Abstract
Objective—
To determine whether subcutaneous fat thickness measured on thoracic radiographs was associated with body condition score (BCS) in dogs.
Animals—
87 client-owned dogs (41 males and 46 females) with a median age of 10.0 years (range, 1 to 16 years) and median weight of 20.3 kg (range, 3.1 to 58.0 kg).
Procedures—
Age, sex, body weight, and breed were recorded. Body condition scores (scale from 1 to 9) and muscle condition scores were assigned by a single investigator. Subcutaneous fat thickness was measured at the level of the eighth rib head on a dorsoventral or ventrodorsal radiographic view of the thorax by a single investigator. Ratios of subcutaneous fat thickness to the width of the midbody of T8 on the ventrodorsal or dorsoventral radiographic view (T8 ratio) and to the length of the midbody of T4 on a right lateral radiographic view (T4 ratio) were calculated and compared with BCS by means of the Spearman correlation method.
Results—
Median BCS was 6 (range, 1 to 9), and all muscle condition scores were represented. There were significant correlations between BCS and T4 ratio (r = 0.86) and between BCS and T8 ratio (r = 0.84).
Conclusions and Clinical Relevance—
Results indicated that in this population, there was a significant association between BCS and subcutaneous fat thickness measured on thoracic radiographs. Findings suggested that measuring subcutaneous fat thickness could aid in the retrospective assignment of BCS in studies involving dogs in which BCS was not recorded in the medical record.
Various quantitative methods for estimating body fat percentage in dogs have been evaluated, including dual-energy X-ray absorptiometry, the deuterium oxide dilution method, and a CT technique.1,2 Limitations of these methods include the need for special equipment or expertise, inconsistency in results, variability between breeds and sizes of dogs, and variability based on hydration status. Additionally, owing to the costs associated with these methods, they are not practical to assess large populations of dogs or for everyday clinical practice.
Body condition scoring is the current practical method for subjectively estimating the amount of fat in dogs, with BCSs most commonly assigned on a scale of 1 through 53 or 1 through 9.4 With the 9-point BCS system, each 1-point increase or decrease in BCS represents an approximately 10% increase or decrease in body weight and an approximately 5% increase or decrease in body fat percentage.5
Although assigning a BCS as a part of every physical examination has been recommended,6 scores are not always assigned or recorded in the medical record. For studies of BCS based on retrospective reviews of medical records, this can mean that large numbers of records have to be excluded from consideration. In addition, because BCSs are subjective, there can be inconsistency among clinicians assigning scores. Thus, a method for retrospectively assigning BCSs or for retrospectively verifying scores assigned by different clinicians would be useful.
Because BCS reflects body fat content, it seems logical that the amount of subcutaneous fat would be associated with BCS. The goal of the study reported here, therefore, was to determine whether subcutaneous fat thickness measured on thoracic radiographs would be associated with BCS in dogs.
Materials and Methods
Dogs examined at the Tufts University Cummings School of Veterinary Medicine’s Foster Hospital for Small Animals for which the diagnostic evaluation included obtaining orthogonal radiographic views of the thorax were eligible for enrollment in the study. The study protocol was approved by the Clinical Studies Review Committee at the Tufts Cummings School of Veterinary Medicine. Owner consent was not required because assigning a BCS was a standard part of every physical examination at this institution. Dogs were excluded if they were < 1 year old or had a history of thoracic surgery or thoracic limb amputation because of the potential that the incision could affect assignment of a BCS or measurement of subcutaneous fat thickness. On the basis of a priori sample size calculations, an attempt was made to enroll 12 dogs in each BCS score category.
For all dogs enrolled in the study, a BCS (scale of 1 to 9) and MCS (severe, moderate, mild, or no muscle wasting) were assigned by a single investigator (DEL). In addition, information on age, sex, body weight, and breed was recorded.
