Abstract
Background:
The Treatment Options for type 2 Diabetes in Adolescent and Youth study, a randomized clinical trial of three treatments for type 2 diabetes (T2DM) in youth, demonstrated treatment failure (defined as sustained HbA1c ≥8%, or inability to wean insulin after 3 months after acute metabolic decomposition) in over half of the participants. Given that binding of mononuclear cells to vascular endothelium, initiated by cellular adhesion molecules and chemokines, is an early step in vascular injury, we sought to evaluate (a) changes in cellular adhesion molecule levels during the trial; (b) effect of diabetes treatment; and (c) association of markers with HbA1c, hypertension, hypercholesterolemia, nephropathy, and retinopathy.
Methods:
Participants (n = 515 of 699) that had baseline assessment of adhesion molecules (monocyte chemoattractant protein-1 [MCP-1], vascular cell adhesion marker [VCAM], intercellular adhesion marker [ICAM], and E-Selectin) and at least one other assessment, measured at month 12, 24, or 36, were included.
Results:
Over 1 to 3 years, significant increases in MCP-1 and decreases in VCAM (both P < .0001) concentrations were found; however, no significant interactions were identified with treatment group for any molecule. For every 1% increase in HbA1c, ICAM increased by 1.8%, VCAM by 1.5%, and E-selectin by 6.8% (all P < .0001). E-selectin increased by 3.7% and 4.2% for every 10 mm Hg increase in systolic and diastolic blood pressure, respectively (both P < .0001). ICAM was 10.2% higher and E-selectin was 15.5% higher in participants with microalbuminuria (both P < .01). There was no significant association of adhesion molecule levels with retinopathy.
Conclusion:
Concentrations of cellular adhesion molecules rise with increasing HbA1c in youth with T2DM, and are associated with blood pressure and microalbuminuria, markers of vascular injury.
Keywords: adhesion molecules, HbA1c, microvascular complications, type 2 diabetes
1 |. INTRODUCTION
Binding of mononuclear cells to vascular endothelium is an early step in the atherosclerotic process and is initiated by cellular adhesion molecules and chemokines,1 which play a key role in endothelial dysfunction and resultant plaque formation.2 Cellular adhesion molecules function in interactions between cells and extracellular membrane proteins to attract immune cells to the site of inflammation.3 Adhesion molecules include four broad classes: cadherins, integrins, selectins, and immunoglobulin family members.3 E-selectin, present on activated endothelial cells, belongs to the class of selectins and is the most integral to recruitment of leukocytes to areas of inflammation due to its ability to upregulate after interleukin 1-beta and tumor necrosis factor alpha (TNF-α) stimulation.4 Both vascular cell adhesion marker (VCAM) and intercellular adhesion marker (ICAM) belong to the immunoglobulin family, which bind to the integrins, modulating the interaction between the endothelial cells and leukocytes, and have increased cell surface expression upon exposure to TNF-α.3 Finally, monocyte chemoattractant protein-1 (MCP-1) is a key chemokine regulating inflammatory monocyte migration and infiltration and has a critical role in obesity-induced adipose tissue inflammation and endothelial dysfunction.5
As children with type 2 diabetes (T2DM) and obesity exhibit a chronic inflammatory state,6,7 the inflammatory effects of adhesion molecules on the endothelium is presumed to be accelerated, but has not been well studied in pediatric populations. In adults, soluble levels of adhesion molecules, including VCAM, ICAM, and E-selectin, have been shown to predict future cardiovascular events and correlate with the inflammatory markers interleukin 6 and C-reactive protein, as well as risk factors, such as hypertension, insulin resistance, T2DM, low high-density lipoprotein-cholesterol, and hypercholesterolemia.8–10 VCAM, ICAM, and E-selectin have also been identified as biomarkers predicting onset or progression of diabetic nephropathy and retinopathy,11,12 whereas circulating levels of MCP-1 have been shown to predict progression of diabetic nephropathy and retinopathy.13,14
The Treatment Options for type 2 Diabetes in Adolescent and Youth (TODAY) study demonstrated that metformin with the addition of rosiglitazone, but not the lifestyle intervention program, was superior to metformin alone in achieving durable glycemic control in youth newly diagnosed with T2DM.15 Overall, over half of the participants demonstrated treatment failure, defined as sustained HbA1c≥8%, or inability to wean from insulin after 3 months after acute metabolic decomposition.16 By the end of the randomized controlled trial, 34% had hypertension, 11% had an elevated LDL cholesterol, 17% had microalbuminuria, and 14% had retinopathy.17
Circulating levels of adhesion molecules have been correlated with both micro- and macrovascular complications in adults with diabetes but have not been studied in youth with T2DM.18–22 In the current study, we used a well-phenotyped cohort of adolescent participants with T2DM to evaluate: (a) the changes in circulating levels of ICAM, VCAM, E-selectin, and MCP-1 over time during the trial; (b) the effects of diabetes treatment; and (c) the association of these adhesion molecule blood levels and chemokines with HbA1c, hypertension, low-density lipoprotein (LDL) dyslipidemia, nephropathy, and retinopathy.
