet.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Setianingsih T.Y., Wiyatno A., Hartono T.S., Hindawati E., Dewantari A.K., Myint K.S., et al. Detection of multiple viral sequences in the respiratory tract samples of suspected Middle East respiratory syndrome coronavirus patients in Jakarta, Indonesia 2015–2016. Int. J. Infect. Dis. 2019;86:102–107. doi: 10.1016/j.ijid.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DiMaio M.A., Sahoo M.K., Waggoner J., Pinsky B.A. Comparison of Xpert Flu rapid nucleic acid testing with rapid antigen testing for the diagnosis of influenza A and B. J. Virol Methods. 2012;186:137–140. doi: 10.1016/j.jviromet.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park Y.J., Jin J.Y., Yang H.J., Lee W.R., Lee D.H., Pyun B.-Y., et al. Clinical characteristics of 2009 pandemic influenza A (H1N1) infection in children and the performance of rapid antigen test. Korean J. Pediatr. 2011;54:405. doi: 10.3345/kjp.2011.54.10.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahony J.B., Petrich A., Smieja M. Molecular diagnosis of respiratory virus infections. Crit. Rev. Clin. Lab Sci. 2011;48:217–249. doi: 10.3109/10408363.2011.640976. [DOI] [PubMed] [Google Scholar]
- 80.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chu D.K., Pan Y., Cheng S.M., Hui K.P., Krishnan P., Liu Y., et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruning A., Aatola H., Toivola H., Ikonen N., Savolainen-Kopra C., Blomqvist S., et al. Rapid detection and monitoring of human coronavirus infections. New Microbes New Infect. 2018;24:52–55. doi: 10.1016/j.nmni.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaunt E.R., Hardie A., Claas E.C., Simmonds P., Templeton K.E. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J. Clin. Microbiol. 2010;48:2940–2947. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho C.H., Lee C.K., Nam M.H., Yoon S.Y., Lim C.S., Cho Y., et al. Evaluation of the AdvanSure™ real-time RT-PCR compared with culture and Seeplex RV15 for simultaneous detection of respiratory viruses. Diagn. Microbiol. Infect. Dis. 2014;79:14–18. doi: 10.1016/j.diagmicrobio.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kashir J., Yaqinuddin A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med. Hypotheses. 2020:109786. doi: 10.1016/j.mehy.2020.109786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li D., Wang D., Dong J., Wang N., Huang H., Xu H., et al. False-negative results of real-time reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: role of deep-learning-based CT diagnosis and insights from two cases. Korean J. Radiol. 2020;21:505–508. doi: 10.3348/kjr.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang W., Yan F. Patients with RT-PCR-confirmed COVID-19 and normal chest CT. Radiology. 2020;295 doi: 10.1148/radiol.2020200702. E3-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chua F., Armstrong-James D., Desai S.R., Barnett J., Kouranos V., Kon O.M., et al. The role of CT in case ascertainment and management of COVID-19 pneumonia in the UK: insights from high-incidence regions. Lancet Respir. Med. 2020;8:438–440. doi: 10.1016/S2213-2600(20)30132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W., et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health. 2020;8(4):e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferretti L., Wymant C., Kendall M., Zhao L., Nurtay A., Abeler-Dörner L., et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020:368. doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cho H., Ippolito D., Yu Y.W. Contact tracing mobile apps for covid-19: privacy considerations and related trade-offs. arXiv preprint arXiv. 2020:200311511. [Google Scholar]
- 93.Silva N.F., Neves M.M., Magalhães J.M., Freire C., Delerue-Matos C. Emerging electrochemical biosensing approaches for detection of Listeria monocytogenes in food samples: an overview. Trends Food Sci. Technol. 2020;99:621–633. [Google Scholar]
- 94.Kumar S., Sharma J.G., Maji S., Malhotra B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016;78:497–504. doi: 10.1016/j.bios.2015.11.084. [DOI] [PubMed] [Google Scholar]
