Abstract
The mitochondrial permeability transition pore (mPTP) or mitochondrial megachannel is arguably one of the most mysterious phenomena in biology today. mPTP has been at the center of ongoing extensive scientific research for the last several decades. In this review we will discuss recent advances in the field that enhance our understanding of the molecular composition of mPTP, its regulatory mechanisms and its pathophysiological role. We will describe our recent findings on the role of ATP synthase c-subunit ring as a central player in mitochondrial permeability transition and as an important metabolic regulator during development and in degenerative diseases.
Introduction
The mitochondrial permeability transition (mPT) was discovered in the 1970s. It was first characterized as an abnormal swelling of mitochondria upon high calcium overload [1-4]. Later, it was shown by electrophysiological recordings that mitochondrial matrix swelling and subsequent rupture of the mitochondrial outer membrane were due to the opening of a high conductance, non-selective channel of the mitochondrial inner membrane, named the “mitochondrial megachannel” by Zoratti’s group or “multiconductance channel” by Kinnally’s group [5-10]. For decades, mPTP was assumed to be a cell death channel that caused irreversible, apoptosis-inducing changes in mitochondria, rather than a pore having any physiological function [11, 12]. Nevertheless, we now know that the mPTP is a pivotal physiological channel, which regulates Ca2+ signaling, [13-15] intracellular Ca2+ homeostasis [16-23] and ATP production efficiency [24-30]. The mechanism of how mPTP converts from a physiological to a pathological channel remains mysterious. Open conformations of mPTP with both low and high conductance states have been reported during electrophysiological recordings of isolated mitochondria [5-8, 10, 18]. A flickering channel corresponding to a low conductance conformation has been reported to have different subconductance levels with a ~300 pS peak conductance, while the large conductance state has a peak conductance of ~1200-1800 pS [5-8]. The low conductance openings were suggested to be brief and reversible and perhaps to contribute to the physiological function of mPTP, while the prolonged openings of the high conductance state may induce irreversible depolarization of the inner membrane, mitochondrial swelling and subsequent cell death [6, 13-15, 31-33].
Nevertheless, the exact molecular mechanism of mPTP opening, the difference between low and high conductance states and the molecular mechanism of conversion between these states remains unknown. The mPT is a complex phenomenon that occurs during a combination of events that also disrupt mitochondrial structure and function. These changes include, but are not limited to, depletion of adenine nucleotides, elevated mitochondrial matrix Ca2+ levels, increases in inorganic phosphate and reactive oxygen species, dissipation of mitochondrial inner membrane potential and matrix swelling [11, 32-36]. The combination of these changes induces mPTP opening and downstream cell death mechanisms.
The molecular composition of mPTP: ATP synthase c-subunit ring as the inner membrane leak channel
The molecular identity of mPTP is still controversial and is a subject of intensive scientific debate. Different mitochondrial proteins, such as the adenine nucleotide translocator (ANT) [37], the voltage-dependent anion channel (VDAC) [38, 39], the phosphate carrier (PiC) [40] and the translocator protein (TSPO) [41-43] have been suggested previously to play a direct role in mPTP formation. Further studies have shown that these proteins regulate channel behavior rather than constitute the pore of the mitochondrial permeability transition (mPTP) [44-47]. Mitochondrial FOF1 ATP synthase was suggested to be a key player in mPTP formation according to several recent reports [28, 30, 48-57]. The peptidyl-prolyl cis-trans isomerase cyclophilin D (CypD) is a well-known regulator of mPTP [58, 59]. Its interaction with ATP synthase OSCP subunit was described by Bernardi’s lab [60], leading them to hypothesize that ATP synthase is involved in mPTP formation. Our lab independently built a hypothetical model of the involvement of ATP synthase in mPT based on our studies in which we reported interaction of the anti-cell death protein Bcl-xL with the ATP synthase β subunit [27, 28].
Multidisciplinary studies performed by Bernardi’s group suggested that the channel is located between ATP synthase dimers with the direct involvement of FO subunits e and g [48-52]. Yeast mutant strains lacking subunits e and g, subunits that are necessary for dimer formation [61, 62], displayed a striking resistance to mPTP opening suggesting at first glance that ATP synthase dimerization is required for pore formation in situ [49, 50]. The Arg-8 residue of subunit e was found to be crucial for stabilizing the ATP synthase dimers and for formation of full conductance mitochondrial megachannels in yeast [51]. Furthermore, channel formation was observed in detergent-solubilized tetramers and dimers but not in monomers of F1FO ATP synthase purified from bovine and porcine hearts [48, 52]. Nevertheless, our studies have recently shown that ATP synthase monomer produces a high multi-conductance channel activity with the biophysical characteristics of mPTP, suggesting that the channel is not located between e and g subunits and ATP synthase dimerization is not required for its activity [30].
If the channel resides within the ATP synthase monomer, the likely location is the membrane embedded c-subunit ring, considering its transmembrane pore-like cavity. Several studies have suggested that the c-subunit comprises the pore [28, 30, 53-57]. The chloroform extract of isolated rat liver mitochondria was reported to have a channel activity similar to the mPTP channel seen in patch-clamp recordings of native mitoplasts [56]. The analysis of biochemical composition of the chloroform-extracted material revealed that it contains a low molecular weight protein, identified to be the ATP synthase c-subunit. The chloroform extract also contained the PHB/polyP/Ca2+ complex, which the authors at that time suggested to be the ion-conducting module of the mPTP [56]. In another study, phosphorylated and non-phosphorylated forms of c-subunit isolated from rat liver mitochondria by chloroform extraction were studied in lipid bilayer experiments [53]. Phosphorylated c-subunit was shown to form cation-selective channels with brief openings in artificial lipid membranes, while Ca2+-induced dephosphorylation of c-subunit led to the formation of non-selective channels, suggesting that the phosphorylation status of c-subunit might determine activation of a structural/regulatory component of mPTP [53]. Genetic manipulation of c-subunit expression levels by siRNA in HeLa cells confirmed that c-subunit is required for mPTP-driven mitochondrial fragmentation and cell death triggered by cytosolic calcium overload and oxidative stress [54].
In our studies, affinity purified, reconstituted human c-subunit was demonstrated to form a large, voltage-sensitive channel in patch-clamp recordings, but its activity was not sensitive to well-known mPTP regulators, CsA and Ca2+, since their binding sites were found to be located in ATP synthase F1 subunits [28]. Depletion of the c-subunit attenuated Ca2+-induced depolarization of the inner mitochondrial membrane while its overexpression sensitized primary hippocampal neurons to cell death. c-subunit was observed to undergo measurable conformational changes by expanding its size upon activation of the channel [28]. Additionally, mutations that loosen the packing of the c-subunit ring led to the formation of c-rings with a larger internal diameter and significantly increased conductance compared to WT [28] and these mutations sensitized cells to death. CypD/Ca2+-mediated and CsA/ADP-inhibited dissociation of ATP synthase F1 subunits from FO was associated with mPT suggesting that unmasking of the c-subunit ring is required for initiation of channel conductance [28]. Further evidence that F1 becomes uncoupled from FO in the presence of high Ca2 is that direct binding of Ca2+ to ATP synthase β subunit [63, 64] dissociates ATP hydrolysis from H+ pumping in the presence of Ca2+ [65]. Interestingly, age-dependent decreases in F1 content compared to FO, decreasing the F1/FO ratio, have been reported in rat brain and heart mitochondria [66, 67].
Nevertheless, the prominent role of the c-subunit ring in mPTP has been recently questioned in several studies [68-70]. One of the main reasons for this controversy is the hydrophobic nature of the c-subunit pore-lining residues. However, different kinds of densities are observed to occupy the c-subunit lumen in recent structures of F1FO ATP synthases from bacteria to eukaryotes. In some studies the c-subunit pore has been reported to be filled with detergents or lipids [71-73], which are predicted to preclude ion conductance through its cavity, although partial occlusion of other known conducting channels with lipids has been reported [74]. Interestingly, c-subunit lumen was shown to be filled by a 40 amino acid-long alpha-helical protein instead of lipids in the recent cryo-EM structure of porcine ATP synthase tetramer [75]. 6.8PL proteolipid subunit of ATP synthase was assigned to this density although further studies are needed to fully reveal the identity of this protein. According to this structure, the C-termini of the four ATP synthase e subunits are bent toward the four c-subunit rings in each tetramer, which allows them to interact with the C-terminal ends of the 6.8PL helices occupying the central pore of the four c-rings [75]. It is possible that the interaction between subunits c, 6.8PL and e is important for keeping the c-subunit lumen sealed and the c-subunit channel closed. This will prevent dissipation of membrane potential under normal physiological conditions, decreasing the probability of channel opening and initiation of permeability transition.
