Abstract
Background
Selection of cancer treatment fundamentally relies on staging of the underlying malignancy. The aim of this study was to evaluate the feasibility and effectiveness of laparoscopic narrow band imaging (NBI) for operative staging and detection of occult peritoneal cancer metastases.
Methods
A randomized, controlled feasibility trial with crossover design evaluating adult patients with gastrointestinal or gynecologic malignancies who have a clinical indication for diagnostic laparoscopy was conducted. Twenty-three patients were randomized to white-light followed by NBI laparoscopy (n = 11) or NBI followed by white-light laparoscopy (n = 12) using the Olympus Evis Exera II system. Three patients were excluded from analysis.
Results
In all 20 study patients, the abdominal cavity was sufficiently illuminated. An enhanced contrast of microvasculature and organ surface pattern was appreciated. Eight of the 20 patients (40 %) were found to have metastases of the peritoneal surface. While NBI did not show any additional peritoneal lesions, 2 of the 63 suspicious-appearing nodules seen on white-light imaging were not visible on NBI (p = 0.50). The median diameter of all the nodules identified was 2 mm (range 1–50 mm) and was identical with each method.
Conclusions
The information from this feasibility study demonstrated that NBI provides adequate illumination of the abdominal cavity and a unique contrast that enhances microvasculature and architectural surface pattern. The results suggest that NBI laparoscopy is not superior in detecting peritoneal metastases compared to standard white-light laparoscopy, but might provide a technology that could be applied for other abdominal pathologies.
Keywords: GI; Cancer, Gynae; Cancer, Pancreato bilio; Cancer, Imaging & VR; Technical
For gastrointestinal and gynecologic malignancies, staging dictates the treatment strategy and defines the role of operative treatment. Despite the improvements in available staging tools, including cross-sectional radiographic imaging and staging laparoscopy, the accuracy of staging varies tremendously, with “under-staging” still being a common problem for many malignancies such as pancreatic, gastric, or gallbladder carcinomas [1–5]. This is reflected by the observation that patients with these particular malignancies have distant progression of cancer in up to 40 % of patients within 12 months after an R0 resection with curative intent, with the peritoneum being a common site for initial recurrence [2, 4, 6]. Since the vast majority of these peritoneal metastases were likely present at the time of operation, but too small to be detected with conventional methods, there is a crucial need for technologic improvements.
Conventional white-light laparoscopy or white-light imaging only detects peritoneal metastases of a certain size. Narrow band imaging (NBI), a form of spectral imaging, works by emitting light bands at a specified wavelength aimed at maximal light absorption of the target tissue. The captured images, therefore, provide a negative contrast of the target tissue compared to the surrounding tissue, resulting in augmented signal-to-background ratios and possibly improved detection of small peritoneal metastases. The only commercially available FDA-approved NBI laparoscope, which was tested in this study, uses light bands that target tissue rich in hemoglobin, such as blood vessels. Since neoplasms frequently promote neovascularization, this NBI system has been proposed for operative cancer staging [7]. The purpose of this study was to determine the feasibility of NBI laparoscopy for detection of peritoneal metastases in adult patients with gastrointestinal and gynecologic malignancies.
Patients and methods
Study design, recruitment, and eligibility criteria
The study was a randomized, controlled, crossover feasibility trial conducted on patients who underwent staging laparoscopy for gastrointestinal or gynecologic malignancies. Adult patients (≥18 years of age) who had been evaluated at Lahey Hospital & Medical Center, Burlington, MA, for a suspected or confirmed diagnosis of gastrointestinal or gynecologic malignancy and were planned to undergo an elective staging laparoscopy as part of standard treatment were recruited. Patients with clinical contraindication to staging laparoscopy, severe cardiopulmonary comorbidities demanding minimization of operative time, pregnancy, impaired capacity to make informed medical decisions, or undergoing an emergent operation were excluded. An informed consent was obtained for each patient. The study proposal was approved by the Lahey Clinic Institutional Review Board and registered on clinicaltrials.gov (NCT01944930).
Patients were randomized to undergo standard white-light laparoscopy followed by NBI laparoscopy (group A) or NBI laparoscopy followed by white-light laparoscopy (group B) using block randomization to prevent imbalance during the study (SAS 9.3 computer software, SAS Institute Inc, Cary, NC).