For measurement of subcutaneous fat thickness, radiographic images of all dogs were downloaded to a radiology workstationa by a single investigator (JSS) who removed identifying information from the images, randomized them using a software program,b and assigned a new numeric identifier. A single investigator (DEL) blinded to identifying information until after radiographic measurements had been completed then measured subcutaneous fat thickness at the level of the eighth rib head on the dorsoventral or ventrodorsal radiographic view of the thorax.c For this measurement, a line was drawn on the left and right sides of the thorax perpendicular to the vertebral column and extending from the lateral aspect of the lung-thoracic wall interface to the skin-air interface. To account for any slight rotation attributable to radiographic positioning, the mean of the lengths of the left- and right-sided measurements was used as the subcutaneous fat thickness. Any dog for which the radiographic view excluded the skin surface was removed from the study. Similarly, any dog with a subcutaneous thoracic mass at the level of the eighth rib head that altered the skin contour was removed from the study.
To account for differences in dog body sizes, 2 subcutaneous fat thickness ratios were calculated. The T8 ratio was calculated as the ratio of subcutaneous fat thickness to the width of the midbody of T8 on the dorsoventral or ventrodorsal radiographic view. The T4 ratio was calculated as the ratio of subcutaneous fat thickness to the length of the midbody of T4 on the right lateral radiographic view.
Statistical analysis—
The Spearman correlation method was used to test whether BCS was correlated with the T8 or T4 ratio, whether MCS was correlated with the T4 or T8 ratio, and whether BCS was correlated with MCS. Linear regression analysis was performed to develop regression equations to predict BCS from the T8 and T4 ratios; validity of the model assumptions of normality and homoscedasticity was evaluated with the Shapiro-Wilk test and standard residual analyses. Standard softwared was used for all analyses; values of P < 0.05 were considered significant.
Results
