Abstract
Background
Lung ultrasound is feasible for assessing lung injury caused by coronavirus disease 2019 (COVID-19). However, the prognostic meaning and time-line changes of lung injury assessed by lung ultrasound in COVID-19 hospitalised patients are unknown.
Methods
Prospective cohort study designed to analyse prognostic value of lung ultrasound in COVID-19 patients by using a quantitative scale (lung ultrasound Zaragoza (LUZ)-score) during the first 72 h after admission. The primary end-point was in-hospital death and/or admission to the intensive care unit. Total length of hospital stay, increase of oxygen flow and escalation of medical treatment during the first 72 h were secondary end-points.
Results
130 patients were included in the final analysis; mean±sd age was 56.7±13.5 years. Median (interquartile range) time from the beginning of symptoms to admission was 6 (4–9) days. Lung injury assessed by LUZ-score did not differ during the first 72 h (21 (16–26) points at admission versus 20 (16–27) points at 72 h; p=0.183). In univariable logistic regression analysis, estimated arterial oxygen tension/inspiratory oxygen fraction ratio (PAFI) (hazard ratio 0.99, 95% CI 0.98–0.99; p=0.027) and LUZ-score >22 points (5.45, 1.42–20.90; p=0.013) were predictors for the primary end-point.
Conclusions
LUZ-score is an easy, simple and fast point-of-care ultrasound tool to identify patients with severe lung injury due to COVID-19, upon admission. Baseline score is predictive of severity along the whole period of hospitalisation. The score facilitates early implementation or intensification of treatment for COVID-19 infection. LUZ-score may be combined with clinical variables (as estimated by PAFI) to further refine risk stratification.
Short abstract
Lung injury caused by coronavirus disease 2019 (COVID-19) can be measured through lung ultrasound. Lung ultrasound identifies COVID-19 patients at a higher risk of complications, and could support clinical-decision making in COVID-19 patients. http://bit.ly/3cQiz6K
Introduction
Coronavirus disease 2019 (COVID-19) is a systemic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1, 2]. The virus emerged in China in late 2019 and quickly spread worldwide, challenging healthcare systems and becoming the most devastating pandemic in over a century [3]. In essence, it is a multisystem disease, with special tropism for lungs, where may lead to a severe respiratory failure, ultimately causing the need for mechanical ventilation and high fatality rates [4]. None of the therapies tested so far, except low-dose systemic corticosteroids in the most severely ill patients, have shown efficacy in reducing mortality [5]. Therefore, early detection of lung involvement, and anticipation of respiratory complications in COVID-19 patients would be of enormous assistance for clinicians in order to individualise patient management and anticipate the need for mechanical ventilation.
Not surprisingly, COVID-19 has resulted in a diagnostic challenge, since many patients present a dissociation between symptoms and radiological findings (e.g. in approximately a third of patients, infiltrates upon chest radiography are absent) [2, 6, 7]. Since first outbreak began [1], attempts have been made to improve early diagnosis and potential complications of COVID-19 infection. Due to its sensitivity, pulmonary computed tomography (CT) has been postulated as the “gold standard” to detect lung involvement [8, 9]. However, lung CT has some limitations, especially when repeated examinations are required: equipment is not always available due to high demand; radiation exposure; the need to move the patient; or the subsequent need for enhanced environmental cleaning after its use; all of which are inconvenient and time-consuming. For all these reasons, lung ultrasound and point-of-care ultrasound [10] have been positioned as potential alternatives for the management of patients with COVID-19.
We hypothesise that lung ultrasound is useful during admission to quantify lung injury produced by COVID-19. Furthermore, we believe that the degree of lung injury might be related to prognosis and, hence, lung ultrasound may help in therapeutic decision-making during the first 72 h. If true, lung ultrasound could help to design more effective and earlier treatment strategies in patients admitted for COVID-19 infection.
The goals of the study were to 1) analyse the prognostic value of lung damage estimated by lung ultrasound at admission; 2) validate a specific quantitative scale for lung injury in patients with COVID-19 using lung ultrasound; 3) analyse whether the changes in lung lesions quantified through lung ultrasound during the first 48–72 h of admission can identify patients with a worse prognosis; and 4) analyse the correlation between analytical and clinical variables and the severity of lung damage quantified by lung ultrasound.
Material and methods
Study design and setting
This was a prospective cohort study carried out in the infectious diseases and internal medicine departments of a tertiary university teaching centre between July and October 2020. The study consisted of two phases. The first aimed to analyse the role of lung ultrasound in COVID-19 patients, and the second aimed to identify blood biomarkers with potential clinical utility. Results included in this article refer to the first phase of the study.