- 95.Yadav A.K., Dhiman T.K., Lakshmi G., Berlina A.N., Solanki P.R. A highly sensitive label-free amperometric biosensor for norfloxacin detection based on chitosan-yttria nanocomposite. Int. J. Biol. Macromol. 2020;151:566–575. doi: 10.1016/j.ijbiomac.2020.02.089. [DOI] [PubMed] [Google Scholar]
- 96.Haji-Hashemi H., Norouzi P., Safarnejad M.R., Ganjali M.R. Label-free electrochemical immunosensor for direct detection of Citrus tristeza virus using modified gold electrode. Sensor. Actuator. B Chem. 2017;244:211–216. [Google Scholar]
- 97.Freitas T.A., Proença C.A., Baldo T.A., Materón E.M., Wong A., Magnani R.F., et al. Ultrasensitive immunoassay for detection of Citrus tristeza virus in citrus sample using disposable microfluidic electrochemical device. Talanta. 2019;205:120110. doi: 10.1016/j.talanta.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 98.Joshi S.R., Sharma A., Kim G.-H., Jang J. Low cost synthesis of reduced graphene oxide using biopolymer for influenza virus sensor. Mater. Sci. Eng. C. 2020;108:110465. doi: 10.1016/j.msec.2019.110465. [DOI] [PubMed] [Google Scholar]
- 99.Navakul K., Warakulwit C., Yenchitsomanus P.T., Panya A., Lieberzeit P.A., Sangma C. A novel method for dengue virus detection and antibody screening using a graphene-polymer based electrochemical biosensor. Nanomedicine: nanotechnology, Biol. Med. 2017;13:549–557. doi: 10.1016/j.nano.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 100.Kaushik A., Yndart A., Kumar S., Jayant R.D., Vashist A., Brown A.N., et al. A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein. Sci. Rep. 2018;8:1–5. doi: 10.1038/s41598-018-28035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar S., Mohan A., Guleria R. Biomarkers in cancer screening, research and detection: present and future: a review. Biomarkers. 2006;11:385–405. doi: 10.1080/13547500600775011. [DOI] [PubMed] [Google Scholar]
- 102.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gong J., Dong H., Xia S.Q., Huang Y.Z., Wang D., Zhao Y., et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. MedRxiv. 2020 doi: 10.1186/s12879-020-05681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiang J., Wen J., Yuan X., Xiong S., Zhou X., Liu C., et al. Potential biochemical markers to identify severe cases among COVID-19 patients. medRxiv. 2020 [Google Scholar]
- 107.Henry B.M., De Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 108.Singh R., Hong S., Jang J. Label-free detection of influenza viruses using a reduced graphene oxide-based electrochemical immunosensor integrated with a microfluidic platform. Sci. Rep. 2017;7:42771. doi: 10.1038/srep42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yetisen A.K., Akram M.S., Lowe C.R. Based microfluidic point-of-care diagnostic devices. Lab Chip. 2013;13:2210–2251. doi: 10.1039/c3lc50169h. [DOI] [PubMed] [Google Scholar]
- 110.Thévenot D.R., Durst R.A., Wilson G.S. Biosens. Bioelectron. 2001;16:121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 111.Mohd Said N.A. University College Cork; 2014. Electrochemical Biosensor Based on Microfabricated Electrode Arrays for Life Sciences Applications. [Google Scholar]
- 112.Mekonen A.A. Near East University; 2019. Design of a Novel H5N1 Electrochemical Biosensor. [Google Scholar]
- 113.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14:5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 114.Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 115.Moitra P., Alafeef M., Dighe K., Frieman M., Pan D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020;14(6):7617–7627. doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu X., Wang X., Han L., Chen T., Wang L., Li H., et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens. Bioelectron. 2020;166:112437. doi: 10.1016/j.bios.2020.112437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao Z., Cui H., Song W., Ru X., Zhou W., Yu X. A simple magnetic nanoparticles-based viral rna extraction method for efficient detection of SARS-CoV-2. bioRxiv. 2020 doi: 10.1016/j.talanta.2023.124479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumar V., Mishra S., Sharma R., Agarwal J., Ghoshal U., Khanna T., et al. Development of RNA-based assay for rapid detection of SARS-CoV-2 in clinical samples. bioRxiv. 2020 doi: 10.1159/000522337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92(9):1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Z., Zhang Z., Zhai X., Li Y., Lin L., Zhao H., et al. Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal. Chem. 2020;92:7226–7231. doi: 10.1021/acs.analchem.0c00784. [DOI] [PubMed] [Google Scholar]