A similar curved region of a presumed FO protein interacting with the detergent micelles occupying the c-subunit cavity was reported earlier for the bovine mitochondrial ATP synthase [76]. Likewise, the recent high-resolution structure of the in meso crystallized c-ring from spinach chloroplasts revealed additional electron densities inside the c-rings, suggested to originate from isoprenoid quinones such as coenzyme Q in mitochondria and plastoquinone in chloroplasts [77]. Coenzyme Q and its analogues are electron carriers of bioenergetic chains, also hypothesized to serve as universal cofactors of ATP synthase, stabilizing the c-ring and preventing ion leakage through it [77]. Additional studies are required to verify the exact nature of the densities contained within the c-subunit pore [77].
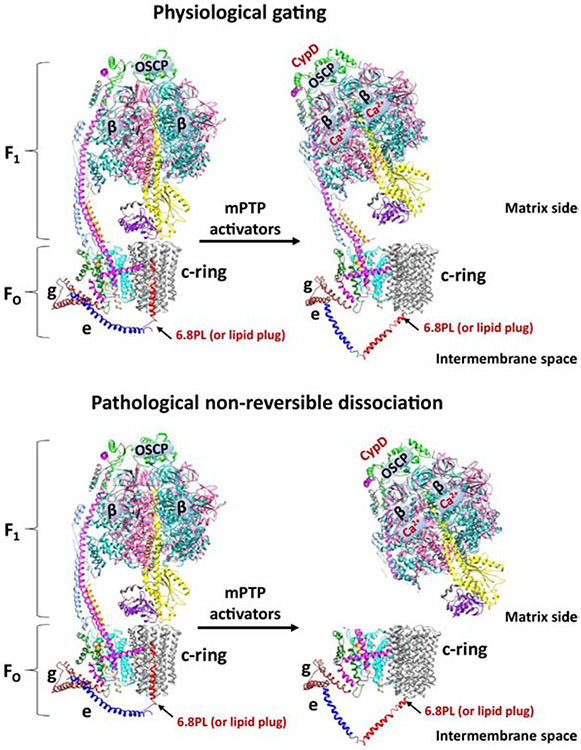
In light of these recent findings we suggest a new “bent-pull” model of c-subunit channel gating (Figure 1a, b). ATP synthase consists of two subcomplexes, the hydrophilic F1, which bears the catalytic nucleotide binding sites on the β subunits, and the membrane-embedded FO [76, 78, 79]. Tight coupling between F1 and FO subcomplexes is required for highly efficient rotational catalysis and occurs through two stalks, the F1 central stalk (consisting of γ, δ, ε subunits in mitochondria) and the FO peripheral stalk (primarily consisting of OSCP, b, h, d subunits ) [76, 78, 79]. OSCP connects FO with F1 through the peripheral stalk and is an important site of modulation of ATP synthase leak channel activity due to its interaction with different endogenous and pharmacological inducers of mPTP [80-82]. During the mPT initiation step, mPTP modulators bind directly to different ATP synthase subunits: CypD binds to OSCP subunit [83-85] and Ca2+ binds to β subunit [63, 64] (Figure 1a). We suggest that these binding steps induce conformational changes in the ATP synthase peripheral stalk subunits, which then modify interactions of c-subunit with membrane embedded FO subunits, including subunit e. The subsequent conformational changes in e subunit then pull the lipid “plug” or the proteins out from the c-subunit lumen to open the channel from the side facing the intermembrane space (Figure 1a). This model highlights both the importance of c-subunit as a pore forming component of mPTP [28, 30] and also the crucial role of ATP synthase subunits e and g in mPTP formation [48-51]. The conformational changes in ATP synthase due to the binding of mPTP inducers may also pull away F1 from the mouth of the c-subunit pore to free the channel from the side facing the matrix. These concurrent events will open the pore. This model represents the reversible, brief openings of the mPTP channel, the type of openings that most likely occur under physiological conditions. We suggest that non-reversible dissociation of F1 from FO occurs during long-lasting openings of mPTP (Figure 1b). This marks the point of no return because swelling as a result of prolonged pore opening triggers outer membrane rupture, release of cytochrome c and activation of downstream cell death pathways in detrimental pathological conditions, such as during brain or heart ischemia or in neurodegenerative diseases.
Figure 1. Proposed “bent-pull” model of F1FO ATP synthase c-subunit channel gating in physiological and pathological conditions.
A. Reversible brief openings of ATP synthase channel. mPTP inducing agents, CypD and Ca2+ bind to the OSCP and β subunits, respectively, inducing conformational changes in ATP synthase peripheral stalk subunits then modifying interactions of membrane embedded FO subunits, including subunit e, with the lipid or protein (6.8PL) “plug” occupying c-subunit. The subsequent conformational changes in e subunit then pull this “plug” out from the c-subunit lumen to open the channel from the side facing the intermembrane space. These conformational changes also pull away F1 from the mouth of the c-subunit pore to open the channel from the side facing the matrix. ATP synthase subunits are drawn as ribbon representations (modified PDB ID code: 6J5I [75]). B. Non-reversible dissociation of F1 from FO occurring during long-lasting openings of c-subunit channel in pathology. F1 dissociates from FO, which marks the point of no return in cell metabolism, since swelling as a result of prolonged pore opening triggers outer membrane rupture in detrimental pathological conditions, such as in brain or heart ischemia or in neurodegenerative diseases. For simplicity only ATP synthase monomer is shown, even though the ATP synthase is present as a dimer in native mitochondrial inner membrane. ATP synthase subunits are drawn as ribbon representations (modified PDB ID code: 6J5I [75]).
Surprisingly, the complete knockout of the ATP synthase c-subunit from HAP1-A12 cells results in no change in sensitivity of the mPT to calcium as studied in calcium retention capacity (CRC) experiments of mitochondria, allowing the authors of this study to conclude that c-subunit and ATP synthase are not required for mPT [70]. The CRC experiments, however, may only indicate a loss of membrane potential and not mPT-induced swelling, which has not been studied in c-subunit knockout HAP1-A12 cells [70]. Swelling indicates an osmotic change induced by solute and water entering into the matrix. It is independent of a loss in mitochondrial membrane potential since, for example, it is known that experimental mitochondrial isolation where membrane potential is absent does not produce appreciable swelling. The driving force for the Ca2+ uptake in CRC experiments is the mitochondrial membrane potential and as long as the c-subunit knockout HAP1-A12 cells are able to maintain a membrane potential, by possible reversal of ANT or other mechanisms, they can have similar Ca2+ uptake compared with the control cells, as has been observed in [70].
Unlike the CRC, patch-clamp recordings are a direct measure of mPTP pore opening by the measurement of its channel activity. Patch-clamp recordings of these same c-subunit knockout mitoplasts (mitochondrial inner membrane preparations lacking the outer membrane) revealed that they lack the high conductance CsA-sensitive mPTP channel activity recorded in WT [57]. A small and CsA-sensitive channel was still found to be present in c-subunit knockout mitoplasts, and this channel was also bongkrekic acid-sensitive suggesting that the ANT could possibly contribute to small conductance inner mitochondrial membrane activity recorded in the absence of c-subunit [57]. In contrast to these findings, the channel activity recorded from mitochondrial inner membranes of wild-type and ANT-deficient yeast strains demonstrated the same conductance, ion selectivity and voltage dependence in both strains [9]. The recorded ion conductance was not sensitive to the specific ANT inhibitor carboxyatractyloside, suggesting that ANT is not responsible for mPTP activity in yeast [9].
