Abstract
To determine whether the permeation capacity and analgesic efficacy of articaine (ATC) could be increased and cytotoxicity decreased by encapsulation in poly(ɛ-caprolactone) nanocapsules (ATCnano), aiming at local or topical anesthesia in dentistry. Cellular viability was evaluated (using the MTT test and fluorescence microscopy) after 1 h and 24 h exposure of HaCaT cells to ATC, ATCnano, ATC with epinephrine (ATCepi), and ATC in nanocapsules with epinephrine (ATCnanoepi). The profiles of permeation of 2% ATC and 2% ATCnano across swine esophageal epithelium were determined using Franz-type vertical diffusion cells. Analgesic efficacy was evaluated with a von Frey anesthesiometer in a postoperative pain model in rats, comparing the 2% ATC, 2% ATCnano, 2% ATCepi, and 2% ATCnanoepi formulations to 4% ATCepi (a commercially available formulation). We show that use of the nanocapsules decreased the toxicity of articaine (P<0.0001) and increased its flux (P = 0.0007). The 2% ATCepi and 4% ATCepi formulations provided higher analgesia success and duration (P<0.05), compared to 2% ATC, 2% ATCnano, and 2% ATCnanoepi. Articaine-loaded poly(ɛ-caprolactone) nanocapsules constitute a promising formulation for intraoral topical anesthesia (prior to local anesthetic injection), although it is not effective when injected in inflamed tissues for pain control, such as irreversible pulpitis.
Introduction
Articaine is an amide local anesthetic containing a thiophene ring and an ester radical in its chemical structure [1]. It is widely used in dentistry due to the fast onset and appropriate duration of analgesia, good diffusibility, and short plasma half-life, compared to other amide-type anesthetics [2].
Articaine has been shown to provide a better quality of pulpal anesthesia in inflammatory hypernociception, such as irreversible pulpites [3,4]. In addition, it is effective in lower molar pulpal anesthesia after its buccal infiltration, which is usually achieved using blockade techniques [4–7]. However, there have reports of paresthesia and transitory post-operative sensitivity associated with the use of articaine, which could be related to the concentration in which the compound is used (4%) [8].
In the last decade, there has been a significant increase in the number of studies concerning local anesthetics associated with bioactive molecules and/or sustained release systems, with the aim of reducing drug toxicity and improving bioavailability [9–11]. Among such systems, polymeric nanoparticles have attracted great interest. These consist of vesicles with diameter smaller than 1 μm, which can be classified as nanospheres or nanocapsules, according to their composition and structural organization. Nanospheres are composed of a dense matrix, while nanocapsules consist of a polymeric coat surrounding an oily nucleus. The drug can be dissolved in the nucleus, dispersed in the matrix, or adsorbed on the particle surface [12–14].
Previous results have shown that polymeric nanoparticles are a promising drug release system for local anesthetics. Poly(ε-caprolactone) nanospheres containing lidocaine presented lower toxicity and improved analgesic action, compared to plain lidocaine [15]. Formulations of articaine-loaded poly(ɛ-caprolactone), poly(ethylene glycol)-poly(ɛ-caprolactone), and alginate/chitosan nanocapsules showed high encapsulation efficiencies and lower cytotoxicity, compared to free local anesthetic solution [16,17]. More recently, hydrogels of articaine-loaded poly(ɛ-caprolactone) nanocapsules were found to present increased in vitro permeability across a cellulose membrane [18] which could result in better clinical performance, since a high correlation between in vitro permeation parameters and in vivo intraoral topical anesthesia effect was previously found [19,20]. In addition, drug delivery systems can act as permeation enhancers and increase transdermal delivery [21,22].
Therefore, in this work, evaluation was made of whether encapsulation of articaine in poly(ε-caprolactone) nanocapsules could decrease its cytotoxicity, and increase permeability and analgesic efficacy, focusing on its possible use in local or topical anesthesia in dentistry.
Materials and methods
Experimental design
The effect of encapsulation in poly(ε-caprolactone) nanocapsules on the cytotoxicity of articaine was evaluated in vitro in viability tests using human keratinocyte (HaCaT) cells, employing the MTT colorimetric assay and fluorescence imaging. The permeation of articaine across swine esophageal epithelium was evaluated using Franz-type vertical diffusion cells. The analgesic efficacy of articaine was determined using a postoperative pain model in rats.
The formulations tested and the respective controls for the cell culture viability tests were as follows: articaine (ATC), articaine with 1:200,000 epinephrine (ATCepi), articaine-loaded poly(ε-caprolactone) nanocapsules (ATCnano), articaine-loaded poly(ε-caprolactone) nanocapsules with 1:200,000 epinephrine (ATCnanoepi), cell culture medium (DMEM), DMEM with epinephrine, DMEM with poly(ε-caprolactone) nanocapsules, and DMEM with poly(ε-caprolactone) nanocapsules and epinephrine. Epinephrine (at a final concentration of 1:200,000) was added to the formulations immediately before the experiment. All the formulations were diluted in DMEM immediately before use.
Articaine at 2% (2% ATC) and 2% articaine-loaded poly(ε-caprolactone) nanocapsules (2% ATCnano) were evaluated using the in vitro permeability model.
The following formulations were evaluated in vivo in terms of analgesic efficacy: 2% articaine-loaded poly(ε-caprolactone) nanocapsules (2% ATCnano), 2% articaine-loaded poly(ε-caprolactone) nanocapsules with 1:200,000 epinephrine (2% ATCnanoepi), 2% articaine (2% ATC), 2% articaine with 1:200,000 epinephrine (2% ATCepi), and 4% articaine with 1:200,000 epinephrine (4% ATCepi). The negative controls were poly(ε-caprolactone) nanocapsules, poly(ε-caprolactone) nanocapsules with 1:200,000 epinephrine, 0.9% sodium chloride solution, and 0.9% sodium chloride solution with 1:200,000 epinephrine. The 4% ATCepi formulation was tested because this is the clinically available formulation of articaine associated with epinephrine. The epinephrine bitartrate salt was purchased from Sigma-Aldrich (St. Louis, MO, USA). Epinephrine was added to the formulations immediately before the experiment.
Preparation of the formulations
Articaine base (in the neutral form) was obtained as described previously [18]. Briefly, articaine hydrochloride (donated by DFL Ind. Com. Ltda., Rio de Janeiro, Brazil) was dissolved in water, the pH was adjusted to pH 8.5, and the aqueous phase was extracted with ethyl acetate. The organic phase was filtered, dried, and evaporated. The oil obtained was crystallized at -18 oC.
