Abstract
Background
Maintaining employment for adults with cancer is important, however, little is known about the impact of surgery for rectal cancer on an individual's capacity to return to work (RTW). This study aimed to determine the impact of laparoscopic vs. open resection on RTW at 12 months.
Methods
Analyses were undertaken among participants randomized in the Australian Laparoscopic Cancer of the Rectum Trial (ALaCaRT), with work status available at baseline (presurgery), and 12 months. Multivariable logistic regression, adjusted for sociodemographic and clinical characteristics estimated the effect of surgery on RTW in any capacity, or return to preoperative work status at 12 months.
Results
About 228 of 449 (51%) surviving trial participants at 12 months completed work status questionnaires; mean age was 62 years, 66% males, 117 of these received laparoscopic resection (51%). Of 228, 120 were employed at baseline (90 full‐time, 30 part‐time). Overall RTW in 120 participants in paid work at baseline was 78% (84% laparoscopic, 70% open surgery). Those employed full‐time were more likely to RTW at 12 months (OR, 3.55; 95% CI, 1.02–12.31). Those with distant metastases at baseline were less likely to RTW (OR, 0.07; 95% CI, <0.01–0.83). Laparoscopic surgery was associated with a higher rate of RTW but did not reach statistical significance (OR 2.88; 95% CI, 0.95–8.76).
Conclusions
Full‐time work presurgery and the presence of metastatic disease predicts RTW status at 12 months. A laparoscopic‐assisted surgical approach to rectal cancer may facilitate more patients to RTW, however, larger sample sizes are likely needed to confirm this result.
Keywords: clinical trial, income, laparoscopy, open abdomen techniques, rectal neoplasms, return to work, socioeconomic factors
Rectal cancer patients with laparoscopic‐assisted surgery have a slightly but not significantly higher return to work rate at 12 months than those with open rectal resection. Results of this randomised controlled trial highlight the importance of discussing employment disruption and retention strategies with adults undergoing surgery for rectal cancer

1. INTRODUCTION
Rectal cancer has become an emerging public health issue among working‐age adults in Australia and New Zealand due to an increased prevalence of obesity, alcohol consumption and dietary intake of red and processed meats. 1 , 2 According to the Australian Institute of Health and Welfare (AIHW) 2015 data, the incidence rate of rectal cancer for ages 30–64 years was 19.3 per 100,000 and the corresponding mortality rate was 6.5. 3 Employed individuals with rectal cancer face many challenges to their work life as treatments are often disruptive to their employment, earnings, and other role activities. 4 , 5 , 6 One study among employed middle‐aged people with colorectal cancer, reported 27% stopped working and 19% reduced their work hours at 1‐year postdiagnosis. 5 Stopping or reducing work may be a source of financial and psychological stress for cancer survivors, 6 , 7 , 8 , 9 which adversely affects their health‐related quality of life (HRQoL) and economic security. 4 , 5 , 8
Laparoscopic‐assisted resection has been widely used in colon but not rectal cancer surgery, since it was first described in 1992, 10 with reported benefits of less intraoperative blood loss, fewer adhesions, shorter hospital stay, and faster return to work (RTW). 11 , 12 , 13 , 14 , 15 , 16 , 17 In achieving better short‐term outcomes, proponents of the laparoscopic approach have persistently advocated this technique for rectal cancer treatment. 18
Using an open laparotomy approach, resection of the rectum often involves a more complicated and morbid procedure than for other gastro‐intestinal cancers and more precise dissection to reduce the chance of cancer recurrence. 18 However, the noninferiority of the laparoscopic approach to conventional open resection for rectal cancer treatment based on pathological outcomes was not established in the Australian Laparoscopic Cancer of the Rectum Trial (ALaCaRT) 19 , 20 or the American College of Surgeons Oncology Group Z6051 trial, 21 even though 2 year oncologic outcomes were not significantly different. 20 In addition to clinical outcomes, the effect of laparoscopic‐assisted surgery on RTW for rectal cancer survivors has never been evaluated in a randomized trial.
The purpose of this analysis was to examine the effect of laparoscopic compared with open resection for rectal cancer on RTW among rectal cancer survivors at 12 months participating in ALaCaRT, after adjusting for sociodemographic and clinical characteristics.
2. METHODS
2.1. Study participants
Data from the first 12 months after enrolment and surgery for ALaCaRT were used in this prospective analysis. ALaCaRT was a multicenter randomized, noninferiority, phase 3 trial evaluating the safety, and efficacy of laparoscopic resection versus open surgery for rectal cancer. 19 , 20 ALaCaRT participants were adults aged 18 years or older, with a histological diagnosis of adenocarcinoma of the rectum within 15 cm of the anal verge, and a life expectancy of at least 12 weeks. 19 Four hundred and seventy‐five patients from 24 hospitals in Australia and New Zealand were recruited between March 2010 and November 2014, with 473 eligible for analysis. 19 All ALaCaRT participants were randomized 1:1 to undergo laparoscopic or open surgery stratified by site of the tumor, the registering surgeon, the planned operative procedure (low anterior resection or abdominoperineal resection), body mass index (BMI), preoperative radiotherapy and distant metastases. Central ethics approval was obtained by the Sydney Local Health District human research ethics committee. Individual sites not covered by the central approval obtained local approval. All participants gave written informed consent before trial randomization. The study protocol has been reported in detail previously. 19
ALaCaRT participants were included in the current analysis if they reported their work status in the “Labour force and Income impacts of illness survey” (the survey) at study baseline prior to surgery and 12 months after surgery. The survey collected participants' socioeconomic data (including family composition, education level, work status, and annual income) at study baseline prior to surgery, 3, 6, and 12 months after surgery. Clinical data and surgical outcomes of participants were extracted from the trial database.