Inclusion criteria were 1) age ≥18 years; 2) informed consent granted; and 3) confirmed diagnosis of SARS-CoV-2 (COVID-19) infection by nasopharyngeal PCR or specific serology (IgM and/or IgG) with sign or symptoms of clinically active respiratory infection. Exclusion criteria were 1) intensive care unit (ICU) admission; 2) refusal of the patient to participate; 3) functional dependence (Barthel index <50 points); 4) moderate/severe cognitive impairment (Pfeiffer scale); 5) advanced COPD (forced expiratory volume in 1 s <30%) or a history of emphysema and/or pulmonary fibrosis; or 6) active cancer.
Variables and definitions
Patients were assessed three times during hospitalisation: 1) “admission” (within first 24 h of admission); 2) “control” (48–72 h later); and 3) “discharge” (the day prior to discharge). At each time point, lung involvement was quantified using a lung ultrasound protocol (see later), vital signs were recorded (blood pressure, heart rate, oxygen saturation and respiratory therapy), PAFI (arterial oxygen tension/inspiratory oxygen fraction (FIO2) ratio) index was estimated (ePAFI) and patient's dyspnoea was quantified using Borg scale (from 1 (minimum) to 10 (maximum)). Routine blood laboratory data (complete blood count, biochemistry, coagulation and blood gasses) were recorded. Additional blood samples were bio-banked, following patient consent, and kept at −80°C for future analysis (Aragón Health System Biobank).
Lung ultrasound
Lung ultrasound examinations were performed using the UPROBE-C5PL wireless ultrasound device (Leleman), convex probe of 3.5–5 MHz, with a gain of 80–100 dB, and a maximum depth of 160–220 mm. Images and videos were stored (iPad 10.2; Apple). The researchers responsible for lung ultrasound were internal medicine specialists, with extensive experience in clinical ultrasound (>2 years and >180 thoracic lung ultrasound explorations) [11–13].
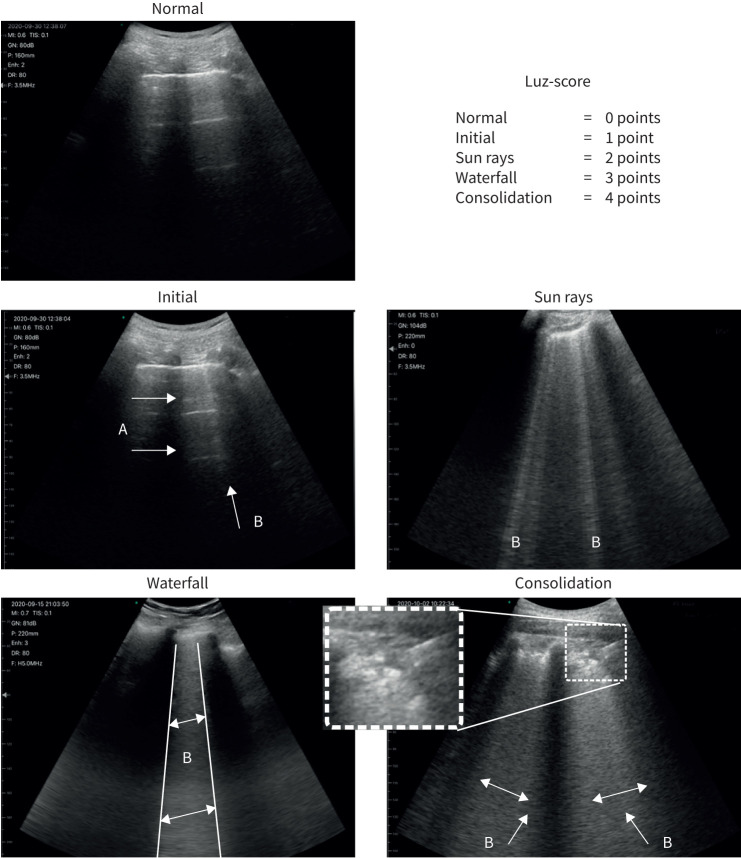
In each examination, 12 areas were analysed according to previous studies [14] (two anterior, two lateral and two posterior for each lung). Given the progressive nature of ultrasound changes in COVID-19, a score between 0 and 4 points was assigned to each quadrant according to the pattern of observed findings, resulting in a total score between 0 and 48 points (0 points: A lines and normal pleural line; 1 point: A lines coexist with isolated and small B lines; 2 points: A lines disappear and multiple B lines are seen alternating with preserved lung parenchymal spaces. Pleural line thickens and small “bites” may be seen; 3 points: B lines merge and form a giant B line that fills the entire intercostal space. Pleural line is blurred, bites appear more frequently; 4 points: Pleural line is broken and subpleural consolidations (1–1.5 cm deep) are observed. “Sun ray” and “waterfall” patterns coexist) (figure 1 and supplementary figure S5). We named this protocol “lung ultrasound Zaragoza (LUZ)-score”. In cases of multiple patterns coexisting in the same lung quadrant (according to the intercostal space analysed), the finding with highest score was annotated. In addition, the number of affected areas, presence of subpleural consolidations and the presence of pleural effusion were recorded.
