Young adults who quit smoking subsequently had lower metabolic risk behaviors. Among those with unmanaged stress, the link was explained by greater readiness to increase fruit and vegetable intake.
Keywords: diet, exercise, health risk behaviors, smoking cessation, stress, young adult
Abstract
Smoking cessation may support changes in metabolic risk behaviors (e.g., high-fat diet, physical inactivity, poor sleep, low fruit and vegetable consumption [FVC]). We examined the association between smoking cessation and metabolic risk behavior profiles, mediated by readiness to change risk behaviors and moderated by stress management. Participants were young adult smokers in a randomized controlled trial of a Facebook smoking cessation intervention. Measures included stage of change for five metabolic risk behaviors: FVC, diet, physical activity, sleep hygiene, and stress management. Moderated mediation was used to examine relationships between smoking cessation at T1 (predictor), readiness to change metabolic risk behaviors at T2 (mediators), stress management at T3 (moderator), and metabolic risk behavior profile at T3 (outcome) over 9 months. T1 smoking abstinence was associated with greater readiness to increase FVC at T2, which predicted lower likelihood of T3 metabolic risk (β = −0.22, 95% confidence interval [CI] [−0.53, −0.03]). This indirect effect was moderated by stress management such that greater readiness to increase FVC at T2 was associated with lower T3 metabolic risk for participants with unmanaged stress (β = −0.90, 95% CI [−1.32, −0.49], p < .001), but not for participants with well-managed stress (β = −.22, 95% CI [−0.48, 0.04], p = .096). Young adults who quit smoking subsequently had lower metabolic risk behaviors. Among participants with unmanaged stress, those who quit smoking had greater readiness to increase FVC and lower likelihood of subsequent metabolic risk. Smoking cessation interventions could aim to teach broadly applicable behavior change skills and build confidence for decreasing metabolic risk.
Implications.
Practice: Smoking cessation interventions for young adults should aim to build self-efficacy for increasing fruit and vegetable consumption and should teach skills that can be applied to both smoking and other health behaviors.
Policy: To maximize improvements to health, public health initiatives aimed at young adults who smoke cigarettes should also emphasize fruit and vegetable consumption.
Research: Future research is needed to determine how best to implement nutrition-related content in smoking cessation interventions for young adults.
INTRODUCTION
Health risk behaviors tend to cluster together, such that people who engage in some risk behaviors (e.g., smoking) are more likely to engage in others [1]. Across a variety of age groups and cultures, smoking is associated with a high-fat diet [2–4], physical inactivity [2–6], low fruit and vegetable consumption (FVC) [4–8], and poor sleep [9]. Clustering of health risk behaviors may be due to common underlying principles of behavior change that can be applied across behaviors [10,11] or due to changes in habit strength, such that when one behavior becomes habitual, self-regulatory resources can be reallocated to another behavior [12]. Among health risk behaviors, smoking is the leading cause of preventable death worldwide [13]. Quitting smoking confers major health benefits, especially when smokers quit during young adulthood [14]. However, some former smokers retain behaviors that put them at risk for metabolic syndrome [15], a grouping of conditions (e.g., hypertension, high cholesterol, insulin resistance) that together raise one’s risk for atherosclerotic cardiovascular disease and type 2 diabetes mellitus [16]. Behavioral risk factors for metabolic syndrome include physical inactivity [17], low FVC [18], high-fat diet [19], poor sleep hygiene [20], and unmanaged stress [21]. As such, improving multiple health risk behaviors is likely to result in greater improvements to metabolic health than quitting smoking alone.
Smoking cessation may be followed by positive changes in metabolic risk behaviors over time if former smokers adopt a healthier lifestyle [15,22]. Alternatively, smoking cessation may be associated with a subsequent increase in metabolic risk behaviors, especially if stress is not managed effectively [23–25]. Stress is a common trigger for smoking [26] and a commonly reported barrier to smoking cessation [25]. Overeating, sedentary activities (e.g., TV, video games) and other coping mechanisms may be used instead of smoking to combat stress. Indeed, smokers who experience unmanaged stress are also more likely to engage in pathological eating behavior [24] and experience food cravings [23], and poor stress management is associated with poor diet in both smokers and nonsmokers [27]. Most research in this area is cross-sectional, and longitudinal research is needed to understand how changes in smoking, readiness to change other behaviors, and stress management can affect subsequent metabolic risk.
Behavior change can be conceptualized as a multistage, longitudinal process. The Transtheoretical Model describes varying stages of readiness to change a specific behavior: precontemplation (not ready to change), contemplation (ready to change in the next 6 months), preparation (ready to change in the next 30 days), action (behavior change has been initiated), and maintenance (behavior change has been sustained for 6 months or more) [28]. Prior research has suggested that multiple behaviors may change sequentially [29]; however, research has not examined readiness to change as a mechanism for the relationships between smoking and metabolic risk, nor the potential moderating role of stress management during this behavior change process.