2.2. Study variables
The main outcome for this analysis was the RTW rate at 12 months following either type of surgery. Figure 1 depicts a causal diagram (i.e. logic model) 22 , 23 of the possible associations with sociodemographic, clinical characteristics, and surgical outcomes on RTW at 12 months. To define “RTW”, two measures were calculated: (a) participants in paid full‐time or part‐time paid employment at 12 months (yes, no); (b) return to preoperative work status or full‐time work at 12 months (yes, no). Participants who were unemployed or not in the labor force at study baseline were excluded from these analyses.
FIGURE 1.
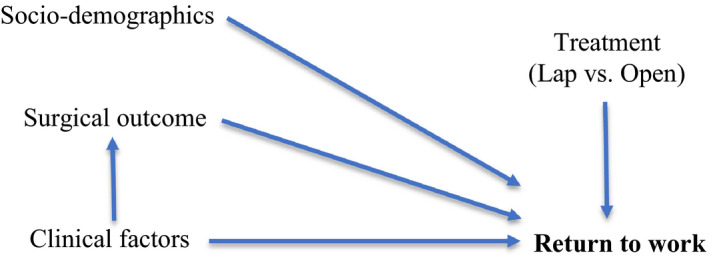
Causal diagram assessing the relationships between sociodemographic and clinical characteristics, treatment type and surgical outcomes on return to work for rectal cancer survivors
Explanatory variables included type of surgery (laparoscopic‐assisted vs. open resection), surgical/pathology composite outcome (successful vs. unsuccessful) and participants' sociodemographic and clinical characteristics at baseline. We used the pathological criterion for successful resection, which comprises complete total mesorectal excision (TME), clear circumferential margins (≥1 mm) and clear distal margins (≥1 mm), as described previously. 19 , 20 Clinical factors included BMI kg/m2 (<25, 25–30, ≥30); site of tumor (high: 10–15 cm from the anal verge, middle: 5–10 cm, low:<5 cm); tumor stage (T1, T2, T3); nodal status (N0, N1, N2); distant metastases (M0, M1); planned operative procedure (low anterior resection, abdominoperineal resection); preoperative radiotherapy (yes, no); performance status measured by Eastern Cooperative Oncology Group Scale (0–4). 24 Socioeconomic factors included age in years at randomization; sex (male, female); family composition (couple with dependent children, single parent, couple only, living alone); work status at baseline (full‐time paid work, part‐time paid work, unemployed looking for work, not in labor force [i.e. do not have a job and not looking for work]); highest educational attainment (university degree, certificate/Diploma, high school, or leaving certificate, year 9 or below/never attended school); usual yearly personal income before tax (<AU$29,999; $30,000–$69,999; $70,000–$149,999; $150,000 or more).
2.3. Statistical methods
Descriptive statistics are presented for participants' sociodemographic and clinical characteristics at baseline, type of surgery (randomization allocation as per intention to treat principle), and surgical outcome. Variables hypothesized to be associated with RTW, were identified a priori and are presented in Figure 1. Univariate analyses included the Pearson chi‐square (χ2) tests for categorical variables and the Student's t‐tests for continuous variables. Multivariate logistic regression models were built using known associated variables and backward elimination methods to estimate the effect of treatment, surgical outcome, and other factors associated with RTW at 12 months after the index procedure (surgery); with adjustment for age, sex, and cancer TNM stage. 25 , 26 , 27 , 28 , 29 , 30 Odds ratios (ORs) with conventional 95% CIs were presented and all quoted p‐values referred to the comparison with the specified referent category, with p < 0.05 for statistical significance. All analyses were conducted in SAS 9.4, Windows version (SAS Institute).
2.4. Sensitivity analyses
To ascertain factors associated with a return to preoperative work status, we performed separate logistic regressions among the participants who had returned to paid work at 12 months, excluding those who did not return to their preoperative work status (sensitivity analysis 1, [SA1]). To investigate the impact of missing data on the sample who returned to work at 12 months, and who completed the work status questionnaire at 3‐ or 6‐month time points (n = 102), we conducted a separate analysis (SA2). To explore whether the inclusion of participants who had died during the 12‐month follow‐up period influenced the impact of type of surgery on RTW, a separate sensitivity analysis was conducted including those who completed the baseline measure (SA3). The 12‐month RTW and return to preoperative work status measure was coded as “No” for deceased participants.
3. RESULTS
Of 473 ALaCaRT participants, 449 survived ≥12 months and 228 of these (51%) completed the work status questionnaire at baseline and 12 months. (Figure 2) To assess the representativeness of this sample, comparisons were conducted between ALaCaRT participants who completed the work status questionnaire and those did not. Respondents in our analysis were slightly younger, had tumor in the middle part of rectum rather than the low part of rectum, were less likely to have tumor‐stage 3 (T3) cancer, and receive preoperative radiotherapy compared with nonrespondents.(Table 1) Baseline characteristics of the study sample by treatment arm (laparoscopic‐assisted surgery n = 117; open surgery n = 111) are shown in Table 2. No significant difference in baseline characteristics by surgery type was identified.
FIGURE 2.