Nanocapsule formulations were prepared by the oil-in-water emulsion/solvent evaporation method [23] based on the composition determined earlier [17]. Briefly, two solutions, one containing 400 mg of poly(ε-caprolactone) (PCL, Sigma-Aldrich, St. Louis, MO, USA) and 200 mg of Myritol® 318 (donated by Chemspecs Comercio e Representaçoes Ltda., São Paulo, Brazil) in 20 mL of chloroform (Labsynth, Diadema, São Paulo, Brazil), and another with 200 mg articaine base dissolved in 10 mL of acetone (Labsynth, Diadema, São Paulo, Brazil), were mixed and sonicated for 1 min at 100 W. This pre-emulsion was added to 50 mL of aqueous solution containing 150 mg of polyvinyl alcohol surfactant (PVA, Sigma-Aldrich, St. Louis, MO, USA), with sonication for 8 min to form the emulsion. The organic solvent was removed in a rotary evaporator and the emulsion was concentrated to a final volume of 10 mL, containing 20 mg/mL articaine [15]. The volume was then adjusted to 18.178 mL and 13.516 mg of articaine hydrochloride were added, in order to obtain an encapsulation efficiency of approximately 50%.
This encapsulation was chosen because a short onset was desired for clinical use, which is not achieved with a higher anesthetic encapsulation. This was confirmed in the pilot study, where all the animals presented lip numbness at the first analgesia assessment (5 min after the injection), using around 50% encapsulation of articaine in poly(ε-caprolactone) nanocapsules.
Characterization of articaine-loaded poly(ε-caprolactone) nanocapsules
The mean diameter (MD), polydispersion index (PI), and pH of the articaine-loaded poly(ε-caprolactone) nanocapsules were evaluated. The MD and PI were determined by dynamic light scattering, using a ZetaSizer Nano ZS 90 instrument (Malvern Instruments, UK). The measurements (in triplicate) were performed at 25°C, with a fixed angle of 90°, using diluted samples (about 1.0x10-3 mol/L) [15–18].
The pH values of the nanocapsules in aqueous suspensions were determined (in triplicate) using a previously calibrated pH meter (Thermo Electron Orion® Model 290A+, Sigma). The stability results for this formulation have been presented previously [18].
The encapsulation efficiency (%EE) of articaine was calculated by subtracting the non-encapsulated (free) local anesthetic from the total local anesthetic present in the initial solution. The amount of non-encapsulated articaine was determined by ultrafiltration (Microcon 10 kDa pore size filter units, Millipore) and centrifugation (14,000 g for 25 min), followed by HPLC quantification, as described below (Articaine analysis). All analyses were performed in triplicate.
Articaine analysis
Articaine was quantified by high performance liquid chromatography (HPLC), employing a Varian ProStar 325 instrument (Agilent Technologies, Lake Forest, California) equipped with an isocratic PS 210 pump, a UV-Vis detector, and an automatic injector, according to a previously validated method [18]. Galaxy Workstation 410 software (Agilent Technologies, Lake Forest, California) was used for data collection.
Briefly, the chromatographic conditions consisted of a C18 reversed-phase column (5 μm, 110 Å, 150 x 4.6 mm; Phenomenex Gemini), a mobile phase of monobasic sodium phosphate (0.02 mol/L, pH 3.0, adjusted with phosphoric acid) and acetonitrile (88:12, v/v), at a flow rate of 1.5 mL/min, and a controlled temperature of 35°C. The mobile phase was filtered and degassed. The injection volume was 100 μL and the detector wavelength was set at 274 nm. Under these conditions, the limits of detection and quantification were 0.007 and 0.023 μg/mL, respectively (R2 = 0.9992).
The HPLC-grade acetonitrile employed in the chromatographic analyses was obtained from JT Baker (Phillipsburg, NJ, USA).
In vitro models
Cell viability assays
Cell culture. Immortalized human keratinocytes (HaCaT) were chosen due to their good proliferative capacity in culture and their use in previous in vitro cytotoxicity studies of local anesthetics and drug delivery systems [24,25]. The HaCaT cells (BCRJ 0100, Rio de Janeiro Cell Bank, Rio de Janeiro, RJ, Brazil) were grown in 75 cm2 TPP® bottles (Techno Plastic Products AG, Trasadingen, Switzerland) containing Dulbecco’s Modified Eagle Medium (DMEM, Vitrocell, Campinas, SP, Brazil) supplemented with 10% (v/v) fetal bovine serum (Vitrocell), 10,000 U/mL penicillin (Vitrocell), and 100 μg/mL streptomycin (Vitrocell), at 37°C, in a humid atmosphere with 5% CO2 [24,25].
MTT colorimetric assay. Semi-confluent cells were seeded into 96-well tissue plates, at approximately 1x104 cells/well, followed by incubation for 48 h, at 37°C, in a humid atmosphere with 5% CO2. Subsequently, the cells were exposed to ATC, ATCepi, ATCnano, and ATCnanoepi, at concentrations in the range from 0.06% to 1%, for 1 h or 24 h. The half-maximal inhibitory concentration (IC50) was determined for all formulations and exposure periods.
Cell viability was evaluated by incubation with 0.3 mg/mL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich, St. Louis, MO, USA) for 3 h at 37°C. The cell viability values were obtained by conversion of the absorbance readings (A570 nm) into percentages of viable cells. The values obtained for DMEM were used as reference controls (100% cell viability).
Cell viability by fluorescence imaging. The fluorescence imaging analysis of cell viability was performed as described previously, using the LIVE/DEAD® assay (Invitrogen, Carlsbad, CA, USA) [25]. Briefly, approximately 1x105 cells were inoculated into 24-well plates and were incubated for 48 h, at 37°C, under a humid atmosphere with 5% CO2. Subsequently, the cells were exposed to ATC, ATCepi, ATCnano, and ATCnanoepi, at the ATC IC50 obtained in the MTT assay. Methanol (70%) was used as the cell death positive control. After exposure to the formulations for 1 h or 24 h, the cells were washed twice with phosphate-buffered saline (PBS), followed by addition of 300 μL of the LIVE/DEAD® solution to each well containing HaCaT cells. The plates were then incubated at room temperature for 30–45 min. Fluorescence images were acquired using an inverted microscope (Axiovert 40 CFL, Carl Zeiss, Germany) coupled to an MEC camera (AxioCam, Carl Zeiss, Germany). Calcein/AM was detected using wavelengths of 450–490 nm (excitation) and 515–565 nm (emission). Cells labeled with EthD-1 were detected using wavelengths of 528–546 nm (excitation) and 590–617 nm (emission) [25].
In vitro permeation assay
The permeation capacities of the articaine formulations were evaluated using a swine esophageal epithelium model. This model has been extensively used as a permeation barrier model in transbuccal delivery studies, due to its similarity to human oral mucosa in terms of histological organization, permeability, and lipid composition [26]. Swine esophagus was obtained from a local slaughterhouse (Frigorífico Angelelli Ltda, Piracicaba, SP, Brazil) and transported (within 10 min) to the laboratory in isotonic phosphate buffer (PBS, pH 7.4). The ends were discarded and the esophageal mucosa was carefully separated from the outer muscle layer with a scalpel. After immersion of the mucosal tissue in a distilled water bath for 2 min, at 60°C, the epithelium was gently separated from the connective tissue and was kept in isotonic saline [26].
Firstly, 1.0 mL of the buffer solution was added to the donor compartment and the cells were allowed to equilibrate for 60 min in a water bath at 37°C. Following the equilibration period, the epithelium barrier integrity was checked and only undamaged specimens presenting tissue resistivity of at least 3 kΩ/cm2 were used [27].
