Abstract
We investigated the effects of regular walking in green and suburban environments on heart rate variability (HRV) and blood pressure (BP) in middle-aged adults. Twenty-three adults participated in a non-randomized crossover experiment comprised of once-weekly 50-min moderate-intensity walking sessions. Separated by a two-week washout period, participants walked for three weeks in each of two treatment conditions (green and suburban) in a local arboretum and suburban sidewalks of Chaska, MN. Eleven participants completed green walking first and 12 suburban walking first. Walks were split into 15-min intra-walk phases, with phases representing each walk’s beginning, middle, and final 15-min. Repeated measures linear mixed models evaluated (1) HRV phase differences between treatments and HRV change within treatments, and (2) pre- and post-walk BP differences between treatments and pre- to post-walk BP changes. Intra-walk phase analyses revealed higher HRV during green walking vs. suburban walking during phase 2 (p < 0.0001) and phase 3 (p = 0.02). Less HRV reduction was seen between intra-walk phases 1 and 2 during green vs. suburban walking (p = 0.02). Pre- to post-walk changes revealed decreased mean systolic BP for both green (p = 0.0002) and suburban (p = 0.003) walking conditions, but not for diastolic BP. Post-walk BP results were similar after both green walking and suburban walking. In summary, walking sessions in a green environment elicited greater beneficial HRV responses compared to a suburban environment. Additionally, walking in either environment, green or suburban, promoted reductions in systolic BP.
Keywords: greenspace, suburban environment, physical activity, heart rate variability, blood pressure
1. Introduction
Autonomic nervous system (ANS) dysfunction has been associated with poor cardiovascular health. Briefly, the parasympathetic nervous system (PNS) and the sympathetic nervous system (SNS) are responsible for maintaining proper ANS influence on the heart. PNS influence is heightened when an individual is in a more relaxed state, while SNS influence increases when an individual is in a state of excitement or stress.1 PNS-SNS balance can be measured via heart rate variability (HRV)—a non-invasive measure of the influence of the PNS and SNS on the heart.2,3 Higher time-domain measures of HRV are considered better (i.e., greater variation in the time between heart beats), with lower HRV (i.e., smaller variation in the time between heart beats) related to increased risk of sudden cardiac death,4 atherosclerotic plaque progression,5 congestive heart failure,6 coronary heart disease,7,8 stroke,9,10 and myocardial infarction.11,12 Notably, regular physical activity participation (i.e., exercise) is known to improve HRV. Randomized trials and epidemiologic studies have suggested that exercise leads to improved time- and frequency-domain HRV parameters in healthy13–16 and clinical populations (e.g., individuals with diabetes mellitus17 and heart failure18,19) compared to their less active counterparts.
Human interactions with nature (i.e., nature-based green environments) have been linked to numerous positive physiological health outcomes.20–22 In a recent systematic review and meta-analysis, interactions with nature were associated with improved HRV, lower cortisol production, and decreased risk of type 2 diabetes.23 To date, however, evidence remains inconclusive about how the environment might interact with exercise to promote beneficial cardiovascular responses and, more specifically, the cardiovascular responses characterizing HRV and blood pressure.24,25 Studies of green exercise (i.e., exercise in nature-based green environments)26 have suggested that exercise in green environments results in greater improvements in cortisol27 as well as blood pressure (BP) and heart rate,28 compared to exercise completed in urban environments. A study of HRV in young adult males suggested a single session of forest walking improved HRV indices relative to a single session of urban walking29, but little research on this topic has been conducted in other populations. Walking in settings that are more suburban or urban areas may induce greater psychosocial stress while also exposing individuals to greater amounts of environmental pollutants (e.g., air and noise pollution) vs. greener environments; all of which may have the effect of lowering HRV and increasing BP. 30–33
Investigating the difference in cardiovascular responses between green environments and suburban environments, the latter representing where middle-aged adults are most likely to live, is important in understanding the benefits of green exercise and the development of green exercise prescriptions for middle-aged adults to prevent or attenuate cardiovascular disease progression. Such studies may assist in not only describing the benefits of green exercise, but also in supporting the advocation for greenspace conservation to improve use of green areas for physical activity and promote community-level health.34 The purpose of this crossover study was to experimentally investigate the effects of repeated moderate-intensity walking sessions completed in green and suburban environments on the HRV and BP of middle-aged adults. Given nature’s demonstrated ability to reduce stress and cortisol levels, and improve mood,35–38 as well as the known BP-lowering effects of exercise,39 we hypothesized that green walking relative to suburban walking would result in: (1) higher time-domain HRV measurements during predetermined phases of the walk; (2) less HRV reduction throughout the course of the walk; and (3) greater pre-post BP reductions after the walk.
2. Methods
2.1. Participants
Twenty-four middle-aged adults (20 females; mean ± SD: 49.3 ± 6.7 years) were recruited for this study. Prospective participants underwent study screening against the following inclusion criteria: (1) aged 35–59 years; (2) not currently taking anti-anxiety or anti-depressant medications; (3) not having diagnosis of, and/or taking medication for, a chronic disease; (4) having no contraindications to moderate-intensity walking; and (5) not currently meeting the recommended levels of weekly physical activity (i.e., ≥150 min/week)39. Study procedures were approved by the Institutional Review Board (IRB) of the University of Minnesota, with the study registered on clinicaltrials.gov (NCT03442998). Following a detailed explanation of study procedures, informed consent was obtained from all participants prior to baseline data collection. All procedures performed with participants were in accordance with the ethical standards of the University of Minnesota IRB and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.40 Participants were compensated on a prorated basis up to $200.00 US for study participation.
2.2. Study Design
This study was a 9-week crossover trial in which all participants were asked to participate in both of the following treatments: (1) green walking: walking on unpaved and secluded nature trails of a local arboretum; and (2) suburban walking: walking on paved sidewalks of medium traffic roads located in a medium-density residential area. Each treatment was three consecutive weeks in duration and completed once a week on the same day and time. A two-week washout period (i.e., no study walking) separated treatments (see Figure 1). Given limited study resources, participants could not be randomized to the two conditions and tested simultaneously. Therefore, the first 12 participants recruited were assigned the following treatment sequence: green walking then suburban walking. The next 12 participants were assigned to the opposite treatment sequence: suburban walking then green walking.
Fig. 1.