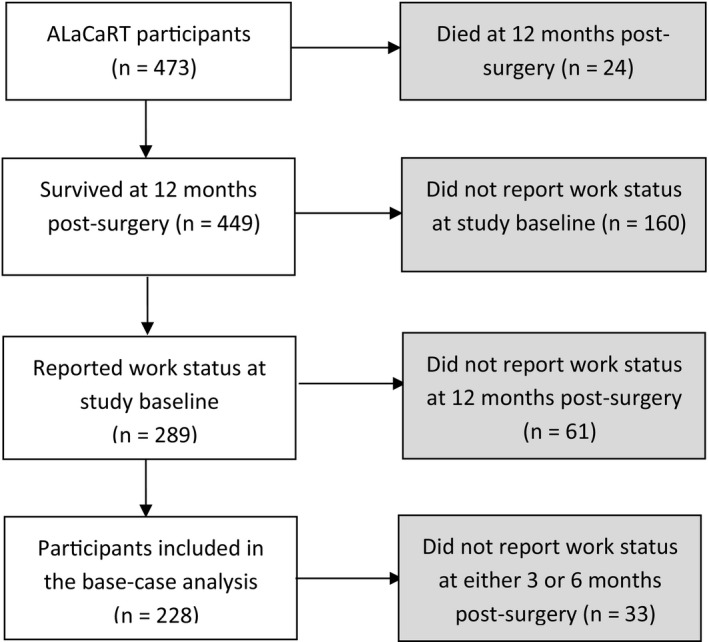
ALaCaRT participants included in the return to work analysis. ALaCaRT, Australian Laparoscopic Cancer of the Rectum Trial
TABLE 1.
Baseline characteristics and surgical outcomes among ALaCaRT participants by whether reported their work status in the labor force and income impacts of illness survey (the survey) at baseline and 12‐month after surgery.
| Variable | Description (%, unless specified) | Included in RTW analysis | All (n = 473) | p‐value | |
|---|---|---|---|---|---|
| Yes (n = 228) | No (n = 245) | ||||
| Sex | Males | 65.8 | 65.7 | 65.8 | 0.986 |
| Females | 34.2 | 34.3 | 34.2 | ||
| Age | Mean age (years) | 61.7 | 64.9 | 63.4 | 0.01 |
| Age group | Below 45 | 8.3 | 6.5 | 7.4 | 0.004 |
| 45–54 | 16.2 | 15.1 | 15.6 | ||
| 55–64 | 33.8 | 22.5 | 27.9 | ||
| 65–75 | 28.5 | 30.6 | 29.6 | ||
| 75 or above | 13.2 | 25.3 | 19.5 | ||
| Treatment arm | Laparoscopic‐assisted surgery | 51.3 | 49.4 | 50.3 | 0.68 |
| Surgical outcome | Successful resection | 86.8 | 83.3 | 85.0 | 0.28 |
| Preoperative radiotherapy | 43.4 | 55.1 | 49.5 | 0.01 | |
| BMI | Below 25 | 31.1 | 36.3 | 33.8 | 0.33 |
| 25–30 | 43.0 | 42.9 | 42.9 | ||
| 30 or above | 25.9 | 20.8 | 23.3 | ||
| Site of tumor | High | 23.3 | 20.4 | 21.8 | 0.01 |
| Middle | 49.1 | 38.0 | 43.3 | ||
| Low | 27.6 | 41.6 | 34.9 | ||
| T‐stage | T1 | 5.3 | 7.4 | 6.4 | 0.04 |
| T2 | 33.9 | 23.8 | 28.7 | ||
| T3 | 60.8 | 68.8 | 64.9 | ||
| N‐stage | N0 | 51.5 | 47.4 | 49.4 | 0.52 |
| N1 | 35.2 | 37.1 | 36.2 | ||
| N2 | 12.8 | 15.5 | 14.2 | ||
| Nx | 0.4 | 0 | 0.2 | ||
| M‐stage | M1 | 5.3 | 4.5 | 4.9 | 0.70 |
| Performance status (ECOG) | 0 | 82.4 | 78.3 | 80.3 | 0.23 |
| 1 | 16.7 | 18.8 | 17.8 | ||
| 2 | 0.9 | 2.9 | 1.9 | ||
| Planned operative procedure | Abdominoperineal resection | 7.0 | 7.8 | 7.4 | 0.76 |
Abbreviation: ALaCaRT, Australian Laparoscopic Cancer of the Rectum Trial; ECOG, Eastern Cooperative Oncology Group Scale.
TABLE 2.
Baseline characteristics of 228 ALaCaRT participants who completed the work status questionnaire at baseline and 12‐months after surgery, by treatment arm.