The buffer solution was replaced by 1.0 mL of the test formulation, applied to the epithelial surface under infinite dose conditions, and the glass chamber was closed. The experiments were conducted under sink conditions. Aliquots of 300 μL were periodically removed from the receptor chamber (at 15, 30, 45, 60, 90, 120, 150, and 180 min), with the volume being immediately made up using fresh buffer solution. The concentration of articaine was determined by HPLC, as described in Articaine analysis.
The cumulative amount of ATC permeated across the swine esophageal epithelium was plotted as a function of time (n = 9 for each formulation). The steady-state flux (Jss) was obtained from the slope of the linear portion of the curve and the lag time was obtained from the intercept of this straight line at the x-axis.
In vivo model
Ethical approval
The analgesic efficacy study in rats was approved on March 26th 2012 by the Ethics Committee on Animal Use of the University of Campinas (CEUA-UNICAMP, protocol #2638–1). The procedures were conducted in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals, issued by the International Association for the Study of Pain [28], and complied with the Principles of Laboratory Animal Care (NIH publication #85–23, revised in 1985).
Animals
Fifty-four adult male Wistar SPF rats (250–300 g) were obtained from the Multidisciplinary Center for Biological Investigation of the University of Campinas (CEMIB/UNICAMP, Campinas, SP, Brazil). The animals were submitted to a 12-h day/night cycle, at 22°C, and were allowed free access to water and food during the study.
Local analgesia efficacy in a postoperative pain model
This experiment consisted of measuring the paw withdrawal response to application of force, using an electronic von Frey anesthesiometer (Insight Equipment Ltda, Ribeirão Preto, Brazil), after induction of hypernociception (postoperative pain), as described previously [29,30]. Briefly, rats were placed in cages divided into compartments (23 cm width x 20 cm depth x 18 cm height) with wire mesh floors and mirrors underneath, allowing 15–30 min for acclimatization. After this period, the anesthesiometer was used to apply progressively greater force, ranging from 0.0073 to 0.456 N, to the plantar surface of the right hind paw, at intervals of 5 min, in order to establish the baseline response, which was calculated as the mean of three measurements.
Following this procedure, an incision (1 cm long x 3 mm deep) closed by three sutures (6–0 nylon, Brasuture Ind. Imp. Exp. Ltda., São Sebastião da Grama, SP, Brazil) was performed in the plantar surface of the right hind paw of the rats, under isoflurane anesthesia (Isoforine, Cristália Chemicals and Pharmaceuticals Ltd., Itapira, SP, Brazil). After allowing 24 h for recovery, the animals were placed in the same cages for acclimatization followed by lateral application of force to the wound, as described previously. Animals presenting 20% reduction in the force required to elicit paw withdrawal were considered as presenting hyperalgesic pain and were submitted to formulations testing [29,30].
These rats were randomly divided into 9 groups (n = 6) and received 0.1 mL injections of the articaine formulations or the controls, at the side of the incision. Five minutes after the injection, force was applied laterally to the wound every 10 min, using the von Frey anesthesiometer. Local analgesia was considered successful when the animal did not withdraw the paw after application of force of 0.456 N. The duration of analgesia was considered to be the period of time between injection of the formulation and the last time that the animal did not withdraw the paw. The formulations were coded by one researcher and the experiments were performed by other investigators blinded for the formulations injected.
Statistical analyses
The results were analyzed using the unpaired t-test (characterization), nonlinear fit analysis (MTT assay), Mann-Whitney test (in vitro permeation parameters), ANOVA and the Student-Newman-Keuls test (analgesia duration), and the log-rank test (analgesia success). The significance level was set at 5%.
Results
Characterization of articaine-loaded poly(ε-caprolactone) nanocapsules
The characteristics of the formulations containing articaine-loaded poly(ε-caprolactone) nanocapsules and empty poly(ε-caprolactone) nanocapsules, determined in the present study, are presented in Table 1. The results related to nano-analysis are published in a previous study of our group [18]. After loading the poly(ε-caprolactone) nanocapsules with articaine, no changes were observed in the nanocapsule diameter or the polydispersion index (P>0.05).
Table 1. Mean diameter, polydispersion index, encapsulation efficiency, and pH values obtained for the poly(ε-caprolactone) nanocapsules.
| Parameter | Articaine-loaded poly(ε-caprolactone) nanocapsules | Poly(ε-caprolactone) nanocapsules |
|---|---|---|
| Mean diameter (nm) | 450.07 ± 27.98 | 432.12 ± 10.07 |
| Polydispersion index | 0.11 ± 0.04 | 0.10 ± 0.02 |
| pH | 6.93 ± 0.06 | 5.55 ± 0.01 |
| Encapsulation efficiency (%) | 51.33 ± 3.06 | ---- |
The mean diameter (MD), polydispersion index (PI), and pH of the articaine-loaded poly(ε-caprolactone) nanocapsules were evaluated. The MD and PI were determined by dynamic light scattering, using a ZetaSizer Nano ZS 90 instrument (Malvern Instruments, UK). The measurements (in triplicate) were performed at 25°C, with a fixed angle of 90°, using diluted samples (about 1.0x10-3 mol/L) [15–18].
The pH values of the nanocapsules in aqueous suspensions were determined (in triplicate) using a previously calibrated pH meter (Thermo Electron Orion® Model 290A+, Sigma). The stability results for this formulation have been presented previously [18].
Cell viability assays
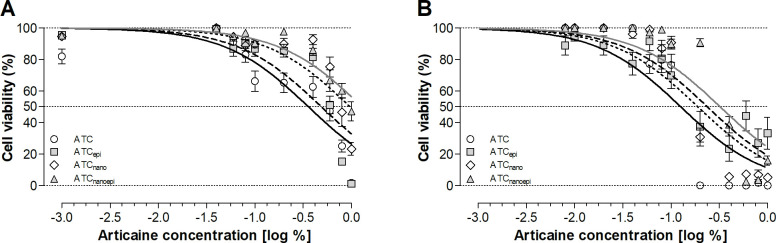
The ATC formulation was the most cytotoxic to HaCaT cells after 1 and 24 h, while ATCnanoepi was the least cytotoxic. The addition of epinephrine or encapsulation in the nanocapsules decreased the articaine cytotoxicity, which increased in the order ATCnanoepi < ATCnano < ATCepi < ATC (Fig 1, Table 2), Nonlinear regression analysis showed a significant difference between all the formulations (Table 2), as illustrated in Figs 2 and 3.
Fig 1.
Percentage (%) cell viability (mean ± SEM) after (A) 1-h and (B) 24-h exposure to the formulations containing articaine at different concentrations, using the MTT colorimetric assay ATC: Articaine; ATCepi: Articaine with 1:200,000 epinephrine; ATCnano: Articaine-loaded poly(ε-caprolactone) nanocapsules; ATCnanoepi: Articaine-loaded poly(ε-caprolactone) nanocapsules with 1:200,000 epinephrine. Nonlinear fit analysis (P<0.0001).