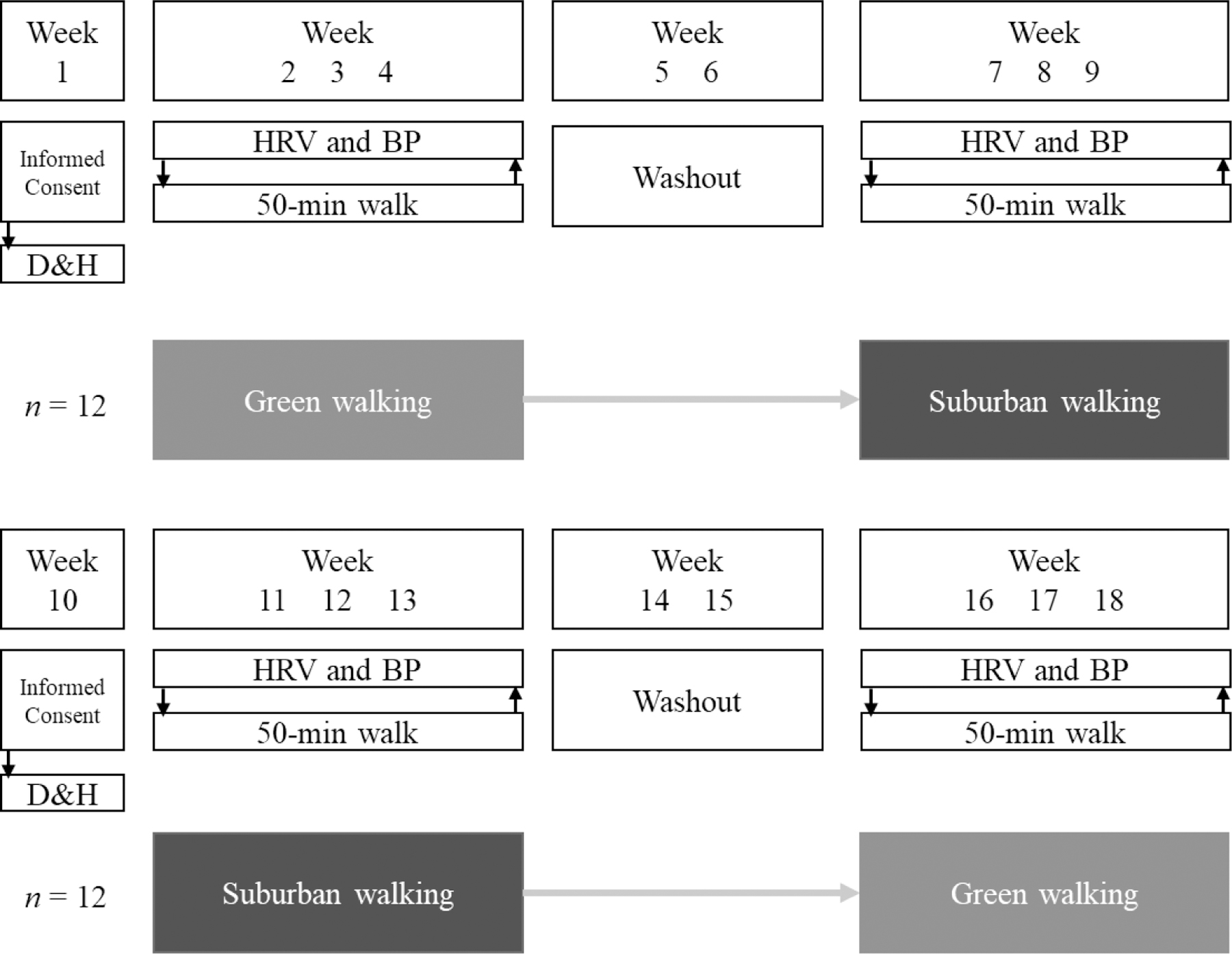
Study design. ≪1.5-column fitting image≫
Note. D&H, Baseline demographic and health assessment; HRV and BP, heart rate variability and blood pressure assessment.
2.3. Environmental Settings
For the green walking condition, we chose a walking path at a nature preserve, with a richness in vegetation and greenness. This location was situated conveniently about 40 kilometers southwest of the St-Paul-Minneapolis, MN metropolitan area. More specifically, participants walked at the Wood Duck Trail of the Minnesota Landscape Arboretum (MLA), which is approximately 1.6 kilometers long. This trail was unpaved, secluded by tall trees and shrubs, contoured a pond, and had minimal inclination. The MLA is part of the University of Minnesota and has more than 1,200 acres of gardens, woods, and prairies in addition to several kilometers of trails. The suburban walks occurred on paved sidewalks approximately three kilometers away from the MLA’s trail, adjacent to medium traffic roads located within a medium-density residential area (town population of ~27,000 residents) and had minimal inclination and few intersections. All participants walked in both green and suburban settings and in the same pre-specified paths.
2.4. Measures
2.4.1. Demographics
At the baseline visit, participants were first consented and then administered a demographic questionnaire for date of birth, sex, and measures of socioeconomic status (i.e., household income, employment status, education).
2.4.2. Anthropometrics
Weight and body fat percentage were measured on a calibrated electronic Tanita TBF-300A Body Composition Analyzer scale (Tanita Corp., Tokyo, Japan) to the nearest 0.1 kg and 0.1%, respectively. Height was measured with a stadiometer (model 437; Seca, Hamburg, Germany) to the nearest 0.1 cm. Body mass index (BMI) was calculated from the weight and height measurements and reported in kg/m2. Participants were measured while wearing light clothing and no shoes.
2.4.3. Heart Rate Variability
HRV was measured using the Zephyr BioHarness™ 3 (Zephyr Technology Ltd., Auckland, New Zealand)—a chest-mounted apparatus which has been validated.41–43 This apparatus (‘BioHarness’) measured HRV at a sampling rate of 250 Hz, with HRV calculated on a rolling 300-second basis. HRV measurements were reported as standard deviations of normal-to-normal RR intervals (SDNN) in milliseconds (ms)—a time-domain measurement which characterizes overall HRV.3 Research assistants moistened the electro-sensing pads and placed the BioHarness around each participant’s chest, and situated the BioHarness’s data collection unit (i.e., the BioModule) on the participant’s left side along the mid-axillary line. The BioHarness also collected data on heart rate (HR) and activity intensity (measured in g-forces; ≥ 0.20 g indicative of moderate-intensity walking). HRV, HR, and activity data were collected prior to, during, and after each walk and these data were downloaded and imported into Zephyr’s OmniSense Analysis program immediately after each participant’s walking sessions.