Using a sample of young adults participating in a randomized controlled trial of a 90-day smoking cessation intervention, the present study examined the relationship between abstinence and metabolic risk over 9 months, mediated by readiness to change metabolic risk behaviors and moderated by stress management. Risk may be conceptualized as the presence of a group of health risk behaviors, and individuals with multiple risk factors are more likely to have negative health outcomes than those with a single risk factor [30]. Changing multiple health risk behaviors (e.g., quitting smoking, increasing physical activity, improving diet) is key to reducing metabolic risk [16]. Therefore, a clustered variable that describes a constellation of relevant risk behaviors is likely to yield a clearer image of an individual’s behavioral metabolic risk factor profile. Prior work from our group identified three latent classes of risk behaviors in the present sample: metabolic risk, substance use risk, and low risk [31]. As such, we used membership in the metabolic risk latent class, determined based on multiple health risk behaviors, as a proxy for metabolic risk.
We predicted that smoking cessation would be associated with increased readiness to change other health risk behaviors and, subsequently, lower metabolic risk. Data for this study come from 3 (T1)-, 6 (T2)-, and 12 (T3)-month outcomes from a randomized controlled trial evaluating a smoking cessation intervention for young adults delivered on Facebook [32]. Our first hypothesis was that abstinence from smoking at T1 would be associated with subsequent positive change in general health orientation, such that successfully quitting smoking would be followed by readiness to change one or more metabolic risk behaviors at T2. Because some studies show mixed associations between smoking and readiness to change different metabolic risk behaviors, such as diet and exercise [7,8], we examined readiness to change each metabolic risk behavior (FVC, diet, physical activity, sleep hygiene, and stress management) separately at T2. Our second hypothesis is that readiness to change at T2 would be associated with lower metabolic risk at T3. However, former smokers who are experiencing unmanaged stress may find it more difficult to change behaviors, even if they feel ready to do so. Unmanaged stress may in turn increase the use of metabolic risk behaviors as coping mechanisms. Therefore, our third hypothesis was that stress management would moderate the relationship between smoking cessation and metabolic risk behaviors, such that smoking cessation would be associated with greater (lower) metabolic risk among participants with unmanaged (managed) stress.
METHODS
Participants, design, and procedure
Participants were 500 young adult smokers in the United States participating in a randomized controlled trial of a novel intervention for young adult smokers delivered on Facebook (ClinicalTrials.gov number NCT02207036) [32,33]. Participants were recruited using a paid ad campaign on Facebook between October 2014 and July 2015, with details reported previously [34]. Facebook users who clicked on the ad were taken to a secure, confidential eligibility survey. Eligibility criteria included age (18–25 years old), ability to read and comprehend English, lifetime use of at least 100 cigarettes, current smoking (3+ days per week), and current Facebook use (4+ days per week). Following eligibility screening and consent, participants’ identities and ages were verified using their Facebook profile or a photo ID. Participants were randomly assigned to the intervention condition (n = 251) or the control condition (in which they were given a referral to smokefree.gov; n = 249). Participants in both the intervention and control conditions were included in analyses.
Intervention condition participants were assigned to “secret” (private) Facebook groups tailored to their baseline stage of change for smoking cessation: precontemplation, contemplation, or preparation [28,35]. In the groups, study staff posted once per day for the 90 days. These Facebook posts were designed based on US Clinical Practice Guidelines and Transtheoretical Model skills for smoking cessation [35,36] and were designed to elicit thought, feedback, and/or action from participants. For example, posts tailored toward participants who were not ready to quit smoking focused on using motivational interviewing techniques to encourage participants to weigh the pros and cons of smoking and to build their motivation to quit. Posts tailored toward participants who were ready to quit focused more on preparing to quit, coping with triggers, and identifying social support. In addition to the Facebook posts, live sessions with a smoking cessation counselor were hosted on Facebook once per week. Participants were compensated with a $20 gift card for completing each of four surveys (baseline, 3 months, 6 months, and 12 months) and a bonus $20 for completing all surveys, for a total of $100. In addition, participants were randomly assigned to receive a monetary incentive for commenting on all 90 Facebook posts up to $90, for total possible compensation of $190. Details of the intervention, clinical trial design, and outcomes have been reported previously [32,33]. This research was approved by the UCSF Institutional Review Board.
Measures
At baseline, 3 months, 6 months, and 12 months, participants completed an online survey using Qualtrics survey software. Relevant measures for the present study are described below. The mediating variables (i.e., readiness to change health risk behaviors), moderating variable (i.e., stress management), and the outcome variable (i.e., latent class membership based on health risk behavior profile) were derived from the Staging Health Risk Assessment (S-HRA), developed by Pro-Change Behavior Systems (South Kingstown, RI). The S-HRA measures a variety of current health risk behaviors and behavioral intentions using one item for each behavior (i.e., diet, physical activity, fruit and vegetable consumption, sleep hygiene, and stress management). Guidelines are defined for each health behavior, and participants indicate their readiness to meet guidelines on a continuum from unready to meet guidelines in the near future to already meeting guidelines.
In this study, risk was conceptualized as current behavior, as measured by endorsement of each behavior on the items measuring readiness to change. For example, a participant who reports that they do not currently adhere to exercise recommendations would be considered “at risk” for physical inactivity, regardless of their intention to exercise in the future. Risk status criteria were based on the Healthy People 2020 goals for the United States [37]. The S-HRA staging algorithm has strong predictive validity of future health risk behaviors [35,38–40]. Examples for each behavior are provided below.