FIGURE 1.
Lung ultrasound Zaragoza (LUZ)-score. Arrows indicate ultrasound findings described for each score.
Outcomes
The primary end-point was defined as the combined occurrence of in-hospital death or transfer to the ICU for invasive mechanical ventilation. The following secondary outcomes were also considered: 1) length of hospital stay until discharge in patients not requiring ICU admission; 2) whether an increase in flow of oxygen during the 72 h following admission was required; and 3) change to more aggressive medical treatment (defined as adding remdesivir or convalescent plasma, or either starting corticosteroids or increasing dexamethasone dose) during the 72 h after admission.
Statistical analysis
Continuous variables were expressed as mean±sd or median (interquartile range (IQR)), as appropriate. Categorical variables were expressed as percentages. To perform the comparative analysis between normal continuous variables, t-test and ANOVA were used. Those variables that did not follow normality were compared using the Mann–Whitney and Kruskal–Wallis U-tests. Categorical variables were compared using the Chi-squared test. The analysis of the different correlations between continuous variables was carried out using the Pearson or Spearman test.
Sample size was calculated based on the number of blood samples required to carry out phase 2 of the study (see Study design section). The final objective was to collect 100 serum samples from patients with complete follow-up (baseline, control and discharge). To account for the consequences of healthcare pressure during the recruitment phase, an approximate percentage of losses of 30% were estimated, marking a final goal of 130 inclusions.
In univariable and multivariable logistic regression analysis, LUZ-score was dichotomised based on a cut-off value selected from receiver operating characteristic analysis of its primary end-point predictive value. A multivariable logistic regression model was designed to identify factors independently associated with the need for ICU transfer during admission or intra-hospital death. Candidate predictors were selected from the univariable analysis when p-value <0.200, and entered at a single step in the multivariable analysis. Age was also included in the model. Bootstrapping with 1000 replicates was performed, testing the stability of the model. Continuous candidate variables were transformed using logarithmic polynomials if necessary.
Confidence intervals included were 95%, establishing statistical significance with a p-value <0.05. Statistical analysis was carried out with Statistical Package for the Social Sciences (SPSS version 24.0 for Windows).
The study complied with the fundamental guidelines of the Helsinki declaration guidelines (CEICA, Ref. C.P.-C.I. PI20/248, 13 May 2020).
Results
Baseline characteristics are provided in table 1.
TABLE 1.
Baseline characteristics according to Lung ultrasound Zaragoza (LUZ)-score at baseline (tertiles)
| Total | LUZ-score | p-value | |||
| <13 | 13–26 | >26 | |||
| Total sample | 130 | 32 (24.6) | 69 (53.1) | 29 (22.3) | |
| Age years | 56.7±13.5 | 54.0±13.2 | 56.3±14.7 | 60.5±10.0 | 0.173 |
| Male n (%) | 80 (61.5) | 20 (62.5) | 43 (62.3) | 17 (58.6) | 0.935 |
| Duration of symptoms days | 6 (5) | 4.0 (5.0) | 6.0 (3.0) | 8.0 (5) | 0.003 |
| Time until COVID-19 confirmation days | −2 (6) | −1.5 (7) | −2.0 (6) | −3.0 (6) | 0.315 |
| Comorbidities n (%) | |||||
| Hypertension | 50 (38.5) | 11 (34.4) | 24 (34.8) | 15 (51.7) | 0.250 |
| Heart failure | 4 (3.1) | 2 (6.3) | 2 (2.9) | 0 (0.0) | 0.366 |
| Dyslipidaemia | 37 (28.5) | 11 (34.4) | 15 (21.7) | 11 (37.9) | 0.187 |
| Coronary artery disease | 5 (3.8) | 2 (6.3) | 2 (2.9) | 1 (3.4) | 0.712 |