The primary endpoint was the feasibility of NBI laparoscopy. The secondary endpoint was the rate of detection of peritoneal metastases with NBI laparoscopy compared to routine white-light laparoscopy.
White-light and NBI laparoscopy
For both white-light laparoscopy and NBI laparoscopy, a standard sterilized 10-mm rigid laparoscope (Olympus EndoEYE surgical videoscope with Olympus Evis Exera II CV-180 video processor and Olympus Evis Exera II CLV-180 light source, Olympus, Center Valley, PA) was used. The peritoneal cavity was inspected in standard white-light imaging (unfiltered xenon light) and NBI modes (filtered xenon light with emission bands at 415 nm and at 540 nm). Image resolution was 1080i. Surgeons were not tested for color vision deficiencies.
Image classification
Images of the peritoneal surface were classified at the time of laparoscopy as normal peritoneum, non-suspicious-appearing mass/nodule, or suspicious-appearing mass/nodule for each imaging modality independently using best medical practice. For any masses/nodules, the size of the lesion was estimated using routine laparoscopic instruments as reference and its shape was described. Histopathology biopsies were obtained of all suspicious-appearing lesions after completion of the white-light and NBI laparoscopy using routine laparoscopic biopsy methods in accordance with best medical practice.
Statistical methods
Demographic and clinical characteristics of the samples were summarized using descriptive statistics. The McNemar and sign tests were utilized for univariate analyses comparing white-light to NBI laparoscopy to account for the pairing between methods on a given lesion. This study was a feasibility trial, and therefore, the sample size was purposefully kept small and not meant to be powered to detect a clinically meaningful difference.
Results
From March 2013 to September 2014, 23 patients met eligibility criteria and were included in the study. Eleven patients were randomized to white-light laparoscopy followed by NBI laparoscopy (group A), and 12 patients were randomized to NBI laparoscopy followed by white-light laparoscopy (group B). One patient from group A was excluded due to unavailability of the NBI light source for the particular operation, and two patients from group B were excluded due to postoperative non-cancer diagnosis, both patients with a gallbladder mass determined to be benign after resection. This resulted in a study cohort of 20 patients for analysis, in whom the results for white-light imaging were compared to NBI (Fig. 1).