2 |. METHODS
2.1 |. TODAY study participants and study design
The TODAY study has been previously described in detail.15 Briefly, the TODAY study enrolled participants aged 10 to 17 with a diagnosis of T2DM for less than 2 years, body mass index (BMI) ≥85th percentile, and an absence of diabetes-related autoimmunity. Participants were randomized to one of three treatment arms and followed longitudinally for 2 to 6 years: metformin alone, metformin with rosiglitazone, or metformin plus a lifestyle intervention program. The primary outcome of the trial was time to treatment failure, defined as a persistently elevated HbA1c (≥8%) over a period of 6 months or inability to wean from insulin after metabolic decompensation. After an average of 3.86 years of follow-up, 319 (45.6%) participants reached the primary outcome. Rates of failure were 51.7%, 46.6%, and 38.6% for metformin alone, metformin plus lifestyle intervention, and metformin plus rosiglitazone, respectively. Participants in the metformin plus rosiglitazone group demonstrated a significantly lower rate of treatment failure than participants in the metformin alone group, (38.6% vs 51.7%), P = .006.15
For the following analyses, we present data from TODAY at baseline (randomization) and at months 12, 24, and 36. As previously described,16 participants had assessments of anthropometric measures (including weight, height, and BMI), blood pressure, and fasting laboratory studies at each study visit.
2.2 |. Laboratory measurements
Measurements of lipids, HbA1c, glucose, insulin, c-peptide, and adhesion molecules and chemokines (ICAM, VCAM, E-selectin, and MCP-1) were performed centrally at The Northwest Lipid Research Laboratory at the University of Washington, Seattle, WA according to standardized procedures. Adhesion molecules levels were measured in plasma as part of a 9-plex assay using reagents from R&D Systems (Minneapolis, MN), on a BioRad Bioplex-100 analyzer. The limit for detection of the assay was 87.9 pg/mL, 238 pg/mL, 18.8 pg/mL, and 9.9 pg/mL for ICAM, VCAM, E-selectin, and MCP-1, respectively. The intra- and inter-assay coefficients of variation for ICAM were 9.06% and 13.93%, respectively, for VCAM were 4.01% and 11.05%, for E-selectin were 9.04% and 10.98%, and for MCP-1 were 6.46% and 9.81%.
2.3 |. Assessment of complications
Hypertension was defined as an average systolic or diastolic blood pressure≥130/80 mm Hg or≥95th percentile for age, sex, and height measured on at least two consecutive study visits23 or on an antihypertensive medication. LDL dyslipidemia was defined as an LDL cholesterol ≥130 mg/dL sustained over at least two consecutive study visits or on lipid-lowering medication.
Urine microalbumin was measured and glomerular filtration rate (eGFR) was calculated at baseline and annually. Microalbuminuria was defined as an albumin-to-creatinine ratio of ≥30 mg/G creatinine on two of three urine samples collected over a 3 month minimal period.24 eGFR was estimated by the full-age-spectrum combined creatinine and cystatin C equation and hyperfiltration was defined as an eGFR ≥135 mL/min/1.73 m2 sustained over two or more consecutive visits.25
Retinopathy evaluations were completed in the last year of the TODAY study. Digital fundus photographs of seven standard stereoscopic fields that were readable in at least one eye were reviewed and scored centrally by The Fundus Photograph Reading Center at the University of Wisconsin, Madison, WI according to an abbreviated and modified version of the Early Treatment Diabetic Retinopathy Study Final Retinopathy Severity Scale for Persons.26 The scale has 17 steps, ranging from no retinopathy in either eye to high-risk proliferative retinopathy in both eyes. Very mild non-proliferative retinopathy or worse was defined as a score of ≥2. There were no participants at the end of TODAY with more than mild scores (4–5).
2.4 |. Statistical Considerations
Separate generalized linear mixed models were used to evaluate the effects of original treatment group assignment or primary outcome status on the mean of each adhesion molecule over repeated time points. The models assumed an unstructured covariance structure. Similar models were constructed to evaluate the association of HbA1c, hypertension, LDL dyslipidemia, nephropathy, and retinopathy with adhesion molecules over repeated time points, adjusting for age, sex, and concurrent BMI. Glycemic variables and comorbidities were measured repeatedly over time and entered the models as time-dependent covariates. Adhesion molecule variables were log-transformed and the percent change in each molecule per unit change in predictor is shown by [100(eβ−1)], where β is the beta estimate coefficient from the mixed model. For binary comorbidities, the percent difference in mean adhesion molecules between groups (comorbidity present vs absent) is presented (Trial Registration: clinicaltrials.gov NCT00081328).
3 |. RESULTS
3.1 |. Baseline characteristics
There were 515 TODAY participants with a baseline measurement and at least one other annual assessment of adhesion molecule levels, measured at month 12, 24, or 36. The majority of participants (57%) had measurements at all four time points, 33% at three time points, and 9% at two time points. At baseline, participants averaged 13.9 ± 2.0 years of age, 33% black non-Hispanic, 43% Hispanic, 20% white non-Hispanic, with a median duration of diabetes of 5 months (Q1,Q3: 4,10), and average BMI 34.4 ± 7.5 kg/m2. There were no differences in any of the anthropometric or biochemical measurements at TODAY baseline between the original cohort of 699 participants and the sub-cohort of 515 participants with ≥2 adhesion molecule measurements. (Table 1).
TABLE 1.