2.4.4. Blood Pressure
Participants sat with feet flat on the floor and legs uncrossed for at least five minutes before BP was measured. A HEM-705CP automatic blood pressure monitor (OMRON, Omron Healthcare Co., Ltd., Kyoto, Japan) measured BP, with the cuff placed on the upper left arm. Systolic and diastolic BP in millimeters of mercury (mmHg) were measured three times before and after each walk and the average of these measurements used for analyses.
2.5. Procedures
Participants arrived at each walking session in a fasted state (6+ hrs.). To calibrate the BioHarness, participants were asked to engage in five and two minutes of sitting and standing, respectively. Blood pressure measurements were subsequently taken following five minutes of quiet rest. Before starting each walk, participants were offered light snacks to break up fasting, and given a stopwatch to time their walks as the use of smartphones and/or smart devices (e.g., smartwatch) was prohibited to avoid distracted walking and facilitate engagement with the environment. The participants were asked to walk alone continuously for 50 minutes and were encouraged to observe their environment (e.g., flora and fauna) and to stop as needed (e.g., at crosswalks, to tie an untied shoe). Although walking speed was participant-controlled, running and/or jogging were not allowed. After returning from each walk, participants were asked once again to engage in five and two minutes of sitting and standing, respectively, per BioHarness protocol, with BP measured after another five minutes of quiet rest. Finally, the BioHarness was taken off each participant, turned off, and data downloaded immediately for later processing.
2.6. Statistical Analysis
We first inspected all data for physiological plausibility and potential data recording/entry errors, followed by normality testing. HRV, HR, and activity data from each walking session were split into three intra-walk phases, with the first, second, and third phases representing each walk’s first 15, middle 15, and final 15 minutes, respectively. We chose this 15-minute intra-walk phase length to ensure that the greatest amount of data during each walk for each participant could be analyzed when accounting for shorter walking times (shortest walk on record: 47 minutes). Two-minute pre- and post-walk sitting phases for HRV and HR were also defined. Congruent with the HRV literature, we adjusted for HR in our HRV analyses at rest and during each walking session.
We employed a repeated measures linear mixed model approach using PROC MIXED in SAS version 9.4 (SAS Institute Inc., Cary, NC). The statistical model treated each participant as a random effect and treatment (green vs. suburban), period, and sequence (green then suburban vs. suburban then green) as fixed effects. This model can be presented mathematically as:
where Yijkl is the outcome due to participant i, treatment j, period k, and sequence l measured at a specific time (walk week), with μ being the intercept, δl the sequence, βi(l) the random effect due to participant i nested within sequence l, αj the treatment, γk the period, and εijkl the random error. Period was included due to the fact we were unable to randomize participants into a treatment sequence and thus needed to adjust for period effects.44 HRV during each intra-walk phase and the pre- and post-walk phases, as well as HRV change between phases, were modeled separately as dependent variables, and treatment, period, and sequence as independent variables. HR was also included in the preceding model as a continuous covariate. A compound symmetry structure was used to model correlations between repeated measurements over time as this structure demonstrated the smallest Akaike information criterion (AIC) value. Differences between conditions were evaluated using Tukey-adjusted least-squared means with 95% CIs. Analyses of pre- and post-walk BP differences, as well as pre- to post-walk BP changes, were analyzed using the same model parameters and structure as specified above, with the only difference being that HR was not a covariate in these analyses. We did not perform formal sample size and power calculations for this pilot study, given that our objective included the assessment of intervention trends in outcome measures and feasibility. Therefore, as suggested in previous literature,45 the analytical sample size of 23 was considered adequate.
3. Results
A modified CONSORT participant flow diagram is shown in Figure 2. One participant dropped out after signing the consent form and was therefore excluded from analyses. Another participant discontinued during the washout period for reasons unrelated to the study but was included in the analyses per intention-to-treat (ITT).46,47 Table 1 presents participant characteristics. The sample was comprised of mostly middle-aged females who were obese, had at least some college-level education, and somewhat elevated resting BP.
Fig. 2.
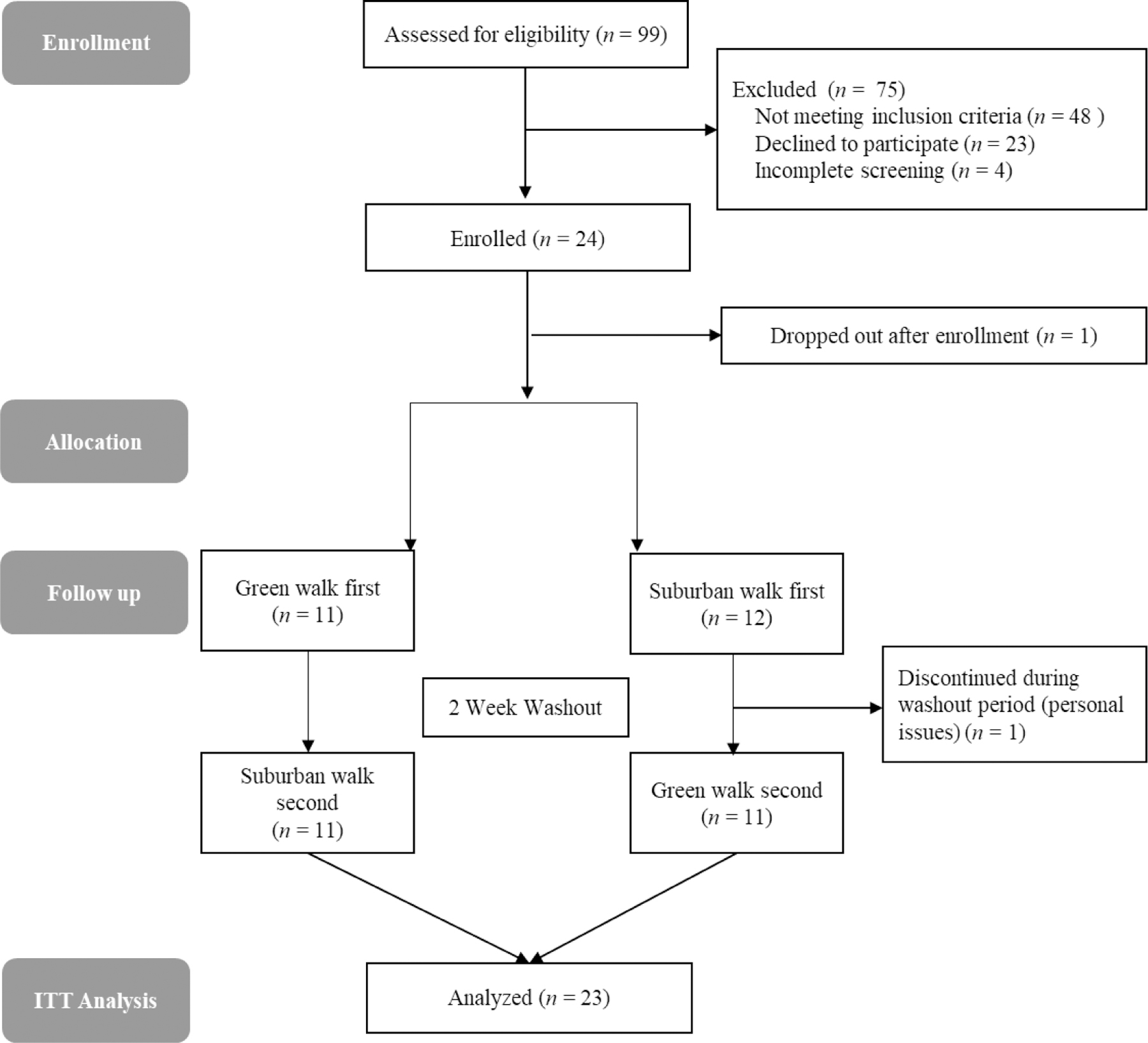
CONSORT Flow Diagram modified for non-randomized crossover trial design. ≪1.5-column fitting image≫
Table 1.