Predictor: abstinence from smoking
Seven-day point prevalence abstinence from smoking was used as a measure of abstinence at 3, 6, and 12 months. Participants answered, “How many cigarettes have you smoked in the past 7 days?”. Those who reported having smoked zero cigarettes were considered abstinent. Abstinence was coded as “1” and continued smoking was coded as “0” at each time point. Point prevalence abstinence is highly correlated with prolonged abstinence and generally produces similar effect sizes in research [41].
Mediators
Stages of change for the following health risk behaviors were measured at time 2. Response options for meeting the behavioral guideline in each domain were: “No, and I do not intend to in the next 6 months” (precontemplation stage of change), “No, but I intend to in the next 6 months” (contemplation), “No, but I intend to in the next 30 days” (preparation), “Yes, I have been, but for less than 6 months” (action), and “Yes, I have been for more than 6 months” (maintenance). Data were coded such that higher numbers represent higher stages of change (1 = precontemplation, 5 = maintenance).
Diet.
Participants were asked about their diet in terms of “healthy eating.” Healthy eating was defined as “eating the number of calories that allows you to reach and maintain a healthy weight and eating a diet that is low in fat.” Examples of these behaviors (e.g., paying attention to serving sizes, eating bread without butter) were provided.
Physical activity.
To measure physical activity, participants answered, “Do you engage in regular exercise?”. Definitions of moderate-intensity, vigorous-intensity, and mixed-intensity exercise were provided. For example, vigorous activity was defined as activity that “causes big increases in your breathing and heart rate and makes conversation difficult (such as jogging or running) for at least 75 minutes each week.”
Fruit and vegetable consumption.
To assess FVC, participants answered, “Do you eat at least 4.5 cups of fruits and vegetables per day?”. Examples of serving sizes considered equivalent to 1 cup (e.g., 1 cup cooked vegetables, 1/2 cup dried fruit) were provided.
Sleep hygiene.
To assess sleep hygiene, participants answered, “Do you have good sleep habits?”. Examples of good sleep habits (e.g., getting at least 7 hr of sleep a night, maintaining a regular bed and wake time) were provided.
Stress management.
To measure stress management, participants answered, “Do you effectively practice stress management in your daily life?”. An additional response option was, “I do not currently have any stress in my life.” Examples of stress management (e.g., regular relaxation, making time for social activities) were provided.
Moderator: stress management
Stress management at T3 was measured using the stage of change item described above. Responses were dichotomized and dummy-coded such that 0 = managed stress (action stage, maintenance stage, or no current stress) and 1 = unmanaged stress (precontemplation, contemplation, or preparation stage). Staging items focused on “stress reduction” and “stress management” appear to be part of the same underlying construct [42]; therefore, a self-reported lack of stress was coded as managed stress.
Outcome: metabolic risk
Latent class analysis was previously used to identify three common patterns of health risk behaviors that were stable across baseline, 3 months, 6 months, and 12 months: metabolic risk (characterized by a high-fat diet, low FVC, physical inactivity, poor sleep hygiene, and poor stress management), substance use risk (characterized by heavy drinking, cannabis use, and other drug use), and low risk [31]. These latent classes were identified at each time point and were quite stable over time. Latent transition analysis showed that relatively few participants transitioned between classes [31]. For these analyses, metabolic risk was operationalized as most likely membership in the metabolic risk class (vs. another class) at time 3.
Participant characteristics
Demographic variables included age, sex (male, female, other), and race/ethnicity (non-Hispanic Caucasian, Native American, African American, Asian, Pacific Islander, Hispanic, more than one race). Smoking characteristics included cigarettes per day (10 or fewer, 11–20, 21–30, 31, or more), days of smoking per week, readiness to quit smoking at baseline (precontemplation, contemplation, preparation), quit attempt in the past year (yes/no), degree of nicotine dependence [43], and daily smoking (yes/no).
Analyses
Hypotheses were tested using mediation and moderated mediation analyses, which allow for an examination of the sequential pathway between abstinence from smoking and subsequent metabolic risk, as well as differences in this pathway based on stress management. All analyses were conducted using IBM SPSS 25 and the PROCESS macro, a regression-based SPSS add-on that allows users to specify and test moderation, mediation, and related models [44].
Mediation
First, Model 4 of the PROCESS macro was used to test the indirect effects of readiness to change risk behaviors at T2 on the relationship between T1 smoking abstinence and T3 metabolic risk. A bootstrap resampling process with 5,000 repetitions created 95% bias-corrected confidence intervals (CIs) for the indirect effect. Indirect effects are considered to be statistically significant and indicative of mediation if their CIs do not contain zero. T2 readiness to change diet, physical activity, FVC, sleep, and stress management were entered into the same model as parallel mediators. Nonsignificant mediators were then removed from the model [44].