Baseline characteristics of all TODAY participants and the subset of participants with adhesion molecules measured
| Characteristics | All TODAY participants (n = 699) | Subset with adhesion molecules (n = 515) |
|---|---|---|
| Age (years) | 14.0 ± 2.0 | 13.9 ± 2.0 |
| Diabetes duration (months) | 5 (4, 10) | 5 (4, 10) |
| Race/ethnicity (%) | ||
| Black non-Hispanic | 32 | 33 |
| Hispanic | 40 | 44 |
| White non-Hispanic | 20 | 20 |
| Other | 7 | 3 |
| Cigarette smoker (%) | 4 | 2 |
| Treatment group (%) | ||
| Metformin only | 33 | 34 |
| Metformin and rosiglitazone | 33 | 32 |
| Metformin and lifestyle | 34 | 34 |
| Physical examination | ||
| BMI (kg/m2) | 34.9 ± 7.6 | 34.4 ± 7.5 |
| Waist circumference (cm) | 108.6 ± 16.7 | 107.8 ± 16.5 |
| Systolic BP (mm Hg) | 113.2 ± 10.9 | 112.7 ± 11.1 |
| Diastolic BP (mm Hg) | 66.7 ± 8.2 | 66.4 ± 8.2 |
| Lipid | ||
| Total cholesterol (mg/dL) | 146.1 ± 29.6 | 145.0 ± 28.7 |
| LDL cholesterol (mg/dL) | 85.0 ± 24.8 | 83.6 ± 24.5 |
| HDL cholesterol (mg/dL) | 38.7 ± 8.6 | 38.9 ± 8.9 |
| Triglycerides (mg/dL) | 114.6 ± 78.2 | 114.5 ± 75.5 |
| Metabolic | ||
| HbA1c (%) | 6.0 ± 0.8 | 6.0 ± 0.7 |
| Fasting glucose (mg/dL) | 111.4 ± 26.0 | 109.9 ± 23.6 |
| Fasting insulin (μU/mL) | 31.6 ± 24.8 | 30.6 ± 22.2 |
| Fasting c-peptide (ng/mL) | 3.8 ± 1.6 | 3.7 ± 1.5 |
Note: Data are mean ± SD, median (Q1, Q3), or %.
Abbreviations: BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TODAY, Treatment Options for type 2 Diabetes in Adolescent and Youth.
3.2 |. Longitudinal changes over time
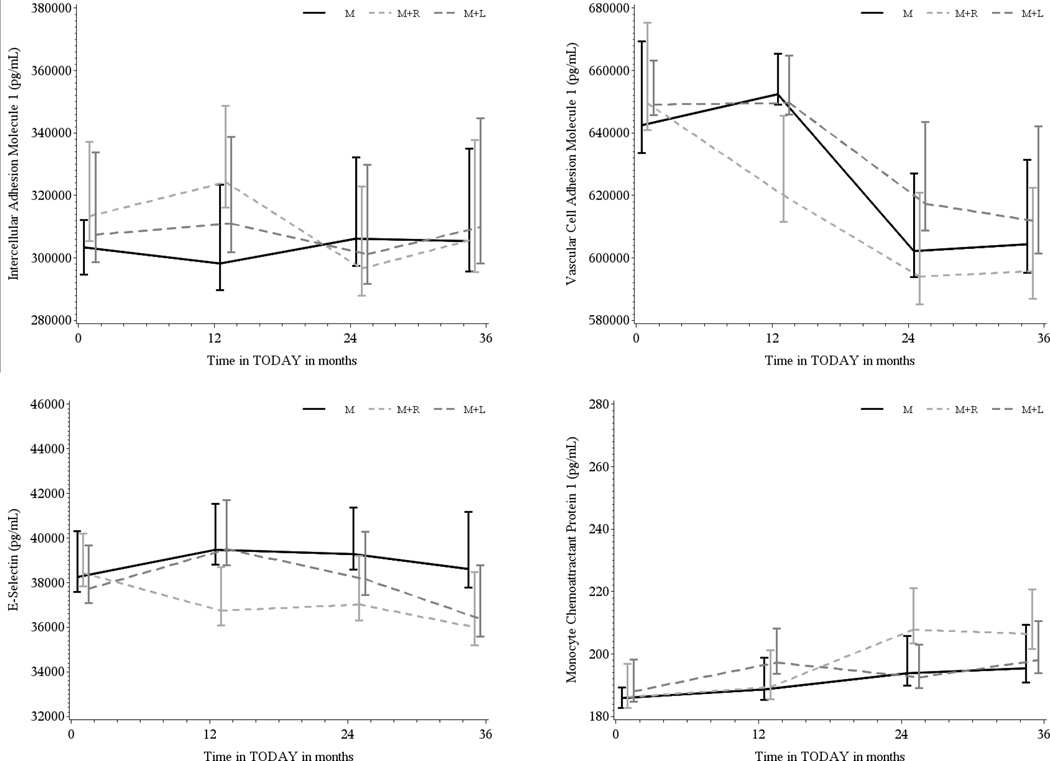
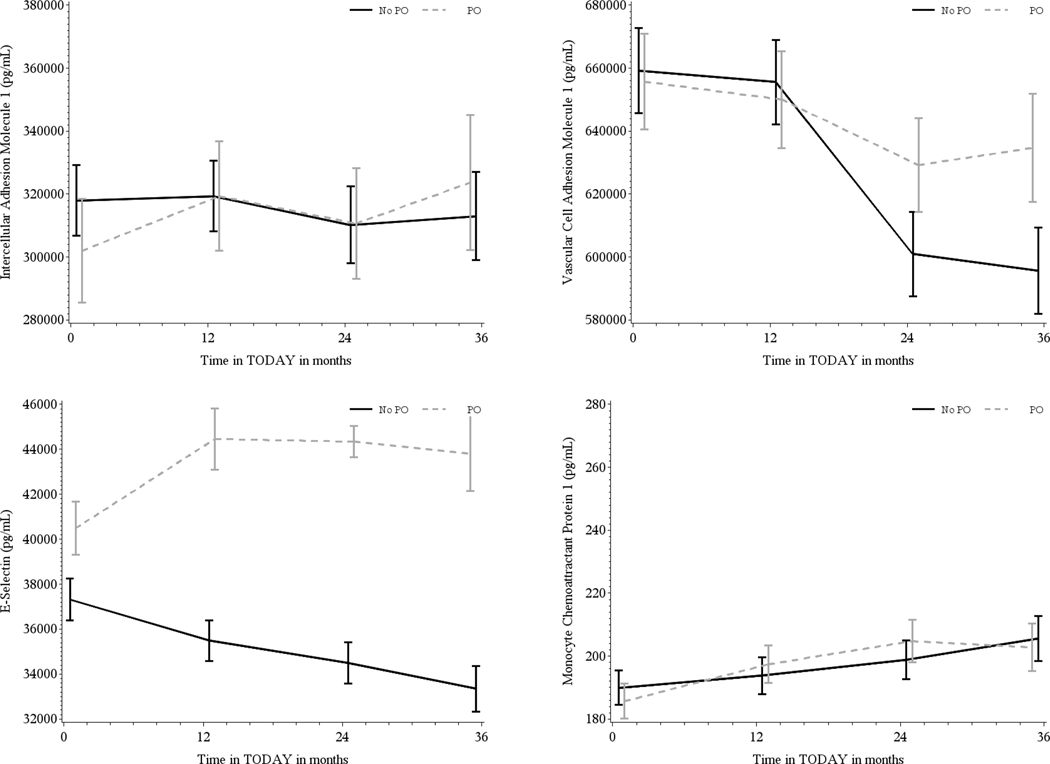
Separately for ICAM, VCAM, E-selectin, and MCP-1, the within-person correlation of the biomarker values overtime was moderate to high (intraclass correlation: 0.93, 0.72, 0.78, and 0.69, respectively). There was a significant increase in MCP-1 (P < .0001) as well as a significant decrease in VCAM (P < .0001) concentrations over the duration of the study. However, there were no significant treatment group differences overtime (Figure 1). Participants who reached the primary outcome of glycemic failure by the end of TODAY had significantly higher E-selectin compared to participants who maintained glycemic control across throughout the study (P < .0001, Figure 2). There were no significant differences in the circulating levels of ICAM, VCAM, or MCP-1 related to treatment failure.