Selected baseline demographics and health characteristics of participants included in the analytical sample (n = 23).
| Characteristic | |
|---|---|
| Age, mean ± SD, y | 49.7 ± 6.5 |
| Female, n (%) | 19 (83) |
| Education, n (%) | |
| College/Some college | 17 (74) |
| Graduate level | 6 (26) |
| Household Income, n (%) | |
| <49,999 | 4 (17) |
| 50,000–99,999 | 8 (35) |
| 100,000 or more | 9 (39) |
| Prefer not to answer | 2 (9) |
| Exercise, mean ± SD, days/week | 1.7 ± 0.6 |
| Body mass index, n (%), kg/m2 | |
| Normal (18.5–24.99) | 8 (33) |
| Overweight (25–29.99) | 6 (25) |
| Obese (≥ 30.0) | 10 (42) |
| Body fat percentage, mean ± SD | 38.4 ± 10.1 |
| Resting blood pressure, mean ± SD | |
| Systolic (mmHg) | 124.7 ± 14.0 |
| Diastolic (mmHg) | 79.5 ± 11.3 |
| Resting HRV, mean ± SD, ms | 45.5 ± 18.4 |
| Resting HR, mean ± SD, beats per minute | 70.3 ± 16.4 |
Note. kg/m2, kilograms/meter squared; mmHg, millimeters of mercury; HRV, heart rate variability; ms, milliseconds; HR, heart rate.
3.1. Heart Rate Variability
Figure 3 presents mean HRV for green and suburban walking conditions across intra-walk phases. Results revealed a somewhat higher mean HRV during the first intra-walk phase for the green walking relative to suburban walking (p = 0.4). Additionally, higher mean HRV during green walking vs. suburban walking during the second (31.0 ± 1.4 vs. 22.6 ± 1.4, respectively; p < 0.0001) and third (33.2 ± 3.0 vs. 24.3 ± 2.9, respectively; p = 0.02) intra-walk phases were observed. Within-walking condition HRV changes between first and second intra-walk phases revealed a greater mean HRV decrease during the suburban walking condition (−13.0 ± 2.0; p < 0.0001) compared to the green walking condition (−7.0 ± 2.1; p = 0.001). Similarly, within-condition HRV changes between the first and third intra-walk phases showed slightly greater mean HRV decrease during the suburban walking condition (−11.6 ± 1.8; p < 0.0001) relative to the green walking condition (−8.7 ± 1.9; p < 0.0001). Changes within-condition between the second and third intra-walk phases showed a slight increase in HRV during the suburban walking condition (1.5 ± 1.0; p = 0.1) compared to the green walking (−1.7 ± 1.1; p = 0.2).
Fig. 3.