Table 2. Half-maximal inhibitory concentration (IC50: Concentration for inhibition of 50% of the cells) and confidence interval (%) after 1-h and 24-h exposure of HaCaT cells to articaine formulations.
| Formulations | IC50 (%) | IC50 confidence interval (%) | ||
|---|---|---|---|---|
| 1-h | 24-h | 1-h | 24-h | |
| ATC | 0.3597 | 0.1176 | 0.3034 to 0.4265 | 0.1082 to 0.1277 |
| ATCepi | 0.5786 | 0.2467 | 0.5469 to 0.6122 | 0.1770 to 0.3439 |
| ATCnano | 0.7692 | 0.1639 | 0.7272 to 0.8137 | 0.1490 to 0.1803 |
| ATCnanoepi | 0.9582 | 0.3487 | 0.8538 to 1.075 | 0.3220 to 0.3776 |
P<0.0001 for all formulations. Different exposure times were analyzed separately.
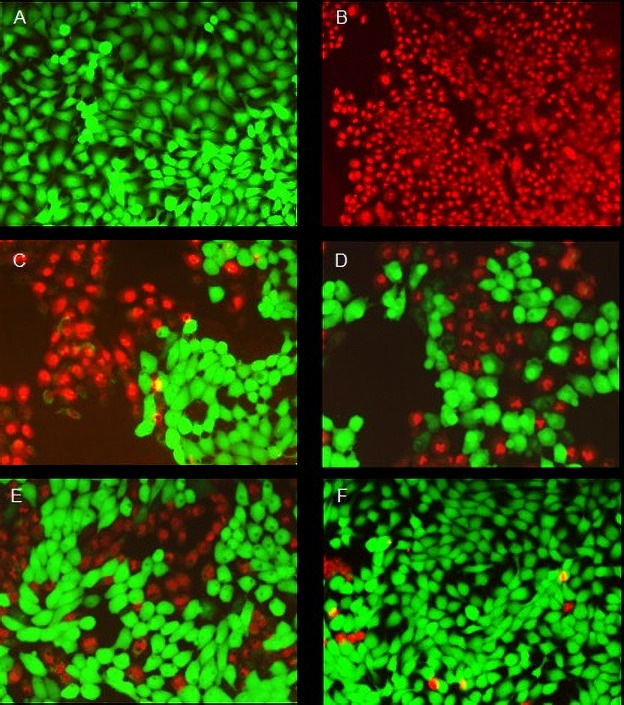
Fig 2. Fluorescence images showing the viability of the HaCaT cells treated with articaine formulations at the IC50 concentration for 1 h at 37°C.
(A) live cells (negative control); (B) dead cells (positive control treated with 70% methanol); treatments with (C) ATC; (D) ATCepi; (E) ATCnano; (F) ATCnanoepi. Each image is composed of two superimposed images taken through different filters for green (calcein) and red (homodimer-1) fluorescence. All images were acquired at the same magnification (10x).
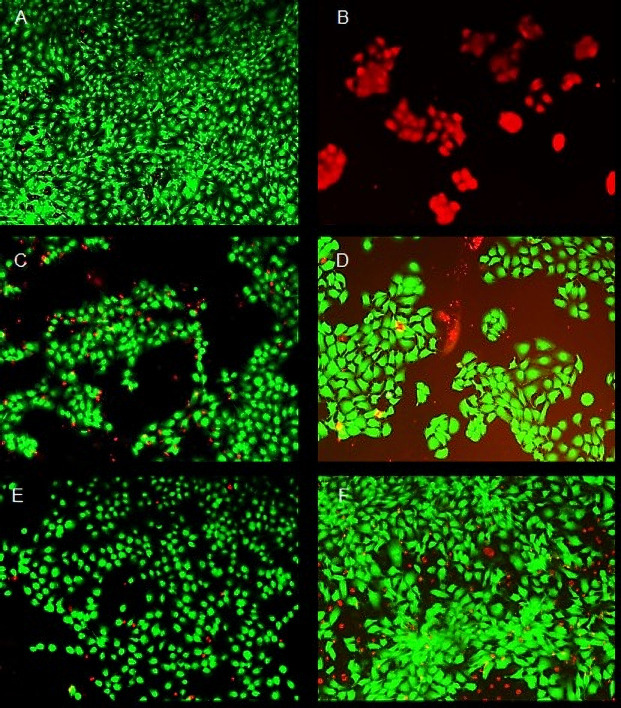
Fig 3. Fluorescence images showing the viability of the HaCaT cells treated with articaine formulations at the IC50 concentration for 24 h at 37°C.
(A) live cells (negative control); (B) dead cells (positive control treated with 70% methanol); treatments with (C) ATC; (D) ATCepi; (E) ATCnano; (F) ATCnanoepi. Each image is composed of two superimposed images taken through different filters for green (calcein) and red (homodimer-1) fluorescence. All images were acquired at the same magnification (10x).
The cell viabilities obtained after 1 h exposure to the control formulations were 98.5% (DMEM with epinephrine), 97.3% (DMEM with poly(ε-caprolactone) nanocapsules), and 97.3% (DMEM with poly(ε-caprolactone) nanocapsules and epinephrine). After 24 h exposure to the control formulations, the cell viabilities were 95.6% (DMEM with epinephrine), 97.8% (DMEM with poly(ε-caprolactone) nanocapsules), and 93.0% (DMEM with poly(ε-caprolactone) nanocapsules and epinephrine).
In vitro permeation assay
The ATCnano formulation presented higher permeation, compared to the ATC formulation (Fig 4, Table 3). There was no lag time (0 h) for either formulation since permeation occurred immediately after experiment started, as the intercept of the straight line at the x-axis presented negative values for either formulations (ATC = -0,79±0,40 and ATCnano = -0,49±0,33).
Fig 4. Permeation profiles (mean ± SD, n = 6–8) of ATC permeated after 3 h, for ATC permeation across fresh swine esophageal epithelium from formulations applied under infinite dose conditions.
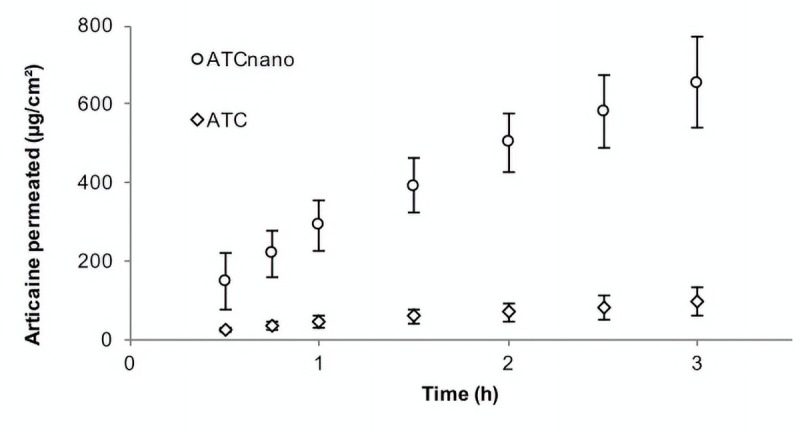
White circles: ATCnano (articaine-loaded poly(ε-caprolactone) nanocapsules; white diamonds: ATC (articaine).