FIGURE 1.
Geometric mean ± SD adhesion molecule levels by treatment group (M = black, M + R = gray, M + L = dark gray) over 36 months, for intracellular adhesion marker, vascular cell adhesion marker (VCAM), E-Selectin, and monocyte chemoattractant protein-1 (MCP-1). There was a significant increase in MCP-1 (P < .0001) as well as a significant decrease in VCAM (P < .0001) over the duration of the study. However, there were no significant treatment group differences over time
FIGURE 2.
Geometric mean ± SD adhesion molecule levels by primary outcome (PO, glycemic failure) status (No PO = black, PO = gray) over 36 months for intracellular adhesion marker (ICAM), vascular cell adhesion marker (VCAM), E-Selectin, and monocyte chemoattractant protein-1 (MCP-1). Participants who reached the PO of glycemic failure by the end of Treatment Options for type 2 Diabetes in Adolescent and Youth (TODAY) had significantly higher E-selectin compared with participants who maintained glycemic control across throughout the study (P < .0001). There were no significant differences in the circulating levels of ICAM, VCAM, or MCP-1 related to treatment failure
3.3 |. Longitudinal association with glycemic control and comorbidities
At the start of the TODAY study, mean HbA1c was 6.0 ± 0.7%, and mean systolic and diastolic blood pressure were 112.7 ± 11.1 and 66.4 ± 8.2 mm Hg, respectively. Additionally, 19% of participants had hypertension, 3% had LDL dyslipidemia, 8% had microalbuminuria, and 13% had hyperfiltration. Table 2 presents the longitudinal association of each adhesion molecule with glycemia, as assessed by HbA1c, and comorbidities. ICAM, VCAM, and E-selectin were all positively correlated with increasing HbA1c overtime. ICAM increased by 1.8% and VCAM by 1.5% for every 1% increase (eg, 7% to 8% or 9% to 10%) in HbA1c, while E-selectin increased by 6.8% for every 1% increase in HbA1c (all P < .0001) (Table 2).
TABLE 2.