Fig. 1.
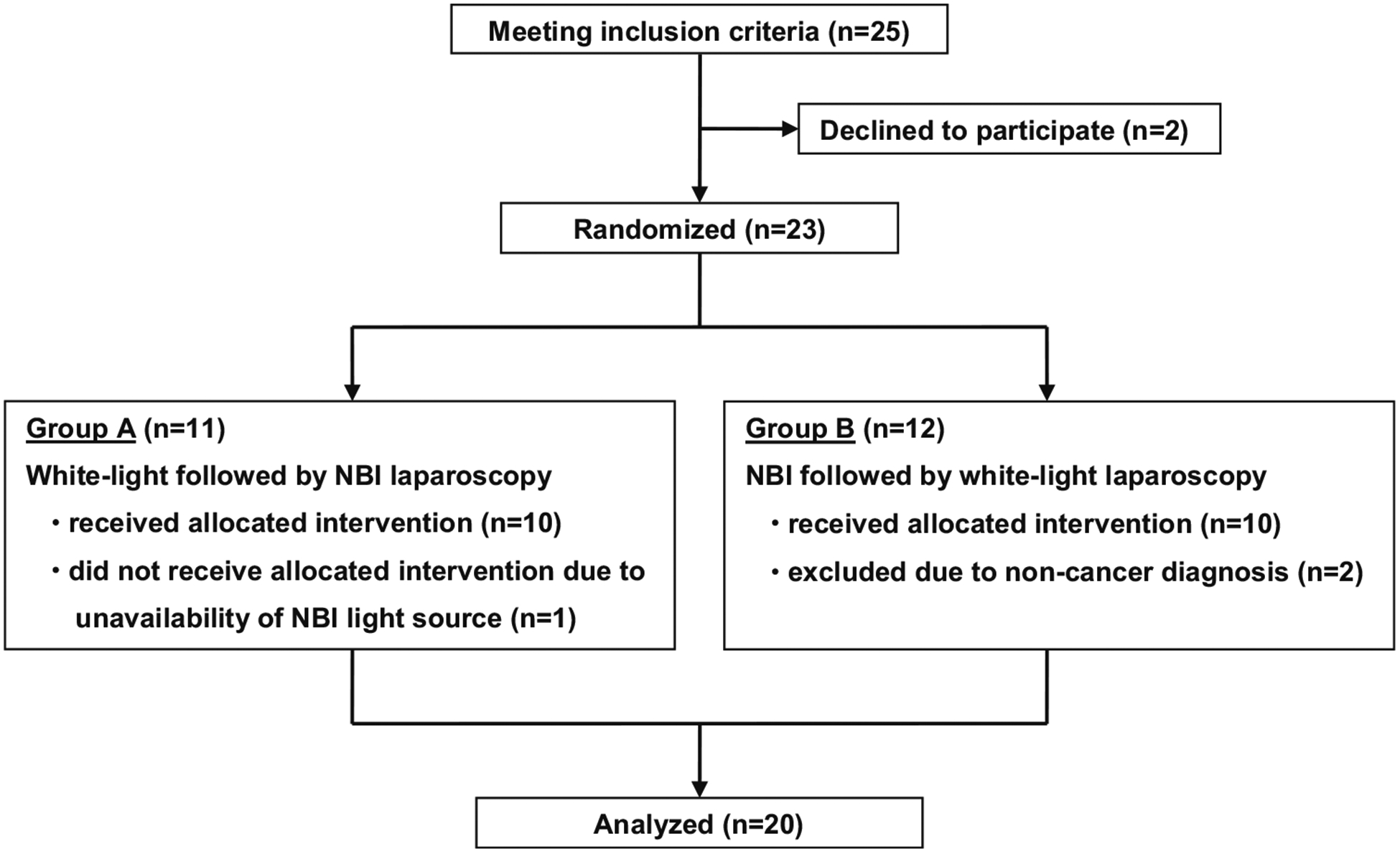
Patient selection and randomization
Patient demographics and preoperative clinical findings of the 20 study patients are listed in Table 1. All 20 underwent CT scan of the abdomen for radiographic staging, while 13 underwent additional MRI, PET-CT, or ultrasonography of the abdomen. Staging radiographic studies revealed a single peritoneal metastasis in one patient. A total of four surgeons performed the laparoscopies, with 50 % being performed by a single surgeon. For each laparoscopic image, interpretation was conducted by the surgeon according to best medical practice. In all patients, the abdominal cavity was sufficiently illuminated with NBI. An enhanced contrast of microvasculature and organ surface pattern was appreciated with NBI compared to white-light imaging (Fig. 2). In the 20 study patients, the median time to complete the white-light laparoscopy was 121 (range 60–224) seconds compared to 113 (range 60–246) seconds for NBI laparoscopy (p = 0.36). The entire greater sac of the abdominal cavity was inspected in 17 patients. Limited view due to adhesions within a single quadrant was experienced in three patients. No intra-operative or in-hospital complications related to the staging laparoscopy were identified (Table 2).
Table 1.
Patient demographics and clinical findings
| Median age | 63 (31–82) years |
|---|---|
| Gender | 11 males |
| 9 females | |
| Primary neoplasm | |
| Pancreatic adenocarcinoma | 8 patients |
| Cholangiocarcinoma | 4 patients |
| Gastric carcinoma | 3 patients |
| Small bowel carcinoma | 2 patients |
| Gallbladder adenocarcinoma | 1 patient |
| Rectal adenocarcinoma | 1 patient |
| Endometrial adenocarcinoma | 1 patient |
| Neoadjuvant treatment | 20 % |
| Preoperative radiographic stagea | |
| Stage I | 12 patients |
| Stage II | 2 patients |
| Stage III | 2 patients |
| Stage IV | 4 patients |
| Preoperative radiographic peritoneal metastases | 5 % |
AJCC cancer stage (seventh edition)
Fig. 2.