Table 3. Calculated steady-state fluxes (Jss) and cumulative amounts of ATC permeated after 3 h (Q3h), for ATC permeation across fresh swine esophageal epithelium from formulations applied under infinite dose conditions.
| Formulation | Jss (μg.cm-2.h-1) | ER* | Q3h (μg) | R2 (0.5 – 3h)** |
|---|---|---|---|---|
| ATCnano | 191.96(148.46–254.55)** | 8.06 | 1075.7(950.46–1595.3)*** | 0,984±0,008 |
| ATC | 23.82(14.47–31.31) | ------ | 160.78(98.49–335.58) | 0,987±0,008 |
Mann-Whitney test: data presented as median (minimum-maximum) n = 6–8
**P<0.001
***P<0.0001. Each permeation parameter was analyzed separately.
*ER: Enhancement ratio, comparing the Jss values for ATCnano and ATC. **R2: mean(±SD) of regression coefficients calculated between 0.5 and 3 h.
In vivo assays of local analgesia in a postoperative pain model
All the animals presented a 20% reduction in the withdrawal response to force application after incision/suture and were included in the experiment. The control formulations did not induce local analgesia. The 2% ATCepi and 4% ATCepi formulations presented similar analgesia success (Fig 5) and duration (Fig 6) (P = 0.59), and both provided higher analgesia success and duration than 2% ATC, 2% ATCnano, and 2% ATCnanoepi, while 2% ATCnanoepi provided higher analgesia success than 2% ATCnano (P = 0.034).
Fig 5. Analgesia success (%) in inflamed tissue after subcutaneous injection.
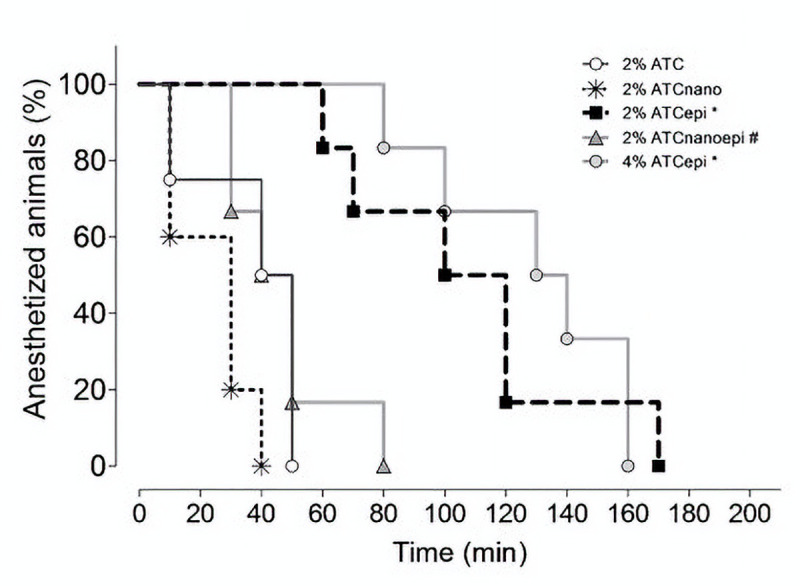
2% articaine (2% ATC), 2% articaine-loaded poly(ε-caprolactone) nanocapsules (2% ATCnano), 2% articaine with 1:200,000 epinephrine (2% ATCepi), 2% articaine-loaded poly(ε-caprolactone) nanocapsules with 1:200,000 epinephrine (2% ATCnanoepi), and 4% articaine with 1:200,000 epinephrine (4% ATCepi) (n = 6 rats/group). Log-rank test: *P<0.01 compared to 2% ATC, 2% ATCnano, and 2% ATCnanoepi; #P = 0.0344 compared to 2% ATCnano.
Fig 6. Analgesia duration (mean±SD, in minutes) after subcutaneous injection.
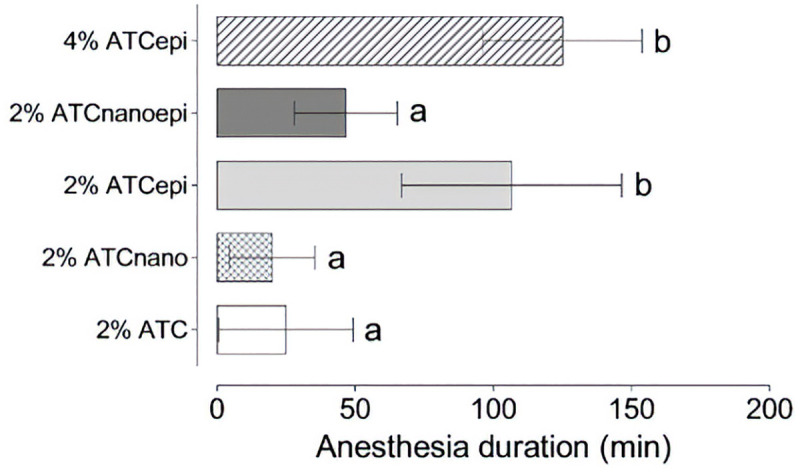
2% articaine (2% ATC), 2% articaine-loaded poly(ε-caprolactone) nanocapsules (2% ATCnano), 2% articaine with 1:200,000 epinephrine (2% ATCepi), 2% articaine-loaded poly(ε-caprolactone) nanocapsules with 1:200,000 epinephrine (2% ATCnanoepi), and 4% articaine with 1:200,000 epinephrine (4% ATCepi) (n = 6 rats/group). ANOVA and LSD t-test: different letters indicate P<0.05.
Discussion
Articaine presents interesting properties and consequently is widely studied in the world. The encapsulation of articaine in poly(ε-caprolactone) nanocapsules is a promising strategy for dental practice since it provides decreased cytotoxicity, which can reduce the risk of nerve cell damage and paresthesia [2,8], together with increased permeability resulting in higher superficial analgesia after topical application at oral mucosa [19,20].
The mean diameter and polydispersion index of the nanocapsules did not change after the incorporation of articaine. The polydispersion index remained at low values, showing good homogeneity of the system, which confirmed the results previously reported by our group [15–18]. The mean diameter obtained for the articaine-loaded poly(ε-caprolactone) nanocapsules (450.07 ± 27.98) was in the range observed previously for articaine encapsulated in poly(ethylene glycol)-poly(ε-caprolactone) nanospheres (569.2 ± 30.5 nm), articaine in alginate/chitosan nanospheres (342.4 ± 20.5 nm), articaine in poly(ε-caprolactone) nanocapsules (445.5 ± 2.1 nm), and lidocaine in poly(ε-caprolactone) nanospheres (449.6 ± 0.5 nm) [15–18] consistent with values observed for colloidal suspensions [15].
Accordingly, the polydispersion index (0.11 ± 0.04) was in the range observed for articaine encapsulated in poly(ethylene glycol)-poly(ε-caprolactone) nanospheres (0.144 ± 0.025), articaine in alginate/chitosan nanospheres (0.236 ± 0.030), articaine in poly(ε-caprolactone) nanocapsules (0.068 ± 0.005), and lidocaine in poly(ε-caprolactone) nanospheres (0.072) [15–18] and was indicative of homogeneity of the nanoparticles.