Longitudinal association of adhesion molecules with glycemic control and comorbidities, adjusted for age, sex, and concurrent BMI
| Adhesion molecules | ||||
|---|---|---|---|---|
| ICAM (pg/mL) | VCAM (pg/mL) | E-selectin (pg/mL) | MCP-1 (pg/mL) | |
| HbA1c (per 1%) | ||||
| % change | 1.8 | 1.5 | 6.8 | 0.8 |
| P-value | <.0001 | <.0001 | <.0001 | .1222 |
| Systolic blood pressure (per 10 mm Hg) | ||||
| % change | 0.7 | 0.1 | 3.7 | −0.6 |
| P-value | .3028 | .9099 | <.0001 | .5348 |
| Diastolic blood pressure (per 10 mm Hg) | ||||
| % change | 1.4 | 0.1 | 4.2 | −0.3 |
| P-value | .0771 | .9366 | <.0001 | .7992 |
| Hypertension (yes vs no) | ||||
| % difference in means | 1.3 | 1.1 | 3.3 | 2.3 |
| P-value | .5767 | .5795 | .1796 | .4265 |
| LDL dyslipidemia (yes vs no) | ||||
| % difference in means | −2.7 | −1.7 | −0.1 | −4.1 |
| P-value | .4400 | .5623 | .9840 | .3463 |
| Microalbuminuria (yes vs no) | ||||
| % difference in means | 10.2 | −1.3 | 15.5 | 4.1 |
| P-value | .0014 | .5966 | <.0001 | .2645 |
| Hyperfiltration (yes vs no) | ||||
| % difference in means | 2.3 | −0.7 | −1.2 | −2.3 |
| P-value | .3828 | .7533 | .6200 | .4386 |
| Very mild NPDR (yes vs no) | ||||
| % difference in means | 12.5 | −3.3 | 5.7 | 2.6 |
| P-value | .2584 | .4157 | .3089 | .6562 |
The bold values represent p-values that are <0.05. Note: Each cell represents a single generalized linear mixed model regressing the log of ICAM, VCAM, E-selection, or MCP-1 on each comorbidity separately, adjusting for age, sex, and concurrent BMI. HbA1C and comorbidities were measured repeatedly over time and entered the models as time-dependent covariates. Adhesion molecule variables were log-transformed and the percent change in each molecule per unit change in predictor is shown by [100(eβ−1)], where β is the beta estimate coefficient from the mixed model. For binary comorbidities, the percent difference between groups (yes vs no) in mean adhesion molecule is presented.
Abbreviations: BMI, body mass index; ICAM, intercellular adhesion marker; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; NPDR, non-proliferative diabetic retinopathy; VACM, vascular cell adhesion marker.
E-selectin was associated with blood pressure overtime and increased by 3.7% and 4.2% for every 10 mm Hg increase in systolic and diastolic blood pressure (both P < .0001), respectively. There were no significant relationships overtime between the presence of study-defined hypertension, LDL dyslipidemia, or hyperfiltration and any of the adhesion molecules (Table 2). ICAM was 10.2% higher and E-selectin was 15.5% higher in participants with microalbuminuria compared to those without (P = .0014 and P < .0001, respectively). For example, a 10.2% mean difference in ICAM was equivalent to 347.6 ng/mL in those with microalbuminuria vs 315.5 ng/mL in those without; and a 15.5% mean difference in E-selectin was equivalent to 44.9 ng/mL for those with microalbuminuria vs 38.8 ng/mL in those without.
Among the 515 participants included in this analysis, 427 had readable retinopathy evaluations at the end of the TODAY study. Among them, 60 (14%) participants had non-proliferative diabetic retinopathy. There were no significant relationships overtime with the presence of mild retinopathy for any of the adhesion molecules.
4 |. DISCUSSION
In the present analysis, serial measurements of circulating adhesion molecules in youth with T2DM demonstrated relationships with glycemic control and diabetes complications. MCP-1 levels increased while VCAM levels decreased overtime. ICAM, VCAM, and E-selection were all positively correlated with increasing HbA1c, and E-selectin was higher in the subgroup of participants who did not maintain glycemic control during TODAY compared to those who had stable control. Additionally, E-selectin was positively associated with blood pressure and both ICAM and E-selectin were associated with microalbuminuria. There were no significant differences in adhesion molecule levels overtime in relationship to the presence of mild retinopathy.
To our knowledge, circulating levels of adhesion molecules have not been previously reported in youth with T2DM. However, higher levels of these markers have been reported in youth with obesity and metabolic syndrome. Kapiotis and colleagues found that circulating E-selectin levels were higher in obese compared to lean youth, whereas no difference was observed for ICAM or VCAM.6 We demonstrate that in the TODAY cohort of youth with T2DM, MCP-1 levels increased overtime, whereas VCAM levels decreased over time. These changes may be associated with changes in glycemic control.