| Diabetes | 22 (16.9) | 5 (15.6) | 9 (13.0) | 8 (27.6) | 0.210 |
| History of smoking | 40 (30.8) | 8 (25.0) | 21 (30.4) | 11 (37.9) | 0.548 |
| COPD/asthma | 13 (10.0) | 5 (15.6) | 6 (8.7) | 2 (6.9) | 0.457 |
| Atrial flutter/fibrillation | 5 (3.8) | 3 (9.4) | 1 (1.4) | 1 (3.4) | 0.155 |
| CKD | 6 (4.6) | 2 (6.3) | 2 (2.9) | 2 (1.5) | 0.607 |
| Clinical variables | |||||
| BMI kg·m−2 | 28.7 (6.2) | 29.0 (5.4) | 28.8 (7.4) | 28.3 (6.0) | 0.625 |
| SBP mmHg | 126.8±16.5 | 129.7±15.9 | 125.2±16.0 | 128.1±14.7 | 0.731 |
| DBP mmHg | 77.2±10.8 | 75.7±12.9 | 76.8±9.8 | 79.5±8.5 | 0.315 |
| Heart rate beats·min−1 | 81.4±2.2 | 80.6±15.6 | 80.7±11.6 | 84.8±9.9 | 0.217 |
| ePAFI mmHg | 382 (92) | 429 (0) | 355 (93) | 346 (73) | <0.001 |
| Borg scale for dyspnoea | 4 (5) | 3 (5) | 4 (6) | 5 (4) | 0.031 |
| Laboratory | |||||
| Urea mg·dL−1 | 34 (20) | 34.5 (23.5) | 33.0 (20.5) | 35.0 (16.0) | 0.448 |
| Creatinine mg·dL−1 | 0.9 (0.29) | 0.97 (0.18) | 0.88 (0.31) | 0.89 (0.30) | 0.241 |
| Aspartate transaminase U·L−1 | 38 (25) | 30 (16) | 39 (26) | 43 (22) | 0.044 |
| Alanine transaminase U·L−1 | 30 (29) | 34 (24) | 30 (33) | 30 (37) | 0.816 |
| Creatine phosphokinase U·L−1 | 94 (91) | 131 (110) | 89 (86) | 83 (64) | 0.460 |
| Lactate dehydrogenase U·L−1 | 306 (146) | 254 (109) | 307 (166) | 394 (183) | <0.001 |
| C-reactive protein mg·L−1 | 64.2 (81.2) | 36.7 (58.8) | 67.5 (75.5) | 86.8 (104.9) | 0.013 |
| Ferritin ng·mL−1 | 729 (855) | 737 (721) | 670 (770) | 954 (1414) | 0.248 |
| Haemoglobin g·dL−1 | 14.2 (2.1) | 14.2 (2.0) | 14.1 (2.4) | 14.1 (1.9) | 0.275 |
| Total leukocytes ×1000 | 5.5 (3.1) | 5.7 (3.8) | 5.9 (3.2) | 5.0 (3.1) | 0.479 |
| Total lymphocytes ×1000 | 0.9 (0.6) | 0.9 (0.7) | 1.0 (0.6) | 0.8 (0.6) | 0.250 |
| D-dimer ng·mL−1 | 731 (661) | 739 (903) | 658 (654) | 891 (743) | 0.037 |
| Fibrinogen mg·dL−1 | 777 (213) | 723 (207) | 793 (220) | 785 (231) | 0.182 |
| Interleukin-6 pg·mL−1 | 42.34 (26.6) | 25.4 (22.5) | 45.4 (28.0) | 46.4 (46.9) | <0.001 |
| Therapies n (%) | |||||
| Colchicine | 8 (6.2) | 2 (6.3) | 4 (5.8) | 2 (6.9) | 0.979 |
| Plasma | 1 (0.8) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0.641 |
| Remdesivir | 45 (34.6) | 4 (12.5) | 28 (40.6) | 13 (44.8) | 0.009 |
| Systemic corticosteroids | 99 (76.2) | 17 (53.1) | 55 (79.7) | 27 (93.1) | 0.001 |
| Medium dose of corticosteroids (dexamethasone mg) | 6 (3) | 6 (1) | 6 (3) | 6 (3) | 0.596 |
| Low-molecular-weight heparin | 124 (95.4) | 28 (87.5) | 67 (97.1) | 28 (96.6) | 0.044 |
Data are presented as n, mean±sd or median (interquartile range), unless otherwise stated. Bold type represents statistical significance. COVID-19: coronavirus disease 2019; CKD: chronic kidney disease (estimated glomerular filtration rate <60 mL·min−1·1.73 m2 using the CKD Epidemiology Collaboration creatinine method); BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ePAFI: estimated arterial oxygen tension/inspiratory oxygen fraction ratio.
Lung ultrasound
A total of 341 examinations were performed (130 at admission, 124 at control and 87 at discharge). Pulmonary involvement observed through LUZ-score did not vary between baseline and control phases (median (IQR) 21 (10) versus 20 (11), respectively; p=0.183), although significant decrease was observed at discharge (13 (12); p≤0.001) (figure 2 and supplementary table S1). Similarly, the number of lung areas affected did not vary significantly during the first 72 h of admission (9±3 versus 9±4; p=0.077). The most affected lung fields were lower right lobe (R6=69.2%) and lower left lobe (L6=65.2%), followed by lateral portion of upper left lobe (L3=58.5%) and lateral portion of upper right lobe (R3=56.2%) (supplementary table S1).
FIGURE 2.
