Abstract
Diazoxide is the first-line drug for treating hyperinsulinism and the only pharmacological agent approved for hyperinsulinism by the Federal Drug Administration. This systemic review and meta-analysis aimed to investigate the efficacy and safety of diazoxide for treating hyperinsulinemic hypoglycemia (HH). The meta-analysis of the efficacy and safety of diazoxide in treating HH was performed by searching relevant studies in the PubMed, Embase, and Cochrane databases. The findings were summarized, and the pooled effect size and its 95% confidence interval (CI) were calculated. A total of 6 cohort studies, involving 1142 participants, met the inclusion criteria. Among the cohort studies, the pooled estimate of the response rate of diazoxide therapy was 71% (95% CI 50%–93%, Pheterogeneity< 0.001, I2 = 98.3%, Peffect< 0.001). The common side effects were hypertrichosis (45%), fluid retention (20%), gastrointestinal reaction (13%), edema (11%), and neutropenia (9%). Other adverse events included pulmonary hypertension (2%) and thrombocytopenia (2%). This meta-analysis suggested that diazoxide was potentially useful in HH management; however, it had some side effects, which needed careful monitoring. Furthermore, well-designed large-scale studies, such as randomized controlled trials, might be necessary in the future to obtain more evidence.
Introduction
Hyperinsulinemic hypoglycemia (HH) describes the condition and effects of low blood glucose caused by excessive insulin. Many cases are reported in childhood as a congenital disorder [1]. Congenital hyperinsulinemia (CHI) is the most common and serious cause of persistent hypoglycemia in newborns and children. It occurs between 1:2500 and 50,000 live births [2]. CHI is characterized by excessive secretion of insulin by pancreatic B cells, which is the most common cause of persistent hypoglycemia in infancy [2]. CHI is a heterogeneous disease in clinical manifestations, imaging, histology, and genetics. So far, mutations in more than 10 different genes (ABCC8, KCNJ11, GLUD1, GCK, HADH, HK1, CACNA1D, FOXA2, UCP2, SLC16A1, HNF4A, HNF1A, PMM2, and PGM1) have been reported in the genetic etiology of CHI [3–6]. CHI has three main histological types: focal, diffuse, and atypical [7,8].
Diazoxide is the first choice for treating CHI. It is a nondiuretic benzothiadiazine originally formulated as a peripheral vasodilator to reduce severe hypertension by smooth muscle relaxation [9]. It was first used to treat CHI in the 1960s [10] and has been the primary treatment since then. Diazoxide is the only drug approved for this indication in the United States, Canada, the United Kingdom, the European Union, China, Australasia, and Japan, [11]. Diazoxide acts by binding to the sulfonylurea receptor-1 subunit in the ATP-sensitive K+ (KATP) channel, causing the channel to open and increase its permeability to potassium ions. This results in the excessive polarization of beta cells, followed by the inhibition of Ca2+-dependent insulin secretion [12].
Several clinical studies investigated the efficacy and safety of diazoxide for treating CHI [13–18]. However, the results remain ambiguous. Meissner et al. [13] reported that 47/114 (41.2%) patients responded to diazoxide. Hu et al. [14] found that 36/44 (81.8%) patients were responsive to diazoxide treatment. This meta-analysis based on six cohort studies was conducted and aimed to investigate the efficacy and safety of diazoxide for treating CHI.
Materials and methods
The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines [19].
Search strategy
The PubMed, Embase, and Cochrane databases were searched for studies published up to January 2021, using the following terms: ‘‘hyperinsulinemic hypoglycemia,” ‘‘Congenital hyperinsulinism,” ‘‘hypoglycemia,” ‘‘neonates,” ‘‘infants,” ‘‘children,” and ‘‘diazoxide” with all possible combinations. The search strategy for the Embase database was included in the supplementary document in S1 Table. Using these parameters, all eligible studies were filtered out, and their reference lists were viewed for more available studies.