We observed that VCAM levels were correlated positively with dysglycemia, yet showed a longitudinal trend of decreasing levels overtime that was unexpected. Marfella and colleagues found that VCAM-1 levels were not different in adults with short duration of T2DM as compared to non-diabetic controls, and did not increase with hyperglycemia in a clamp model.27 Our cohort also had a relatively short duration of T2DM; however, in our study higher VCAM levels were associated with hyperglycemia. Insulin has been shown to increase VCAM and E-selectin expression, and in the setting of insulin resistance downstream of the insulin receptor, cellular adhesion molecules increase.28 However, in a euglycemic-hyperinsulinemic clamp study by Targher and colleagues, VCAM-1 was not associated with insulin sensitivity.29 A decrease in VCAM might be expected if insulin resistance were improving across this time period; however, our previous analyses demonstrated that insulin sensitivity was only improved in the group that received rosiglitazone, with no change in the other two groups.30 Overall, the relationships between VCAM and diabetes and glycemic control are complex, and may vary in adult vs pediatric populations.
In an adult population with T2DM, ICAM was positively associated with increasing HbA1c31 similar to what we observed in youth with T2DM (Table 2). Marfella and colleagues have shown in a hyperglycemic clamp experiment that ICAM is increased by hyperglycemia, and this increase may be altered by nitric oxide bioavailability.27 Targher and colleagues assessed the relationship of insulin sensitivity to circulating adhesion molecules and found that although initially ICAM and E-selectin were negatively correlated with insulin sensitivity, this relationship disappeared in a multiple regression model controlling for BMI.29
MCP-1 has a well-established association with T2DM32 and positively correlates with HbA1c in adult cohorts.33,34 In obese children, MCP-1 levels were predictive of insulin resistance.35 In our cohort, ICAM, VCAM, and E-selectin, but surprisingly not MCP-1, were positively associated with HbA1c. Although data in pediatric T2DM are lacking, a study in youth with type 1 diabetes (T1DM) demonstrated no association of circulating MCP-1 levels with HbA1c, though levels were higher in those with multiple complications.36
Hypertension is a comorbidity of obesity and T2DM that was present in over 30% of the cohort at the end of TODAY. In adults with essential hypertension, but no diabetes, ICAM and VCAM have been shown to be higher than in patients who are normotensive.37 E-selectin and VCAM have been shown to correlate with systolic and diastolic blood pressure in hypertensive and normotensive adults.38 Ciobanu and colleagues found that ICAM and VCAM were significantly elevated in adults with T2DM and uncontrolled blood pressure.39 In a study of youth with T1DM, E-selectin was found to be positively correlated with diastolic blood pressure.40 Similar to these studies in adults with hypertension and youth with T1DM, E-selectin was correlated with both systolic and diastolic blood pressure in TODAY.
In adults with T2DM, vascular adhesion molecules were associated with increase in urinary albumin excretion over a 10 year period, independent of blood pressure or glucose control.21 Unsurprisingly, given the key role of inflammation and endothelial dysfunction in the process, adhesion molecules are also implicated in diabetic nephropathy. ICAM, VCAM, and MCP-1 were all found to be increased in adults with T2DM who also had microalbuminuria.20,41,42 We found, similar to the studies of adults, that ICAM is increased in participants with microalbuminuria. In addition, E-selectin is also increased in these participants, which may suggest that E-selectin is also associated with microvascular disease in youth.
In a study of adults with T2DM conducted by Blum and colleagues, VCAM and E-selectin increased across the retinopathy spectrum from no retinopathy to proliferative retinopathy.43 Nowak and colleagues found that VCAM and ICAM were increased in patients with T2DM and any level of retinopathy.44 In a young adult cohort with T2DM and retinopathy, MCP-1 was demonstrated to be increased in those with proliferative diabetic retinopathy.45 Most studies evaluating the difference in circulating adhesion molecules only demonstrated significant findings at the stage of proliferative retinopathy. No TODAY participants had proliferative retinopathy, which may explain the lack of association with the adhesion molecules in the current study.
Diabetic therapies have effects on circulation adhesion molecules. In adults, thiazolidinedione treatment is associated with reduced levels of VCAM, E-selection, and MCP-1.46 In the present study, circulating levels of adhesion molecules did not differ by treatment arm. While the addition of rosiglitazone was associated with improved glycemic control, the duration of therapy may not have been long enough to appreciate changes in vascular adhesion molecule levels. Also, in adults with T2DM, the addition of insulin therapy has been associated with decrease in E-selectin in proportion to the improvement in glycemic control.33,47 This is consistent with our findings that participants who did not maintain glycemic control had higher E-selectin levels than those who did maintain glycemic control. MCP-1 levels also reduce with insulin therapy; in our cohort, this phenomenon may obscure the relationship of MCP-1 to glycemic control,48 as participants with poor control had insulin therapy initiated per study protocol.
This study does have limitations. The measurement of circulating adhesion molecule levels was a secondary analysis performed on stored samples; therefore, some of the analyses have reduced sample size. While significant associations were still found, we may be underpowered to determine all possible associations and changes. The longitudinal analysis thus far only includes 3 years. As the study continues, additional longitudinal data will be available, providing more insight into the associations of the circulating adhesion molecules with glycemic control and future complications.
In conclusion, we have demonstrated that in youth with T2DM, circulating adhesion molecules levels change over time with an increase in MCP-1 and decrease in VCAM. E-selectin, ICAM, and VCAM were related to glycemic control and E-selectin was related to blood pressure. Further studies will be needed to determine if the circulating adhesion molecules could be used to predict risk for micro-and macrovascular disease in youth onset T2DM.