- 121.Tian B., Gao F., Fock J., Dufva M., Hansen M.F. Homogeneous circle-to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosens. Bioelectron. 2020:112356. doi: 10.1016/j.bios.2020.112356. [DOI] [PubMed] [Google Scholar]
- 122.Mahari S., Roberts A., Shahdeo D., Gandhi S. eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2. bioRxiv. 2020 [Google Scholar]
- 123.Mavrikou S., Moschopoulou G., Tsekouras V., Kintzios S. Development of a portable, ultra-rapid and ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 spike protein antigen. Sensors. 2020;20:3121. doi: 10.3390/s20113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahmadivand A., Gerislioglu B., Ramezani Z., Kaushik A., Manickam P., Ghoreishi S.A. Femtomolar-level detection of SARS-CoV-2 spike proteins using toroidal plasmonic metasensors. arXiv preprint arXiv. 2020:200608536. doi: 10.1016/j.bios.2021.112971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vadlamani B.S., Uppal T., Verma S.C., Misra M. Functionalized TiO2 nanotube-based electrochemical biosensor for rapid detection of SARS-CoV-2. Sensors. 2020;20:5871. doi: 10.3390/s20205871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murugan D., Bhatia H., Sai V., Satija J. P-FAB: a fiber-optic biosensor device for rapid detection of COVID-19. Trans. Indian Nat. Acad. Eng. 2020;5:211–215. doi: 10.1007/s41403-020-00122-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nag P., Sadani K., Mukherji S. Optical fiber sensors for rapid screening of COVID-19. Trans. Indian Nat. Acad. Eng. 2020;5:233–236. doi: 10.1007/s41403-020-00128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park G.S., Ku K., Baek S.H., Kim S.J., Kim S.I., Kim B.T., et al. Development of reverse transcription loop-mediated isothermal amplification (RT-LAMP) assays targeting SARS-CoV-2. J. Mol. Diagn. 2020;22(6):729–735. doi: 10.1016/j.jmoldx.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Djaileb A., Charron B., Jodaylami M.H., Thibault V., Coutu J., Stevenson K., et al. 2020. A Rapid and Quantitative Serum Test for SARS-CoV-2 Antibodies with Portable Surface Plasmon Resonance Sensing. [Google Scholar]
- 130.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020:1–5. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Calucho E. Biosensors for pandemics May 06, 2020. 2020. Lateral flow devices for COVID-19-related biomarkers; p. 29. [Google Scholar]
- 132.Lin D., Liu L., Zhang M., Hu Y., Yang Q., Guo J., et al. Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. Eur. J. Clin. Microbiol. Infect. Dis. 2020:1–7. doi: 10.1007/s10096-020-03978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pan H., Zhang Z., Cao Q., Wang X., Lin Z. Smart Structures and NDE for Industry 40, Smart Cities, and Energy Systems. International Society for Optics and Photonics; 2020. Conditional assessment of large-scale infrastructure systems using deep learning approaches (Conference Presentation) p. 113820T. [Google Scholar]
- 134.Poon L.L., Leung C.S., Tashiro M., Chan K.H., Wong B.W., Yuen K.Y., et al. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop-mediated isothermal amplification assay. Clin. Chem. 2004;50:1050–1052. doi: 10.1373/clinchem.2004.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pyrc K., Milewska A., Potempa J. Development of loop-mediated isothermal amplification assay for detection of human coronavirus-NL63. J. Virol Methods. 2011;175:133–136. doi: 10.1016/j.jviromet.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mori Y., Nagamine K., Tomita N., Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001;289:150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 137.Shirato K., Yano T., Senba S., Akachi S., Kobayashi T., Nishinaka T., et al. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP) Virol. J. 2014;11:139. doi: 10.1186/1743-422X-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thai H.T.C., Le M.Q., Vuong C.D., Parida M., Minekawa H., Notomi T., et al. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2004;42:1956–1961. doi: 10.1128/JCM.42.5.1956-1961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Njiru Z.K. Loop-mediated isothermal amplification technology: towards point of care diagnostics. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shirato K., Semba S., El-Kafrawy S.A., Hassan A.M., Tolah A.M., Takayama I., et al. Development of fluorescent reverse transcription loop-mediated isothermal amplification (RT-LAMP) using quenching probes for the detection of the Middle East respiratory syndrome coronavirus. J. Virol Methods. 2018;258:41–48. doi: 10.1016/j.jviromet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang P., Wang H., Cao Z., Jin H., Chi H., Zhao J., et al. A rapid and specific assay for the detection of MERS-CoV. Front. Microbiol. 2018;9:1101. doi: 10.3389/fmicb.2018.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y., Odiwuor N., Xiong J., Sun L., Nyaruaba R.O., Wei H., et al. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus rna using colorimetric LAMP. MedRxiv. 2020 [Google Scholar]
- 143.El-Tholoth M., Bau H.H., Song J. A single and two-stage, closed-tube, molecular test for the 2019 novel coronavirus (COVID-19) at home, clinic, and points of entry. ChemRxiv. 2020 [Google Scholar]
- 144.Song J., Liu C., Mauk M.G., Rankin S.C., Lok J.B., Greenberg R.M., et al. Two-stage isothermal enzymatic amplification for concurrent multiplex molecular detection. Clin. Chem. 2017;63:714–722. doi: 10.1373/clinchem.2016.263665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lamb L.E., Bartolone S.N., Ward E., Chancellor M.B. 2020. Rapid detection of novel coronavirus (COVID19) by reverse transcription-loop-mediated isothermal amplification. Available at SSRN 3539654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nair M., Jayant R.D., Kaushik A., Sagar V. Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev. 2016;103:202–217. doi: 10.1016/j.addr.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shafiee H., Wang S., Inci F., Toy M., Henrich T.J., Kuritzkes D.R., et al. Emerging technologies for point-of-care management of HIV infection. Annu. Rev. Med. 2015;66:387–405. doi: 10.1146/annurev-med-092112-143017. [DOI] [PubMed] [Google Scholar]
- 148.Kaushik A., Vabbina P.K., Atluri V., Shah P., Vashist A., Jayant R.D., et al. Electrochemical monitoring-on-chip (E-MoC) of HIV-infection in presence of cocaine and therapeutics. Biosens. Bioelectron. 2016;86:426–431. doi: 10.1016/j.bios.2016.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang S.-B., Tang D.-Y. Electrochemical immune-biosensor for immunoglobulin G based bioelectrocatalytic reaction on micro-comb electrodes. Bioproc. Biosyst. Eng. 2008;31:385–392. doi: 10.1007/s00449-007-0173-5. [DOI] [PubMed] [Google Scholar]
- 150.Grieshaber D., MacKenzie R., Vörös J., Reimhult E. Electrochemical biosensors-sensor principles and architectures. Sensors. 2008;8:1400–1458. doi: 10.3390/s80314000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kaushik A., Vasudev A., Arya S.K., Pasha S.K., Bhansali S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014;53:499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 152.Kaushik A., Jayant R.D., Tiwari S., Vashist A., Nair M. Nano-biosensors to detect beta-amyloid for Alzheimer's disease management. Biosens. Bioelectron. 2016;80:273–287. doi: 10.1016/j.bios.2016.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kaushik A., Tiwari S., Jayant R.D., Marty A., Nair M. Towards detection and diagnosis of Ebola virus disease at point-of-care. Biosens. Bioelectron. 2016;75:254–272. doi: 10.1016/j.bios.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Skottrup P.D., Nicolaisen M., Justesen A.F. Towards on-site pathogen detection using antibody-based sensors. Biosens. Bioelectron. 2008;24:339–348. doi: 10.1016/j.bios.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 155.Xiao Y., Lubin A.A., Heeger A.J., Plaxco K.W. Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew. Chem. 2005;117:5592–5595. doi: 10.1002/anie.200500989. [DOI] [PubMed] [Google Scholar]