The role of ANT in mPTP formation was further studied by using the Ant-triple-null mice and quadruple-null mice, for Ant1, Ant2, Ant4 and Ppif. Genetic ablation of all three ANT isoforms inhibited mPTP opening in mouse embryonic fibroblasts (MEFs) of Ant-triple-null mice but not in liver cells. mPTP opening was completely inhibited only after genetic deletion of Ant and Ppif in the liver mitochondria, suggesting that ANT may constitute the main pore forming component of mPTP in MEFs while another, CypD-dependent protein is required for mPTP formation in liver cells [86]. In contrast to this implication, another interpretation suggests that inhibition of mPTP in MEFs does not necessarily mean that ANT itself is the pore-forming component in this cell type, but instead it could be a regulatory component by maintaining the physiological balance of adenine nucleotides between the matrix and cytosol. For example, if complete deletion of ANT leads to the accumulation of the ATP/ADP pool in the matrix, then this will completely inhibit mPTP channel activity, which would be observed as an inability to depolarize the inner membrane in a calcium dependent manner, coupled to a decrease in channel activity in patch-clamp recordings of ANT depleted mitoplasts as was described in the ANT KO studies [86]. Future ANT/c-subunit double KO studies will be required to reach a conclusion. Based on these findings we suggest that ANT is an important regulator of mPTP in all cell types as has been reported for CypD [19, 32, 60, 87].
Physiological relevance of the ATP synthase leak channel in synaptic plasticity
We have reported earlier that mPTP channel openings serve a physiological function and contribute to synaptic plasticity in neurons [88, 89]. Recordings of mitochondrial ion channel activity inside living neuronal synapses demonstrate that presynaptic nerve stimulation results in Ca2+ dependent channel openings in the mitochondrial membranes [88, 89]. The study is consistent with the requirement for the opening of an inner membrane Ca2+ activated conductance to allow Ca2+ to flow between the matrix and the cytosol during and for a short time after high frequency presynaptic activity [89-91]. We reported that anti-apoptotic protein Bcl-xL regulates this activity, since application of the recombinant protein enhances mitochondrial channel activity, increases certain forms of short term presynaptic plasticity [92], and restores stimulus dependent postsynaptic depolarization and action potential firing from depleted synapses. Therefore, a Bcl-xL-regulated, Ca2+-dependent conductance spanning the two mitochondrial membranes is required for short term plasticity of presynaptic neurotransmitter release. The Ca2+ release occurring coincident with this opening is required for synaptic strengthening [90, 91].
Bcl-xL could also mediate an important metabolic change brought on by high frequency activity. This was supported by the finding that injection of Bcl-xL or ATP into the presynapse caused synaptic strengthening beyond the pre-stimulation level. Such plasticity of neurotransmitter release after intense activity prevents neurotransmitter depletion during high frequency firing, is crucial to brain development [93] and is required for long term changes in synaptic plasticity that underlie memory formation and learning [94, 95].
Mitochondrial plasticity is linked to structural and functional synaptic remodeling. Overexpressed Bcl-xL in isolated neurons localizes mostly to mitochondria but also to synaptic vesicle membranes [96, 97]. Neurons overexpressing Bcl-xL have increased numbers of presynaptic vesicle clusters [96], synapses and mitochondria. The largest mitochondria reside close to the expanded vesicle pools [96]. Bcl-xL coordinates with the mitochondrial fission protein, Drp-1 and the biogenesis factor PGC-1alpha to achieve mitochondrial positioning, but fusion is not required, suggesting that mitochondrial growth at a more proximal location [98, 99], followed by mitochondrial fission, supplies the enlarged synapses with newly minted mitochondria [100, 101]. Bcl-xL and Drp-1 work together directly on synaptic vesicles to bring about activity dependent vesicle pool refilling within the first few minutes after high frequency action potential firing [97]. ATP production is markedly increased by Bcl-xL overexpression in the same neurons where synapses are larger and vesicle recycling is enhanced [27], suggesting that Bcl-xL also affects metabolism to enhance synaptic growth.
Despite the increased activity of neurons overexpressing Bcl-xL, oxygen consumption is decreased [27]. This paradox was resolved by the finding that Bcl-xL is bound directly to the ATP synthase β subunit [27, 102], where it increases ATP synthesis enzymatic rate and reduces inner mitochondrial membrane leak, enhancing the efficiency of ATP production by the ATP synthase (Figure 2). In contrast to overexpression, depletion of Bcl-xL causes instability of mitochondrial membrane potential and energy wasting, consistent with a leak that dissipates the membrane potential [102]. Bcl-xL KO causes embryonic lethality just before E12.5 [103], but conditional KO mice lacking Bcl-xL in neuronal progenitors survive, albeit with a highly malformed cortex and severe loss of neurite growth and synaptogenesis [102].
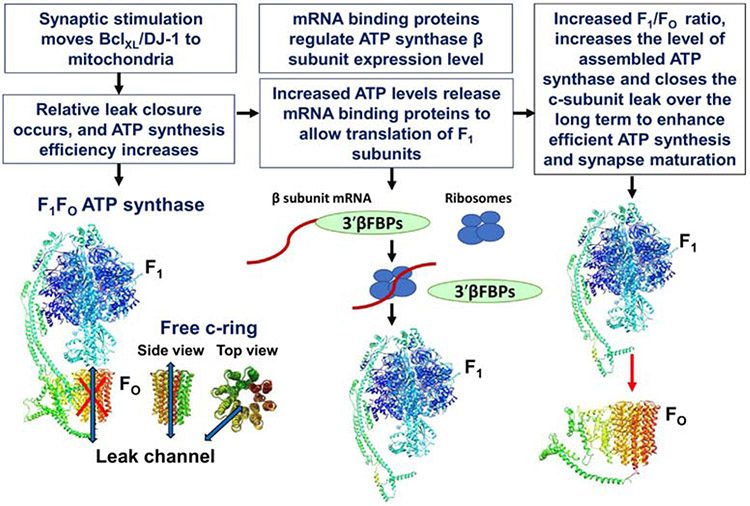
Fig. 2. Ratio of ATP synthase F1:FO is determined by Bcl-xL and DJ-1.
In response to synaptic stimulation, Bcl-xL and DJ-1 move to mitochondria to close the leak. ATP production by mitochondria causes the removal of the 3′UTR β-F1-ATPase mRNA-binding proteins (3′βFBPs): p129, p89, p61, p59, p54, and p51, from the 3’UTR of the mRNA of F1 β subunit. This allows F1 synthesis which leads to a long-lasting change in F1/FO ATP synthase ratio by reducing the level of free, uncomplexed c-subunits in mitochondria; this further decreases the probability of leak opening. DJ-1 and Bcl-xL are required for adjusting F1/FO ATP synthase ratio and neurite outgrowth in dopaminergic neurons. Closure of the leak supports neuronal differentiation and synapse maturation. ATP synthase subunits are drawn as ribbon representations (modified PDB ID code: 6J5I [75]).
These findings led us to assume that the likely place for the Bcl-xL regulated mitochondrial inner membrane leak is the ATP synthase itself, since the Bcl-xL inhibitor ABT-737 [27], and the even more selective Bcl-xL inhibitor WEHI-539, induce inner membrane leak formation during patch clamp recordings or during measurements of mitochondrial membrane potential [104].
ATP synthase leak channel modulates cardiac and neuronal development
If Bcl-xL and perhaps other pro-survival molecules enhance the function of the ATP synthase by enhancing coupling in oxidative phosphorylation, this suggests that ATP synthase leak channel activity or open probability may be graded to adjust metabolism even in healthy cells. Relative leak closure could occur progressively during embryonic development and during the process of synaptogenesis. Bcl-xL expression increases rapidly during these developmental stages in the early embryo from E8.5-E11.5, at the time that the heart and brain are forming. The fact that embryos die at this stage in the absence of Bcl-xL makes a striking argument for the critical role of ATP synthase leak closure as causative of developmental maturation.
Much previous evidence supports the notion that metabolic changes are crucial to different developmental periods in the embryo [105]. Very early (preimplantation) embryos produce mitochondrial ATP and TCA products [106, 107]. They also regulate development by balancing redox state to manage oxidant load [106]. Just before implantation, glucose use is turned on by low ATP/ADP ratios. Subsequently both glycolysis and mitochondrial oxidative phosphorylation increase.
After the morula stage, glucose uptake continues to increase. Glucose transporters increase on the cell membranes and there is increase in lactate production and a decrease in pyruvate uptake [108]. During the subsequent blastocyst stage where the embryo forms a cavity, glucose uptake is dramatically increased, followed by an increase in oxidative phosphorylation and an increase in pyruvate use again. Mitochondrial oxidative metabolism is required during the early post-implantation period, despite the evidence that the environment is relatively hypoxic. The rapid growth of the embryo during this stage of development and the requirement for reducing equivalents and NAD make a convincing case for aerobic glycolysis plus TCA use as the primary form of metabolism during this period.