| Variable | Description (%, unless specified) | Treatment arm | p‐value | |
|---|---|---|---|---|
| Laparoscopic‐assisted (n = 117) | Open (n = 111) | |||
| Sex | Males | 68.4 | 63.1 | 0.40 |
| Females | 31.6 | 36.9 | ||
| Age | Mean age (years) | 61.7 | 61.7 | 0.99 |
| Age group (years) | Below 45 | 7.7 | 9.0 | 0.20 |
| 45–54 | 17.1 | 15.3 | ||
| 55–64 | 36.8 | 30.6 | ||
| 65–74 | 22.2 | 35.1 | ||
| 75 or above | 16.2 | 9.9 | ||
| Surgical outcome | Successful resection | 82.9 | 91.0 | 0.07 |
| Preoperative radiotherapy | Yes | 47.0 | 39.6 | 0.26 |
| BMI | Below 25 | 30.8 | 31.5 | 0.98 |
| 25–30 | 43.6 | 42.3 | ||
| 30 or above | 25.6 | 26.1 | ||
| Site of tumor | High | 24.8 | 21.6 | 0.83 |
| Middle | 48.7 | 49.6 | ||
| Low | 26.5 | 28.8 | ||
| T‐stage | T1 | 7.7 | 2.7 | 0.24 |
| T2 | 32.5 | 35.5 | ||
| T3 | 59.8 | 61.8 | ||
| N‐stage | N0 | 43.9 | 59.5 | 0.08 |
| N1 | 38.8 | 31.5 | ||
| N2 | 16.4 | 9.0 | ||
| Nx | 0.9 | 0 | ||
| M‐stage | M1 | 6.8 | 3.6 | 0.27 |
| Performance status | 0 | 78.6 | 86.5 | 0.16 |
| 1 | 19.7 | 13.5 | ||
| 2 | 1.7 | 0 | ||
| Planned operative procedure | Abdominoperineal resection | 8.6 | 5.4 | 0.35 |
| Family composition | Couple with dependent children | 18.8 | 23.6 | 0.63 |
| One person with dependent children | 1.7 | 1.8 | ||
| Couple only | 53.9 | 55.5 | ||
| Living alone | 25.6 | 19.1 | ||
| Education level | University degree | 28.5 | 19.3 | 0.45 |
| Certificate/ diploma | 25.0 | 29.4 | ||
| High school | 33.6 | 37.6 | ||
| Year 9 or below | 12.9 | 13.8 | ||
| Usual yearly personal income before tax (AU$) | Less than $30,000 | 43.6 | 48.7 | 0.21 |
| $30,000–$69,999 | 30.8 | 32.4 | ||
| $70,000–$149,999 | 16.2 | 16.2 | ||
| More than $150,000 | 9.4 | 2.7 | ||
| Main source of income | Wage/salaries | 35.3 | 40.0 | 0.14 |
| Self‐employed | 17.2 | 11.8 | ||
| Government benefits | 25.9 | 30.9 | ||
| Farm | 2.6 | 0 | ||
| Other a | 13.8 | 16.4 | ||
| No source | 5.2 | 0.9 | ||
| Work status | Full‐time paid work | 39.3 | 39.6 | 0.89 |
| Part‐time paid work | 14.5 | 11.7 | ||
| Unemployed | 2.6 | 1.8 | ||
| Not in labor force | 43.6 | 46.9 | ||
| Main reason for not working | Own ill health—cancer related | 16.7 | 12.7 | 0.40 |
| Own ill health—other illness | 9.3 | 5.5 | ||
| Care‐giving for sick/disabled person | 1.9 | 5.5 | ||
| Retired | 68.5 | 76.4 | ||
| Others b | 3.7 | 0 | ||
Abbreviation: ALaCaRT, Australian Laparoscopic Cancer of the Rectum Trial.
Other sources of income included the Age Pension, Veterans’ pension, superannuation, rental income, and share dividends.
Other reasons included redundancy and work‐based issues.
3.1. Change in work status distribution
The distribution of work status between the two treatment arms across the study assessments is shown in Figure 3. For rectal cancer survivors in the laparoscopic‐assisted surgery group, the proportion not in the labor force increased from 44% at baseline to 53% (i.e. 9% dropped out of the workforce) 12 months after surgery. This change was mainly driven by a reduction in the proportion of participants in part‐time paid work from 15% to 9% over the same period. The proportion of people receiving open resection and not in the labor force at 12 months increased from 47% at baseline to 60% (13% dropped out of the workforce). This change was mostly due to a reduction in the proportion of participants in full‐time paid work from 40% to 23% over the same period.
FIGURE 3.
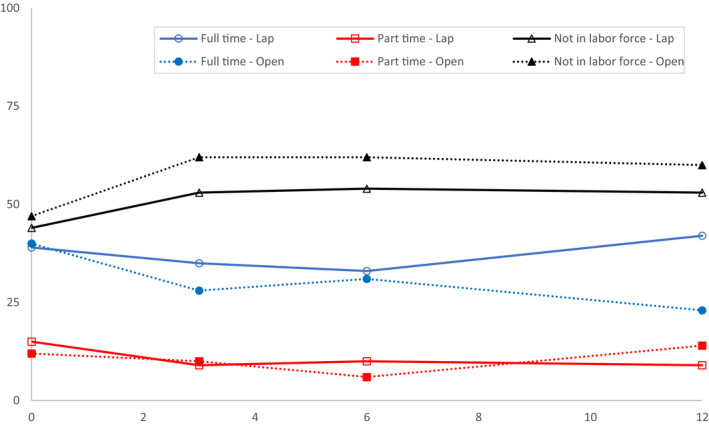
Proportion of ALaCaRT participants by treatment arm, employed full‐time, part‐time or not in the labor force at baseline, 3, 6 and 12‐months (n = 228). Number and proportion of ALaCaRT participants by treatment arm, employed full‐time, part‐time or not in the labor force at baseline, 3, 6 and 12‐months. ALaCaRT, Australian Laparoscopic Cancer of the Rectum Trial
3.2. Return to work
Table 3 illustrates that work status at baseline was a strong predictor of participants' RTW at 12 months. A high proportion (82%) of participants in full‐time paid work at baseline returned to work 12 months after surgery. Sixty‐three percent of participants in part‐time paid work at baseline returned to work over the same period. Two participants (2%) who were not working at baseline (either unemployed or not in the labor force) entered the workforce 12 months after surgery.
TABLE 3.
Change in work status from baseline to 12 months.
| Work status at baseline | Work status at 12 months | ||
|---|---|---|---|
| Full‐time paid work (n = 68) | Part‐time paid work (n = 27) | Unemployed/not in labor force (n = 133) | |
| Full‐time paid work (n = 90) | 63 (70.0%) | 11 (12.0%) | 16 (18.0%) |
| Part‐time paid work (n = 30) | 5 (16.7%) | 14 (46.7%) | 11 (36.7%) |
| Unemployed/ not in labor force (n = 108) | 0 (0%) | 2 (1.9%) | 106 (98.1%) |
Results of the logistic regression analysis for RTW and return to preoperative work status at 12 months are shown in Table 4. The unadjusted OR of RTW at 12 months for participants receiving laparoscopic resection was 2.25 (95% CI, 0.93–5.44). After adjustment for relevant sociodemographic and clinical factors, the effect of laparoscopic‐assisted surgery on RTW was higher but not statistically significant (OR, 2.88; 95% CI, 0.95–8.76). Multivariate analyses showed participants in full‐time paid work at baseline were more likely to RTW at 12 months (OR, 3.55; 95% CI, 1.02–12.31), while those with distant metastases (OR, 0.07; 95% CI, 0.01–0.83) were less likely to RTW during the 12‐month follow‐up period.