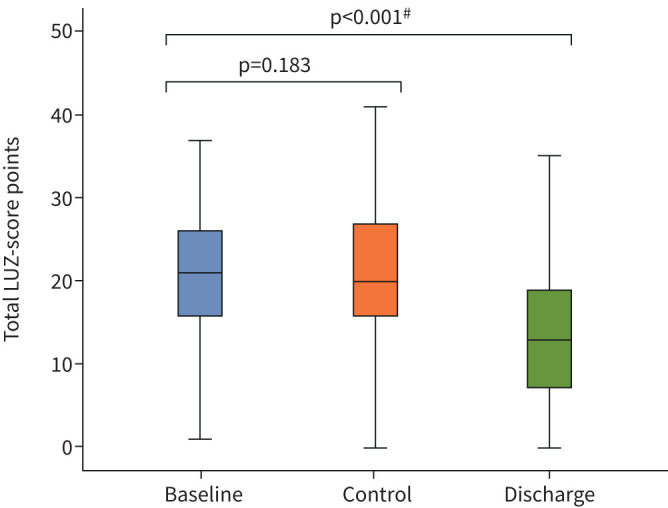
Box plots showing lung ultrasound Zaragoza (LUZ)-score distribution at baseline, control and discharge. #: Mann–Whitney U-test between LUZ-score at baseline and LUZ-score at baseline and discharge.
Stratification of population according to LUZ-score (tertiles) did not show significant differences in baseline characteristics or in comorbidities (table 1). However, those patients with greater pulmonary involvement (LUZ-score >75th percentile) presented a higher degree of respiratory failure by ePAFI (p≤0.001), as well as a higher dyspnoea score by Borg scale (p=0.031). Patients with greater lung ultrasound alterations (LUZ-score >75th percentile) presented higher concentrations at admission of aspartate transaminase (p=0.044), lactate dehydrogenase (p≤0.001), d-dimer (p=0.037), C-reactive protein (p=0.013) and interleukin-6 (p≤0.001). The proportion of patients who received treatment with systemic steroids (p=0.001) and remdesivir (0.009) was higher among patients with greater initial lung involvement quantified by LUZ-score (table 1).
LUZ-score showed significant correlations with variables related to respiratory function, such as ePAFI (r=−0.516; p≤0.001) or Borg scale (r=0.228; p=0.009); lung-tissue biomarkers, such as lactate dehydrogenase (r=0.395; p≤0.001); and with markers of systemic inflammation such as C-reactive protein (r=0.286; p=0.001) or IL-6 (r=0.383; p≤0.001) (supplementary table S2 and figure S2).
Outcomes
13 (10.1%) out of 130 patients reached the primary end-point: 12 patients required admission to ICU for invasive mechanical ventilation, and another died of bacteraemia associated with a central venous catheter. A baseline LUZ-score of 22 was the point of maximum sensitivity for the primary end-point (sensitivity 76.9%, specificity 62.1%, area under the curve 0.693; p=0.023) (supplementary figure S3).
The median (IQR) length of hospital stay for patients not requiring admission to ICU was 8 (6) days. At control phase, oxygen administration had to be increased in 37 (32.7%) patients, and 44 (37.9%) needed to change to more aggressive medical treatment. Patients with a baseline LUZ-score >75th percentile presented a significantly higher proportion of events for the primary end-point (25%; p=0.016), a longer length of stay without being transferred to intensive care (median 9 days, IQR 6 days; p=0.003), and required a significant increase in oxygen administration at control (41.7%; p=0.037) (table 2). Kaplan–Meier curves showing how baseline LUZ-score stratification might help predicting patients at higher risk of primary end-point can be found in supplementary figure S4.
TABLE 2.
Outcomes by lung ultrasound Zaragoza (LUZ)-score at baseline
| Total | LUZ-score | p-value | |||
| <25th percentile | 25–75th percentile | >75th percentile | |||
| Primary outcome | |||||
| ICU admission and/or death | 13 10.1) | 3 (9.4) | 3 (4.3) | 7 (25.0) | 0.016 |
| Secondary outcomes | |||||
| Length of stay days | 8±6 | 5±6 | 8±4 | 9±6 | 0.003 |
| Necessity of increased oxygen therapy at 48/72 h | 42 (34.1) | 5 (16.1) | 27 (39.7) | 10 (41.7) | 0.037 |
| Necessity to escalate COVID-19 treatment at 48/72 h | 52 (40.9) | 9 (29.0) | 29 (42.0) | 14 (51.9) | 0.199 |
| Necessity of increased oxygen therapy or escalation of COVID-19 treatment at 48/72 h | 62 (50.4) | 11 (35.5) | 35 (51.5) | 16 (66.7) | 0.067 |
Data are presented as n (%) or mean±sd, unless otherwise stated. ICU: intensive care unit; COVID-19: coronavirus disease 2019. Bold type represents statistical significance.
Univariable logistic regression analysis identified estimated ePAFI (hazard ratio (HR) 0.99, 95% CI 0.98–0.99; p=0.027) and LUZ-score at baseline >22 (HR 5.45, 1.42–20.90; p=0.013) as potential predictors for the primary end-point (supplementary table S3). In the multivariable logistic regression, after adjusting for confounders and bootstrapping (table 3), LUZ-score at baseline >22 points (cut-off with highest sensitivity) was identified as an independent predictor for the primary end-point (HR 5.25, 0.84–32.84; p=0.038) (table 3).