Moderated mediation
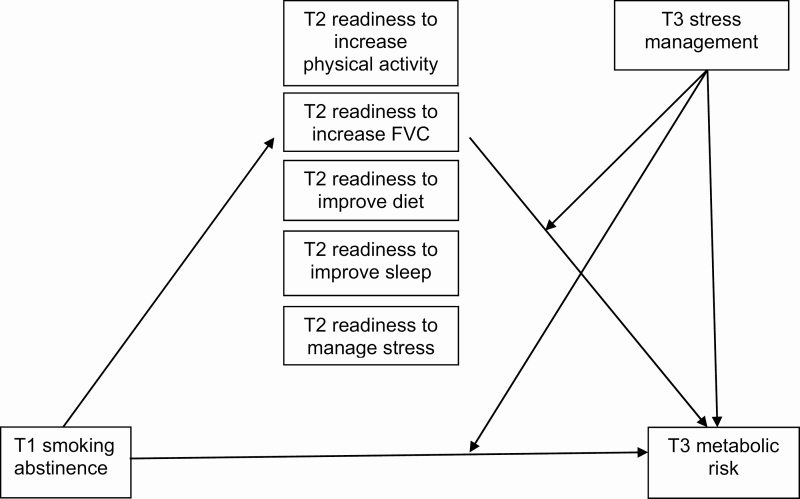
Second, after identifying significant mediator(s) using Model 4, Model 15 of the PROCESS macro [44] was used to test the moderating effect of T3 stress management on the aforementioned mediation model. The hypothesized model (with all possible mediators) is presented in Figure 1. Nonsignificant mediators were from Model 4 were not included in Model 15.
Fig 1.
| Hypothesized moderated mediation model.
RESULTS
Participants were 500 young adults age 18–25. See Table 1 for participant characteristics. Retention rates for completion of health risk behavior measures were 71% (354/500) at T1 (3 months), 65% (323/500) at T2 (6 months), and 69% (343/500) at T3 (12 months). Retention did not differ by treatment group, daily smoking, stage of change, stress management, or membership in the metabolic risk class (p’s > .05) at baseline. Intervention condition was not related to the outcome (i.e., being in the metabolic risk class at T3; χ 2 = 1.01, p = .316), and the pattern of results remained the same when intervention condition was included or excluded from the models. Therefore, to reduce the number of parameters in the models, final analyses did not control for intervention condition.
Table 1.
| Participant characteristics at baseline (N = 500)
| Variable | M (SD) or %/n |
|---|---|
| Age (M/SD) | 20.9 (2.0) |
| Sex (%/n) | |
| Male | 44.8 (224) |
| Female | 54.6 (273) |
| Gender minority | 0.6 [3] |
| Race or ethnicitya (%/n) | |
| Non-Hispanic Caucasian | 73.8 (366) |
| Native American | 1 [5] |
| African American | 2.6 [13] |
| Asian/Pacific Islander | 1.2 [6] |
| Hispanic | 6.9 [34] |
| More than one | 14.5 (72) |
| Cigarettes per day (%/n) | |
| 10 or fewer | 48.0 (240) |
| 11–20 | 46.6 (233) |
| 21–30 | 4.0 [20] |
| 31 or more | 1.4 [7] |
| Cigarettes per day (M/SD) | 11.6 (6.8) |
| Days per week smoked (M/SD) | 6.8 (0.86) |
| Stage of change for smoking at baseline (%/n) | |
| Precontemplation | 30.0 (150) |
| Contempation | 48.6 (243) |
| Preparation | 21.4 (107) |
| Past year 24-hr quit attempt (% yes/SD) | 62.2 (311) |
| FTCD (M/SD) | 3.2 (2.1) |
| Smoke within first 30 min of waking (% yes/n) | 53.2 (266) |
| Daily smoking (% yes/n) | 86.6% (433) |
FTCD, Fagerström Test of Cigarette Dependence.
a n = 496.
Descriptive characteristics
Seven-day point prevalence abstinence rates in the intervention (control) groups were 13.6% (7.5%) at T1, 18.6% (14.5%) at T2, and 21.6% (20.5%) at T3. Readiness to change metabolic risk behaviors at time 2 is reported in Table 2. Among participants who reported smoking at T1, 53.9% were in the metabolic risk group at T3, 32.5% were in the low-risk group, and 13.7% were in the substance use risk group. Among those who were abstinent at T1, 66.7% were in the low-risk group at T3, 25.9% were in the metabolic risk group, and 7.4% were in the substance use risk group.
Table 2.