TABLE 4.
Multivariable analysis of return to work and return to preoperative work status for ALaCaRT participants at 12 months.
| Variable | Return to work at 12 months (n = 120) | Return to preoperative work status or full‐time work at 12 months (n = 120) | |||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Treatment arm | Open | 1 | Reference | 1 | Reference |
| Laparoscopic assisted | 2.88 | 0.95–8.76 | 2.01 | 0.83–4.86 | |
| Surgical outcome | Unsuccessful resection | 1 | Reference | 1 | Reference |
| Successful resection | 3.81 | 0.81–17.84 | 2.00 | 0.57–7.00 | |
| Sex | Females | 1 | Reference | 1 | Reference |
| Males | 1.20 | 0.35–4.15 | 1.81 | 0.73–4.51 | |
| Age (years) at randomization | 0.95 | 0.90–1.01 | 0.98 | 0.95–1.03 | |
| Tumor stage | T1 | 1 | Reference | 1 | Reference |
| T2 | 2.21 | 0.31–15.63 | 0.83 | 0.14–4.94 | |
| T3 | 1.21 | 0.17–8.45 | 0.39 | 0.06–2.34 | |
| Nodal status | N0 | 1 | Reference | 1 | Reference |
| N1 | 0.61 | 0.16–2.29 | 1.64 | 0.54–4.96 | |
| N2 | 10.42 | 0.64–170.05 | 6.03 | 0.96–37.88 | |
| Distant metastasis | M0 | 1 | Reference | 1 | Reference |
| M1 | 0.07 | 0.01–0.83 | 0.38 | 0.05–2.97 | |
| Performance status | ECOG score: 0 | 1 | Reference | — | — |
| ECOG score: 1 or 2 | 0.32 | 0.07–1.47 | — | — | |
| Work status at baseline | Part‐time | 1 | Reference | — | — |
| Full‐time | 3.55 | 1.02–12.31 | — | — | |
Logistic regression models with the use of backward selection to identify the independent variables. Assumed that a significance level of <0.25 was required for a variable to stay in the model (SLSTAY=0.25).
Included 120 ALaCaRT participants on full‐time / part‐time paid work at baseline.
Abbreviations: ALaCaRT, Australian Laparoscopic Cancer of the Rectum Trial; ECOG, Eastern Cooperative Oncology Group Scale; OR, odds ratio.
3.3. Return to preoperative work status or full‐time work
Eighty‐two participants (68%) (laparoscopic n = 49; open surgery n = 33) returned to their preoperative work status or full‐time work at 12 months. The unadjusted OR for laparoscopic resection was 2.55 (95% CI, 1.15–5.63). However, when adjusted for sociodemographic and clinical factors, the effect of laparoscopic‐assisted surgery was not statistically significant (OR, 2.01; 95% CI, 0.83–4.86). (Table 4).
3.4. Sensitivity analysis
In sensitivity analyses, among the 93 participants who returned to work at 12 months, age at randomization (OR, 1.09; 95% CI, 1.01–1.18) was positively associated with return to preoperative work status or full‐time work at 12 months. Both treatment type and surgical outcome had no significant effect on return to preoperative work status or full‐time at 12 months. (Table S1).
Including participants with complete labor force surveys at additional time points of 3 and 6 months (n = 102), sensitivity analyses (Table S2) showed participants in paid work at baseline were more likely to RTW at 12 months with laparoscopic‐assisted surgery than open surgery (OR, 6.68; 95% CI, 1.12–39.96). Participants with a BMI of ≥30 kg/m2 at baseline compared with those with a BMI of <25 kg/m2 (OR, 24.50; 95% CI, 1.67–358.92) were also more likely to RTW. Those with distant metastases (OR, 0.03; 95% CI, <0.01–0.75) were less likely to RTW during the 12‐month follow‐up period. The effect of a successful resection on RTW was not statistically significant (OR, 5.40; 95% CI, 0.43–68.02). Regression analyses also showed that in this group of rectal cancer survivors who completed surveys at all assessment points, those who received laparoscopic‐assisted surgery (OR, 3.81; 95% CI, 1.09–13.36) compared with those receiving open surgery, and participants who lived with a spouse and dependent children (OR, 18.69; 95% CI, 2.39–145.82) compared with those that lived with their spouse only, were more likely to return to their preoperative work status or full‐time work by 12 months.
Three participants in paid work at baseline died at 12 months and were included in the third sensitivity analysis. Results of logistic regression analysis (Table S3) among 123 participants in paid work at baseline showed those with laparoscopic‐assisted surgery compared with those receiving open surgery were more likely to RTW at 12 months (OR, 3.06; 95% CI, 1.03–9.11). Participants with a successful resection (OR, 4.84; 95% CI, 1.13–20.80) compared with those with an unsuccessful resection and those in full‐time paid work (OR, 4.26; 95% CI, 1.25–14.51) compared with those in part‐time paid work at baseline were more likely to RTW. Those with distant metastases (OR, 0.08; 95% CI, 0.01–0.76) and older participants (OR, 0.95; 95% CI, 0.89–1.00) were less likely to RTW at 12 months. Regression analyses also showed that participants receiving laparoscopic‐assisted surgery (OR, 2.80; 95% CI, 1.07–7.32) compared with open, participants with a successful resection (OR, 5.39; 95% CI, 1.30–22.37) compared with an unsuccessful resection, participants who lived with a spouse and dependent children (OR, 4.44; 95% CI, 1.15–17.13) compared with those that lived with their spouse only, were more likely to return to preoperative work status or full‐time work at 12 months.