This Warburg-style metabolism of developing embryos at the post-implantation stage is also observed in highly proliferating cancer cells [105, 109-114]. The Warburg effect [115] is characterized by increased glycolytic activity despite the presence of adequate environmental oxygen concentration. Even though ATP generation from carbon sources is less efficient compared with oxidative phosphorylation, aerobic glycolysis at this developmental period efficiently meets demands for the production of macromolecules by the TCA cycle, useful to facilitate cell proliferation [105, 116-118].
It has been proposed that the shift during embryonic development to aerobic glycolysis sustains the dramatic increase in body mass observed during the stages just prior to terminal differentiation of cardiac and nervous system cells [111]. Aerobic glycolysis, however, may also be required for signaling, regulating differentiation spatially and temporally even after proliferation has ceased [105, 110, 119]. We suggest that the harboring of a leak channel within the ATP synthase enables cells to quickly shift between oxidative phosphorylation and aerobic glycolysis by placing mitochondrial ATP synthase dependent ATP production “off-line” to increase glycolysis and TCA cycle-dependent biosynthesis. How much off-line will depend on the species, the presence of regulators such as Bcl-xL and crucially, the ratio of F1 subunits to FO. The evidence for this is that the c-subunit leak relatively “closes” during the period of central nervous system and cardiac development between E8.5 and E11.5 [120, 121]. In the heart, it was found that mitochondria are normally depolarized and certain electron complexes are relatively quiescent at E9.5, but that by E11.5, the ATP synthase begins producing ATP and the membrane potential becomes properly hyperpolarized [120]. These dates coincide with the onset of increased oxygen consumption and cardiac muscle contraction, suggesting that oxidative phosphorylation becomes more active at the time of inner membrane leak closure [120]. Indeed the mPTP inhibitor CsA hastens cardiac development and depletion of the main activator of the mPTP, CypD, causes cardiac myocyte differentiation to shift earlier [48, 60, 87, 122-124]. These developmental studies suggest that closure of the ATP synthase (c-subunit) leak by developmentally timed ATP synthase assembly (of FO with F1) [27, 102] could control development of the heart between E8.5-E11.5 (Figure 2).
In the developing mammalian nervous system there is an increase in oxidative enzymes associated with downregulation of MYC during neuronal differentiation [121]. Acid production (from lactate) but also oxygen consumption are high in vitro in explanted cells at E8.5 but by E10.5 as nervous system cells begin to differentiate and the neural tube forms, both acid production and oxygen consumption shift down dramatically [121].
Immunoblotting at this time (E10.5) demonstrates an upregulation of fully assembled Complex IV and V but it is clear from RNA sequencing of the explants comparing E8.5 to E10.5 that all three isoforms of ATP synthase c-subunit are downregulated by E10.5 [121]. This suggests that a change in stoichiometry of the ATP synthase, favoring fully assembled ATP synthase over uncomplexed (free) c-subunit, is causative of a reduction in both glycolysis and oxygen consumption and increases the coupling of oxidative phosphorylation. A similar change in ATP synthase stoichiometry was suggested previously in a large study of the flexibility of cancer cells upon switching to different metabolic states under varied environmental conditions [125].
These studies highlight that changes in metabolic phenotype during development are complex. In the pre-differentiation period, when cells are moving rapidly into position, they upregulate glycolytic enzymes and mitochondrial metabolism so that TCA cycle may be used for anabolism [124, 126-128]. Most importantly, upregulation of glycolytic and mitochondrial pathways at this time balances the NAD+/NADH ratio. Mitochondria are therefore not silent when glycolytic metabolism is favored [129] but are required for TCA cycle anabolic pathways to regenerate adequate supplies of reducing equivalents and NAD+ as well as intermediates for biosynthetic pathways. During differentiation, however, oxidative phosphorylation increases, the stoichiometry of the ATP synthase changes to favor fully formed ATP synthase, and molecules like Bcl-xL close the leak for proper cardiac and nervous system development. We argue that, during the pre-differentiation period, the ATP synthase (c-subunit) leak is required for anaplerosis and maintenance of the NAD+/NADH ratio. If the inner membrane leak is too low, mitochondrial inner membrane potential builds up and NADH levels become high, feeding back to shut down TCA cycle function. To continue to run TCA and provide anaplerotic intermediates, NAD+ must be remade from the activity of the NADH dehydrogenase. As Vander Heiden argues in his recent review, “continued nutrient oxidation [for the purpose of biosynthesis] requires cycling of NADH back to NAD+, which necessitates transfer of electrons to an electron acceptor such as oxygen” [118].
ATP synthase leak closure regulates synaptic development and cancer cell growth
An even further decrease in mitochondrial inner membrane leak occurs again later in nervous system development when neurons begin to make contact with other neuronal partners to form mature synaptic connections. This period of synaptogenesis begins to occur just after E10 in the rodent and continues exuberantly during the early postnatal period up to the time of weaning. We find that ATP synthase leak closure during synaptic development heralds the onset of mature synapse formation.
The time of formation of mature synapses is accompanied by a decrease in glycolytic enzymes and an increase in use of oxidative phosphorylation for ATP production. In contrast to the normal developmental metabolic changes in brain, by examining the metabolism of an autism spectrum disorder known as Fragile X (FX) Syndrome, we discovered a persistent glycolytic phenotype [130]. Fragile X Syndrome is caused by a CGG repeat sequence that forms in the X-linked gene Fmr1 which codes for FMRP [131, 132]. The repeat sequence causes the gene for FMRP to become hypermethylated and inactivated [133]. This results in complete absence of the FMRP protein, severe intellectual disability and features of autism including neuronal hyperexcitability, sensory misperceptions and seizures [134-138]. The disorder is modeled in the mouse and in drosophila by Fmr1 gene deletion. Striking cellular features of the disorder in all animals during development include high protein synthesis rates [139], marked abnormalities of synaptic phenotype [135] and decreases in brain connectivity [140].
Our studies of young FMRP-lacking mice at the time of normally high expression levels of FMRP (P10), confirmed that body temperature and lactate production were elevated, consistent with an aerobic glycolytic phenotype. Indeed, closer studies of mitochondria isolated from the brains of the KO animals using modular kinetic techniques [141] identified an inner membrane leak that caused enhanced oxygen consumption in a depolarization-dependent manner [130]. The leak was reminiscent of the futile cycling of the membrane potential we had identified previously in neurons depleted of Bcl-xL [102]. We found that the likely cause of the leak was enhanced depolarization-dependent opening of the mPTP channel [130]. The closure of this leak with CoQ10 on P9 acutely induced changes in morphology and density of dendritic spines in Fmr1 KO mouse forebrain suggesting that the mPTP regulates synapse maturation and development and that an open pore maintains the spines in an immature state [130]. It is known that Fmr1yY synaptoneurosomes are unable to synthesize proteins in response to stimulation, which leads to significantly reduced number of synapses in Fmr1 KO mice in the first few weeks of life [142]. Additionally, mitochondria were shown to be necessary for stimulus-induced synaptic protein translation and synaptic plasticity [143, 144]. Consistent with these findings, we reported significantly lower rates of protein synthesis in the 10-day-old Fmr1 KO mouse forebrain during synaptogenesis, which was restored after closing the pathological mitochondrial leak channel by CoQ10 in Fmr1 mutant mice [130]. This suggests that increasing the efficiency of oxidative phosphorylation due to mPTP leak closure in neurons of developing brain is important for meeting the substantial protein synthesis needs and metabolic demands during synaptogenesis.
The timing of synthesis of proteins involved in oxidative phosphorylation, in particular the timing of the synthesis of ATP synthase F1 catalytic domain subunits, is crucial to mitochondrial biogenesis during growth and development. Cuezva lab working in cancer cells and our lab working in synapses found that the mechanism regulating the accretion of ATP synthase β subunit during cellular proliferation and synapse formation, respectively, is controlled at the level of mRNA translation [145]. Cuezva lab described that the translation of ATP synthase β subunit is dependent on the 3’UTR of the transcript and it is being regulated by the common mechanism both in the fetal liver and cancer cells. The mRNAs that accumulate in the fetal liver and cancer cells are in a translation-repressed state. The fetal liver [146] and cancer cells [147] contain a set of proteins, the 3′UTR β-F1-ATPase mRNA-binding proteins (3′βFBPs): p129, p89, p61, p59, p54, and p51 that specifically bind the 3’UTR of the mRNA. This interaction prevents mRNA translation at the ribosome leading to decreased expression of ATP synthase β subunit both during fetal development and in liver carcinogenesis.