| Readiness to change health risk behaviors at time 2 by smoking status at time 1
| Smoking at time 1 (n = 269) | Abstinent at time 1 (n = 26) | |||||||
|---|---|---|---|---|---|---|---|---|
| Precontemplation | Contemplation | Preparation | Action/maintenance (low risk) | Precontemplation | Contemplation | Preparation | Action/maintenance (low risk) | |
| Diet | 73 (27.2%) | 93 (34.7%) | 48 (17.9%) | 54 (20.1%) | 7 (26.9%) | 6 (23.1%) | 5 (19.2%) | 8 (30.8%) |
| Physical activity | 24 (9.0%) | 60 (22.4%) | 41 (15.3%) | 143 (53.3%) | 2 (7.7%) | 3 (11.5%) | 1 (3.8%) | 20 (76.9%) |
| FVC | 44 (16.4%) | 81 (30.2%) | 51 (19.0%) | 92 (34.3%) | 1 (3.8%) | 4 (15.4%) | 8 (30.8%) | 13 (50%) |
| Sleep hygiene | 52 (19.4%) | 65 (24.3%) | 47 (17.5%) | 104 (38.8%) | 1 (3.8%) | 4 (15.4%) | 6 (23.1%) | 15 (57.7%) |
| Stress management | 30 (11.2%) | 42 (15.7%) | 47 (17.5%) | 149 (55.6%) | 0 (0%) | 3 (11.5%) | 1 (3.8%) | 22 (84.7%) |
Mediation
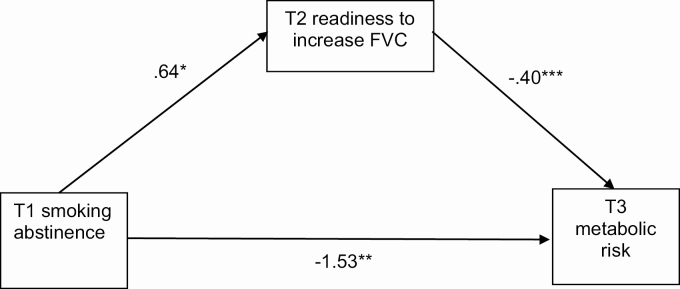
There was a significant direct effect of T1 smoking abstinence on T3 membership in the metabolic risk latent class, such that abstinence at T1 predicted lower likelihood of T3 metabolic risk (β = −1.55, 95% CI [−2.72, −0.37], p = .010). This effect was mediated by readiness to increase FVC at T2 (β = −0.22, 95% CI [−0.53, −0.03], p < .05). Specifically, T1 smoking abstinence was associated with greater readiness to increase FVC at T2, which predicted lower likelihood of T3 metabolic risk. Diet (β = 0.04, 95% CI [−0.04, 0.26]), physical activity (β = −0.13, 95% CI [−0.40, 0.09]), sleep hygiene (β = −0.05, 95% CI [−0.27, 0.08]), and stress management (β = −0.02, 95% CI [−0.20, 0.14]) at T2 were not significant mediators (p’s > .05). T1 smoking abstinence was directly associated with T2 readiness to increase FVC (β = 0.64, 95% CI [0.06, 1.21], p = .030), improve stress management (β = 0.74, 95% CI [0.14, 1.34], p = .016), and improve sleep hygiene (β = 0.73, 95% CI [0.12, 1.34], p = .019). However, T1 smoking abstinence was not associated with readiness to increase physical activity (β = 0.35, 95% CI [−0.22, 0.92], p = .229) or improve diet (β = 0.22, 95% CI [−0.26, 0.69], p = .367). The model including only FVC, the significant mediator, is presented in Figure 2.
Fig 2.
| Mediation model for fruit and vegetable consumption.
Moderated mediation
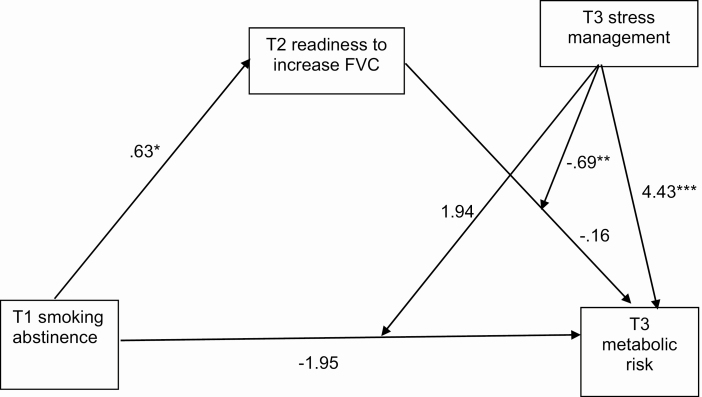
The index of moderated mediation (a test of the equivalence of an indirect effect across different levels of a moderator) [45] was statistically significant, suggesting that the mediating effect of FVC differed by stress management (β = −0.43, 95% CI [−1.13, −0.04], p < .05). Specifically, there was a conditional indirect effect of T1 smoking abstinence on T3 metabolic risk for participants with unmanaged stress (β = −0.53, 95% CI [−1.24, −0.08], p < .05), but not managed stress (β = −0.10, 95% CI [−0.40, 0.04], p > .05). Follow-up moderation analysis (PROCESS Model 1) showed that for participants with unmanaged stress, readiness to increase FVC at T2 was associated with lower metabolic risk at T3 (β = −0.90, 95% CI [−1.32, −0.49], p < .001). For participants with managed stress, readiness to increase FVC at T2 was not significantly associated with metabolic risk at T3 (β = −0.22, 95% CI [−0.48, 0.04], p = .096). Unmanaged stress was associated with greater likelihood of metabolic risk in the full moderated mediation model (β = 4.43, 95% CI [2.66, 6.19], p < .001). Path coefficients for the full model are shown in Figure 3.
Fig 3.
| Moderated mediation model for fruit and vegetable consumption. Six participants who were abstinent from smoking at T1 reported smoking again at T2 and T3. When those participants were excluded from analyses, the standard error of the abstinence X stress interaction term could not be identified. Upon further examination, the issue was caused by 1 participant having a unique combination of abstinence, stress management, and metabolic risk. Removing the other 5 participants did not alter the pattern of results. Therefore, all participants are included in the reported results.
DISCUSSION
This study examined the longitudinal relationship between smoking cessation, metabolic risk behaviors, and stress management among young adult smokers participating in a smoking cessation intervention trial. Over a 9-month period, readiness to increase fruit and vegetable consumption (FVC) mediated the relationship between abstinence from smoking and decreased metabolic risk behaviors. Abstinence from smoking at T1 was associated with greater readiness to increase FVC at T2 and lower likelihood of engaging in metabolic risk behaviors at T3. Unexpectedly, moderated mediation analysis showed that this relationship was largely driven by participants with unmanaged stress, for whom readiness to increase FVC was subsequently associated with lower likelihood of metabolic risk behaviors.