Ninety-seven dogs were enrolled in the study and had BCSs and MCSs assigned and radiographic images prepared for evaluation. At the time of radiographic assessment by the blinded investigator, 10 dogs were excluded because the skin surface was not visible on the radiographic image or superimposed masses interfered with the required radiographic measurements.
Of the 87 dogs that remained in the study, 41 were male and 46 were female. Subcutaneous fat thickness was measured at the level of the eighth rib head on the dorsoventral radiographic view in 62 dogs and on the ventrodorsal radiographic view in 25 dogs. Median age was 10.0 years (range, 1 to 16 years; mean, 8.9 years), and median weight was 20.3 kg (range, 3.1 to 58.0 kg; mean, 22.3 kg). There were 10 mixed-breed dogs, with the remaining dogs representing 38 breeds. The most common breeds were Labrador Retriever (n = 9) and Boxer (5). Median BCS was 6 (range, 1 to 9; mean, 5.7), with 4 dogs assigned a BCS of 1, 6 assigned a BCS of 2, 6 assigned a BCS of 3, 11 assigned a BCS of 5, and 12 each assigned BCSs of 4, 6, 7, 8, and 9. All MCSs were represented, with 21 dogs having severe muscle wasting, 14 having moderate muscle wasting, 26 having mild muscle wasting, and 26 having normal muscle condition or no muscle wasting (Figure 1).
Figure 1—
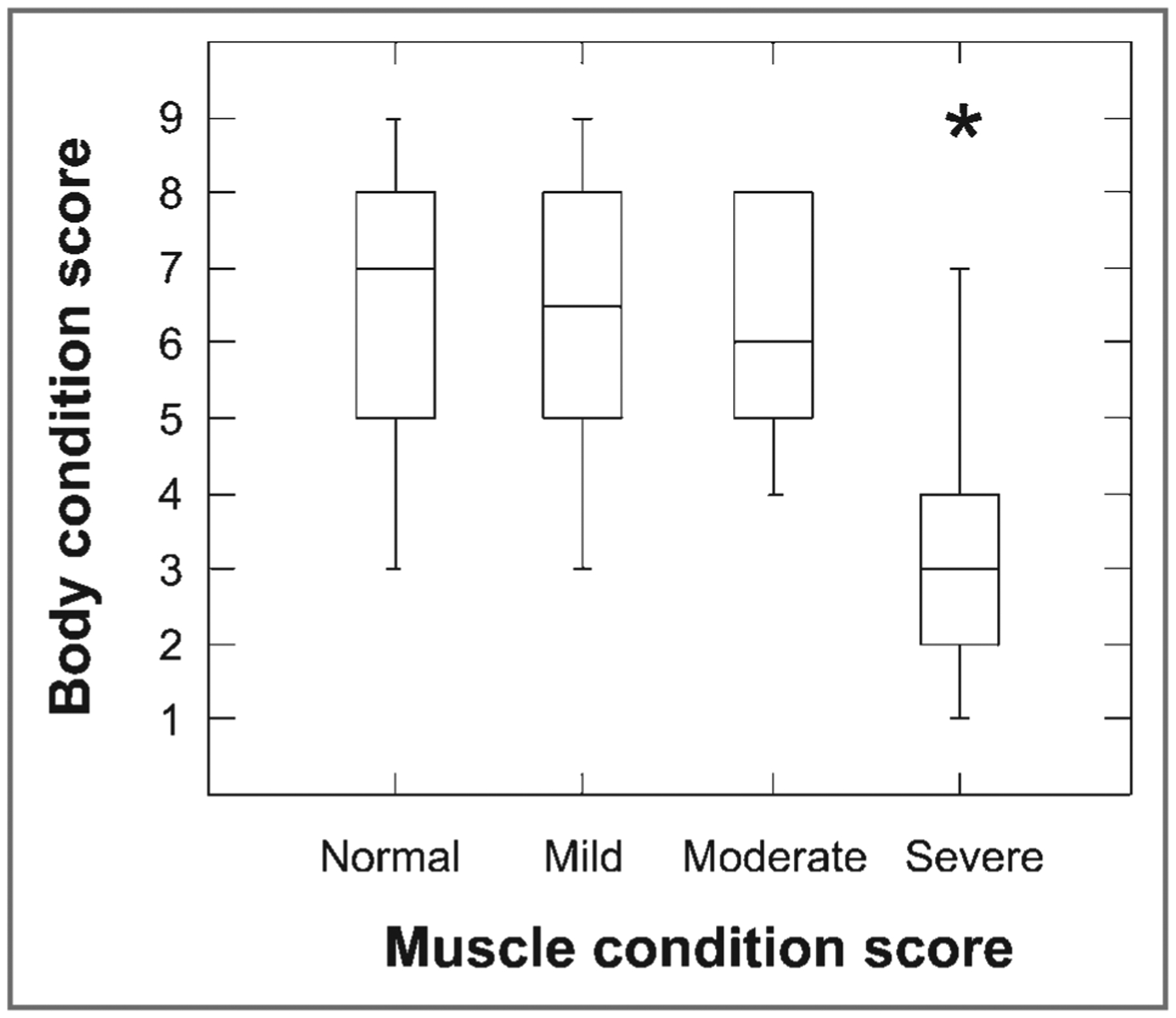
Box plots of BCS as a function of MCS (no [normal], mild, moderate, or severe muscle wasting) in 87 dogs. For each plot, the box represents the central 50% of the values. The horizontal line in each box represents the median, and the bars indicate the median ± 1.5 × interquartile range. *Outlier value (value > 1.5 times interquartile range)
Median T4 ratio was 1.2 (range, 0.2 to 3.5), and median T8 ratio was 1.3 (range, 0.2 to 3.7; Table 1). There were significant correlations between BCS and the T4 ratio (r = 0.86; P < 0.001) and between BCS and the T8 ratio (r = 0.84; P < 0.001). There was also a significant correlation between BCS and MCS (r = −0.47; P < 0.001). There were variable T4 and T8 ratios for all BCS categories, especially BCS of 9 (Figure 2). For dogs with a BCS of 9, the median T4 ratio was 2.58 (range, 1.48 to 3.45) and median T8 ratio was 2.68 (range, 1.16 to 3.70). There were significant but weak correlations between MCS and the T4 ratio (r = −0.39; P < 0.001) and between MCS and the T8 ratio (r = −0.37; P < 0.001). There was no interaction between MCS and the T4 ratio (P = 0.33) or between MCS and the T8 ratio (P = 0.53) in predicting BCS.