The expression of ATP synthase β subunit regulates the assembly of F1. It was demonstrated that inactivation of the ATP2 gene expression, coding for the catalytic subunit β, by RNA interference in the green alga Chlamydomonas reinhardtii impaired the assembly of full ATP synthase [148].
Successful translation of the β subunit mRNA to produce fully formed ATP synthase is also dependent on mechanisms that participate in ATP synthase β subunit mRNA localization to sites of mRNA translation [145]. In synapses, this is crucial since the site of synaptic mRNA translation is often very distant from the nucleus. An RNA binding particle (ribonuclear particle, RNP) containing several different RNA binding proteins and ATP synthase β subunit mRNA has been described. Most likely, based on studies in yeast and mammalian liver, this particle is bound to ribosomes and localized to the mitochondrial outer membrane at the site of local translation of β subunit in response to mitochondrial metabolic signals [149-151]. Based on this information we suggest that timed increased in expression of ATP synthase β subunit leads to an increase in the F1/FO ratio, which increases the level of assembled ATP synthase and closes the c-subunit leak. The closure of c-subunit leak enhances efficient ATP synthesis to support neuronal differentiation and synapse maturation (Figure 2).
Stoichiometry change in ATP synthase F1 subunits to FO as a critical cause of neurodegenerative disease
Mitochondrial dysfunction has been widely implicated in neurodegenerative disease, but we and others are increasingly focused on changes in function and in regulation of the ATP synthase. Alzheimer’s Disease (AD) is one of the most commonly occurring neurodegenerative diseases, accounting for up to 80% of all dementia cases. It is characterized by progressive cognitive and motor deterioration, and affects memory, attention, speech and behavior. Over many pre-clinical and symptomatic (clinical) years, AD pathology causes synaptic dysfunction followed by the death of brain cells, leading to significant tissue shrinkage [152, 153]. Oxidative damage and redox imbalance produce stress that is most severe for mitochondria, where most reactive oxygen species are produced [154, 155]. It was noticed in human pathological samples of early AD that mitochondrial components suffered oxidative damage, impairing their function [156-160]. The α subunit of the mitochondrial ATP-synthase was recognized as the most common lipoxidized protein in the entorhinal cortex of all AD cases at stages I/II of the disease [161]. Cyclophilin D (CypD) knock out mice have a reduced propensity toward mitochondrial permeability transition (mPT) and crossing the CypD−/− mice with an AD model mouse reduced mPT, enhanced calcium handling by neuronal mitochondria and protected the mice from loss of function in tasks of learning and memory [162, 163]. A decrease in levels of the oligomycin sensitivity conferring protein (OSCP) subunit of the F1FO-ATP synthase was attributed to binding of the aggregated protein amyloid beta (Aβ) with OSCP in the brains of AD individuals and in an AD mouse model [164]. Decreases in OSCP levels were pronounced in neuronal mitochondria [164]. The loss of OSCP led to reduced ATP production, elevated oxidative stress and activated mPT and these abnormalities were alleviated in the mouse by resupply of OSCP [164]. When Aβ enters mitochondria, cyclin-dependent kinase-1 (Cdk1) is activated and cyclin B1 is stabilized [165]. The Cyclin B1–Cdk1 complex in the mitochondria phosphorylates Bcl-xL, leading to its dissociation from the β subunit of F1FO–ATP synthase. This inhibits ATP synthase activity and causes mitochondrial depolarization [165] that activates mPT.
We have now found that loss of the ATP synthase β subunit occurs in a Parkinson’s Disease (PD) mouse model [166]. Rare mutations in the gene encoding DJ-1 are found in about 1% of familial cases of PD. DJ-1 is a peptidase C56 family protein with known and uncharacterized cellular functions [167]. DJ-1 mutant animals show increased sensitivity to neuronal toxins, and in different species DJ-1 is required for normal life span, motor function, and neuronal resistance to oxidative damage [168-170]. Defects in DJ-1 alter mitochondrial morphology and function [171]. DJ-1 translocates to mitochondria from the cytosol in response to mitochondrial stress [172-174], suggesting that DJ-1 may assist Parkin and PINK1 in protein trafficking. DJ-1 also regulates mitochondrial metabolism: DJ-1 mutant cells have impaired ATP production and abnormal respiration [170]. The mutant mitochondria are sensitive to mPT [175] and DJ-1 deficient mitochondria demonstrate a leaky mitochondrial inner membrane [166, 170]. We found that the leak of DJ−/− mitochondria is produced by the ATP synthase; WT DJ-1 binds tightly to the β subunit causing leak closure in mitochondrial recordings (Figure 2) [166]. Mutant DJ-1 fails to close the leak despite persistent binding to the β subunit. The abnormalities of the ATP synthase in DJ-1−/− animals are associated with reduced enzymatic function, reduced ATP levels and impaired neurite extension in isolated dopaminergic neurons, suggesting that normal ATP synthase function is critical for growth and targeting of dopaminergic neuron endings [166]. Levels of the ATP synthase β subunit are low in aged DJ-1−/− mouse brain and in patient cell lines even though levels of the c-subunit are normal, suggesting that this degenerative disease is associated with a reduction in F1/FO ratio with resultant c-subunit leak channel formation.
Concluding remarks
Here we discussed some current concepts and controversies in the field of mitochondrial pathophysiology and the key role of ATP synthase c-subunit in forming a mitochondrial inner membrane leak channel, which has physiological as well as pathological functions in cell metabolism, in embryonic and synaptic development and during aging-related degenerative diseases. We have proposed a new model of mPTP formation, which describes that brief, physiological openings of ATP synthase leak channel are due to the reversible conformational changes in ATP synthase structure. We have also suggested a model of long-lasting pathological openings of mPTP; these persistent openings under pathological conditions result in non-reversible dissociation of ATP synthase F1 subcomplex from FO. While our models are supported by structural and functional findings and corroborative findings in disease models, further studies are needed to understand mPTP structure and the molecular mechanisms of its regulation in health and disease.
Highlights.
ATP synthase c-subunit is the leak channel of mitochondrial permeability transition.
c-subunit channel is the master regulator of cell metabolism in embryonic and synaptic development.
F1/FO stoichiometry change is critical for c-subunit regulatory function.
Non-reversible dissociation of ATP synthase F1 from FO triggers age-related ischemic and degenerative diseases.