4. DISCUSSION
Our research identified several factors that were associated with RTW, including being employed in a full‐time rather than part‐time capacity presurgery, and not having distant metastases at time of surgery. As a minimally invasive surgery, faster RTW, which contributes to income, a sense of structure and social recovery, 31 , 32 , 33 has been one of the main objectives of undergoing laparoscopic‐assisted surgery for abdominal and pelvic procedures. Type of surgery, age, sex, and surgical outcome (successful versus unsuccessful), were not significant predictors of RTW for rectal cancer patients in most scenarios. Although the RTW rate was somewhat higher for those who received laparoscopic‐assisted rather than open rectal surgery, this result was not statistically significant in the main analysis, but significant in sensitivity analyses for sub‐groups who completed the labor force survey at all assessment points, and when those who died before 12 months were included.
Findings of this analysis extend our knowledge of laparoscopic surgery for the treatment of rectal cancer. In the context of ALaCaRT results, noninferiority for a surrogate outcome of pathological outcomes was not shown. 19 , 20 This analysis showed that laparoscopic‐assisted surgery had no significant improvement on RTW at 12 months, therefore, one might question the ongoing role of laparoscopic‐assisted surgery for the treatment of rectal cancer. However, this approach remains useful for colon cancer, 11 , 12 , 13 , 14 , 15 , 16 , 17 gastric cancer, 34 prostate cancer, 35 and kidney cancer patients. 36 The survival and recurrence differences in both ALaCaRT and other trials have shown no significant difference between open and laparoscopic approaches. 20 Given that maintaining employment is an important patient‐centered outcome for cancer survivors, their family members and society, 31 , 32 , 33 the potential impact of laparoscopic‐assisted surgery on RTW could be promoted to new patients.
With some evidence of having less postoperative pain, shorter recovery time and equivalent long‐term outcomes, the laparoscopic approach has become a gold standard for colon cancer, 37 prostate cancer, 38 benign ovarian tumors, 39 endometriosis, 40 and other surgeries on the organs in the abdominal and pelvic area. For rectal cancer treatment, a Cochrane review based on evidence from nonrandomized studies showed that laparoscopic surgery offered some short‐term benefits in patients with less blood loss, a quicker return to normal diet, less pain, less narcotic use, and less immune response. 41 In a recent systematic review and meta‐analysis on 13 RCTs, laparoscopic surgery significantly reduced the rate of surgical site infections, blood loss, length of hospital stay and time to first bowel movement, despite it had longer operative time. 42 This analysis reported the medium‐term impact of such surgical approaches on RTW at 12 months for rectal cancer through a randomized controlled trial.
Novel therapeutic approaches and initiatives such as patient education and group discussion, multidisciplinary intervention through physical exercise and counseling, workplace accommodations through job flexibility, coworker support, and employment‐based health insurance have been implemented to assist cancer survivors to RTW after treatment. 27 , 32 , 37 , 38 , 39 This provides a sense of “normality,” a feeling of social belonging, 40 and relieving financial stress of cancer patients and their family for cancer treatment and other bills, 43 , 44 which can improve cancer survivors’ quality of life. 45 , 46 Second, with the rising costs of cancer care, 47 any policies or practices that improve the likelihood of a person maintaining their employment after cancer treatment deserve thoughtful consideration.
To our knowledge, this is the first evaluative study worldwide using a multicenter randomized controlled trial design to examine the effect of laparoscopic‐assisted surgery on RTW for the treatment of rectal cancer. Importantly, nearly two‐thirds of trial participants were of working age (i.e. 66 years or younger, the current threshold for the Aged Pension in Australia), which is reflective of contemporary issues facing adults with new rectal cancer diagnoses. There are, however, some limitations to acknowledge. First, the study had a modest response rate to the labor force and income impacts of illness questionnaire. Fifty‐one percent of ALaCaRT participants reporting their work status at baseline and 12 months, and some patients had missing data at the 3 and 6 month time points. This was due in part to the late addition of this survey when the trial was already recruiting (after 72 patients). Second, we did not have data about participants’ income protection, where this cover is likely to influence a cancer survivor's decision to RTW. Third, due to the ALaCaRT exclusion criteria, study participants may be healthier than the average rectal cancer survivors in the community; having no T4 tumors, no involvement of the circumferential resection margin, and no concurrent or previous invasive pelvic malignant tumors within 5 years before study enrollment. 19 Results of this study, therefore, may not be generalizable to all adults treated for rectal cancer. Fourth, the finding of greater RTW for laparoscopic‐assisted surgery may need to be interpreted with caution as there was no evidence this surgical approach had a significant impact on intermediate outcomes (i.e. 2‐year disease‐free survival). 20
5. CONCLUSIONS
Ongoing employment is a critical concern for many rectal cancer patients and treatment options that enhance RTW prospects should be discussed. Full‐time work and the presence of metastatic disease are likely to predict RTW at 12 months. A laparoscopic‐assisted surgical approach to rectal cancer may facilitate more patients to RTW, however, larger sample sizes are likely needed to confirm this result.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Law: Conceptualization, formal analysis, methodology, software, visualization and writing—original draft; Brewer: Data curation and writing—review and editing; Brown: Data curation, methodology and validation; Wilson: Data curation and project administration; Bailey: Data curation and project administration; Hague: Funding acquisition, investigation, writing—review and editing; Simes: Funding acquisition, investigation, writing—review and editing; Stevenson: Funding acquisition, investigation, writing—review and editing; Solomon: Funding acquisition, investigation, writing—review and editing; Morton: Conceptualization, methodology, supervision, writing—review and editing.