- 156.Fan C., Plaxco K.W., Heeger A.J. Electrochemical interrogation of conformational changes as a reagentless method for the sequence-specific detection of DNA. Proc. Natl. Acad. Sci. Unit. States Am. 2003;100:9134–9137. doi: 10.1073/pnas.1633515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J. Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis. 2005;17:7–14. [Google Scholar]
- 158.Solanki P.R., Kaushik A., Agrawal V.V., Malhotra B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011;3:17–24. [Google Scholar]
- 159.Labib M., Sargent E.H., Kelley S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016;116:9001–9090. doi: 10.1021/acs.chemrev.6b00220. [DOI] [PubMed] [Google Scholar]
- 160.Cao Q., Kim H.-s., Pimparkar N., Kulkarni J.P., Wang C., Shim M., et al. Medium-scale carbon nanotube thin-film integrated circuits on flexible plastic substrates. Nature. 2008;454:495–500. doi: 10.1038/nature07110. [DOI] [PubMed] [Google Scholar]
- 161.Seker E., Berdichevsky Y., Begley M.R., Reed M.L., Staley K.J., Yarmush M.L. The fabrication of low-impedance nanoporous gold multiple-electrode arrays for neural electrophysiology studies. Nanotechnology. 2010;21:125504. doi: 10.1088/0957-4484/21/12/125504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Squires T.M., Quake S.R. Microfluidics: fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005;77:977. [Google Scholar]
- 163.Patterson A.S., Hsieh K., Soh H.T., Plaxco K.W. Electrochemical real-time nucleic acid amplification: towards point-of-care quantification of pathogens. Trends Biotechnol. 2013;31:704–712. doi: 10.1016/j.tibtech.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 164.Hsieh K., Ferguson B.S., Eisenstein M., Plaxco K.W., Soh H.T. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc. Chem. Res. 2015;48:911–920. doi: 10.1021/ar500456w. [DOI] [PubMed] [Google Scholar]
- 165.Arugula M.A., Zhang Y., Simonian A.L. Biosensors as 21st century technology for detecting genetically modified organisms in food and feed. Anal. Chem. 2014;86:119–129. doi: 10.1021/ac402898j. [DOI] [PubMed] [Google Scholar]
- 166.Moschopoulou G., Vitsa K., Bem F., Vassilakos N., Perdikaris A., Blouhos P., et al. Engineering of the membrane of fibroblast cells with virus-specific antibodies: a novel biosensor tool for virus detection. Biosens. Bioelectron. 2008;24:1027–1030. doi: 10.1016/j.bios.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 167.Pejcic B., De Marco R., Parkinson G. The role of biosensors in the detection of emerging infectious diseases. Analyst. 2006;131:1079–1090. doi: 10.1039/b603402k. [DOI] [PubMed] [Google Scholar]
- 168.Niemz A., Ferguson T.M., Boyle D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011;29:240–250. doi: 10.1016/j.tibtech.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wei Y., Wong L.P., Toh C.-S. Fuel cell virus sensor using virus capture within antibody-coated nanochannels. Anal. Chem. 2013;85:1350–1357. doi: 10.1021/ac302942y. [DOI] [PubMed] [Google Scholar]
- 170.Cheng M.S., Ho J.S., Tan C.H., Wong J.P.S., Ng L.C., Toh C.-S. Development of an electrochemical membrane-based nanobiosensor for ultrasensitive detection of dengue virus. Anal. Chim. Acta. 2012;725:74–80. doi: 10.1016/j.aca.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 171.Cavalcanti I.T., Guedes M.I., Sotomayor M.D., Yamanaka H., Dutra R.F. A label-free immunosensor based on recordable compact disk chip for early diagnostic of the dengue virus infection. Biochem. Eng. J. 2012;67:225–230. [Google Scholar]
- 172.Nguyen B.T.T., Peh A.E.K., Chee C.Y.L., Fink K., Chow V.T., Ng M.M., et al. Electrochemical impedance spectroscopy characterization of nanoporous alumina dengue virus biosensor. Bioelectrochemistry. 2012;88:15–21. doi: 10.1016/j.bioelechem.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 173.Tang D., Tang J., Su B., Ren J., Chen G. Simultaneous determination of five-type hepatitis virus antigens in 5 min using an integrated automatic electrochemical immunosensor array. Biosens. Bioelectron. 2010;25:1658–1662. doi: 10.1016/j.bios.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 174.de la Escosura-Muñiz A., Maltez-da Costa M., Sánchez-Espinel C., Díaz-Freitas B., Fernández-Suarez J., González-Fernández Á., et al. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosens. Bioelectron. 2010;26:1710–1714. doi: 10.1016/j.bios.2010.07.069. [DOI] [PubMed] [Google Scholar]