Table 1—
Radiographic measurements obtained for 87 dogs.
| Variable | Median (range) | Mean ± SD |
|---|---|---|
| Subcutaneous fat thickness (mm)* | 19.3 (3.7–55.1) | 22.0 ± 12.3 |
| Length of T4 (mm) | 18.8 (7.6–27.1) | 17.8 ± 4.5 |
| Width of T8 (mm) | 19.5 (8.6–31.3) | 19.2 ± 5.4 |
| Ratio of subcutaneous fat thickness to T4 length (T4 ratio) | 1.2 (0.2–3.5) | 1.3 ± 0.8 |
| Ratio of subcutaneous fat thickness to T8 width (T8 ratio) | 1.1 (0.2–3.7) | 1.2 ± 0.8 |
Measured on the dorsoventral or ventrodorsal radiographic projection at the level of the eighth rib head; for each dog, the mean value for measurements of the left and right sides was used.
Figure 2—
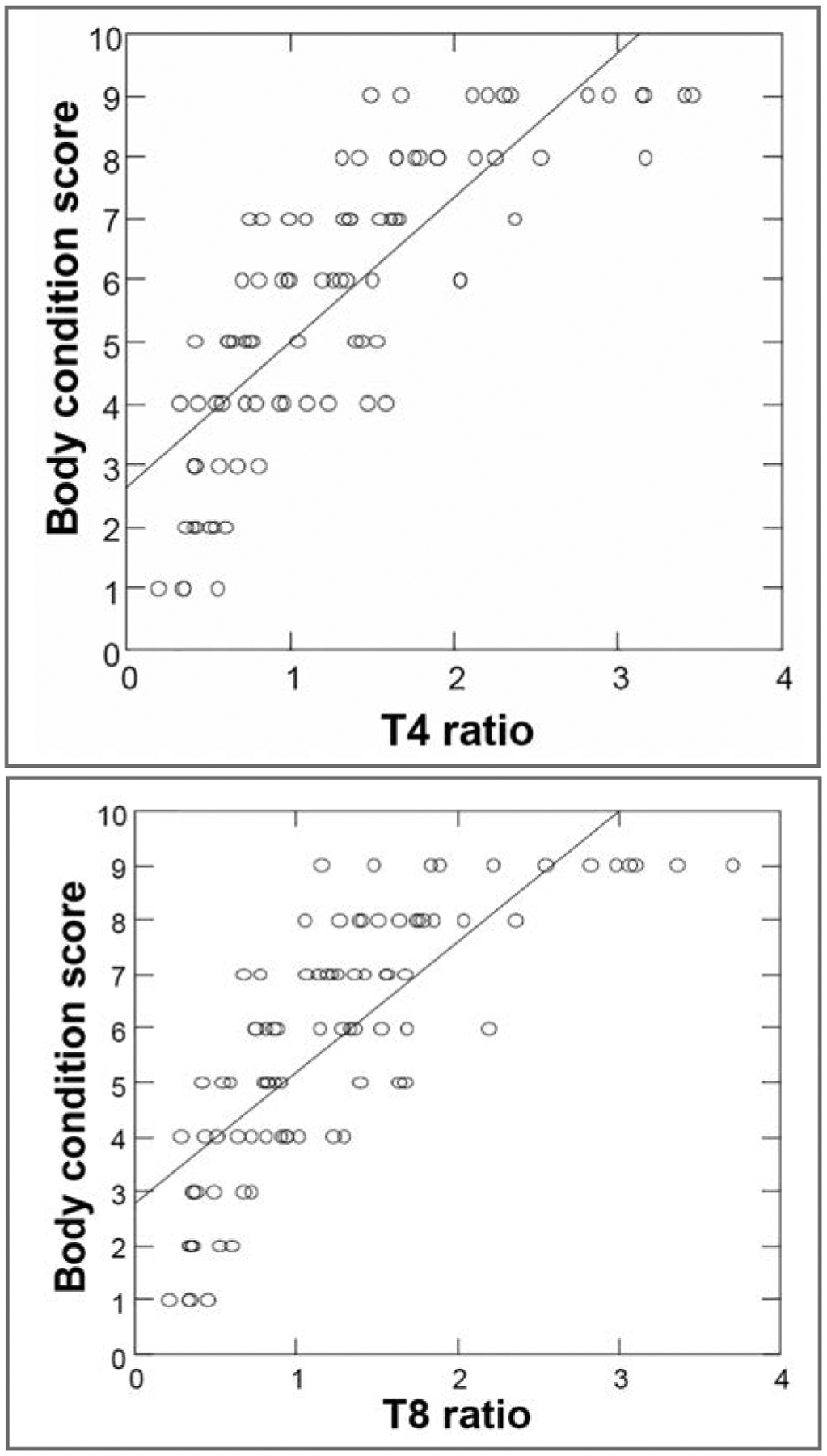
Scatterplots of (A) BCS versus T4 ratio (ie, subcutaneous fat thickness at the level of the eighth rib head on a dorsoventral or ventrodorsal radiographic view of the thorax divided by the length of the midbody of T4 on a lateral radiographic view; top) and (B) BCS versus T8 ratio (ie, subcutaneous fat thickness at the level of the eighth rib head on a dorsoventral or ventrodorsal radiographic view of the thorax divided by the width of the midbody of T8 on the same radiographic view; bottom) for 87 dogs. There were significant correlations between BCS and T4 ratio (r = 0.86; P < 0.001) and between BCS and T8 ratio (r = 0.84; P < 0.001). In each plot, the solid line represents the best-fit line for these data.