TABLE 3.
Univariable and multivariable logistic regression model for the primary combined end-point all-cause mortality and/or intensive care unit admissions
| Univariable | Multivariable | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.02 (0.97–1.06) | 0.351 | 1.03 (0.97–1.10) | 0.287 |
| Dyslipidaemia | 2.35 (0.73–7.53) | 0.151 | 1.53 (0.32–7.20) | 0.586 |
| Diabetes | 2.42 (0.67–8.70) | 0.176 | 0.99 (0.15–6.44) | 0.992 |
| ePAFI | 0.99 (0.98–0.99) | 0 . 027 | 0.99 (0.98–1.00) | 0.640 |
| CPK | 1.00 (1.00–1.00) | 0.098 | 1.00 (0.99–1.00) | 0.109 |
| Total lymphocytes | 1.06 (0.97–1.17) | 0.175 | 1.03 (0.92–1.15) | 0.563 |
| Baseline LUZ-score >22 # | 5.45 (1.42–20.90) | 0.013 | 5.25 (0.84–32.84) | 0.038¶ |
Bold type represents statistical significance. ePAFI: estimated arterial oxygen tension/inspiratory oxygen fraction ratio; CPK: creatine phosphokinase; LUZ: lung ultrasound Zaragoza. #: point of highest sensitivity and specificity (sensitivity 76.9%, specificity 62.1%, area under the curve 0.693; p=0.023); ¶: significant after a bootstrap of 1000 replicates.
Discussion
In this study we monitored lung injury through lung ultrasound in patients hospitalised due to COVID-19. We propose a quantitative score based on lung ultrasound to estimate severity of the disease. Our main results show that LUZ-score at admission identifies patients with more severe lung injury and can accurately predict poor outcomes. The score does not change over the first 72 h of hospital stay, meaning that it is fully informative upon admission.
Given the extensive lung involvement in COVID-19 patients, lung ultrasound may have some potential utility in the management of acutely ill patients [15, 16]. Some studies have reported an improvement in lung involvement assessment in COVID-19 by using lung ultrasound in the context of the emergency department and the ICU [17, 18], but we only have found two prospective studies analysing the prognostic value of lung ultrasound during the first pandemic wave of COVID-19 (March and April 2020) [19, 20]. The first included 80 patients (17 outpatients, 42 hospitalised patients and 21 patients with orotracheal intubation or death), initially admitted to the emergency department [19]. The authors used a quantitative scale (lung ultrasound score) based on measurements in 10 areas of the chest identifying those patients with the greatest probability of admission to the ICU. The second study included 120 patients, using a similar quantitative scale, based only in six areas, and including COVID-19 patients admitted either to a medical ward or ICU [20]. Of these patients, only 20 (16.6%) were monitored during the study. The authors concluded that lung ultrasound rapidly identifies pulmonary involvement and provides risk stratification. Despite the novelty and importance of these findings, both studies had some limitations. Samples were heterogeneous, as both outpatients and those hospitalised in a medical ward or at the ICU were included. Furthermore, they did not provide data about changes in treatment guided by the score, nor about follow-up in lung ultrasound score. (Lichter et al. [20] only monitored 20 patients.) In addition, these studies were carried out during the first pandemic wave, and thus their results may not translate effectively to the current situation, since some effective therapies, such as dexamethasone [21] and remdesivir [22] are now being used in a more systematic fashion.
In our patients, LUZ-score did not change during the first 72 h after admission (p=0.183), which underlines the importance of the early assessment of the lung. Postero-inferior and supero-lateral areas of both lungs were the regions more commonly and more severely involved. This pattern is similar to that described in other studies using lung ultrasound in COVID-19 [18, 19, 23]. One striking finding from our study, not previously reported, was that although lung injury decreased significantly at discharge (median (IQR) 21 (10) points at admission versus 13 (12) points at discharge; p≤0.001), most patients still had lung ultrasound findings. Whether these findings indicate an active or evolving injury remains to be clarified. Whatever the meaning, this is a clear expression of the heterogeneity of the clinical picture of COVID-19 infection and the dissociation between clinical and radiological development. Furthermore, the persistence of lung artefacts at discharge should prompt the medical community to address the follow-up of these patients from a comprehensive perspective, which must include a close monitoring of lung function and potential residual lesions.
In our cohort, patients with the highest LUZ-score (>75th percentile) at baseline had longer duration of symptoms, more dyspnoea self-reported via the Borg scale, a significantly lower ePAFI and higher concentration of lactate dehydrogenase, C-reactive protein and interleukin-6. In keeping with the perception of a greater degree of severity, patients with a higher LUZ-score had been treated at admission more often with systemic steroids (93.1%, p=0.001) and remdesivir (44.8%, p=0.009). In short, lung ultrasound and quantification of lung damage using the LUZ-score identified the most severely affected patients, as shown by significant correlation with other measures of severity such as clinical or analytical parameters and indirectly through the treatment they received.