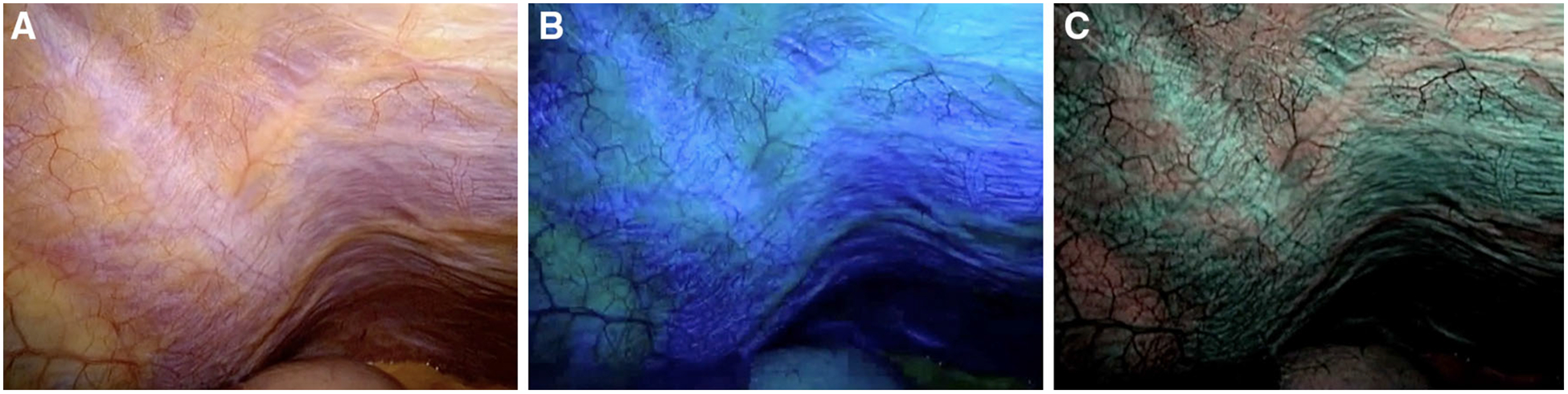
Peritoneal surface seen with white light (A), blue light (B), and NBI with blue/green light (C)
Table 2.
Peritoneal nodules seen by white-light versus NBI laparoscopy
| White light (n = 88) | NBI (n = 86) | p value | |
|---|---|---|---|
| Image patdology classification (number of lesions) | |||
| Suspicious-appearing | 63 | 61 | 0.50 |
| Biopsy malignant | 12 | 12 | N/A |
| Biopsy benign | 10 | 10 | N/A |
| Biopsy not performed in patients with known peritoneal metastases | 34 | 32 | 0.50 |
| Biopsy not performed in patients without known peritoneal metastases | 7 | 7 | N/A |
| Non-suspicious-appearing | 25 | 25 | N/A |
| Not visualized | 0 | 2 | 0.50 |
| Median estimated diameter | 2 (1–50) mm | 2 (1–50) mm | N/A |
| Shape of lesion (number of lesions) | 0.99 | ||
| Round and flat | 32 | 32 | |
| Round and raised | 31 | 30 | |
| Irregular and flat | 11 | 11 | |
| Irregular and raised | 14 | 13 |
N/A not available since results were identical in group comparison
A total of 63 suspicious-appearing nodules were seen on white-light imaging [median 1 (range 0–17) per patient] (Fig. 3). The median diameter of nodules in this subgroup was 2 mm (range 1–50 mm). No additional suspicious-appearing nodules were seen using NBI, but two suspicious-appearing nodules in patients with later proven peritoneal metastases were missed with NBI (both 3 mm in diameter). The suspicious-appearing nodules seen on NBI were not statistically different in number (white light 63 vs. NBI 61, p = 0.50) or shape (p = 0.99) to those seen using white-light imaging. The shape classification between each method only differed for one nodule. The estimated diameter between each method was identical for each nodule. Twenty-two representative suspicious-appearing nodules were biopsied confirming metastases in 12 and benign disease in 10.
Fig. 3.