Regression equations to estimate BCS from the T4 and T8 ratios were developed:
The adjusted R2 values for these 3 equations were 0.73, 0.72, and 0.73, respectively. However, the P value was lower and SE was higher for the combined model (SE = 0.881) than for the models that incorporated the T4 ratio and T8 ratio alone (SE = 0.20 and 0.205, respectively). There were no significant relationships between the T4 ratio or T8 ratio and age, sex, or body weight.
Discussion
Results of the present study indicated that in this population, there was a significant association between BCS and subcutaneous fat thickness measured on thoracic radiographs. Specifically, there were strong correlations between BCS and the T4 ratio (ie, subcutaneous fat thickness at the level of the eighth rib head on a dorsoventral or ventrodorsal radiographic view of the thorax divided by the length of the midbody of T4 on a lateral radiographic view) and between BCS and the T8 ratio (ie, subcutaneous fat thickness at the level of the eighth rib head on a dorsoventral or ventrodorsal radiographic view of the thorax divided by the width of the midbody of T8 on the same radiographic view). These correlations warrant further investigation into the use of these measurements to estimate body composition in studies of obesity.
Muscle condition score was significantly, but weakly, associated with BCS and both the T4 and T8 ratios in the present study. However, there were no interactions between MCS and the T4 and T8 ratios in predicting BCS. Because MCS had a weaker relationship with BCS, we do not recommend using MCS to estimate BCS.
In the present study, we elected to use thoracic radiographic views to measure subcutaneous fat thickness because the ribcage is one of the main sites used for assessing BCS in dogs and the investigators hypothesized that fat thickness in this area would have the highest correlation with BCS. Also, this allowed for measurement of only subcutaneous and not visceral fat. The lateral thoracic wall also does not have large amounts of musculature and is not routinely used in muscle condition scoring, so it was also assumed that differences in muscle condition would minimally affect assessments of fat thickness at this location. Finally, thoracic radiography is a common clinical test in hospitalized dogs, which should make it useful for retrospective assignment of BCS.
In the present study, the ratio of subcutaneous fat thickness to fixed skeletal measurements was used to account for variations in dogs’ size. The length of T4 was selected owing to the ease of measurement, consistency of measurements obtained, minimal distortion of T4 on the lateral radiographic view (compared with distortion of other vertebrae), and accepted use of T4 in the vertebral heart score.7 The width of T8 was chosen because T8 is caudal enough that it is rarely obscured by thoracic limb skin folds, but cranial enough that it is consistently included in thoracic radiographs.
With the data for the 87 dogs of the present study, equations that can be used to estimate BCS were calculated. However, a prospective study with a large number of dogs would need to be performed to validate these equations and assess whether they can be used predict BCS. If this method is shown to be valid, it could potentially be useful to retrospectively estimate BCS in cases where scores were not assigned or not recorded or where an objective method is needed to reduce interobserver variability in assigned scores. Importantly, the lower P value and higher SE when both the T4 and T8 ratios were included in the regression equation suggested that these 2 variables were colinear. In essence, the T4 and T8 ratios were explaining much of the same variance in BCS. Therefore, including both of them in the regression equation was not useful.