Acknowledgments
Work was supported by NIH Grants NS045876, NS112706, NS081746 (to E.A.J.), NIA Grant K01AG054734 (to N.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- [1].Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–7. [DOI] [PubMed] [Google Scholar]
- [2].Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–77. [DOI] [PubMed] [Google Scholar]
- [3].Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–9. [DOI] [PubMed] [Google Scholar]
- [4].Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251:5069–77. [PubMed] [Google Scholar]
- [5].Petronilli V, Szabo I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989;259:137–43. [DOI] [PubMed] [Google Scholar]
- [6].Szabo I, Zoratti M. The mitochondrial megachannel is the permeability transition pore. J Bioenerg Biomembr. 1992;24:111–7. [DOI] [PubMed] [Google Scholar]
- [7].Kinnally KW, Campo ML, Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr. 1989;21:497–506. [DOI] [PubMed] [Google Scholar]
- [8].Zorov DB, Kinnally KW, Perini S, Tedeschi H. Multiple conductance levels in rat heart inner mitochondrial membranes studied by patch clamping. Biochim Biophys Acta. 1992;1105:263–70. [DOI] [PubMed] [Google Scholar]
- [9].Lohret TA, Murphy RC, Drgon T, Kinnally KW. Activity of the mitochondrial multiple conductance channel is independent of the adenine nucleotide translocator. J Biol Chem. 1996;271:4846–9. [DOI] [PubMed] [Google Scholar]
- [10].Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–76. [DOI] [PubMed] [Google Scholar]
- [11].Bernardi P. The permeability transition pore. Control points of a cyclosporin A-sensitive mitochondrial channel involved in cell death. Biochim Biophys Acta. 1996;1275:5–9. [DOI] [PubMed] [Google Scholar]
- [12].Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 2011;1813:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–53. [DOI] [PubMed] [Google Scholar]
- [14].Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta. 1998;1366:33–50. [DOI] [PubMed] [Google Scholar]
- [15].Selivanov VA, Ichas F, Holmuhamedov EL, Jouaville LS, Evtodienko YV, Mazat JP. A model of mitochondrial Ca(2+)-induced Ca2+ release simulating the Ca2+ oscillations and spikes generated by mitochondria. Biophys Chem. 1998;72:111–21. [DOI] [PubMed] [Google Scholar]
- [16].Mnatsakanyan N, Beutner G, Porter GA, Alavian KN, Jonas EA. Physiological roles of the mitochondrial permeability transition pore. J Bioenerg Biomembr. 2017;49:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Crompton M, Virji S, Doyle V, Johnson N, Ward JM. The mitochondrial permeability transition pore. Biochem Soc Symp. 1999;66:167–79. [DOI] [PubMed] [Google Scholar]
- [18].Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, et al. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, et al. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Perez MJ, Quintanilla RA. Development or disease: duality of the mitochondrial permeability transition pore. Dev Biol. 2017;426:1–7. [DOI] [PubMed] [Google Scholar]
- [21].Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. [DOI] [PubMed] [Google Scholar]
- [22].Duan Y, Gross RA, Sheu SS. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol. 2007;585:741–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hurst S, Hoek J, Sheu SS. Mitochondrial Ca(2+) and regulation of the permeability transition pore. J Bioenerg Biomembr. 2017;49:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Levy RJ. Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock. 2007;28:24–8. [DOI] [PubMed] [Google Scholar]
- [25].Levy RJ, Deutschman CS. Deficient mitochondrial biogenesis in critical illness: cause, effect, or epiphenomenon? Crit Care. 2007;11:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Griffiths KK, Levy RJ. Evidence of Mitochondrial Dysfunction in Autism: Biochemical Links, Genetic-Based Associations, and Non-Energy-Related Mechanisms. Oxid Med Cell Longev. 2017;2017:4314025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S, et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol. 2011;13:1224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mnatsakanyan N, Llaguno MC, Yang Y, Yan Y, Weber J, Sigworth FJ, et al. A mitochondrial megachannel resides in monomeric F1FO ATP synthase. Nat Commun. 2019;10:5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nicolli A, Petronilli V, Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry. 1993;32:4461–5. [DOI] [PubMed] [Google Scholar]
- [32].Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992;267:8834–9. [PubMed] [Google Scholar]
- [33].Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992;267:2934–9. [PubMed] [Google Scholar]
- [34].Carraro M, Bernardi P. Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium. 2016;60:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Petronilli V, Costantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem. 1994;269:16638–42. [PubMed] [Google Scholar]
- [36].Szabo I, Bernardi P, Zoratti M. Modulation of the mitochondrial megachannel by divalent cations and protons. J Biol Chem. 1992;267:2940–6. [PubMed] [Google Scholar]
- [37].Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–25. [DOI] [PubMed] [Google Scholar]
- [38].Szabo I, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. I. Binary structure and voltage dependence of the pore. FEBS Lett. 1993;330:201–5. [DOI] [PubMed] [Google Scholar]
- [39].Szabo I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993;330:206–10. [DOI] [PubMed] [Google Scholar]
- [40].Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, Wang X, et al. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014;21:1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pastorino JG, Simbula G, Gilfor E, Hoek JB, Farber JL. Protoporphyrin IX, an endogenous ligand of the peripheral benzodiazepine receptor, potentiates induction of the mitochondrial permeability transition and the killing of cultured hepatocytes by rotenone. J Biol Chem. 1994;269:31041–6. [PubMed] [Google Scholar]
- [42].Chelli B, Falleni A, Salvetti F, Gremigni V, Lucacchini A, Martini C. Peripheral-type benzodiazepine receptor ligands: mitochondrial permeability transition induction in rat cardiac tissue. Biochem Pharmacol. 2001;61:695–705. [DOI] [PubMed] [Google Scholar]
- [43].Li J, Wang J, Zeng Y. Peripheral benzodiazepine receptor ligand, PK11195 induces mitochondria cytochrome c release and dissipation of mitochondria potential via induction of mitochondria permeability transition. Eur J Pharmacol. 2007;560:117–22. [DOI] [PubMed] [Google Scholar]
- [44].Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gutierrez-Aguilar M, Douglas DL, Gibson AK, Domeier TL, Molkentin JD, Baines CP. Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J Mol Cell Cardiol. 2014;72:316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sileikyte J, Blachly-Dyson E, Sewell R, Carpi A, Menabo R, Di Lisa F, et al. Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (Translocator Protein of 18 kDa (TSPO)). J Biol Chem. 2014;289:13769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Carraro M, Giorgio V, Sileikyte J, Sartori G, Forte M, Lippe G, et al. Channel formation by yeast F-ATP synthase and the role of dimerization in the mitochondrial permeability transition. J Biol Chem. 2014;289:15980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carraro M, Checchetto V, Sartori G, Kucharczyk R, di Rago JP, Minervini G, et al. High-Conductance Channel Formation in Yeast Mitochondria is Mediated by F-ATP Synthase e and g Subunits. Cell Physiol Biochem. 2018;50:1840–55. [DOI] [PubMed] [Google Scholar]
- [51].Guo L, Carraro M, Carrer A, Minervini G, Urbani A, Masgras I, et al. Arg-8 of yeast subunit e contributes to the stability of F-ATP synthase dimers and to the generation of the full-conductance mitochondrial megachannel. J Biol Chem. 2019;294:10987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Urbani A, Giorgio V, Carrer A, Franchin C, Arrigoni G, Jiko C, et al. Purified F-ATP synthase forms a Ca(2+)-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat Commun. 2019;10:4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Azarashvili T, Odinokova I, Bakunts A, Ternovsky V, Krestinina O, Tyynela J, et al. Potential role of subunit c of F0F1-ATPase and subunit c of storage body in the mitochondrial permeability transition. Effect of the phosphorylation status of subunit c on pore opening. Cell Calcium. 2014;55:69–77. [DOI] [PubMed] [Google Scholar]
- [54].Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, et al. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Morciano G, Preti D, Pedriali G, Aquila G, Missiroli S, Fantinati A, et al. Discovery of Novel 1,3,8-Triazaspiro[4.5]decane Derivatives That Target the c Subunit of F1/FO-Adenosine Triphosphate (ATP) Synthase for the Treatment of Reperfusion Damage in Myocardial Infarction. J Med Chem. 2018;61:7131–43. [DOI] [PubMed] [Google Scholar]
- [56].Pavlov E, Zakharian E, Bladen C, Diao CT, Grimbly C, Reusch RN, et al. A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys J. 2005;88:2614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Neginskaya MA, Solesio ME, Berezhnaya EV, Amodeo GF, Mnatsakanyan N, Jonas EA, et al. ATP Synthase C-Subunit-Deficient Mitochondria Have a Small Cyclosporine A-Sensitive Channel, but Lack the Permeability Transition Pore. Cell Rep. 2019;26:11–7 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death.[see comment]. Nature. 2005;434:658–62. [DOI] [PubMed] [Google Scholar]