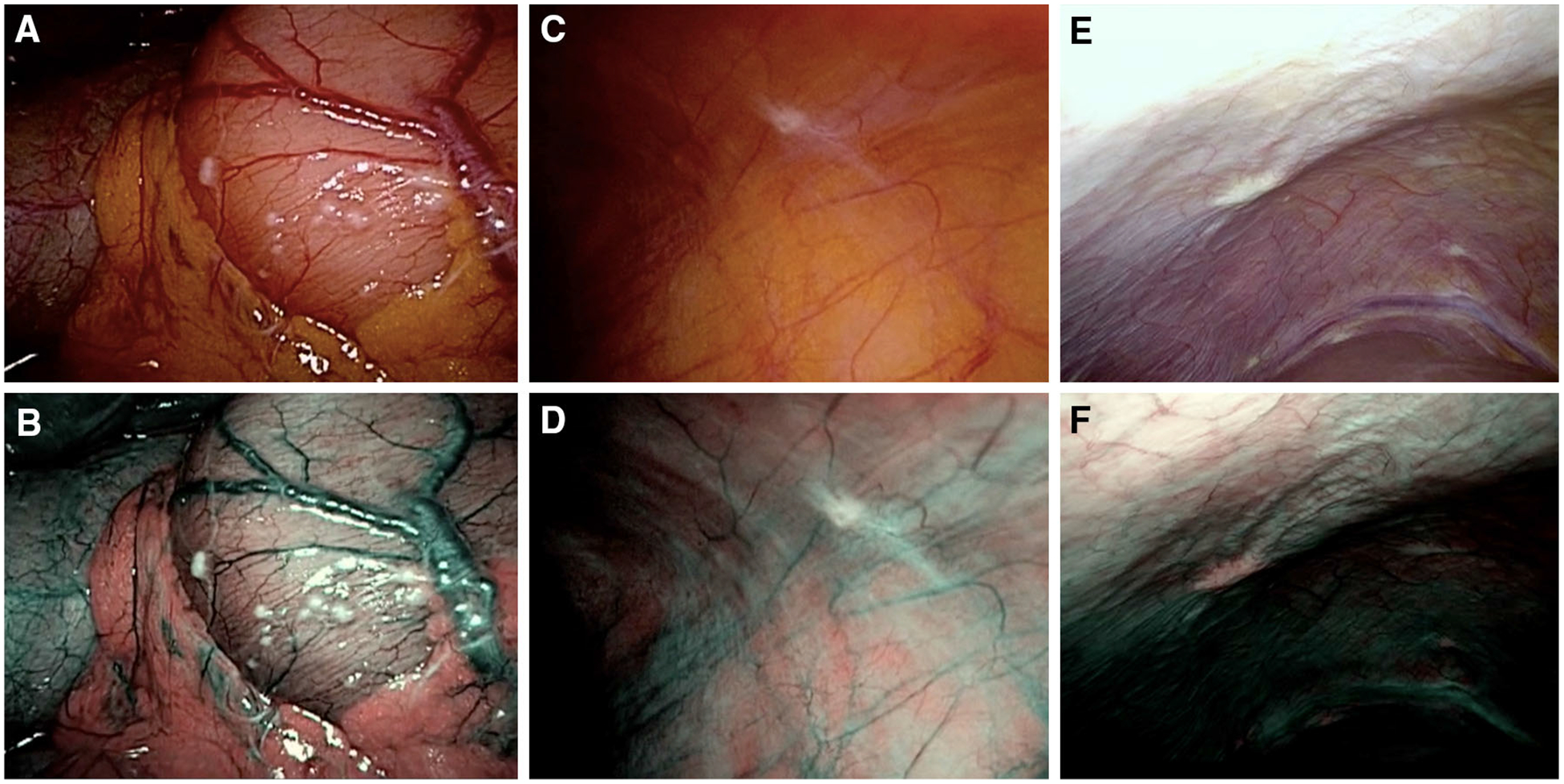
Imaging using white-light (top row) and NBI (bottom row) laparoscopy showing peritoneal metastases equally visualized with either methods in patients with gastric signet ring cell carcinoma (A, B), gastric adenocarcinoma (C, D), and pancreatic adenocarcinoma (E, F)
A total of 25 non-suspicious-appearing nodules were seen [median 1 (range 0–6) per patient], 12 of them in patients with known peritoneal metastases. The median diameter of these non-suspicious-appearing nodules was 2 mm (range 1–5 mm). White-light imaging and NBI detected the same non-suspicious-appearing nodules; the estimated diameter and shape classifications were identical for each method for all nodules. None of the non-suspicious-appearing nodules were biopsied (Fig. 3).