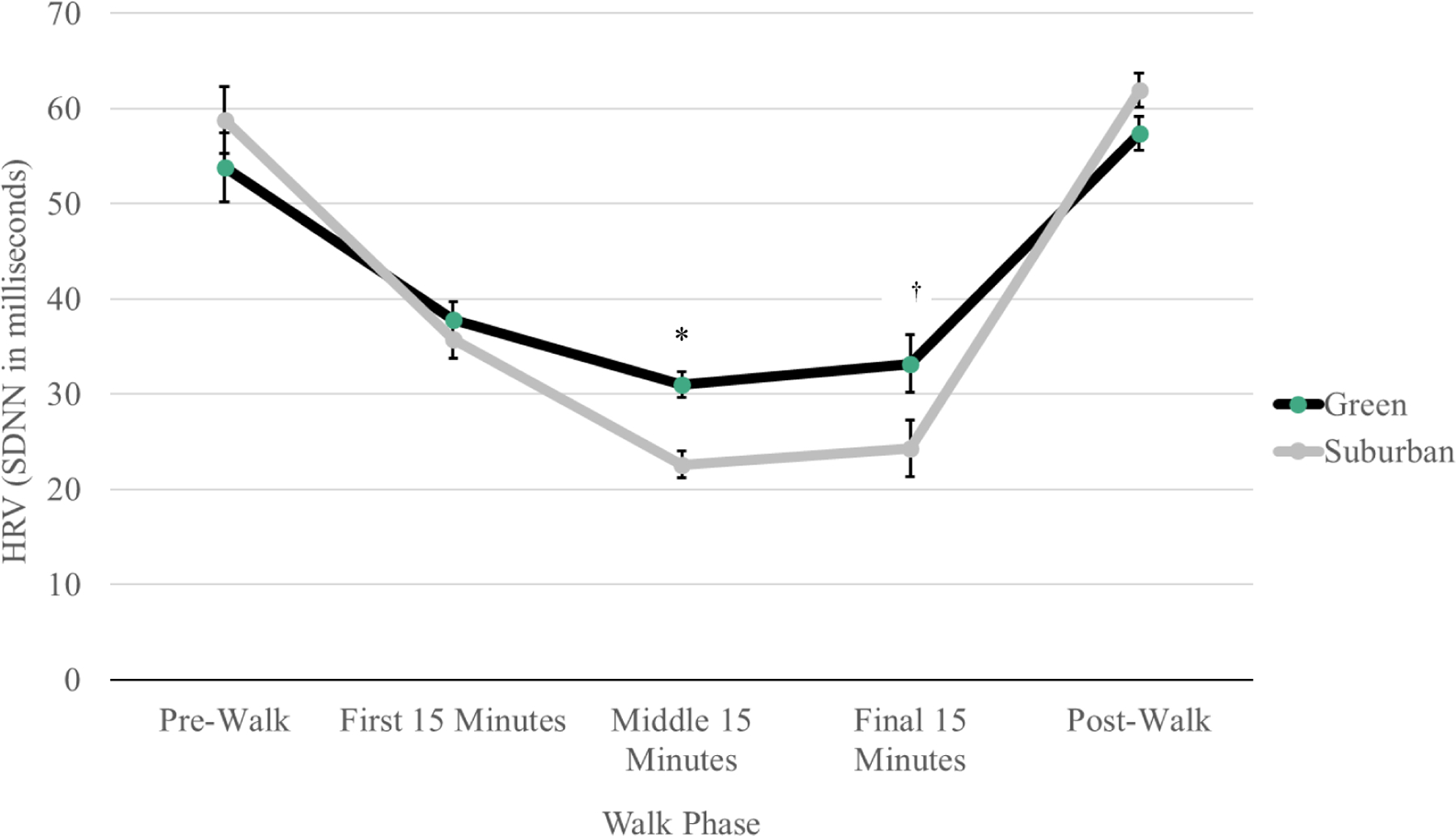
Line graph shows average heart rate variability (ms) change across walk phases by green and suburban conditions (n = 23). ≪1.5-column fitting image≫
Note. Data are presented as mean ± SE. Between-condition contrasts, mean (95% CI): pre-walk: −5.0 (−9.9, −0.08); first 15 min: 2.1 (−2.6, 6.8); second 15 min: 8.4 (6.0, 10.9)*; final 15 min: 8.9 (1.2, 16.6) †; post-walk: −4.5 (−8.0, −1.1).
3.2. Blood Pressure
Table 2 presents pre- and post-walk mean systolic and diastolic BP by green and suburban conditions. Pre-walk systolic and diastolic BP were similar between conditions. Pre- to post-walk changes revealed similarly decreased mean systolic BP for both green (−3.5 ± 0.9 mmHg; p = 0.0002) and suburban (−2.8 ± 0.9 mmHg; p = 0.003) walking conditions. As expected physiologically, diastolic BP changed minimally during the walk. Post-walk results revealed similar mean systolic BP (mean difference: −0.1, 95% CI [−1.9, 1.7]) and mean diastolic BP (mean difference: 0.2, 95% CI [−1.2, 1.7]) for green walking relative to suburban walking.
Table 2.
Pre- and post-walk mean systolic and diastolic blood pressure (mmHg) by green and suburban conditions (n = 23).
| Green |
Suburban |
|||
|---|---|---|---|---|
| Pre-walk | Post-walk | Pre-walk | Post-walk | |
| Blood pressure | mean ± SE | mean ± SE | ||
| Systolic | 120.8 ± 3.2 | 117.3 ± 2.9 | 120.2 ± 3.2 | 117.4 ± 2.9 |
| Diastolic | 74.1 ± 2.1 | 75.1 ± 2.3 | 74.6 ± 2.1 | 74.9 ± 2.3 |
4. Discussion
Our observations suggested that green walking exercise resulted in higher HRV and less HRV reduction compared to suburban walking exercise, after adjustment for HR. These observations were consistent with our first and second hypotheses, respectively. Exercise is known to improve cardiac autonomic regulation by lessening sympathetic and enhancing parasympathetic influence,48,49 with improved HRV indices associated with improved cardiovascular health and outcomes.4,7,10,50 Empirical literature has also revealed the consistent beneficial health effects moderate-intensity exercises like walking have among clinical populations with previous myocardial infarction,11 chronic heart failure,51 angina pectoris,52 and diabetes mellitus.53 Despite these benefits, few studies have investigated how different environmental exposures influence HRV during walking exercise. For example, prior studies have observed that sitting and/or walking in nature environments—termed “forest bathing”—elicited higher HRV compared to sitting and/or walking in city environments.54 Controlled indoor experimental studies have also noted increased HRV after viewing nature-based pictures compared to viewing pictures depicting urban environments.55,56 Similar to our study, a two-day field experiment with young adult males reported that a 15-minute self-paced forest walking session elicited greater PNS activity and lower SNS activity—resulting in improved HRV—compared with an urban walking session.29
Our results may be indicative of two mechanisms. First, walking in suburban or urban areas (i.e., grayer environments) may induce greater psychosocial stress relative to walking in greener environments, with decrements to HRV associated with environmentally-induced stress or anxiety.30 Second, walking in grayer environment may expose individuals to greater amounts of environmental pollutants vs. greener environments. Air pollution has been established as particularly influential on physiological stress responses. Indeed, air pollutants interact with specific receptors in the lungs and vasculature in a manner that increases SNS activity, thus lowering HRV, while also increasing systemic inflammation that may result in higher BP.31–33,57 Neither of the preceding outcomes is desired. Taken together, it would appear that exercise in green environments may benefit ANS and vascular functioning beyond that of exercise completed in urban and suburban environments. In middle-aged adults with conditions such as prediabetes or diabetes that result in impaired autonomic and vascular functioning,58,59 green exercise may be beneficial in lessening the negative health impact of these conditions. Future studies should investigate how these environments’ inherent characteristics (e.g., visual and olfactory stimuli, air quality) may impact psychosocial (e.g., anxiety, stress) and physiological (e.g., blood glucose control) mechanisms and determine whether and/or how these factors contribute to improved HRV.