Results of the mediation analysis are consistent with the extant literature on general health orientation and multiple behavior change. Although the present study did not explicitly assess strategies used for multiple behavior change, extant research suggests that as individuals practice behavior change and achieve success, they become more willing to change other behaviors and are more likely to be successful in changing those behaviors [10–12]. Indeed, smoking abstinence predicted lower metabolic risk 9 months later, as determined by a composite of behaviors (i.e., FVC, physical activity, diet, sleep hygiene, and stress management).
The relationship between smoking abstinence and decreased metabolic risk behaviors was mediated by increased readiness to change FVC. This is consistent with extant research that found a stronger relationship between smoking and FVC than smoking and other diet and/or exercise-related variables [7,8]. Of the metabolic behaviors examined, increasing FVC may be the least daunting change for smokers to make. Resisting temptation to eat high-fat, high-calorie foods may be overwhelming for young adults who are also trying to resist smoking. Moreover, increasing physical activity may be particularly difficult for smokers due to diminished cardiovascular fitness resulting from smoking. Interestingly, abstinence from smoking predicted increased readiness to improve stress management and sleep hygiene, as well as readiness to increase FVC. However, readiness to increase FVC at T2 was the only significant mediator of the relationship between abstinence from smoking at T1 and lower metabolic risk behaviors at T3. This suggests that intentions to change multiple behaviors did not necessarily lead to a decreased behavioral risk profile overall. Increasing FVC may be a more attainable target behavior for young adults who recently quit smoking.
The time interval between measurements of smoking cessation and readiness to change metabolic risk behaviors was only 3 months. A longer follow-up period may have produced greater readiness to change diet and physical activity; however, readiness to change FVC was associated with lower metabolic risk overall. This suggests that former smokers who are ready to increase FVC may subsequently change other metabolic risk behaviors, including following a lower-fat, lower-calorie diet, improving sleep quality, and/or being more physically active. Multiple behavior change may occur regardless of perceived readiness to change, and readiness to change behavior may have occurred at time points that were not measured, as motivation can shift frequently [42]. Multiple behavior change may proceed differently for different individuals. For instance, success in improving metabolic risk behaviors may, in turn, encourage smoking cessation. Extant research suggests that even vulnerable groups of smokers, such as low-income individuals [46] and pregnant women [47], can increase their physical activity without decreasing their chances of quitting smoking. Further research is needed to examine long-term bidirectional associations between smoking, FVC, and other metabolic risk behaviors.
Contrary to hypotheses, the indirect relationship between T1 smoking abstinence and T3 metabolic risk was found to be significant for participants with unmanaged stress, but not those with managed stress. This finding was driven by a significant interaction between stress management and readiness to increase FVC. It is possible that young adults who intend to improve their FVC despite experiencing unmanaged stress place more importance on FVC. These participants may have been more successful in reducing metabolic risk than those who decided to increase FVC with fewer barriers. Overall, unmanaged stress was associated with higher metabolic risk, as expected. Taken together, these results suggest that participants with unmanaged stress who aimed to increase FVC may have been fundamentally different from other participants in some ways (e.g., motivation to improve nutrition). Notably, the association between smoking cessation and metabolic risk was not moderated by stress management. More research is needed to understand the moderating role of stress management in the relationship between readiness to increase FVC and subsequent metabolic risk. When participants with unmanaged and well-managed stress were analyzed together, those who quit smoking at T1 had lower metabolic risk at T3, suggesting a positive change in general health orientation and behavior.
Limitations and Future Directions
Strengths of this study include its longitudinal design and diverse sample of young adult smokers across the United States. This study also had a few notable limitations. First, the cognitive mechanisms of multiple behavior change are unclear. It is possible that quitting smoking increased the confidence, self-regulatory resources, or skills needed to change other behaviors; however, these possibilities were not directly assessed. Future research could address possible mechanisms of multiple health risk behavior change in young adult smokers. Second, measures used in this study were retrospective self-report measures. Devices that track health behaviors such as exercise and sleep may be more precise [48]. Third, lack of membership in the metabolic risk class at time 3 could reflect substance use risk, rather than low risk. Substance use risk following smoking cessation would not be indicative of a positive change in health behavior profiles. However, only two participants who were abstinent from smoking at T1 were in the substance use risk group at T3, and no participants transitioned from metabolic risk to substance use risk. Taken together, this suggests that smoking cessation was associated with subsequent positive change in health risk behaviors. Finally, stress management could have been both a mediator (T2) and a moderator (T3), introducing possible issues of collinearity. However, because stress management was not a significant mediator of the relationship between T1 abstinence from smoking and T3 metabolic risk, the two stress management variables were not included in the same model.