During the operation, 4 patients (20 %) had no apparent peritoneal nodules, 8 patients (40 %) had at least one benign nodule, and 8 patients (40 %) were diagnosed with metastatic disease, including four patients with a new diagnosis. The number of patients in each category was identical for white-light imaging and NBI.
Discussion
This study assessed the feasibility of NBI laparoscopy for detection of early, otherwise occult peritoneal metastases in a limited set of patients with gastrointestinal and gynecologic malignancies. The information from this feasibility study demonstrated that NBI with the Olympus Evis Exera II system provides adequate illumination of the abdominal cavity and a unique contrast that enhances microvasculature, potentially even organ perfusion, and architectural surface patterns. However, the results suggest that NBI laparoscopy might not be superior in detecting peritoneal metastases compared to standard white-light laparoscopy. Future studies unlikely will demonstrate NBI technique to be an innovative screening test that will surpass the sensitivity of white-light laparoscopy for detection of peritoneal metastases.
NBI with the Olympus Evis Exera II system illuminates tissue with filtered xenon light that provides two emission bands limited to 415 and 540 nm, respectively, wavelengths where hemoglobin has maximal light absorption. The resulting images do provide a negative contrast of the microvasculature, possibly organs with greater degree of blood perfusion, and disruption of peritoneal surface pattern, features that are thought to distinguish peritoneal metastases from healthy peritoneum. In clinical use, NBI is selectively utilized to detect premalignant lesions in esophagoscopy, colonoscopy, and urinary cystoscopy [7]. Due to encouraging results in endoscopy, NBI has been tested for cancer staging of the pleural cavity in humans in two small non-randomized feasibility trials [8, 9]. Using NBI thoracoscopy, these trials showed enhanced visualization of the vascularity of pleural implants evaluating 73 and at least 19 lesions in 45 and 26 patients, respectively. Similar to this study, NBI thoracoscopy did not reveal any additional lesions when compared to white-light imaging. With regard to the use of NBI laparoscopy for peritoneal cancer staging, there are three single-case reports published suggesting similar results to this study [7, 10, 11]. Kikuchi et al. [12] recently published a retrospective study on 26 patients with gastric cancer who were found to have at least 37 peritoneal lesions that were examined with white-light and NBI laparoscopy. The video images of the laparoscopy were examined retrospectively. The study focused on the specificity of differentiating benign from malignant peritoneal nodules, but did not provide data on the rate of visualized lesions for each method. It, however, suggested that NBI is seemingly better at detecting abnormal microvascular findings suggestive of the presence of metastases. Yet, no data were described whether NBI is clinically superior in detecting peritoneal lesions. Due to its theoretic properties of detecting metastases and due to the encouraging results in identifying cancers during endoscopy, expectations were raised for this technique.
Given the images provided, the study was able to eliminate concerns of poor light intensity for optimal imaging and concerns of a learning curve of interpreting the provided images. Although this imaging method generates a darker emission than traditional white-light imaging, the system’s automatic brightness adjustment corrects for most of this shortcoming. In all patients, the abdominal cavity seemed to be sufficiently illuminated. The greatest limitation for this study is its sample size, which was purposefully kept low for this feasibility trial. To hypothetically calculate the necessary sample size to provide sufficient statistical power, we assume that white-light imaging will detect peritoneal metastases in 10 % of the gastrointestinal and gynecologic cancer population; the rate is higher in this study due to the presence of higher-risk patients. A clinically meaningful difference could be provided by increasing the detection rate to 20 %. Assuming a two-sided test with α = 0.05 and 80 % power, the number of observations needed is 215 and therefore greater than the 174 observations made in this study. Therefore, the study is limited in its ability to reliably determine the effectiveness of NBI. Yet, the results appear obvious in regard to NBI appearing not to be superior to white-light imaging. In addition, since patients had to undergo two subsequent imaging modalities with a risk of coercion during interpretation of the second imaging modality depending on the results from the first modality, confirmation bias is a potential factor influencing the results. To minimize this bias, the order of the crossover was randomized. Blinding would have been preferred to minimize observer bias, but could not be performed due to the recognizable appearance of the imaging modality. The concern for optical heterogeneity due to a mix of different cancers with different molecular compositions was likely lessened through the study’s crossover design.