Our results reveal a new tool to assist in the management of patients with COVID-19. Quantification of lung injury through an objective measure such as the LUZ-score offers the opportunity for early identification of the most severe patients, and, as a consequence, the early implementation and proper allocation of most intense treatment in those COVID-19 patients.
Relevant information can be extracted from our study. First, patients with more severe respiratory symptoms at admission had higher LUZ-scores (>75th percentile) and more frequently exhibited death from all causes or admission to the ICU (25%, p=0.009). Second, baseline LUZ-score >22 points and ePAFI were identified in the univariable logistic regression model as predictors for the primary end-point (table 3). LUZ-score >22 points remained significant in the final multivariable model, after adjusting for potential confounders and bootstrapping (HR 5.25, 95% CI 0.84–33.84; p=0.038). The fact that confidence intervals were wide and included the unit deserves some consideration. It is probable that a higher power would have been obtained with a larger sample size, but we were limited by the availability of ultrasound equipment and trained staff, the need for higher safety precautions, and the work overload associated with the current pandemic situation. Sample size was designed based on the collection of samples for biomarker analysis, and limited by available funding. It should also be taken into account that the study's primary end-point was hard (death and/or ICU admission). Conversely, the fact that the result is significant after bootstrapping reflects its consistency and strongly supports a potential utility of ultrasound along with other clinical variables, such as ePAFI. This is especially important in a disease with highly variable clinical expression, and frequently dissociated from data yielded by other complementary examinations.
We suggest LUZ-score as an easy, simple and fast point-of-care ultrasound tool in patients with COVID-19 to stratify risk upon admission in combination with other clinical and analytical variables. According to our results, admission lung ultrasound can help clinicians to implement COVID-19 treatment (either by the early increase of oxygen flow or by escalating other therapies) in those cases more severely involved. Given the advantages of lung ultrasound, this technique can be repeated as many times as needed and everywhere, which confer additional advantages for its clinical use.
Limitations
The study was carried out in a single centre, so the results cannot be generalisable. We did not analyse correlations between lung ultrasound and CT, due to the study design. The sample size was designed based on the collection of samples for biomarker analysis, which could have underestimated the power of the multivariable logistic regression analysis. Finally, although all physicians who took lung ultrasound images had great previous experience in lung ultrasound, this technique is operator-dependent, and could have influenced the final results.
Conclusions
Lung ultrasound and LUZ-score allow the quantification of the degree of pulmonary involvement in patients with COVID-19. There are no changes in the score during the first 72 h of admission, which reinforces the importance of the very first ultrasound assessment, which should be performed soon after admission. A baseline admission LUZ-score >22 is a predictor of ICU admission or in-hospital death. Despite the improvement in clinical condition, ultrasound lung artefacts remain at discharge in a proportion of patients. This particular finding has not been reported previously and its significance is not clear.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-04283-2020.SUPPLEMENT (566.3KB, pdf)
Shareable PDF
Acknowledgements
To all the staff, physicians, nurses and technicians, of the internal medicine and infectious diseases departments (Clinical Hospital “Lozano Blesa”, Zaragoza, Spain). To the patients who agreed to participate in the study. To all of the patients who suffered and died from the pandemic and health workers who looked after them.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Conflict of interest: J. Rubio-Gracia has nothing to disclose.
Conflict of interest: I. Giménez-López has nothing to disclose.
Conflict of interest: V. Garcés-Horna has nothing to disclose.
Conflict of interest: D. López-Delgado has nothing to disclose.
Conflict of interest: J.L. Sierra-Monzón has nothing to disclose.
Conflict of interest: L. Martínez-Lostao has nothing to disclose.
Conflict of interest: C. Josa-Laorden has nothing to disclose.
Conflict of interest: F. Ruiz-Laiglesia has nothing to disclose.
Conflict of interest: J.I. Pérez-Calvo has nothing to disclose.
Conflict of interest: S. Crespo-Aznarez has nothing to disclose.
Conflict of interest: J. García-Lafuente has nothing to disclose.
Conflict of interest: N. Peña-Fresneda has nothing to disclose.
Conflict of interest: B. Amores Arriaga has nothing to disclose.
Conflict of interest: B. Gracia-Tello has nothing to disclose.
Conflict of interest: M. Sánchez-Marteles has nothing to disclose.