CONCLUSIONS AND IMPLICATIONS
This study found that young adults who quit smoking had lower likelihood of engaging in metabolic risk behaviors 9 months later. Those who were readier to increase their FVC had lower subsequent metabolic risk, regardless of their reported readiness to change other behaviors. Further research is needed to replicate results and to clarify the underlying mechanisms of multiple health risk behavior change in young adult smokers. For example, results may be driven by mastery of behavior change skills that can be applied across domains, changes in habit strength, or both. If replicated, results suggest that future smoking cessation interventions could subsequently or simultaneously target improvements in metabolic risk behaviors. Young adults who are experiencing unmanaged stress may benefit the most from these intervention components.
Acknowledgments
The authors wish to thank Drs. Kevin Delucchi, Johannes Thrul, and Judith Prochaska for consultation on the analytic approach.
Funding
This study and preparation of this manuscript were supported by the National Institute on Drug Abuse (K23 DA032578, P50 DA09253, and T32 DA007250) and the California Tobacco Related Diseases Research Program (28FT-0015).
Compliance with Ethical Standards
Conflict of Interest: Danielle Ramo has consulted to Carrot, Inc., which makes a tobacco cessation device. Erin Vogel declares no conflicts of interest.
Authors’ Contributions: All authors were involved in the preparation of this manuscript and read and approved the final version.
Human Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1994 Helsinki declaration and its later amendments or comparable ethical standards. Approval was granted by the University of California, San Francisco Institutional Review Board.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: An introduction and overview. Prev Med. 2008;46:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emmons KM, Marcus BH, Linnan L, Rossi JS, Abrams DB. Mechanisms in multiple risk factor interventions: Smoking, physical activity, and dietary fat intake among manufacturing workers. Prev Med. 1994;23:481–489. [DOI] [PubMed] [Google Scholar]
- 3. French SA, Hennrikus DJ, Jeffery RW. Smoking status, dietary intake, and physical activity in a sample of working adults. Health Psychol. 1996;15:448–454. [DOI] [PubMed] [Google Scholar]
- 4. Lee B, Yi Y. Smoking, physical activity, and eating habits among adolescents. West J Nurs Res. 2016;38:27–42. [DOI] [PubMed] [Google Scholar]
- 5. Lee CG, Seo D-C, Middlestadt SE, Lin H-C. Does the relationship between cigarette smoking and other key health behaviors vary by geographic area among US young adults? A multilevel analysis. Int J Behav Med. 2015;22:481–488. [DOI] [PubMed] [Google Scholar]
- 6. Wilson DB, Smith BN, Speizer IS, et al. Differences in food intake and exercise by smoking status in adolescents. Prev Med. 2005;40:872–879. [DOI] [PubMed] [Google Scholar]
- 7. de Vries H, Kremers S, Smeets T, Reubsaet A. Clustering of diet, physical activity and smoking and a general willingness to change. Psychol Health. 2008;23:265–278. [DOI] [PubMed] [Google Scholar]
- 8. Garrett NA, Alesci NL, Schultz MM, et al. The relationship of stage of change for smoking cessation to stage of change for fruit and vegetable consumption and physical activity in a health plan population. Am J Health Prom. 2004;19:118–127. [DOI] [PubMed] [Google Scholar]
- 9. Patterson F, Malone SK, Lozano A, Grandner MA, Hanlon AL. Smoking, screen-based sedentary behavior, and diet associated with habitual sleep duration and chronotype: Data from the UK Biobank. Ann Behav Med. 2016;50:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. The strength model of self-regulation failure and health-related behaviour. Health Psychol Rev. 2009;3:208–238. [Google Scholar]
- 11. Lippke S, Nigg CR, Maddock JE. Health-promoting and health-risk behaviors: Theory-driven analyses of multiple health behavior change in three international samples. Int J Behav Med. 2012;19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleig L, Lippke S, Pomp S, Schwarzer R. Intervention effects of exercise self-regulation on physical exercise and eating fruits and vegetables: A longitudinal study in orthopedic and cardiac rehabilitation. Prev Med. 2011;53:182–187. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Smoking & Tobacco Use. Atlanta, GA: U.S. Department of Health & Human Services; 2018. [Google Scholar]
- 14. Pirie K, Peto R, Green J, et al. The 21st century hazards of smoking and benefits of stopping: A prospective study of one million women in the UK. Lancet. 2013;381:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heggen E, Svendsen M, Tonstad S. Smoking cessation improves cardiometabolic risk in overweight and obese subjects treated with varenicline and dietary counseling. Nutr Metab Cardiovasc Dis. 2017;27:335–341. [DOI] [PubMed] [Google Scholar]
- 16. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 17. Zhang D, Liu X, Liu Y, et al. Leisure-time physical activity and incident metabolic syndrome: A systematic review and dose-response meta-analysis of cohort studies. Metabolism. 2017;75:36–44. [DOI] [PubMed] [Google Scholar]
- 18. Tian Y, Su L, Wang J, Duan X. Fruit and vegetable consumption and risk of the metabolic syndrome: A meta-analysis. Public Health Nutr. 2018;21:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baxter AJ, Coyne T, McClintock C. Dietary patterns and metabolic syndrome – A review of epidemiologic evidence. Asia Pac J Clin Nutr. 2006;15:134–142. [PubMed] [Google Scholar]