Local anesthetics have been tested in different kind of cells, as and fibroblasts, neuroblastoma, mesenchymal stem, keratinocytes [18,31–33]. The immortalized human keratinocytes (HaCaT) have a good proliferative capacity in culture, being used in in vitro studies of cytotoxicity [24,33,34] and other cellular responses to stimulus [35,36].
The MTT assay results showed that encapsulation reduced the toxicity of articaine to HaCaT cells exposed for 1 h and 24 h, which was confirmed by the results of the LIVE/DEAD® assay. The fact that the cytotoxicity of articaine was greatly reduced by its encapsulation is an important finding, since this local anesthetic has been associated with cell injury and paresthesia after local injections in dental procedures [2,8].
The ability of polymeric nanoparticles to reduce the cytotoxicity of local anesthetics has been reported previously. After exposure to plain articaine, 3T3 fibroblast cells showed a 50% reduction in viability, while the exposure of these cells to articaine-loaded poly(ethylene glycol)-poly(ε-caprolactone) nanocapsules and to articaine-loaded alginate/chitosan nanospheres resulted in viability losses of 20% and 30%, respectively [16]. It was also found that the incorporation of bupivacaine into alginate/chitosan nanoparticles reduced its toxicity towards 3T3 fibroblasts [37]. This reduced cytotoxicity might be related to the percentage of encapsulated local anesthetic, which resulted in a reduced amount of free local anesthetic available to interact with the cells.
Furthermore, the combination of epinephrine and articaine-loaded poly(ε-caprolactone) nanocapsules resulted in a further reduction in cytotoxicity. This effect was observed for all the formulations containing this vasoconstrictor, for both exposure periods. The protective effect of epinephrine was previously demonstrated in human articular chondrocytes exposed to 1% lidocaine with epinephrine, in comparison to 1% lidocaine, with the protective effect of epinephrine being attributed to its interaction with the local anesthetic [38]. In the present study, epinephrine may also have interacted with the nanocapsules, reducing the release of articaine. However, the kinetics of articaine release from the nanocapsules was not tested in the presence of epinephrine.
All the anesthetic formulations tested in the present study showed increased cytotoxicity (lower IC50) with longer treatment periods. The same behavior was observed elsewhere for Schwann cells after 4, 24, 48, and 72 h exposure to lidocaine, mepivacaine, chloroprocaine, ropivacaine, and bupivacaine [39] as well as for human articular chondrocytes exposed to lidocaine for 15, 30, and 60 min [38].
Articaine presents useful clinical properties including high diffusibility, lower plasma half-life than other anesthetics of the amide group, and appropriate interaction with lipid membranes [2,40] The high diffusibility of articaine is demonstrated by the fact that this local anesthetic is able to induce pulpal anesthesia in posterior mandibular teeth by buccal infiltration, which is currently achieved with nerve block for other anesthetic solutions [4–7].
However, increased tissue diffusibility does not necessarily reflect increased ability to cross the epithelium barrier after a topical application. In the present study, the permeation degree of articaine was significantly lower than that of the encapsulated local anesthetic, which revealed its poor ability to penetrate the barrier when applied topically. Drug delivery systems such as liposomes, nanoparticles, and nanocapsules are considered promising drug delivery carriers in topical application on the skin [22]. Several studies have demonstrated that these nanocarriers are able to modify drug permeation profiles, due to their permeation-enhancing effects [21]. This was confirmed by the present results showing that articaine encapsulation improved the permeation profile, with an approximately 8-fold increase in the articaine flux across the mucosal barrier. Similarly, it has also been reported that encapsulation in liposomes increased the permeation of lidocaine and benzocaine through swine palatal and esophageal epithelia, compared to commercially available formulations containing the free local anesthetics [19,20].
It has been suggested that in vitro permeation is a valuable tool for prediction of analgesic efficacy during preclinical tests, since an in vitro/in vivo correlation was observed [19,20] Although topical articaine is not commonly used in dentistry, the increased permeation achieved by encapsulation in poly(ε-caprolactone) nanocapsules suggests the possibility of its future application in intraoral topical anesthesia, so in vivo studies should be undertaken to confirm this hypothesis.
In addition to its higher diffusibility, articaine has been demonstrated to provide greater pulpal anesthesia success in teeth with symptomatic pulpitis (hyperalgesic teeth) [3,4]. However, the encapsulation of articaine in polymeric nanoparticles did not improve efficacy following injection in an inflamed tissue model. The present results showed that hyperalgesia due to the surgical wound did not affect the onset of analgesia after infiltration of the articaine formulations, since 100% of the animals showed local analgesia (absence of paw withdrawal) in the first assessment period (5 min), indicating that a sufficient amount of articaine was available to block the free nerve endings.
However, the duration of analgesia was significantly shorter, compared to that achieved with articaine at 2 or 4% associated with epinephrine. This could be attributed to the vasodilator action of articaine, as shown by other local anesthetics, such as lidocaine. As a consequence, most local anesthetics are not available as plain solutions for clinical use in dentistry. Inflamed tissues present increased vasodilation and vascular permeability, so effective local vasoconstriction is required in order to allow sustained analgesia in these tissues. Epinephrine at 1:200,000 or higher concentrations provides adequate vasoconstriction and improves the duration of dental anesthesia [41].
Based on the present findings, it was not clear why the formulation 2% ATCnanoepi presented lower analgesic effect when compared to 2% ATCepi, since the amount of vasoconstrictor was the same in both formulations. We hypothesize that an interaction between epinephrine and the nanocapsules could have occurred, which decreased the amount of epinephrine available to act on the vessels. Therefore, a lower degree of vasoconstriction in inflamed tissue would result in low analgesia success, comparable to that observed for the anesthetic without additives. Such interaction between nanoparticles and epinephrine should be confirmed in future studies.
The amount of articaine did not seem to influence its efficacy in inducing analgesia, since 2% and 4% articaine with epinephrine presented similar duration and success. Previous work also found that 2% and 4% articaine associated with 1:200,000 epinephrine provided similar efficacy for use in non-inflamed tooth extractions in humans [42]. In third molar surgery, efficacy and safety also were tested and articaine at 2 or 4% associated with epinephrine, administered in equal volumes, showed no significant differences [43].
These findings suggest that a formulation with a 50% lower concentration could be clinically used as an alternative to the commercially available 4% articaine, even in inflamed tissue, combining a similar efficacy with a reduction in local toxicity, considering the toxicity to be concentration-related [8]. The systemic toxicity may also be reduced with 2% articaine, compared to 4% articaine (both associated with 1:200,000 epinephrine), as observed in children [44].
Conclusions
Poly(ε-caprolactone) nanocapsules constitute a promising release system for articaine in intraoral topical anesthesia, providing decreased cytotoxicity and increased permeation across a mucosal epithelium model. However, the system was not effective in a postoperative pain model.
The association of polymeric nanocapsules with epinephrine provided a further decrease in cytotoxicity. However, neither ATCnano nor ATCnanoepi were able to improve local analgesia in inflamed tissue. Epinephrine improved analgesic efficacy at both articaine concentrations (2% and 4%), so evaluation of the lower concentration of use in inflamed tissues might be of utility.