Supplementary Material
ACKNOWLEDGEMENTS
This work was completed with funding from NIDDK and the NIH Office of the Director through grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The NIDDK project office was involved in all aspects of the study, including: design and conduct; collection, management, analysis, and interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication. The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi Aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribes and the Indian Health Service.
Funding information
Sanofi Aventis; Pfizer; LifeScan, Inc.; GlaxoSmithKline; Eli Lilly and Company; Bristol-Myers Squibb; Becton, Dickinson and Company
Footnotes
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13062.
Guarantor Statement: Dr. Barbara H. Braffett is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. [DOI] [PubMed] [Google Scholar]
- 2.Richter V, Rassoul F, Purschwitz K, Hentschel B, Reuter W, Kuntze T. Circulating vascular cell adhesion molecules VCAM-1, ICAM-1, and E-selectin in dependence on aging. Gerontology. 2003;49(5):293–300. [DOI] [PubMed] [Google Scholar]
- 3.Petruzzelli L, Takami M, Humes HD. Structure and function of cell adhesion molecules. Am J Med. 1999;106(4):467–476. [DOI] [PubMed] [Google Scholar]
- 4.Silva M, Videira PA, Sackstein R. E-Selectin ligands in the human mononuclear phagocyte system: implications for infection, inflammation, and immunotherapy. Front Immunol. 2017;8:1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapiotis S, Holzer G, Schaller G, et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol. 2006; 26(11):2541–2546. [DOI] [PubMed] [Google Scholar]
- 7.Levitt Katz LE, Bacha F, Gidding SS, et al. Lipid profiles, inflammatory markers, and insulin therapy in youth with type 2 diabetes. J Pediatr. 2018;196:208–16 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brondani R, Rieder CR, Valente D, Araujo LF, Clausell N. Levels of vascular cell adhesion molecule-1 and endothelin-1 in ischemic stroke: a longitudinal prospective study. Clin Biochem. 2007;40(3–4):282–284. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94(9):3171–3182. [DOI] [PubMed] [Google Scholar]
- 10.Liu JJ, Yeoh LY, Sum CF, et al. Vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1, is associated with diabetic kidney disease in Asians with type 2 diabetes. J Diabetes Complications. 2015;29(5):707–712. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Yan J, Liu P, Wang Z. Effects of thiazolidinedione therapy on inflammatory markers of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS One. 2015;10(4):e0123703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erem C, Ozbas HM, Nuhoglu I, Deger O, Civan N, Ersoz HO. Comparison of effects of gliclazide, metformin and pioglitazone monotherapies on glycemic control and cardiovascular risk factors in patients with newly diagnosed uncontrolled type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122(5):295–302. [DOI] [PubMed] [Google Scholar]
- 13.Tam FW, Riser BL, Meeran K, Rambow J, Pusey CD, Frankel AH. Urinary monocyte chemoattractant protein-1 (MCP-1) and connective tissue growth factor (CCN2) as prognostic markers for progression of diabetic nephropathy. Cytokine. 2009;47(1):37–42. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Z, Hennein L, Xu Y, Bao N, Coh P, Tao L. Elevated serum monocyte chemoattractant protein-1 levels and its genetic polymorphism is associated with diabetic retinopathy in Chinese patients with type 2 diabetes. Diabet Med. 2016;33(1):84–90. [DOI] [PubMed] [Google Scholar]
- 15.The TODAY Study Group, Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The TODAY Study Group, Zeitler P, Epstein L, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetics. 2007;8:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tryggestad JB, Willi SM. Complications and comorbidities of T2DM in adolescents: findings from the TODAY clinical trial. J Diabetes Complications. 2015;29(2):307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jude EB, Douglas JT, Anderson SG, Young MJ, Boulton AJ. Circulating cellular adhesion molecules ICAM-1, VCAM-1, P- and E-selectin in the prediction of cardiovascular disease in diabetes mellitus. Eur J Intern Med. 2002;13(3):185–189. [DOI] [PubMed] [Google Scholar]
- 19.Kistorp C, Chong AY, Gustafsson F, et al. Biomarkers of endothelial dysfunction are elevated and related to prognosis in chronic heart failure patients with diabetes but not in those without diabetes. Eur J Heart Fail. 2008;10(4):380–387. [DOI] [PubMed] [Google Scholar]
- 20.Koga M, Otsuki M, Kubo M, Hashimoto J, Kasayama S. Relationship between circulating vascular cell adhesion molecule-1 and microvascular complications in type 2 diabetes mellitus. Diabet Med. 1998;15 (8):661–667. [DOI] [PubMed] [Google Scholar]
- 21.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157–1165. [DOI] [PubMed] [Google Scholar]