- 20. Zohal M, Ghorbani A, Esmailzadehha N, Ziaee A, Mohammadi Z. Association of sleep quality components and wake time with metabolic syndrome: The Qazvin Metabolic Diseases Study (QMDS), Iran. Diabetes Metab Synd Clin Res Rev. 2017;11S:S377–S380. [DOI] [PubMed] [Google Scholar]
- 21. Bergmann N, Gyntelberg F, Faber J. The appraisal of chronic stress and the development of metabolic syndrome: A systematic review of prospective cohort studies. Endocr Connect. 2014;3:R55–R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. King TK, Marcus BH, Pinto BM, Emmons KM, Abrams DB. Cognitive-behavioral mediators of changing multiple behaviors: Smoking and a sedentary lifestyle. Prev Med. 1996;25:684–691. [DOI] [PubMed] [Google Scholar]
- 23. Chao AM, White MA, Grilo CM, Sinha R. Examining the effects of cigarette smoking on food cravings and intake, depressive symptoms, and stress. Eat Behav. 2017;24:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koopmann A, Dinter C, Grosshans M, et al. Psychological and hormonal features of smokers at risk to gain weight after smoking cessation – Results of a multicenter study. Horm Behav. 2011;60:58–64. [DOI] [PubMed] [Google Scholar]
- 25. Mansyur CL, Pavlik VN, Hyman DJ, Taylor WC, Goodrick GK. Self-efficacy and barriers to multiple behavior change in low-income African Americans with hypertension. J Behav Med. 2013;36:75–85. [DOI] [PubMed] [Google Scholar]
- 26. Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology. 1995;119:171–178. [DOI] [PubMed] [Google Scholar]
- 27. Lipschitz JM, Paiva AL, Redding CA, Butterworth S, Prochaska JO. Co-occurrence and coaction of stress management with other health risk behaviors. J Health Psychol. 2015;20:1002–1012. [DOI] [PubMed] [Google Scholar]
- 28. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. [DOI] [PubMed] [Google Scholar]
- 29. James E, Freund M, Booth A, et al. Comparative efficacy of simultaneous versus sequential multiple health risk behavior change interventions among adults: A systematic review of randomised trials. Prev Med. 2016;89:211–223. [DOI] [PubMed] [Google Scholar]
- 30. Xu W-H, Zhang X-L, Gao Y-T, et al. Joint effect of cigarette smoking and alcohol consumption on mortality. Prev Med. 2007;45:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramo DE, Thrul J, Vogel EA, Delucchi K, Prochaska JJ. Multiple health risk behaviors in young adult smokers: Stages of change and stability over time. Ann Behav Med, 2019; doi:10.1093/abm/kaz025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramo DE, Thrul J, Delucchi KL, et al. A randomized controlled evaluation of the Tobacco Status Project, a Facebook intervention for young adults. Addiction. 2018;113:1683–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramo DE, Thrul J, Delucchi KL, et al. The Tobacco Status Project (TSP): Study protocol for a randomized controlled trial of a Facebook smoking cessation intervention for young adults. BMC Public Health. 2015;15:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramo DE, Rodriguez TM, Chavez K, Sommer MJ, Prochaska JJ. Facebook recruitment of young adult smokers for a cessation trial: Methods, metrics, and lessons learned. Internet Interv. 2014;1:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DiClemente CC, Prochaska JO, Fairhurst SK, et al. The process of smoking cessation: An analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol. 1991;59:295–304. [DOI] [PubMed] [Google Scholar]
- 36. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update: Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 37. The Secretary’s Advisory Committee on National Health Promotion and Disease Prevention Objectives for 2020. Recommendations for the Framework and Format of Healthy People 2020. Washington, DC: US Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2008. [Google Scholar]
- 38. Sarkin JA, Johnson SS, Prochaska JO, Prochaska JM. Applying the transtheoretical model to regular moderate exercise in an overweight population: Validation of a stages of change measure. Prev Med. 2001;33:462–469. [DOI] [PubMed] [Google Scholar]
- 39. Prochaska JO, Velicer WF, Rossi JS, et al. Multiple risk expert systems interventions: Impact of simultaneous stage-matched expert system interventions for smoking, high-fat diet, and sun exposure in a population of parents. Health Psychol. 2004;23:503–516. [DOI] [PubMed] [Google Scholar]
- 40. Evers KE, Prochaska JO, Johnson JL, et al. A randomized clinical trial of a population- and transtheoretical model-based stress-management intervention. Health Psychol. 2006;25:521–529. [DOI] [PubMed] [Google Scholar]
- 41. Hughes JR, Carpenter MJ, Naud S. Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic review. Nicotine Tob Res. 2010;12:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Velicer WF, Prochaska JO, Fava JL, Norman GJ, Redding CA. Smoking cessation and stress management: Applications of the Transtheoretical Model of behavior change. Homeostasis. 1998;38:216–233. [Google Scholar]
- 43. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 44. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. 1st ed. New York: The Guilford Press; 2013. [Google Scholar]
- 45. Hayes AF. An index and test of linear moderated mediation. Multivar Behav Res. 2015;50:1–22. [DOI] [PubMed] [Google Scholar]
- 46. Nair US, Patterson F, Rodriguez D, Collins BN. A telephone-based intervention to promote physical activity during smoking cessation: A randomized controlled proof-of-concept study. TBM. 2017;7:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ussher M, Lewis S, Aveyard P, et al. Physical activity for smoking cessation in pregnancy: Randomised controlled trial. BMJ. 2015;350:h2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]