Support statement: The study was funded through a COVID-19 2020 crowdfunding campaign launched by the Aragon Health Research Institute (https://www.iisaragon.es/utilidad-de-la-ecografia-clinica-y-el-uso-de-biomarcadores-sericos-en-la-estratificacion-del-riesgo-de-pacientes-con-infeccion-por-sars-cov2-covid-19/). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Ramanathan K, Antognini D, Combes A, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W, Ni Z, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenthal D, Fowler EJ, Abrams M, et al. . Covid-19 – implications for the health care system. N Engl J Med 2020; 383: 1483–1488. doi: 10.1056/NEJMsb2021088 [DOI] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al. . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan H, Peto R, Henao-Restrepo AM, et al. . Repurposed antiviral drugs for Covid-19 – interim WHO Solidarity trial results. N Engl J Med 2021; 384: 497–511. doi: 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao W, He L, Tang H, et al. . The relationship between chest imaging findings and the viral load of COVID-19. Front Med 2020; 7: 558539. doi: 10.3389/fmed.2020.558539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Chen T, Yang H, et al. . Clinical and radiological changes of hospitalised patients with COVID-19 pneumonia from disease onset to acute exacerbation: a multicentre paired cohort study. Eur Radiol 2020; 30: 5702–5708. doi: 10.1007/s00330-020-06916-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan F, Ye T, Sun P, et al. . Time course of lung changes on chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020; 295: 715–721. doi: 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombi D, Petrini M, Maffi G, et al. . Comparison of admission chest computed tomography and lung ultrasound performance for diagnosis of COVID-19 pneumonia in populations with different disease prevalence. Eur J Radiol 2020; 133: 109344. doi: 10.1016/j.ejrad.2020.109344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med 2017; 69: e27–e54. doi: 10.1016/j.annemergmed.2016.08.457 [DOI] [PubMed] [Google Scholar]
- 11.Josa-Laorden C, Giménez-López I, Rubio-Gracia J, et al. . Valor pronóstico de la medición del diámetro y colapso inspiratorio de la vena cava inferior en la insuficiencia cardiaca aguda. [Prognostic value of measuring the diameter and inspiratory collapse of the inferior vena cava in acute heart failure]. Rev Clin Esp 2016; 216: 183–190. doi: 10.1016/j.rce.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 12.Rubio-Gracia J, Giménez-López I, Sánchez-Marteles M, et al. . Intra-abdominal pressure and its relationship with markers of congestion in patients admitted for acute decompensated heart failure. Heart Vessels 2020; 35: 1545–1556. doi: 10.1007/s00380-020-01634-9 [DOI] [PubMed] [Google Scholar]
- 13.Josa-Laorden C, Cleland JG, Butcher C, et al. . Natural history and prognostic value of ultrasound lung artefacts (“comet tail” sign) in patients admitted to hospital for worsening heart failure. Br J Heart Dis 2018; 1: 112–118. [Google Scholar]
- 14.Volpicelli G, Elbarbary M, Blaivas M, et al. . International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38: 577–591. doi: 10.1007/s00134-012-2513-4 [DOI] [PubMed] [Google Scholar]
- 15.Yadav R, Sahoo D, Graham R. Thoracic imaging in COVID-19. Cleve Clin J Med 2020; 87: 469–476. doi: 10.3949/ccjm.87a.ccc032 [DOI] [PubMed] [Google Scholar]
- 16.Castelao J, Graziani D, Soriano JB, et al. . Findings and prognostic value of lung ultrasound in COVID-19 pneumonia. J Ultrasound Med 2020; 40: 1315–1324. doi: 10.1002/jum.15508 [DOI] [PubMed] [Google Scholar]
- 17.Narinx N, Smismans A, Symons R, et al. . Feasibility of using point-of-care lung ultrasound for early triage of COVID-19 patients in the emergency room. Emerg Radiol 2020; 27: 663–670. doi: 10.1007/s10140-020-01849-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iodice V, Pisaturo M, Fusco FM, et al. . Use of lung ultrasound in COVID-19: comparison with ultra-high-resolution computed tomography among 29 patients at “D. Cotugno” hospital, Naples, Italy. Infez Med 2020; 28: 346–350. [PubMed] [Google Scholar]
- 19.Brahier T, Meuwly J-Y, Pantet O, et al. . Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study. Clin Infect Dis 2020; in press [ 10.1093/cid/ciaa1408]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichter Y, Topilsky Y, Taieb P, et al. . Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med 2020; 46: 1873–1883. doi: 10.1007/s00134-020-06212-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horby P, Shen Lim W, Emberson JR, et al. . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–714. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman JD, Lye DCB, Hui DS, et al. . Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020; 383: 1827–1837. doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tung-Chen Y, Martí de Gracia M, Díez-Tascón A, et al. . Correlation between chest computed tomography and lung ultrasonography in patients with coronavirus disease 2019 (COVID-19). Ultrasound Med Biol 2020; 46: 2918–2926. doi: 10.1016/j.ultrasmedbio.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-04283-2020.SUPPLEMENT (566.3KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-04283-2020.Shareable (236.9KB, pdf)