- 22.Piemonti L, Calori G, Lattuada G, et al. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care. 2009;32(11):2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 24.TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: The TODAY clinical trial. Diabetes Care. 2013;36(6):1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjornstad P, Snell-Bergeon JK, Rewers M, et al. Early diabetic nephropathy: a complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care. 2013;36(11):3678–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TODAY Study Group. Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36(6):1772–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marfella R, Esposito K, Giunta R, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101(19):2247–2251. [DOI] [PubMed] [Google Scholar]
- 28.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Targher G, Bonadonna RC, Alberiche M, Zenere MB, Muggeo M, Bonora E. Relation between soluble adhesion molecules and insulin sensitivity in type 2 diabetic individuals: role of adipose tissue. Diabetes Care. 2001;24(11):1961–1966. [DOI] [PubMed] [Google Scholar]
- 30.TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care. 2013;36(6):1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karimi Z, Kahe F, Jamil A, et al. Intercellular adhesion molecule-1 in diabetic patients with and without microalbuminuria. Diabetes Metab Syndr. 2018;12(3):365–368. [DOI] [PubMed] [Google Scholar]
- 32.Panee J. Monocyte Chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radhakrishnan P, Srikanth P, Seshadri KG, Barani R, Samanta M. Serum monocyte chemoattractant protein-1 is a biomarker in patients with diabetes and periodontitis. Indian J Endocrinol Metab. 2014;18(4):505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papatheodorou K, Papanas N, Papazoglou D, et al. Monocyte chemoattractant protein 1 is correlated with glycemic control and peripheral arterial disease in type 2 diabetic patients with metabolic syndrome. Angiology. 2013;64(3):223–229. [DOI] [PubMed] [Google Scholar]
- 35.Rivera P, Martos-Moreno GA, Barrios V, et al. A novel approach to childhood obesity: circulating chemokines and growth factors as biomarkers of insulin resistance. Pediatr Obes. 2019;14(3):e12473. [DOI] [PubMed] [Google Scholar]
- 36.Guan R, Purohit S, Wang H, et al. Chemokine (C-C motif) ligand 2 (CCL2) in sera of patients with type 1 diabetes and diabetic complications. PloS One. 2011;6(4):e17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cottone S, Mule G, Nardi E, et al. Microalbuminuria and early endothelial activation in essential hypertension. J Hum Hypertens. 2007;21(2):167–172. [DOI] [PubMed] [Google Scholar]
- 38.Boulbou MS, Koukoulis GN, Makri ED, Petinaki EA, Gourgoulianis KI, Germenis AE. Circulating adhesion molecules levels in type 2 diabetes mellitus and hypertension. Int J Cardiol. 2005;98(1):39–44. [DOI] [PubMed] [Google Scholar]
- 39.Ciobanu DM, Mircea PA, Bala C, Rusu A, Vesa S, Roman G. Intercellular adhesion molecule-1 (ICAM-1) associates with 24-hour ambulatory blood pressure variability in type 2 diabetes and controls. Cytokine. 2019;116:134–138. [DOI] [PubMed] [Google Scholar]
- 40.Maggio AB, Farpour-Lambert NJ, Montecucco F, et al. Elevated E-selectin and diastolic blood pressure in diabetic children. Eur J Clin Invest. 2012;42(3):303–309. [DOI] [PubMed] [Google Scholar]
- 41.Bruno CM, Valenti M, Bertino G, et al. Plasma ICAM-1 and VCAM-1 levels in type 2 diabetic patients with and without microalbuminuria. Minerva Med. 2008;99(1):1–5. [PubMed] [Google Scholar]
- 42.Siddiqui K, Joy SS, Al-Rubeaan K. Association of urinary monocyte chemoattractant protein-1 (MCP-1) and kidney injury molecule-1 (KIM-1) with risk factors of diabetic kidney disease in type 2 diabetes patients. Int Urol Nephrol. 2019;51:1379–1386. [DOI] [PubMed] [Google Scholar]
- 43.Blum A, Pastukh N, Socea D, Jabaly H. Levels of adhesion molecules in peripheral blood correlate with stages of diabetic retinopathy and may serve as bio markers for microvascular complications. Cytokine. 2018;106:76–79. [DOI] [PubMed] [Google Scholar]
- 44.Nowak M, Wielkoszynski T, Marek B, et al. Blood serum levels of vascular cell adhesion molecule (sVCAM-1), intercellular adhesion molecule (sICAM-1) and endothelial leucocyte adhesion molecule-1 (ELAM-1) in diabetic retinopathy. Clin Exp Med. 2008;8(3):159–164. [DOI] [PubMed] [Google Scholar]
- 45.Reddy S, Amutha A, Rajalakshmi R, et al. Association of increased levels of MCP-1 and cathepsin-D in young onset type 2 diabetes patients (T2DM-Y) with severity of diabetic retinopathy. J Diabetes Complications. 2017;31(5):804–809. [DOI] [PubMed] [Google Scholar]
- 46.Dolezalova R, Haluzik MM, Bosanska L, et al. Effect of PPAR-gamma agonist treatment on markers of endothelial dysfunction in patients with type 2 diabetes mellitus. Physiol Res. 2007;56(6):741–748. [DOI] [PubMed] [Google Scholar]
- 47.Ryysy L, Yki-Jarvinen H. Improvement of glycemic control by 1 year of insulin therapy leads to a sustained decrease in sE-selectin concentrations in type 2 diabetes. Diabetes Care. 2001;24(3):549–554. [DOI] [PubMed] [Google Scholar]
- 48.Lin Y, Ye S, He Y, Li S, Chen Y, Zhai Z. Short-term insulin intensive therapy decreases MCP-1 and NF-kappaB expression of peripheral blood monocyte and the serum MCP-1 concentration in newlydiagnosed type 2 diabetics. Arch Endocrinol Metab. 2018;62(2):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.