- 175.Heo N.S., Zheng S., Yang M., Lee S.J., Lee S.Y., Kim H.-J., et al. Label-free electrochemical diagnosis of viral antigens with genetically engineered fusion protein. Sensors. 2012;12:10097–10108. doi: 10.3390/s120810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ma C., Xie G., Zhang W., Liang M., Liu B., Xiang H. Label-free sandwich type of immunosensor for hepatitis C virus core antigen based on the use of gold nanoparticles on a nanostructured metal oxide surface. Microchim. Acta. 2012;178:331–340. [Google Scholar]
- 177.Hejazi M., Pournaghi-Azar M., Ahour F. Electrochemical detection of short sequences of hepatitis C 3a virus using a peptide nucleic acid-assembled gold electrode. Anal. Biochem. 2010;399:118–124. doi: 10.1016/j.ab.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 178.Pournaghi-Azar M., Ahour F., Hejazi M. Direct detection and discrimination of double-stranded oligonucleotide corresponding to hepatitis C virus genotype 3a using an electrochemical DNA biosensor based on peptide nucleic acid and double-stranded DNA hybridization. Anal. Bioanal. Chem. 2010;397:3581–3587. doi: 10.1007/s00216-010-3875-5. [DOI] [PubMed] [Google Scholar]
- 179.Sowole M.A., Kraatz H.B. Electrochemical detection of hepatitis C viral NS3-4A protease. Analyst. 2012;137:1120–1124. doi: 10.1039/c2an15881g. [DOI] [PubMed] [Google Scholar]
- 180.Park J.Y., Lee Y.S., Chang B.Y., Kim B.H., Jeon S., Park S.M. Label-free impedimetric sensor for a ribonucleic acid oligomer specific to hepatitis C virus at a self-assembled monolayer-covered electrode. Anal. Chem. 2010;82:8342–8348. doi: 10.1021/ac1019232. [DOI] [PubMed] [Google Scholar]
- 181.Kheiri F., Sabzi R., Jannatdoust E., Shojaeefar E., Sedghi H. A novel amperometric immunosensor based on acetone-extracted propolis for the detection of the HIV-1 p24 antigen. Biosens. Bioelectron. 2011;26:4457–4463. doi: 10.1016/j.bios.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 182.Zheng L., Jia L., Li B., Situ B., Liu Q., Wang Q., et al. A sandwich HIV p24 amperometric immunosensor based on a direct gold electroplating-modified electrode. Molecules. 2012;17:5988–6000. doi: 10.3390/molecules17055988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Labib M., Martić S., Shipman P.O., Kraatz H.B. Electrochemical analysis of HIV-1 reverse transcriptase serum level: exploiting protein binding to a functionalized nanostructured surface. Talanta. 2011;85:770–778. doi: 10.1016/j.talanta.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 184.Hassen W.M., Duplan V., Frost E., Dubowski J.J. Quantitation of influenza A virus in the presence of extraneous protein using electrochemical impedance spectroscopy. Electrochim. Acta. 2011;56:8325–8328. [Google Scholar]
- 185.Li F., Mei L., Li Y., Zhao K., Chen H., Wu P., et al. Facile fabrication of magnetic gold electrode for magnetic beads-based electrochemical immunoassay: application to the diagnosis of Japanese encephalitis virus. Biosens. Bioelectron. 2011;26:4253–4256. doi: 10.1016/j.bios.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 186.Huy T.Q., Hanh N.T.H., Thuy N.T., Van Chung P., Nga P.T., Tuan M.A. A novel biosensor based on serum antibody immobilization for rapid detection of viral antigens. Talanta. 2011;86:271–277. doi: 10.1016/j.talanta.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta. 2019;186:224. doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nidzworski D., Siuzdak K., Niedziałkowski P., Bogdanowicz R., Sobaszek M., Ryl J., et al. A rapid-response ultrasensitive biosensor for influenza virus detection using antibody modified boron-doped diamond. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-15806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lee W.S., Kang T., Kim S.H., Jeong J. An antibody-immobilized silica inverse opal nanostructure for label-free optical biosensors. Sensors. 2018;18:307. doi: 10.3390/s18010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaushik A., Tiwari S., Jayant R.D., Vashist A., Nikkhah-Moshaie R., El-Hage N., et al. Electrochemical biosensors for early stage Zika diagnostics. Trends Biotechnol. 2017;35:308–317. doi: 10.1016/j.tibtech.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vasudev A., Kaushik A., Tomizawa Y., Norena N., Bhansali S. An LTCC-based microfluidic system for label-free, electrochemical detection of cortisol. Sensor. Actuator. B Chem. 2013;182:139–146. [Google Scholar]