Supporting information
Table S1‐S3
ACKNOWLEDGMENTS
The Australasian Gastro‐Intestinal Trials Group (AGITG), Sydney Australia was the legal sponsor of ALaCaRT. We thank Professor Deborah Schofield for her contribution to the design of the Labor force and Income impacts of illness survey used in this trial.
Funding information
RLM was funded by an Australian National Health and Medical Research Council (NHMRC) TRIP Fellowship and a Robinson Fellowship from the University of Sydney. ALaCaRT was supported by grants from the Colorectal Surgical Society of Australia and New Zealand (CSSANZ) Foundation, NHMRC Project Grants (1009973 and 1078113), and the Queensland Cancer Council. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The Australasian Gastro‐Intestinal Trials Group (AGITG) was the legal sponsor and trial coordination was performed by the NHMRC Clinical Trials Centre. The trial was registered with the Australian and New Zealand Clinical Trial Registry, ACTRN12609000663257.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Global Cancer Observatory . Cancer Fact Sheets: Rectum 69372 Lyon CEDEX 08, France International Agency for Research on Cancer, World Health Organaization. 2019. Available from http://gco.iarc.fr/today/data/factsheets/cancers/9‐Rectum‐fact‐sheet.pdf. Accessed 18 Feb 2020
- 2. Feletto E, Yu XQ, Lew JB, et al. Trends in colon and rectal cancer incidence in Australia from 1982 to 2014: analysis of data on over 375,000 cases. Cancer Epidemiol Biomarkers Prev. 2019;28(1):83‐90. [DOI] [PubMed] [Google Scholar]
- 3. Cancer Data in Australia: ACIM Books & ACD Pivot Table. Australian Institute of Health and Welfare, Australian Government; 2019. Available from https://www.aihw.gov.au/reports/cancer/cancer‐data‐in‐australia/acim‐books. Accessed 23 Mar 2020 [Google Scholar]
- 4. Gordon L, Lynch BM, Newman B. Transitions in work participation after a diagnosis of colorectal cancer. Aust Nz J Publ Heal. 2008;32(6):569‐574. [DOI] [PubMed] [Google Scholar]
- 5. Gordon LG, Beesley VL, Lynch BM, et al. The return to work experiences of middle‐aged Australian workers diagnosed with colorectal cancer: a matched cohort study. BMC Public Health. 2014;14:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGrath C, Mihala G, Beesley VL, Lynch BM, Graves N, Gordon LG. "Cancer put my life on hold": work‐related challenges among middle‐aged adults 12 months after a diagnosis of colorectal cancer. Cancer Nurs. 2017;40(2):160‐167. [DOI] [PubMed] [Google Scholar]
- 7. Gordon LG, Beesley VL, Mihala G, Koczwara B, Lynch BM. Reduced employment and financial hardship among middle‐aged individuals with colorectal cancer. Eur J Cancer Care (Engl). 2017;26(5). [DOI] [PubMed] [Google Scholar]
- 8. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10(5):332‐338. [DOI] [PubMed] [Google Scholar]
- 9. Sharp L, Timmons A. Pre‐diagnosis employment status and financial circumstances predict cancer‐related financial stress and strain among breast and prostate cancer survivors. Support Care Cancer. 2016;24(2):699‐709. [DOI] [PubMed] [Google Scholar]
- 10. Phillips EH, Franklin M, Carroll BJ, Fallas MJ, Ramos R, Rosenthal D. Laparoscopic colectomy. Ann Surg. 1992;216(6):703‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clinical Outcomes of Surgical Therapy Study Group , Nelson H, Sargent DJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050‐2059. [DOI] [PubMed] [Google Scholar]
- 12. Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short‐term outcomes of a randomised trial. Lancet Oncol. 2005;6(7):477‐484. [DOI] [PubMed] [Google Scholar]
- 13. Lacy AM, García‐Valdecasas JC, Delgado S, et al. Laparoscopy‐assisted colectomy versus open colectomy for treatment of non‐metastatic colon cancer: a randomised trial. Lancet. 2002;359(9325):2224‐2229. [DOI] [PubMed] [Google Scholar]
- 14. Guillou PJ, Quirke P, Thorpe H, et al. Short‐term endpoints of conventional versus laparoscopic‐assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718‐1726. [DOI] [PubMed] [Google Scholar]
- 15. Angst E, Hiatt JR, Gloor B, Reber HA, Hines OJ. Laparoscopic surgery for cancer: a systematic review and a way forward. J Am Coll Surg. 2010;211(3):412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long‐term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev. 2008;34(6):498‐504. [DOI] [PubMed] [Google Scholar]
- 17. Senagore AJ, Delaney CP, Brady KM, Fazio VW. Standardized approach to laparoscopic right colectomy: outcomes in 70 consecutive cases. J Am Coll Surg. 2004;199(5):675‐679. [DOI] [PubMed] [Google Scholar]
- 18. Indar A, Efron J. Laparoscopic surgery for rectal cancer. Perm J. 2009;13(1):47‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stevenson ARL, Solomon MJ, Lumley JW, et al. Effect of laparoscopic‐assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356‐1363. [DOI] [PubMed] [Google Scholar]
- 20. Stevenson ARL, Solomon MJ, Brown CSB, et al. Disease‐free survival and local recurrence after laparoscopic‐assisted resection or open resection for rectal cancer: the Australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg. 2019;269(4):596‐602. [DOI] [PubMed] [Google Scholar]