Acknowledgments
The authors thank DFL Ind. Com. Ltda. (Rio de Janeiro, Brazil) and Chemspecs Comercio e Representaçoes Ltda. (São Paulo, Brazil) for donation of articaine and Myritol 318, respectively. The authors thank Frigorífico Angelelli Ltda. (Piracicaba, São Paulo, Brazil) for the donation of swine esophagi. The authors thank Professor Dr. Eneida de Paula for the supply of HPLC equipment for articaine analysis.
Data Availability
All relevant data are within the manuscript files.
Funding Statement
Financial support for this study was provided by the São Paulo Research Foundation (FAPESP, grants #2012/06974-4 and #2012/07310-2). Camila Batista da Silva acknowledges a scholarship provided by FAPESP (grant #2012/02539-1).
References
- 1.Malamed SF, Gagnon S, Leblanc D. Articaine hydrochloride: A study of the safety of a new amide local anesthetic. J Am Dent Assoc [Internet]. 2001;132(2):177–85. Available from: 10.14219/jada.archive.2001.0152 [DOI] [PubMed] [Google Scholar]
- 2.Paxton K, Thome DE. Efficacy of Articaine Formulations: Quantitative Reviews. Dent Clin North Am [Internet]. 2010;54(4):643–53. Available from: 10.1016/j.cden.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 3.Ashraf H, Kazem M, Dianat O, Noghrehkar F. Efficacy of articaine versus lidocaine in block and infiltration anesthesia administered in teeth with irreversible pulpitis: A prospective, randomized, double-blind study. J Endod [Internet]. 2013;39(1):6–10. Available from: 10.1016/j.joen.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 4.Nydegger B, Nusstein J, Reader A, Drum M, Beck M. Anesthetic comparisons of 4% concentrations of articaine, lidocaine, and prilocaine as primary buccal infiltrations of the mandibular first molar: A prospective randomized, double-blind study. J Endod [Internet]. 2014;40(12):1912–6. Available from: 10.1016/j.joen.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Corbett IP, Kanaa MD, Whitworth JM, Meechan JG. Articaine Infiltration for Anesthesia of Mandibular First Molars. J Endod. 2008;34(5):514–8. 10.1016/j.joen.2008.02.042 [DOI] [PubMed] [Google Scholar]
- 6.Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. Articaine and lidocaine mandibular buccal infiltration anesthesia: A prospective randomized double-blind cross-over study. J Endod. 2006;32(4):296–8. 10.1016/j.joen.2005.09.016 [DOI] [PubMed] [Google Scholar]
- 7.Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J. 2009;42(3):238–46. 10.1111/j.1365-2591.2008.01507.x [DOI] [PubMed] [Google Scholar]
- 8.Hillerup S, Jensen RH, Ersbøll BK. Trigeminal nerve injury associated with injection of local anesthetics Needle lesion or neurotoxicity? J Am Dent Assoc [Internet]. 2011;142(5):531–9. Available from: 10.14219/jada.archive.2011.0223 [DOI] [PubMed] [Google Scholar]
- 9.de Paula E, Cereda C, Tofoli G, Franz-Montan M, Fraceto L, de Araujo D. Drug Delivery Systems for Local Anesthetics. Recent Pat Drug Deliv Formul. 2009;4(1):23–34. [DOI] [PubMed] [Google Scholar]
- 10.De Paula E, Cereda CM, Fraceto LF, De Araújo DR, Franz-Montan M, Tofoli GR, et al. Micro and nanosystems for delivering local anesthetics. Expert Opin Drug Deliv. 2012;9(12):1505–24. 10.1517/17425247.2012.738664 [DOI] [PubMed] [Google Scholar]
- 11.Franz-Montan M, Ribeiro LN de M, Volpato MC, Cereda CMS, Groppo FC, Tofoli GR, et al. Recent advances and perspectives in topical oral anesthesia. Expert Opin Drug Deliv. 2017;14(5):673–84. 10.1080/17425247.2016.1227784 [DOI] [PubMed] [Google Scholar]
- 12.Preetz C, Rübe A, Reiche I, Hause G, Mäder K. Preparation and characterization of biocompatible oil-loaded polyelectrolyte nanocapsules. Nanomedicine Nanotechnology, Biol Med. 2008;4(2):106–14. 10.1016/j.nano.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 13.Schaffazick SR, Guterres SS, De Lucca Freitas L, Pohlmann AR. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Quim Nova. 2003;26(5):726–37. [Google Scholar]
- 14.Mishra B, Patel BB, Tiwari S. Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine Nanotechnology, Biol Med [Internet]. 2010;6(1):9–24. Available from: 10.1016/j.nano.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 15.Ramos Campos EV, Silva de Melo NF, Guilherme VA, de Paula E, Rosa AH, de Araújo DR, et al. Preparation and Characterization of Poly(ε-Caprolactone) Nanospheres Containing the Local Anesthetic Lidocaine. J Pharm Sci. 2013;102(1):215–26. 10.1002/jps.23350 [DOI] [PubMed] [Google Scholar]
- 16.Melo NFS de, Campos EVR, Gonçalves CM, de Paula E, Pasquoto T, de Lima R, et al. Development of hydrophilic nanocarriers for the charged form of the local anesthetic articaine. Colloids Surfaces B Biointerfaces. 2014;121:66–73. 10.1016/j.colsurfb.2014.05.035 [DOI] [PubMed] [Google Scholar]
- 17.Ramos de Campos EV, de Melo NFS, de Paula E, Rosa AH, Fraceto LF. Screening of conditions for the preparation of poly(ε-caprolactone) nanocapsules containing the local anesthetic articaine. Colloids Surfaces B Biointerfaces. 2013;2:1–6. [Google Scholar]
- 18.Melo NFS de, Campos EVR, Franz-Montan M, Paula E de, Silva CMG da, Maruyama CR, et al. Characterization of Articaine-Loaded Poly (ε -caprolactone) Nanocapsules and Solid Lipid Nanoparticles in Hydrogels for Topical Formulations. J Nanosci Nanotechnol. 2018;18(6):4428–38. 10.1166/jnn.2018.15235 [DOI] [PubMed] [Google Scholar]
- 19.Franz-Montan M, Cereda CMS, Gaspari A, Da Silva CMG, De Araújo DR, Padula C, et al. Liposomal-benzocaine gel formulation: Correlation between in vitro assays and in vivo topical anesthesia in volunteers. J Liposome Res. 2013;23(1):54–60. 10.3109/08982104.2012.742536 [DOI] [PubMed] [Google Scholar]
- 20.Franz-Montan M, Baroni D, Brunetto G, Sobral VRV, Da Silva CMG, Venâncio P, et al. Liposomal lidocaine gel for topical use at the oral mucosa: Characterization, in vitro assays and in vivo anesthetic efficacy in humans. J Liposome Res. 2015;25(1):11–9. 10.3109/08982104.2014.911315 [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Mottaleb MMA, Neumann D, Lamprecht A. Lipid nanocapsules for dermal application: A comparative study of lipid-based versus polymer-based nanocarriers. Eur J Pharm Biopharm [Internet]. 2011;79(1):36–42. Available from: 10.1016/j.ejpb.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 22.Cevc G. Lipid vesicles and other colloids as drug carriers on the skin. Adv Drug Deliv Rev. 2004;56(5):675–711. 10.1016/j.addr.2003.10.028 [DOI] [PubMed] [Google Scholar]