- 192.Kaushik A., Vasudev A., Arya S.K., Bhansali S. Mediator and label free estimation of stress biomarker using electrophoretically deposited Ag@ AgO–polyaniline hybrid nanocomposite. Biosens. Bioelectron. 2013;50:35–41. doi: 10.1016/j.bios.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 193.Kaushik A., Yndart A., Jayant R.D., Sagar V., Atluri V., Bhansali S., et al. Electrochemical sensing method for point-of-care cortisol detection in human immunodeficiency virus-infected patients. Int. J. Nanomed. 2015;10:677. doi: 10.2147/IJN.S75514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cruz A.F.D., Norena N., Kaushik A., Bhansali S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014;62:249–254. doi: 10.1016/j.bios.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 195.Maduraiveeran G., Sasidharan M., Ganesan V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018;103:113–129. doi: 10.1016/j.bios.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 196.Swayamsiddha S., Mohanty C. Application of cognitive internet of medical things for COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020 doi: 10.1016/j.dsx.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang T., Gentile M., Shen C.-F., Cheng C.-M. Multidisciplinary Digital Publishing Institute; 2020. Combining Point-Of-Care Diagnostics and Internet of Medical Things (IoMT) to Combat the COVID-19 Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adly A.S., Adly A.S., Adly M.S. Approaches based on artificial intelligence and the internet of intelligent things to prevent the spread of COVID-19: scoping review. J. Med. Internet Res. 2020;22 doi: 10.2196/19104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Singh R.P., Javaid M., Haleem A., Suman R. Internet of things (IoT) applications to fight against COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(4):521–524. doi: 10.1016/j.dsx.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vaishya R., Javaid M., Khan I., Haleem A. Artificial intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(4):337–339. doi: 10.1016/j.dsx.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kaushik A.K., Dhau J.S., Gohel H., Mishra Y.K., Kateb B., Kim N.-Y., et al. Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Appl. Bio Mater. 2020;3(11):7306–7325. doi: 10.1021/acsabm.0c01004. [DOI] [PubMed] [Google Scholar]
- 202.Hamet P., Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69:S36–S40. doi: 10.1016/j.metabol.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 203.Miller D.D., Brown E.W. Artificial intelligence in medical practice: the question to the answer? Am. J. Med. 2018;131:129–133. doi: 10.1016/j.amjmed.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 204.Yu K., Beam A., Kohane I. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018;2:719–731. doi: 10.1038/s41551-018-0305-z. [DOI] [PubMed] [Google Scholar]
- 205.Albahri A., Hamid R.A. Role of biological data mining and machine learning techniques in detecting and diagnosing the novel coronavirus (COVID-19): a systematic review. J. Med. Syst. 2020:44. doi: 10.1007/s10916-020-01582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zheng X., Sun S., Mukkamala R.R., Vatrapu R., Ordieres-Meré J. Accelerating health data sharing: a solution based on the internet of things and distributed ledger technologies. J. Med. Internet Res. 2019;21 doi: 10.2196/13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shen C., Chen A., Luo C., Zhang J., Feng B., Liao W. Using reports of symptoms and diagnoses on social media to predict COVID-19 case counts in Mainland China: observational infoveillance study. J. Med. Internet Res. 2020;22 doi: 10.2196/19421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nathani B., Vijayvergia R. 2017 International Conference on Intelligent Communication and Computational Techniques (ICCT) IEEE; 2017. The Internet of intelligent things: an overview; pp. 119–122. [Google Scholar]