While results of the present study supported an association between BCS and subcutaneous fat thickness, there were a number of limitations that are important to consider. Subcutaneous fat thickness was measured at the level of the eighth rib head because of the minimal amount of overlying musculature. However, measurements from the thoracic wall to the skin may have included some muscle, and this may have had some effect in reducing the accuracy of the measurement. Thus, additional research to develop more accurate measures of fat thickness is needed. Also, subcutaneous fat thickness was measured on the dorsoventral view in some dogs and on the ventrodorsal view in others owing to variability in the radiographic views available for each dog. Future studies to further evaluate the effect of this factor would be useful. Dogs of various breeds and underlying medical conditions were used in the present study, but additional investigation into the effect of these factors on these measurements is warranted. Another limitation was the inability to enroll 12 dogs from every BCS score category, and only 16 dogs were enrolled with BCSs of 1 (n = 4), 2 (6), and 3 (6) because of a paucity of dogs with these BCSs examined at our hospital.
Some dogs had to be excluded from the present study because collimation of the radiographic projection prevented measurement of the subcutaneous fat thickness. These were most often dogs of a larger body size owing to size limitations of the radiographic detector. Therefore, there may have been some bias in including dogs of smaller sizes, even though dogs weighing up to 58 kg were enrolled. Although the present study population represented a wide range of body sizes and BCSs, future prospective studies should take into account radiographic collimation.
An important finding in the present study was the wide variation in T4 and T8 ratios for dogs with a BCS of 9. With the 9-point BCS system, dogs that are obese and dogs that are morbidly obese are all assigned a score of 9, making it difficult to appreciate the variation in obesity severity among dogs with a BCS of 9. Future studies could build on these findings to further stratify the BCS of 9 as an aid in the assessment of severe obesity.
Acknowledgments
Dr. Linder’s faculty appointment was supported by Royal Canin USA.
The authors thank Robin Ruthazer, Associate Director of the Tufts CTSI Research Design Center/Biostatistics Research Center (supported by the National Center for Research Resources Grant No. UL1 RR025752 and the National Center for Advancing Translational Sciences, National Institutes of Health, Grant No. UL1 TR000073) for assistance with statistical analyses.
Abbreviations
- BCS
Body condition score
- MCS
Muscle condition score
Footnotes
Presented in abstract form at the 2012 American Academy of Veterinary Nutrition Clinical Nutrition and Research Symposium, New Orleans, May 2012.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Osirix Imaging Software, version 4.1.2, Antoine Rosset, Geneva, Switzerland.
Microsoft Excel, version 14.0.6123.5001, Microsoft Corp, Redmond, Wash.
Weasis DICOM viewer, version 1.1.2, Perennity Americas LLC, Miramar, Fla.
Systat, version 13.0, Systat, Chicago, Ill.
References
- 1.Ishioka K, Okumura M, Sagawa M, et al. Computed tomographic assessment of body fat in Beagles. Vet Radiol Ultrasound 2005;46:49–53. [DOI] [PubMed] [Google Scholar]
- 2.Mawby D, Bartges J, d’Avignon A, et al. Comparison of various methods for estimating body fat in dogs. J Am Anim Hosp Assoc 2004;40:109–114. [DOI] [PubMed] [Google Scholar]
- 3.Thatcher CD, Hand MS, Remillard RL. Small animal clinical nutrition: an iterative process In: Hand M, Thatcher, Remillard R, et al. , eds. Small animal clinical nutrition. 5th ed. Topeka, Kan: Mark Morris Institute, 2010;3–20. [Google Scholar]
- 4.Development Laflamme D. and validation of a body condition score system for dogs. Canine Pract 1997;22(4):10–15. [Google Scholar]
- 5.German AJ, Holden SL, Bissot T, et al. Use of starting condition score to estimate changes in body weight and composition during weight loss in obese dogs. Res Vet Sci 2009;87:249–254. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin K, Bartges J, Buffington T, et al. AAHA nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc 2010;46:285–296. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama H, Nakayama T, Hamlin RL. Correlation of cardiac enlargement as assessed by vertebral heart size and echocardiographic and electrocardiographic findings in dogs with evolving cardiomegaly due to rapid ventricular pacing. J Vet Intern Med 2001;15:217–221. [DOI] [PubMed] [Google Scholar]