- [59].Karch J, Molkentin JD. Identifying the components of the elusive mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10396–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, et al. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem. 2009;284:33982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Arselin G, Vaillier J, Salin B, Schaeffer J, Giraud MF, Dautant A, et al. The modulation in subunits e and g amounts of yeast ATP synthase modifies mitochondrial cristae morphology. J Biol Chem. 2004;279:40392–9. [DOI] [PubMed] [Google Scholar]
- [62].Paumard P, Vaillier J, Coulary B, Schaeffer J, Soubannier V, Mueller DM, et al. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002;21:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V, et al. Ca(2+) binding to F-ATP synthase beta subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017;18:1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hubbard MJ, McHugh NJ. Mitochondrial ATP synthase F1-beta-subunit is a calcium-binding protein. FEBS Lett. 1996;391:323–9. [DOI] [PubMed] [Google Scholar]
- [65].Nesci S, Trombetti F, Ventrella V, Pirini M, Pagliarani A. Kinetic properties of the mitochondrial F1FO-ATPase activity elicited by Ca(2+) in replacement of Mg(2). Biochimie. 2017;140:73–81. [DOI] [PubMed] [Google Scholar]
- [66].Guerrieri F, Capozza G, Kalous M, Papa S. Age-related changes of mitochondrial F0F1 ATP synthase. Ann N Y Acad Sci. 1992;671:395–402. [DOI] [PubMed] [Google Scholar]
- [67].Guerrieri F, Capozza G, Kalous M, Zanotti F, Drahota Z, Papa S. Age-dependent changes in the mitochondrial F0F1 ATP synthase. Arch Gerontol Geriatr. 1992;14:299–308. [DOI] [PubMed] [Google Scholar]
- [68].Zhou W, Marinelli F, Nief C, Faraldo-Gomez JD. Atomistic simulations indicate the c-subunit ring of the F1FO ATP synthase is not the mitochondrial permeability transition pore. Elife. 2017;6. [Google Scholar]
- [69].He J, Carroll J, Ding S, Fearnley IM, Walker JE. Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc Natl Acad Sci U S A. 2017;114:9086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc Natl Acad Sci U S A. 2017;114:3409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Oberfeld B, Brunner J, Dimroth P. Phospholipids occupy the internal lumen of the c ring of the ATP synthase of Escherichia coli. Biochemistry. 2006;45:1841–51. [DOI] [PubMed] [Google Scholar]
- [72].Meier T, Matthey U, Henzen F, Dimroth P, Muller DJ. The central plug in the reconstituted undecameric c cylinder of a bacterial ATP synthase consists of phospholipids. FEBS Lett. 2001;505:353–6. [DOI] [PubMed] [Google Scholar]
- [73].Matthies D, Preiss L, Klyszejko AL, Muller DJ, Cook GM, Vonck J, et al. The c13 ring from a thermoalkaliphilic ATP synthase reveals an extended diameter due to a special structural region. J Mol Biol. 2009;388:611–8. [DOI] [PubMed] [Google Scholar]
- [74].Fan C, Choi W, Sun W, Du J, Lu W. Structure of the human lipid-gated cation channel TRPC3. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gu J, Zhang L, Zong S, Guo R, Liu T, Yi J, et al. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science. 2019;364:1068–75. [DOI] [PubMed] [Google Scholar]
- [76].Zhou A, Rohou A, Schep DG, Bason JV, Montgomery MG, Walker JE, et al. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. Elife. 2015;4:e10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Vlasov AV, Kovalev KV, Marx SH, Round ES, Gushchin IY, Polovinkin VA, et al. Unusual features of the c-ring of F1FO ATP synthases. Sci Rep. 2019;9:18547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–8. [DOI] [PubMed] [Google Scholar]
- [79].Menz RI, Walker JE, Leslie AG. Structure of bovine mitochondrial F(1)-ATPase with nucleotide bound to all three catalytic sites: implications for the mechanism of rotary catalysis. Cell. 2001;106:331–41. [DOI] [PubMed] [Google Scholar]
- [80].Antoniel M, Giorgio V, Fogolari F, Glick GD, Bernardi P, Lippe G. The oligomycin-sensitivity conferring protein of mitochondrial ATP synthase: emerging new roles in mitochondrial pathophysiology. Int J Mol Sci. 2014;15:7513–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Giorgio V, Fogolari F, Lippe G, Bernardi P. OSCP subunit of mitochondrial ATP synthase: role in regulation of enzyme function and of its transition to a pore. Br J Pharmacol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rees DM, Leslie AG, Walker JE. The structure of the membrane extrinsic region of bovine ATP synthase. Proc Natl Acad Sci U S A. 2009;106:21597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Alavian KN, Dworetzky SI, Bonanni L, Zhang P, Sacchetti S, Li H, et al. The mitochondrial complex V-associated large-conductance inner membrane current is regulated by cyclosporine and dexpramipexole. Mol Pharmacol. 2015;87:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Starke I, Glick GD, Borsch M. Visualizing Mitochondrial FOF1-ATP Synthase as the Target of the Immunomodulatory Drug Bz-423. Front Physiol. 2018;9:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Johnson KM, Chen X, Boitano A, Swenson L, Opipari AW Jr., Glick GD. Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem Biol. 2005;12:485–96. [DOI] [PubMed] [Google Scholar]
- [86].Karch J, Bround MJ, Khalil H, Sargent MA, Latchman N, Terada N, et al. Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci Adv. 2019;5:eaaw4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Parks RJ, Menazza S, Holmstrom KM, Amanakis G, Fergusson M, Ma H, et al. Cyclophilin D-mediated regulation of the permeability transition pore is altered in mice lacking the mitochondrial calcium uniporter. Cardiovasc Res. 2019;115:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Jonas EA, Knox RJ, Kaczmarek LK. Giga-ohm seals on intracellular membranes: a technique for studying intracellular ion channels in intact cells. Neuron. 1997;19:7–13. [DOI] [PubMed] [Google Scholar]
- [89].Jonas EA, Buchanan J, Kaczmarek LK. Prolonged activation of mitochondrial conductances during synaptic transmission. Science. 1999;286:1347–50. [DOI] [PubMed] [Google Scholar]
- [90].Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994;14:4007–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–91. [DOI] [PubMed] [Google Scholar]
- [92].Jonas EA, Hoit D, Hickman JA, Brandt TA, Polster BM, Fannjiang Y, et al. Modulation of synaptic transmission by the BCL-2 family protein BCL-xL. Journal of Neuroscience. 2003;23:8423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Krueger SR, Kolar A, Fitzsimonds RM. The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron. 2003;40:945–57. [DOI] [PubMed] [Google Scholar]
- [94].Bell ME, Bourne JN, Chirillo MA, Mendenhall JM, Kuwajima M, Harris KM. Dynamics of nasce nt and active zone ultrastructure as synapses enlarge during long-term potentiation in mature hippocampus. J Comp Neurol. 2014;522:3861–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bourne JN, Chirillo MA, Harris KM. Presynaptic ultrastructural plasticity along CA3-->CA1 axons during long-term potentiation in mature hippocampus. J Comp Neurol. 2013;521:3898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Li H, Alavian KN, Lazrove E, Mehta N, Jones A, Zhang P, et al. A Bcl-xL-Drp1 complex regulates synaptic vesicle membrane dynamics during endocytosis. Nat Cell Biol. 2013;15:773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–78. [DOI] [PubMed] [Google Scholar]
- [99].Yamaoka S, Leaver CJ. EMB2473/MIRO1, an Arabidopsis Miro GTPase, is required for embryogenesis and influences mitochondrial morphology in pollen. Plant Cell. 2008;20:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB 3rd, Goebbels S, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. Journal of Cell Biology. 2009;184:707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB 3rd, Goebbels S, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol. 2009;184:707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. Journal of Cell Biology. 2011;195:263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–10. [DOI] [PubMed] [Google Scholar]
- [104].Park HA, Licznerski P, Mnatsakanyan N, Niu Y, Sacchetti S, Wu J, et al. Inhibition of Bcl-xL prevents pro-death actions of DeltaN-Bcl-xL at the mitochondrial inner membrane during glutamate excitotoxicity. Cell Death Differ. 2017;24:1963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Miyazawa H, Aulehla A. Revisiting the role of metabolism during development. Development. 2018; 145. [DOI] [PubMed] [Google Scholar]
- [106].Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol. 2007;77:21–49. [DOI] [PubMed] [Google Scholar]
- [107].MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. [DOI] [PubMed] [Google Scholar]
- [108].Johnson MT, Mahmood S, Patel MS. Intermediary metabolism and energetics during murine early embryogenesis. J Biol Chem. 2003;278:31457–60. [DOI] [PubMed] [Google Scholar]
- [109].Agathocleous M, Love NK, Randlett O, Harris JJ, Liu J, Murray AJ, et al. Metabolic differentiation in the embryonic retina. Nat Cell Biol. 2012;14:859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bulusu V, Prior N, Snaebjornsson MT, Kuehne A, Sonnen KF, Kress J, et al. Spatiotemporal Analysis of a Glycolytic Activity Gradient Linked to Mouse Embryo Mesoderm Development. Dev Cell. 2017;40:331–41 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Homem CCF, Steinmann V, Burkard TR, Jais A, Esterbauer H, Knoblich JA. Ecdysone and mediator change energy metabolism to terminate proliferation in Drosophila neural stem cells. Cell. 2014;158:874–88. [DOI] [PubMed] [Google Scholar]