NBI is based on a novel technology developed relatively recently for imaging of neoplasms in other areas of health care. This new technology had been thought of as a hopeful candidate to not only provide good detection rates for larger peritoneal metastases, but also be able to screen for early, small, otherwise occult metastases, something that is thought to be essential for treatment planning in patients with gastrointestinal and gynecologic malignancies. The results of this study provided material for future improvements and modifications of NBI and spectral imaging laparoscopy techniques. Successful introduction of some form of image-enhanced laparoscopy technique into the clinical setting for cancer staging is expected for the foreseeable future and has the potential for significant improvements in treatment selection and potentially affecting survival rates through earlier and more focused treatment of distant metastases.
Acknowledgments
The study is registered in ClinicalTrials.gov identifier: NCT01944930. This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number UL1 TR000073, through Tufts Clinical and Translational Science Institute (CTSI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures Drs. Schnelldorfer, Jenkins, Birkett, Wright, Price, and Georgakoudi have no conflicts of interest or financial ties to disclose.
References
- 1.Hariharan D, Constantinides VA, Froeling FE, Tekkis PP, Kocher HM (2010) The role of laparoscopy and laparoscopic ultrasound in the preoperative staging of pancreatico-biliary cancers: a meta-analysis. Eur J Surg Oncol 36(10):941–948 [DOI] [PubMed] [Google Scholar]
- 2.Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, Gullerud RE, Donohue JH, Nagorney DM, Farnell MB (2008) Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg 247(3):456–462 [DOI] [PubMed] [Google Scholar]
- 3.de Graaf GW, Ayantunde AA, Parsons SL, Duffy JP, Welch NT (2007) The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol 33(8):988–992 [DOI] [PubMed] [Google Scholar]
- 4.Roviello F, Marrelli D, de Manzoni G, Morgagni P, Di Leo A, Saragoni L, De Stefano A, Italian Research Group for Gastric Cancer (2003) Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 90(9):1113–1119 [DOI] [PubMed] [Google Scholar]
- 5.Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR (2002) Staging laparoscopy in patients with extrahepatic biliary carcinoma. Ann Surg 235(3):392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho SY, Kim SH, Park SJ, Han SS, Kim YK, Lee KW, Lee WJ, Woo SM, Kim TH (2010) Adjuvant chemoradiation therapy in gallbladder cancer. J Surg Oncol 102(1):87–93 [DOI] [PubMed] [Google Scholar]
- 7.Schnelldorfer T (2012) Image-enhanced laparoscopy: a promising technology for detection of peritoneal micrometastases. Surgery 151(3):345–350 [DOI] [PubMed] [Google Scholar]
- 8.Ishida A, Ishikawa F, Nakamura M, Miyazu YM, Mineshita M, Kurimoto N, Koike J, Nishisaka T, Miyazawa T, Astoul P (2009) Narrow band imaging applied to pleuroscopy for the assessment of vascular patterns of the pleura. Respiration 78(4):432–439 [DOI] [PubMed] [Google Scholar]
- 9.Schonfeld N, Schwarz C, Kollmeier J, Blum T, Bauer TT, Ott S (2009) Narrow band imaging (NBI) during medical thoracoscopy: first impressions. J Occup Med Toxicol 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanfani F, Gallotta V, Rossitto C, Fagotti A, Scambia G (2010) Narrow band imaging in borderline ovarian tumor. J Minim Invasive Gynecol 17(2):146–147 [DOI] [PubMed] [Google Scholar]
- 11.Fanfani F, Rossitto C, Fagotti A, Gallotta V, Gagliardi ML, Scambia G (2011) Narrow-band imaging in laparoscopic management of cervical carcinoma. J Minim Invasive Gynecol 18(2):146–147 [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi H, Kamiya K, Hiramatsu Y, Miyazaki S, Yamamoto M, Ohta M, Baba S, Konno H (2014) Laparoscopic narrow-band imaging for the diagnosis of peritoneal metastasis in gastric cancer. Ann Surg Oncol 21(12):3954–3962 [DOI] [PubMed] [Google Scholar]