Results indicated notable pre- to post-walk systolic blood pressure reductions within both walking conditions but not between walking conditions. While these reductions were encouraging and aligned with known blood pressure-lowering effects of exercise, the lack of between condition differences was incongruent with our third hypothesis and the mechanistic pathways previously mentioned. These observations were unable to support Pretty et al.’s60 findings that exercise while viewing green environments led to greater blood pressure reductions relative to exercise completed while viewing built environments (i.e., urban settings)—similar to observations made by Park et al.54 Congruent with our results, Lee and colleagues29 observed decreased systolic blood pressure in young adult males from pre- to post-walk when completing one forest and urban walking session each but no difference between walking environments. These latter observations and the current study’s findings are aligned with literature which has previously suggested that exercise leads to clinically meaningful systolic blood pressure reductions in adults regardless of the exercise environment.61 Further investigation should aim to comprehensively measure other factors that might also affect blood pressure (e.g., air and noise pollution) in order to determine potential environmental effects on blood pressure parameters.
The present study extends the paucity of green exercise literature in at least two ways. First, our study was conducted among middle-aged adults who were, on average, obese and had somewhat elevated blood pressure. Younger and normal weight participants, and with cardiometabolic profiles within the recommended ranges typically comprised the population samples of past studies on this topic. Having less restrictive inclusion criteria allowed us to include in our sample more middle-aged overweight adults with somewhat elevated blood pressure, who were more likely to benefit from the positive effects of green walking relative to healthier populations. Second, our study noted differences in HRV between green and suburban environments as opposed to between green and urban environments. Given the smaller differences in ‘greenness’ between green and suburban environments relative to between green and urban environments, our findings add to the literature which has shown the distinct health benefits of nature-based green environments on health. Future studies are needed to continue discerning where these differences arise.
The current study has several strengths. First, to our knowledge, this is the first study that investigated the effects of repeated once-weekly, 50-minute moderate-intensity walking sessions in green and suburban environments on HRV and blood pressure in middle-aged adults. Second, this study used a balanced and uniform crossover design, with a two-week washout period between treatment arms. This design and the implementation of the washout period enhances the level of statistical precision and power for model parameter estimates that can be obtained from small samples in addition to minimizing any potential carryover effects. Third, the chosen exercise modality (i.e., walking) is easily accessible, low impact, inexpensive and thus readily incorporated into people’s daily routines. However, there are limitations to this study. First, we did not perform simultaneous random assignment to treatment sequences. However, the crossover experiment is a type of self-matched design (i.e., each participant served as his or her own control) that reduces considerably the possibility of confounding and increases statistical precision for effect estimates.62 Second, the study sample is limited in generalizability as it was comprised of mostly women of middle - to high-income sampled from a single geographic area. Third, our study results are likely only generalizable to green and suburban settings with similar characteristics as the walking paths chosen for this study. Fourth, because we designated this investigation as a small pilot trial, we were not powered for, nor did we identify, a priori effect modifiers for analysis. Future larger and well-powered trials should consider identifying and examining a priori effect modifiers (e.g., participants’ residential level of greenness) to enhance our understanding of the examined relationships.
5. Conclusions
This crossover study examined the effects of repeated walking sessions in green and suburban environments on HRV and blood pressure in a sample of middle-aged adults. Results suggested that 50-minute moderate-intensity walking sessions in a green environment elicited greater beneficial HRV responses compared to a suburban environment. Additionally, walking in either environment, green or suburban, promoted reductions in systolic blood pressure. Given these observations, future studies are needed to explore the longer-term interactive effects of walking exercise in green and other outdoor environments (e.g., grey, blue) on HRV, blood pressure, and additional cardiometabolic markers. Replication of our findings along with current green exercise literature discoveries might foster the development of not only nature-based green exercise prescriptions for disease prevention but also to the continued advocacy for greenspace conservation— particularly within more suburban or urban environments.
Acknowledgements
The authors want to thank all the participants for their time and the research assistant Sarah Seidl for her dedication.
Funding
This work was supported by the University of Minnesota Office of the Vice President for Research’s Grant-in-Aid Program (PI: MAP). The HRSA Maternal and Child Health Bureau provided support for the training of JNB (Grant No. 5 T79MC00007–31-00). The National Heart, Lung, and Blood Institute of the National Institutes of Health supports the training of ZCP under Award Number T32 HL007779. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of these funding sources.
Abbreviations:
- HRV
Heart rate variability
- BP
blood pressure
- ANS
autonomic nervous system
- PNS
parasympathetic nervous system
- SNS
sympathetic nervous system
Footnotes
The Institutional Review Board (IRB) of the University of Minnesota approved all study procedures. Following a detailed explanation of study procedures, informed consent was obtained from all participants prior to baseline data collection. All procedures performed with participants were in accordance with the ethical standards of the University of Minnesota IRB and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Trial registration identification number: NCT03442998
Conflict of interest
Authors have no conflicts of interest to report.
Contributor Information
Junia N. de Brito, Division of Epidemiology & Community Health, University of Minnesota, 1300 S 2nd St, Suite 300, Minneapolis, MN 55455, USA.
Zachary C. Pope, Division of Epidemiology & Community Health, University of Minnesota, 1300 S 2nd St, Suite 300, Minneapolis, MN 55455, USA.
Nathan R. Mitchell, Division of Epidemiology & Community Health, University of Minnesota, 1300 S 2nd St, Suite 300, Minneapolis, MN 55455, USA.
Ingrid E. Schneider, Department of Forest Resources, University of Minnesota, 1530 Cleveland Ave North, Suite 301b, St. Paul, MN 55108, USA.
Jean M. Larson, Minnesota Landscape Arboretum, Bakken Center for Spirituality & Healing, University of Minnesota, 3675 Arboretum Drive, Chaska, MN 55318, USA.
Teresa H. Horton, Department of Anthropology, Northwestern University, 1819 Hinman Avenue, Evanston, IL 60208, USA.
Mark A. Pereira, Division of Epidemiology & Community Health, University of Minnesota, 1300 S 2nd St, Suite 300, Minneapolis, MN 55455, USA.