- [112].Oginuma M, Moncuquet P, Xiong F, Karoly E, Chal J, Guevorkian K, et al. A Gradient of Glycolytic Activity Coordinates FGF and Wnt Signaling during Elongation of the Body Axis in Amniote Embryos. Dev Cell. 2017;40:342–53 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Slaninova V, Krafcikova M, Perez-Gomez R, Steffal P, Trantirek L, Bray SJ, et al. Notch stimulates growth by direct regulation of genes involved in the control of glycolysis and the tricarboxylic acid cycle. Open Biol. 2016;6:150155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tennessen JM, Thummel CS. Coordinating growth and maturation - insights from Drosophila. Curr Biol. 2011;21:R750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Warburg O On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- [116].Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. [DOI] [PubMed] [Google Scholar]
- [117].Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell. 2015;57:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between Metabolism and Cancer Biology. Cell. 2017;168:657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ozbudak EM, Tassy O, Pourquie O. Spatiotemporal compartmentalization of key physiological processes during muscle precursor differentiation. Proc Natl Acad Sci U S A. 2010;107:4224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hom JR, Quintanilla RA, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu SS, et al. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev Cell. 2011;21:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Fame RM, Shannon ML, Chau KF, Head JP, Lehtinen MK. A concerted metabolic shift in early forebrain alters the CSF proteome and depends on MYC downregulation for mitochondrial maturation. Development. 2019;146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, et al. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. Journal of Biological Chemistry. 2009;284:33982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Beutner G, Alanzalon RE, Porter GA Jr. Cyclophilin D regulates the dynamic assembly of mitochondrial ATP synthase into synthasomes. Sci Rep. 2017;7:14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Magni G, Orsomando G, Raffelli N, Ruggieri S. Enzymology of mammalian NAD metabolism in health and disease. Front Biosci. 2008;13:6135–54. [DOI] [PubMed] [Google Scholar]
- [125].Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17:1721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Li C, Zhang G, Zhao L, Ma Z, Chen H. Metabolic reprogramming in cancer cells: glycolysis, glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for cancer. World J Surg Oncol. 2016; 14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, et al. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Griffiths KK, Wang A, Wang L, Tracey M, Kleiner G, Quinzii CM, et al. Inefficient thermogenic mitochondrial respiration due to futile proton leak in a mouse model of fragile X syndrome. FASEB J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–7. [DOI] [PubMed] [Google Scholar]
- [132].Tassone F, Iong KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012;4:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85:503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Brown MR, El-Hassar L, Zhang Y, Alvaro G, Large CH, Kaczmarek LK. Physiological modulators of Kv3.1 channels adjust firing patterns of auditory brain stem neurons. J Neurophysiol. 2016;116:106–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, et al. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13:819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Strumbos JG, Brown MR, Kronengold J, Polley DB, Kaczmarek LK. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J Neurosci. 2010;30:10263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Jacquemont S, Pacini L, Jonch AE, Cencelli G, Rozenberg I, He Y, et al. Protein synthesis levels are increased in a subset of individuals with fragile X syndrome. Hum Mol Genet. 2018;27:2039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Goncalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci. 2013;16:903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci U S A. 2001;98:7101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rangaraju V, Lauterbach M, Schuman EM. Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity. Cell. 2019;176:73–84 e15. [DOI] [PubMed] [Google Scholar]
- [144].Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. [DOI] [PubMed] [Google Scholar]
- [145].Willers IM, Cuezva JM. Post-transcriptional regulation of the mitochondrial H(+)-ATP synthase: a key regulator of the metabolic phenotype in cancer. Biochim Biophys Acta. 2011;1807:543–51. [DOI] [PubMed] [Google Scholar]
- [146].Izquierdo JM, Cuezva JM. Control of the translational efficiency of beta-F1-ATPase mRNA depends on the regulation of a protein that binds the 3' untranslated region of the mRNA. Mol Cell Biol. 1997;17:5255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].de Heredia ML, Izquierdo JM, Cuezva JM. A conserved mechanism for controlling the translation of beta-F1-ATPase mRNA between the fetal liver and cancer cells. J Biol Chem. 2000;275:7430–7. [DOI] [PubMed] [Google Scholar]
- [148].Lapaille M, Thiry M, Perez E, Gonzalez-Halphen D, Remacle C, Cardol P. Loss of mitochondrial ATP synthase subunit beta (Atp2) alters mitochondrial and chloroplastic function and morphology in Chlamydomonas. Biochim Biophys Acta. 2010;1797:1533–9. [DOI] [PubMed] [Google Scholar]
- [149].Saint-Georges Y, Garcia M, Delaveau T, Jourdren L, Le Crom S, Lemoine S, et al. Yeast mitochondrial biogenesis: a role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS One. 2008;3:e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–99. [DOI] [PubMed] [Google Scholar]
- [151].Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–61. [DOI] [PubMed] [Google Scholar]
- [152].Selkoe DJ. Resolving controversies on the path to Alzheimer's therapeutics. Nat Med. 2011;17:1060–5. [DOI] [PubMed] [Google Scholar]
- [153].Cadonic C, Sabbir MG, Albensi BC. Mechanisms of Mitochondrial Dysfunction in Alzheimer's Disease. Mol Neurobiol. 2015. [DOI] [PubMed] [Google Scholar]
- [154].Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–48. [DOI] [PubMed] [Google Scholar]
- [155].Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. [DOI] [PubMed] [Google Scholar]
- [156].Du H, Guo L, Yan SS. Synaptic mitochondrial pathology in Alzheimer's disease. Antioxid Redox Signal. 2012;16:1467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Du H, Yan SS. Mitochondrial permeability transition pore in Alzheimer's disease: cyclophilin D and amyloid beta. Biochim Biophys Acta. 2010;1802:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Du H, Yan SS. Mitochondrial medicine for neurodegenerative diseases. Int J Biochem Cell Biol. 2010;42:560–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Cenini G, Lloret A, Cascella R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid Med Cell Longev. 2019;2019:2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–67. [DOI] [PubMed] [Google Scholar]
- [161].Terni B, Boada J, Portero-Otin M, Pamplona R, Ferrer I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer's disease pathology. Brain Pathol. 2010;20:222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nature Medicine. 2008;14:1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2011;32:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Beck SJ, Guo L, Phensy A, Tian J, Wang L, Tandon N, et al. Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer's disease. Nat Commun. 2016;7:11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Veas-Perez de Tudela M, Delgado-Esteban M, Maestre C, Bobo-Jimenez V, Jimenez-Blasco D, Vecino R, et al. Regulation of Bcl-xL-ATP Synthase Interaction by Mitochondrial Cyclin B1-Cyclin-Dependent Kinase-1 Determines Neuronal Survival. J Neurosci. 2015;35:9287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Chen R, Park HA, Mnatsakanyan N, Niu Y, Licznerski P, Wu J, et al. Parkinson's disease protein DJ -1 regulates ATP synthase protein components to increase neuronal process outgrowth. Cell Death Dis. 2019;10:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ -1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–9. [DOI] [PubMed] [Google Scholar]
- [168].Biosa A, Sandrelli F, Beltramini M, Greggio E, Bubacco L, Bisaglia M. Recent findings on the physiological function of DJ-1: Beyond Parkinson's disease. Neurobiol Dis. 2017;108:65–72. [DOI] [PubMed] [Google Scholar]
- [169].Malgieri G, Eliezer D. Structural effects of Parkinson's disease linked DJ-1 mutations. Protein Sci. 2008;17:855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Hao LY, Giasson BI, Bonini NM. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Larsen NJ, Ambrosi G, Mullett SJ, Berman SB, Hinkle DA. DJ-1 knock-down impairs astrocyte mitochondrial function. Neuroscience. 2011;196:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2009;87:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Weinert M, Millet A, Jonas EA, Alavian KN. The mitochondrial metabolic function of DJ-1 is modulated by 14-3-3beta. FASEB J. 2019;33:8925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Giaime E, Yamaguchi H, Gautier CA, Kitada T, Shen J. Loss of DJ-1 does not affect mitochondrial respiration but increases ROS production and mitochondrial permeability transition pore opening. PLoS One. 2012;7:e40501. [DOI] [PMC free article] [PubMed] [Google Scholar]