- 209.Long E., Lin H., Liu Z., Wu X., Wang L., Jiang J., et al. An artificial intelligence platform for the multihospital collaborative management of congenital cataracts. Nat. Biomed. Eng. 2017;1:1–8. [Google Scholar]
- 210.McCall B. COVID-19 and artificial intelligence: protecting health-care workers and curbing the spread. Lancet Digit. Health. 2020;2:e166–e167. doi: 10.1016/S2589-7500(20)30054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang Z., Zeng Z., Wang K., Wong S.-S., Liang W., Zanin M., et al. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J. Thorac. Dis. 2020;12:165. doi: 10.21037/jtd.2020.02.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mujawar M.A., Gohel H., Bhardwaj S.K., Srinivasan S., Hickman N., Kaushik A. Aspects of nano-enabling biosensing systems for intelligent healthcare; towards COVID-19 management. Mater. Today Chem. 2020:100306. doi: 10.1016/j.mtchem.2020.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shi F., Wang J., Shi J., Wu Z., Wang Q., Tang Z., et al. IEEE Reviews in Biomedical Engineering. 2020. Review of artificial intelligence techniques in imaging data acquisition, segmentation and diagnosis for covid-19. [DOI] [PubMed] [Google Scholar]
- 214.Alimadadi A., Aryal S., Manandhar I., Munroe P.B., Joe B., Cheng X. American Physiological Society; Bethesda, MD: 2020. Artificial Intelligence and Machine Learning to Fight COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. N. Engl. J. Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 216.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hurt B., Kligerman S., Hsiao A. Deep learning localization of pneumonia: 2019 coronavirus (COVID-19) Outbreak. J. Thorac. Imaging. 2020;35:W87–W89. doi: 10.1097/RTI.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chun A. In a time of coronavirus, Chinas investment in AI is paying off in a big way. South China Morning Post. 2020:18. [Google Scholar]
- 219.Effenberger M., Kronbichler A., Shin J.I., Mayer G., Tilg H., Perco P. Association of the COVID-19 pandemic with internet search volumes: a google trendstm analysis. Int. J. Infect. Dis. 2020;95:192–197. doi: 10.1016/j.ijid.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ting D.S.W., Carin L., Dzau V., Wong T.Y. Digital technology and COVID-19. Nat. Med. 2020;26:459–461. doi: 10.1038/s41591-020-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen J., See K.C. Artificial intelligence for COVID-19: rapid review. J. Med. Internet Res. 2020;22 doi: 10.2196/21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Alsalem M., Zaidan A., Zaidan B., Hashim M., Albahri O., Albahri A., et al. Systematic review of an automated multiclass detection and classification system for acute Leukaemia in terms of evaluation and benchmarking, open challenges, issues and methodological aspects. J. Med. Syst. 2018;42:204. doi: 10.1007/s10916-018-1064-9. [DOI] [PubMed] [Google Scholar]
- 223.Alsalem M., Zaidan A., Zaidan B., Albahri O., Alamoodi A., Albahri A., et al. Multiclass benchmarking framework for automated acute Leukaemia detection and classification based on BWM and group-VIKOR. J. Med. Syst. 2019;43:212. doi: 10.1007/s10916-019-1338-x. [DOI] [PubMed] [Google Scholar]
- 224.Singh R.P., Javaid M., Haleem A., Vaishya R., Al S. Internet of medical things (IoMT) for orthopaedic in COVID-19 pandemic: roles, challenges, and applications. J. Clin. Orthop. Trauma. 2020 doi: 10.1016/j.jcot.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lin B., Wu S. COVID-19 (Coronavirus Disease 2019): opportunities and challenges for digital health and the internet of medical things in China. OMICS A J. Integr. Biol. 2020;24:231–232. doi: 10.1089/omi.2020.0047. [DOI] [PubMed] [Google Scholar]
- 226.Joyia G.J., Liaqat R.M., Farooq A., Rehman S. Internet of Medical Things (IOMT): applications, benefits and future challenges in healthcare domain. J. Commun. 2017;12:240–247. [Google Scholar]
- 227.Srinivasan B., Tung S. Development and applications of portable biosensors. J. Lab. Autom. 2015;20:365–389. doi: 10.1177/2211068215581349. [DOI] [PubMed] [Google Scholar]