REFERENCES
- 1.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol 2004;97(2):731–738. doi: 10.1152/japplphysiol.00076.2004 [DOI] [PubMed] [Google Scholar]
- 2.Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R. Heart rate variability today. Prog Cardiovasc Dis 2012;55(3):321–331. doi: 10.1016/J.PCAD.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 3.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Heal 2017;5(September):1–17. doi: 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maheshwari A, Norby FL, Soliman EZ, et al. Low heart rate variability in a 2-minute electrocardiogram recording is associated with an increased risk of sudden cardiac death in the general population: The atherosclerosis risk in communities study. PLoS One. 2016;11(8):1–12. doi: 10.1371/journal.pone.0161648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huikuri HV, Jokinen V, Syvänne M, et al. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol 1999;19(8):1979–1985. doi: 10.1161/01.ATV.19.8.1979 [DOI] [PubMed] [Google Scholar]
- 6.Musialik-Łydka A, Sredniawa B, Pasyk S. Heart rate variability in heart failure. In: Kardiologia Polska Vol 58; 2003:10–16. [PubMed] [Google Scholar]
- 7.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes. Circulation. 2000;102(11):1239–1244. doi: 10.1161/01.cir.102.11.1239 [DOI] [PubMed] [Google Scholar]
- 8.Cha SA, Park YM, Yun JS, et al. Time- and frequency-domain measures of heart rate variability predict cardiovascular outcome in patients with type 2 diabetes. Diabetes Res Clin Pract 2018;143:159–169. doi: 10.1016/j.diabres.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaren A, Kerr S, Allan L, et al. Autonomic function is impaired in elderly stroke survivors. Stroke. 2005;36(5):1026–1030. doi: 10.1161/01.STR.0000160748.88374.ce [DOI] [PubMed] [Google Scholar]
- 10.Fyfe-johnson AL, Muller CJ, Alonso A, et al. Heart rate variability and incident stroke: The atherosclerosis risk in communities study. Stroke. 2016;47(6):1452–1458. doi: 10.1161/STROKEAHA.116.012662.Heart [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8 [DOI] [PubMed] [Google Scholar]
- 12.Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–171. doi: 10.1161/01.CIR.85.1.164 [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith RL, Bigger JT, Steinman RC, Fleiss JL. Comparison of 24-hour parasympathetic activity in endurance-trained and untrained young men. J Am Coll Cardiol 1992;20(3):552–558. doi: 10.1016/0735-1097(92)90007-A [DOI] [PubMed] [Google Scholar]
- 14.Fessi MS, Moalla W. Postmatch perceived exertion, feeling, and wellness in professional soccer players. Int J Sport Physiol Perform. 2018;13(5):631–637. doi: 10.1123/ijspp.2017-0725 [DOI] [PubMed] [Google Scholar]
- 15.Buchheit M, Simon C, Charloux A, Doutreleau S, Piquard F, Brandenberger G. Heart rate variability and intensity of habitual physical activity in middle-aged persons. Med Sci Sports Exerc 2005;37(9):1530–1534. doi: 10.1249/01.mss.0000177556.05081.77 [DOI] [PubMed] [Google Scholar]
- 16.Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M. Effects of moderate and vigorous physical activity on heart rate variability in a british study of civil servants. Am J Epidemiol 2003;158(2):135–143. doi: 10.1093/aje/kwg120 [DOI] [PubMed] [Google Scholar]
- 17.Villafaina S, Collado-Mateo D, Fuentes JP, Merellano-Navarro E, Gusi N. Physical exercise improves heart rate variability in patients with type 2 diabetes: a systematic review. Curr Diab Rep 2017;17(11):110. doi: 10.1007/s11892-017-0941-9 [DOI] [PubMed] [Google Scholar]
- 18.Pearson MJ, Smart NA. Exercise therapy and autonomic function in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 2018;23(1):91–108. doi: 10.1007/s10741-017-9662-z [DOI] [PubMed] [Google Scholar]
- 19.Murad K, Brubaker PH, Fitzgerald DM, et al. Exercise training improves heart rate variability in older patients with heart failure: a randomized, controlled, single-blinded trial. Congest Hear Fail 2012;18(4):192–197. doi: 10.1111/j.1751-7133.2011.00282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pretty J, Peacock J, Hine R, Sellens M, South N, Griffin M. Green exercise in the UK countryside: Effects on health and psychological well-being, and implications for policy and planning. J Environ Plan Manag 2007;50(2):211–231. doi: 10.1080/09640560601156466 [DOI] [Google Scholar]
- 21.Morita E, Fukuda S, Nagano J, et al. Psychological effects of forest environments on healthy adults: Shinrin-yoku (forest-air bathing, walking) as a possible method of stress reduction. Public Health. 2007;121(1):54–63. doi: 10.1016/j.puhe.2006.05.024 [DOI] [PubMed] [Google Scholar]
- 22.Gladwell VF, Brown DK, Wood C, Sandercock GR, Barton JL. The great outdoors: How a green exercise environment can benefit all. Extrem Physiol Med 2013;2(1):1. doi: 10.1186/2046-7648-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Twohig-Bennett C, Jones A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ Res 2018;166:628–637. doi: 10.1016/J.ENVRES.2018.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowler DE, Buyung-Ali LM, Knight TM, Pullin AS. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health. 2010;10:456. doi: 10.1186/1471-2458-10-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gidlow CJ, Jones MV, Hurst G, et al. Where to put your best foot forward: Psycho-physiological responses to walking in natural and urban environments. J Environ Psychol 2016;45:22–29. doi: 10.1016/J.JENVP.2015.11.003 [DOI] [Google Scholar]
- 26.Pretty J, Griffin M, Sellens M, Pretty C. Green exercise: Complementary roles of nature, exercise and diet in physical and emotional well-being and implications for public health policy. CES Occas Pap Univ Essex 2003;1:1–39. [Google Scholar]
- 27.Thompson Coon J, Boddy K, Stein K, Whear R, Barton J, Depledge MH. Does participating in physical activity in outdoor natural environments have a greater effect on physical and mental wellbeing than physical activity indoors? A systematic review. Environ Sci Technol 2011;45:1761–1772. doi: 10.1021/es102947t [DOI] [PubMed] [Google Scholar]
- 28.Gladwell VF, Brown DK, Wood C, Sandercock GR, Barton JL. The great outdoors: How a green exercise environment can benefit all. Extrem Physiol Med 2013;2(1):1–7. doi: 10.1186/2046-7648-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Tsunetsugu Y, Takayama N, et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid Based Complement Altern Med 2014;2014:834360. doi: 10.1155/2014/834360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig 2018;15(3):235–245. doi: 10.30773/pi.2017.08.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Block ML, Calderón-Garcidueñas L. Air pollution: Mechanisms of neuroinflammation & CNS disease. Trend Neurosci 2009;32(9):506–516. doi: 10.1016/j.tins.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieters N, Plusquin M, Cox B, Kicinski M, Vangronsveld J, Nawrot TS. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: A meta-analysis. Heart. 2012;98(15):1127–1135. doi: 10.1136/heartjnl-2011-301505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundbäck M, Mills NL, Lucking A, et al. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part Fibre Toxicol 2009;6:1–6. doi: 10.1186/1743-8977-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.APHA. Policy Statement: Improving Health and Wellness through Access to Nature.; 2013.