- 23.Zhou Z, Ye J, Chen L, Ma A, Zou F. Simultaneous determination of ropivacaine, bupivacaine and dexamethasone in biodegradable PLGA microspheres by high performance liquid chromatography. Yakugaku Zasshi. 2010;130(8):1061–8. 10.1248/yakushi.130.1061 [DOI] [PubMed] [Google Scholar]
- 24.Ferreira LEN, Muniz BV, Dos Santos CP, Volpato MC, De Paula E, Groppo FC. Comparison of liposomal and 2-hydroxypropyl-β-cyclodextrin-lidocaine on cell viability and inflammatory response in human keratinocytes and gingival fibroblasts. J Pharm Pharmacol. 2016;68(6):791–802. 10.1111/jphp.12552 [DOI] [PubMed] [Google Scholar]
- 25.Ferreira LEN, Muniz BV, Burga-Sánchez J, Volpato MC, de Paula E, Rosa EAR, et al. The effect of two drug delivery systems in ropivacaine cytotoxicity and cytokine release by human keratinocytes and fibroblasts. J Pharm Pharmacol. 2017;69(2):161–71. 10.1111/jphp.12680 [DOI] [PubMed] [Google Scholar]
- 26.Del Consuelo ID, Pizzolato GP, Falson F, Guy RH, Jacques Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J Pharm Sci. 2005;94(12):2777–88. 10.1002/jps.20409 [DOI] [PubMed] [Google Scholar]
- 27.Cubayachi C, Couto RO, de Gaitani CM, Pedrazzi V, de Freitas O, Lopez RFV. Needle-free buccal anesthesia using iontophoresis and amino amide salts combined in a mucoadhesive formulation. Colloids surfaces B Biointerfaces. 2015;136:1193–201. 10.1016/j.colsurfb.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10. 10.1016/0304-3959(83)90201-4 [DOI] [PubMed] [Google Scholar]
- 29.Da Silva CB, Groppo FC, Dos Santos CP, Serpe L, Franz-Montan M, De Paula E, et al. Anaesthetic efficacy of unilamellar and multilamellar liposomal formulations of articaine in inflamed and uninflamed tissue. Br J Oral Maxillofac Surg [Internet]. 2016;54(3):295–300. Available from: 10.1016/j.bjoms.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 30.Prado AR, Yokaichiya F, Franco MKKD, Silva CMG da, Oliveira-Nascimento L, Franz-Montan M, et al. Complexation of oxethazaine with 2-hydroxypropyl-β-cyclodextrin: increased drug solubility, decreased cytotoxicity and analgesia at inflamed tissues. J Pharm Pharmacol. 2017;69(6):652–62. 10.1111/jphp.12703 [DOI] [PubMed] [Google Scholar]
- 31.Albalawi F, Lim JC, DiRenzo K V., Hersh E V., Mitchell CH. Effects of lidocaine and articaine on neuronal survival and recovery. Anesth Prog. 2018;65(2):82–8. 10.2344/anpr-65-02-02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahnama R, Wang M, Dang AC, Kim HT, Kuo AC. Cytotoxicity of local anesthetics on human mesenchymal stem cells. J Bone Jt Surg—Ser A. 2013;95(2):132–7. 10.2106/JBJS.K.01291 [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro LN de M, Franz-Montan M, Breitkreitz MC, da Silva GHR, de Castro SR, Guilherme VA, et al. Nanohybrid hydrogels designed for transbuccal anesthesia. Int J Nanomedicine. 2018;13:6453–63. 10.2147/IJN.S180080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boonkaew B, Kempf M, Kimble R, Cuttle L. Cytotoxicity testing of silver-containing burn treatments using primary and immortal skin cells. Burns [Internet]. 2014;40(8):1562–9. Available from: 10.1016/j.burns.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 35.Portugal-Cohen M, Numa R, Yaka R, Kohen R. Cocaine induces oxidative damage to skin via xanthine oxidase and nitric oxide synthase. J Dermatol Sci [Internet]. 2010;58(2):105–12. Available from: 10.1016/j.jdermsci.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 36.Jiao Q, Wang H, Hu Z, Zhuang Y, Yang W, Li M, et al. Lidocaine inhibits staphylococcal enterotoxin-stimulated activation of peripheral blood mononuclear cells from patients with atopic dermatitis. Arch Dermatol Res. 2013;305(7):629–36. 10.1007/s00403-013-1339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grillo R, De Melo NFS, De Arajo DR, De Paula E, Rosa AH, Fraceto LF. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. J Drug Target. 2010;18(9):688–99. 10.3109/10611861003649738 [DOI] [PubMed] [Google Scholar]
- 38.Jacobs TF, Vansintjan PS, Roels N, Herregods SS, Verbruggen G, Herregods LL, et al. The effect of Lidocaine on the viability of cultivated mature human cartilage cells: An in vitro study. Knee Surgery, Sport Traumatol Arthrosc. 2011;19(7):1206–13. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, Abrahams MS, Hurn PD, Grafe MR, Kirsch JR. Local anesthetic schwann cell toxicity is time and concentration dependent. Reg Anesth Pain Med. 2011;36(5):444–51. 10.1097/AAP.0b013e318228c835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lygre H, Moe G, Nerdal W, Holmsen H. Interaction of articaine hydrochloride with prokaryotic membrane lipids. Acta Odontol Scand. 2009;67(1):1–7. 10.1080/00016350802443466 [DOI] [PubMed] [Google Scholar]
- 41.Tófoli GR, Ramacciato JC, de Oliveira PC, Volpato MC, Groppo FC, Ranali J. Comparison of effectiveness of 4% articaine associated with 1: 100,000 or 1: 200,000 epinephrine in inferior alveolar nerve block. Anesth Prog. 2003;50(4):164–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Hintze A, Paessler L. Comparative investigations on the efficacy of articaine 4% (epinephrine 1:200,000) and articaine 2% (epinephrine 1:200,000) in local infiltration anaesthesia in dentistry—A randomised double-blind study. Clin Oral Investig. 2006;10(2):145–50. 10.1007/s00784-005-0025-0 [DOI] [PubMed] [Google Scholar]
- 43.Senes AM, Calvo AM, Colombini-Ishikiriama BL, Gonçalves PZ, Dionísio TJ, Santana E, et al. Efficacy and Safety of 2% and 4% Articaine for Lower Third Molar Surgery. J Dent Res. 2015;94(September):166S–173S. 10.1177/0022034515596313 [DOI] [PubMed] [Google Scholar]
- 44.Jakobs W, Ladwig B, Cichon P, Ortel R, Kirch W. Serum levels of articaine 2% and 4% in children. Anesth Prog. 1995;42(3–4):113–5. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript files.