- 21. Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic‐assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weng HY, Hsueh YH, Messam LL, Hertz‐Picciotto I. Methods of covariate selection: directed acyclic graphs and the change‐in‐estimate procedure. Am J Epidemiol. 2009;169(10):1182‐1190. [DOI] [PubMed] [Google Scholar]
- 23. Keele L, Stevenson RT, Elwert F. The causal interpretation of estimated associations in regression models. Polit Sci Res Meth. 2020;8(1):1‐13. [Google Scholar]
- 24. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649‐655. [PubMed] [Google Scholar]
- 25. Short PF, Vasey JJ, Tunceli K. Employment pathways in a large cohort of adult cancer survivors. Cancer. 2005;103(6):1292‐1301. [DOI] [PubMed] [Google Scholar]
- 26. Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population‐based national sample. J Natl Cancer Inst. 2004;96(17):1322‐1330. [DOI] [PubMed] [Google Scholar]
- 27. Bouknight RR, Bradley CJ, Luo Z. Correlates of return to work for breast cancer survivors. J Clin Oncol. 2006;24(3):345‐353. [DOI] [PubMed] [Google Scholar]
- 28. Bradley CJ, Neumark D, Luo Z, Bednarek H, Schenk M. Employment outcomes of men treated for prostate cancer. J Natl Cancer Inst. 2005;97(13):958‐965. [DOI] [PubMed] [Google Scholar]
- 29. Taskila T, Lindbohm ML. Factors affecting cancer survivors’ employment and work ability. Acta Oncol. 2007;46(4):446‐451. [DOI] [PubMed] [Google Scholar]
- 30. Islam T, Dahlui M, Majid HA, et al. Factors associated with return to work of breast cancer survivors: a systematic review. BMC Public Health. 2014;14(Suppl 3):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehnert A, de Boer A, Feuerstein M. Employment challenges for cancer survivors. Cancer. 2013;119(Suppl 11):2151‐2159. [DOI] [PubMed] [Google Scholar]
- 32. Chen YY, Wang CC, Wu WT, et al. Trajectories of returning to work and its impact on survival in survivors with oral cancer: a 5‐year follow‐up study. Cancer. 2020;126(6):1225‐1234. [DOI] [PubMed] [Google Scholar]
- 33. Mehnert A. Employment and work‐related issues in cancer survivors. Crit Rev Oncol Hematol. 2011;77(2):109‐130. [DOI] [PubMed] [Google Scholar]
- 34. Chevallay M, Jung M, Berlth F, Seung‐Hun C, Morel P, Monig S. Laparoscopic surgery for gastric cancer: the European point of view. J Oncol. 2019;2019:8738502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ilic D, Evans SM, Allan CA, Jung JH, Murphy D, Frydenberg M. Laparoscopic and robotic‐assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst Rev. 2017;9:CD009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Janzen NK, Perry KT, Schulam PG. Laparoscopic radical nephrectomy and minimally invasive surgery for kidney cancer. Cancer Treat Res. 2003;116:99‐117. [DOI] [PubMed] [Google Scholar]
- 37. Hassett MJ, O'Malley AJ, Keating NL. Factors influencing changes in employment among women with newly diagnosed breast cancer. Cancer. 2009;115(12):2775‐2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lamore K, Dubois T, Rothe U, et al. Return to work interventions for cancer survivors: a systematic review and a methodological critique. Int J Environ Res Public Health. 2019;16(8):1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Boer AG, Taskila TK, Tamminga SJ, Feuerstein M, Frings‐Dresen MH, Verbeek JH. Interventions to enhance return‐to‐work for cancer patients. Cochrane Database Syst Rev. 2015. 10.1002/14651858.cd007569.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stergiou‐Kita M, Grigorovich A, Tseung V, et al. Qualitative meta‐synthesis of survivors’ work experiences and the development of strategies to facilitate return to work. J Cancer Surviv. 2014;8(4):657‐670. [DOI] [PubMed] [Google Scholar]
- 41. Breukink S, Pierie J, Wiggers T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2006;2006(4):CD005200 10.1002/14651858.CD005200.pub2 [DOI] [PubMed] [Google Scholar]
- 42. Małczak P, Mizera M, Torbicz G, et al. Is the laparoscopic approach for rectal cancer superior to open surgery? A systematic review and meta‐analysis on short‐term surgical outcomes. Wideochir Inne Tech Maloinwazyjne. 2018;13(2):129‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perrone F, Jommi C, Di Maio M, et al. The association of financial difficulties with clinical outcomes in cancer patients: secondary analysis of 16 academic prospective clinical trials conducted in Italy. Ann Oncol. 2016;27(12):2224‐2229. [DOI] [PubMed] [Google Scholar]
- 44. Seifart U, Schmielau J. Return to work of cancer survivors. Oncol Res Treat. 2017;40(12):760‐763. [DOI] [PubMed] [Google Scholar]
- 45. Tamminga SJ, Bultmann U, Husson O, Kuijpens JL, Frings‐Dresen MH, de Boer AG. Employment and insurance outcomes and factors associated with employment among long‐term thyroid cancer survivors: a population‐based study from the PROFILES registry. Qual Life Res. 2016;25(4):997‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mols F, Vingerhoets AJ, Coebergh JW, van de Poll‐Franse LV. Quality of life among long‐term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41(17):2613‐2619. [DOI] [PubMed] [Google Scholar]
- 47. Hong SJ, Li EC, Matusiak LM, Schumock GT. Spending on antineoplastic agents in the United States. J Oncol Pract. 2018;14(11):e683‐e691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
Research data are not shared.