- 35.Pretty J, Peacock J, Sellens M, Griffin M. The mental and physical health outcomes of green exercise. Int J Env Heal Res 2005;15(5):319–337. doi: 10.1080/09603120500155963 [DOI] [PubMed] [Google Scholar]
- 36.Beil K, Hanes D, Beil K, Hanes D. The influence of urban natural and built environments on physiological and psychological measures of stress— A pilot study. Int J Environ Res Public Health. 2013;10(4):1250–1267. doi: 10.3390/ijerph10041250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olafsdottir G, Cloke P, Vögele C. Place, green exercise and stress: An exploration of lived experience and restorative effects. Health Place. 2017;46:358–365. doi: 10.1016/J.HEALTHPLACE.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 38.Marselle MR, Irvine KN, Warber SL. Walking for well-being: Are group walks in certain types of natural environments better for well-being than group walks in urban environments? Int J Environ Res Public Health. 2013;10(11):5603–5628. doi: 10.3390/ijerph10115603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans: 2nd Edition. Washington, DC; 2008. [Google Scholar]
- 40.World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 41.Johnstone JA, Ford PA, Hughes G, Watson T, Garrett AT. Bioharness(TM) multivariable monitoring device: part. I: validity. J Sports Sci Med 2012;11(3):400–408. http://www.ncbi.nlm.nih.gov/pubmed/24149346. Accessed October 12, 2018. [PMC free article] [PubMed] [Google Scholar]
- 42.Johnstone JA, Ford PA, Hughes G, Watson T, Garrett AT. Bioharness(TM) Multivariable Monitoring Device: Part. II: Reliability. J Sports Sci Med 2012;11(3):409–417. http://www.ncbi.nlm.nih.gov/pubmed/24149347. Accessed October 12, 2018. [PMC free article] [PubMed] [Google Scholar]
- 43.Nazari G, Bobos P, Macdermid JC, Sinden KE, Richardson J, Tang A. Psychometric properties of the Zephyr bioharness device: A systematic review. doi: 10.1186/s13102-018-0094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senn S Cross-over Trials in Clinical Research. J. Wiley; 2002. [Google Scholar]
- 45.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4(4):287–291. doi: 10.1002/pst.185 [DOI] [Google Scholar]
- 46.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8(1):18. doi: 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta S Intention-to-treat concept: A review. Perspect Clin Res 2011;2(3):109. doi: 10.4103/2229-3485.83221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malfatto G, Facchini M, Sala L, Branzi G, Bragato R, Leonetti G. Effects of cardiac rehabilitation and beta-blocker therapy on heart rate variability after first acute myocardial infarction. Am J Cardiol 1998;81(7):834–840. doi: 10.1016/S0002-9149(98)00021-6 [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto Y, Hughson RL, Peterson JC. Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J Appl Physiol 1991;71(3):1136–1142. doi: 10.1152/jappl.1991.71.3.1136 [DOI] [PubMed] [Google Scholar]
- 50.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.CIR.94.11.2850 [DOI] [PubMed] [Google Scholar]
- 51.Shah SA, Kambur T, Chan C, Herrington DM, Liu K, Shah SJ. Relation of short-term heart rate variability to incident heart failure (From the multi-ethnic study of atherosclerosis). Am J Cardiol 2015;112(4):533–540. doi:doi: 10.1016/j.amjcard.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanza GA, Sgueglia GA, Cianflone D, et al. Relation of heart rate variability to serum levels of c-reactive protein in patients with unstable angina pectoris. Am J Cardiol 2006;97:1702–1706. doi: 10.1016/j.amjcard.2006.01.029 [DOI] [PubMed] [Google Scholar]
- 53.Liao D, Cai J, Brancatic FL, et al. Association of vagal tone with serum insulin, glucose, and diabetes mellitus- The ARIC study. Diabetes Res Clin Pract 1995;30:211–221. [DOI] [PubMed] [Google Scholar]
- 54.Park BJ, Tsunetsugu Y, Kasetani T, Kagawa T, Miyazaki Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Env Heal Prev Med 2009;15(1):18–26. doi: 10.1007/s12199-009-0086-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gladwell VF, Brown DK, Barton JL, et al. The effects of views of nature on autonomic control. Eur J Appl Physiol 2012;112(9):3379–3386. doi: 10.1007/s00421-012-2318-8 [DOI] [PubMed] [Google Scholar]
- 56.Brown DK, Barton JL, Gladwell VF. Viewing nature scenes positively affects recovery of autonomic function following acute-mental stress. Environ Sci Technol 2013;47(11):5562–5569. doi: 10.1007/s00216-015-8858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai DH, Riediker M, Berchet A, et al. Effects of short- and long-term exposures to particulate matter on inflammatory marker levels in the general population. Environ Sci Pollut Res 2019;26(19):19697–19704. doi: 10.1007/s11356-019-05194-y [DOI] [PubMed] [Google Scholar]
- 58.Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig 2013;4(1):4–18. doi: 10.1111/jdi.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bassi D, Arakelian VM, Mendes RG, et al. Poor glycemic control impacts linear and non-linear dynamics of heart rate in DM type 2. Rev Bras Med do Esporte 2015;21(4):313–317. doi: 10.1590/1517-869220152104150003 [DOI] [Google Scholar]
- 60.Pretty J, Peacock J, Sellens M, Griffin M. The mental and physical health outcomes of green exercise. Int J Environ Health Res 2005;15(5):319–337. doi: 10.1080/09603120500155963 [DOI] [PubMed] [Google Scholar]
- 61.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med 2002;136(7):493–503. doi: 10.7326/0003-4819-136-7-200204020-00006 [DOI] [PubMed] [Google Scholar]
- 62.Weiss N Clinical Epidemiology. In: Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:641–651. [Google Scholar]