Study selection
The inclusion criteria were as follows: (1) cohort studies focusing on the efficacy and safety of diazoxide in treating HH; (2) studies reporting clinical outcomes such as response rates and complications; (3) studies available with the full text; and (4) if more than one study was published using the same case series, selection of the study with the largest sample size. The exclusion criteria were as follows: (1) experimental studies of animal models or cell lines; (2) similar studies involving repetition of patients; and (3) abstract or inappropriate types of publications, such as reviews, guides, or case reports. The selection had no language restrictions. Two investigators independently assessed the eligibility of each study and resolved any differences through discussion.
Data extraction and quality assessment
Two investigators (Xiaohong Chen and Lifang Feng) independently extracted all available data from the included studies based on the description provided by the authors of these studies. Subsequently, any differences were resolved through discussions with the third author. The following information was extracted from all relevant studies: first author, year of publication, country, sex, mean age, number of patients, follow-up time, and assessment results. The quality of the cohort study was assessed using the 9-star Newcastle–Ottawa Scale [20]. High-quality studies were defined as a study with more than 7 stars [20].
Statistical analysis
The meta-analysis was conducted using Stata 12 (Stata-Corp, TX, USA). A meta-analysis of cohort studies based on the random-effects model was conducted to evaluate the clinical response rate (patients responsive to diazoxide/all patients treated with diazoxide) and complications using effect size (response or incidence rate) and its corresponding 95% confidence interval (CI). The heterogeneity of the studies was assessed using Cochran’s Q test and quantified using the I2 statistic (considered high heterogeneity for I2 > 50%) [21,22]. Publication bias was evaluated by the visual inspection of the symmetry of the funnel plot and assessment of Begg’s and Egger’s tests [23]. The trim-and-fill analysis was applied in the case of any publication bias [24].
Results
Study selection
The electronic search identified 348 studies. Three additional studies were found by hand searching from the reference lists of other review studies. According to the inclusion criteria, 227 studies remained after removing the duplicates. Subsequently, 193 irrelevant studies were excluded. Of the remaining 34 studies, 15 were letters, reviews, and meta-analyses, and hence excluded. The remaining 19 studies were systematically reviewed and qualified for full-text reading. After full-text reading, five studies not focusing on children, three lacking usable data, and five case reports were excluded. Finally, 6 studies involving 1142 patients were included in the present meta-analysis [13–18]. The flow chart of the selection of studies and reasons for exclusion is presented in Fig 1.
Fig 1. Flow diagram of study identification.

Characteristics of the studies
The main characteristics of the eligible studies are shown in Table 1. Six cohort studies were included in the meta-analysis. The studies were performed in 4 countries (Germany, China, Japan, and the United States), and the study size ranged from 44 to 384 patients. The patients’ age ranged from 1 day to 17 years. The methodological quality of cohort studies included in the meta-analysis is shown in Table 2. The quality of the cohort studies included in the meta-analysis was generally high: two studies had seven stars, three studies had six stars, and one study had five stars.
Table 1. Characteristics of the studies included in this meta-analysis.
| Authors/Year of publication | Country | Male (%) | Age | Birth Weight (g) | Diazoxide (mg/(kg · day)] | Number of patients | Study design | Follow-up | Outcomes assessed |
|---|---|---|---|---|---|---|---|---|---|
| Meissner/2003 [12] | Germany | 52.6 | 1 D−17 Y | 3670 | 15 | 114 | Retrospective cohort | 6.6Y | Respond to diazoxide |
| Hu/2012 [13] | China | 68.2 | 1 D−2 Y | 2200−5100 | 5−15 | 44 | Retrospective cohort | NA | Respond to diazoxide, Fluid retention, Gastrointestinal reaction, Hypertrichosis |
| Wang/2017 [14] | China | 55.1 | NA | 1900−5800 | NA | 140 | Retrospective case | NA | Respond to diazoxide |
| Fukutomi/2018 [15] | Japan | 61.2 | 0 M−15 Y | NA | 0.3−17.4 | 384 | Special survey | 7Y | Respond to diazoxide, Pulmonary hypertension, Edema, Thrombocytopenia, Fluid retention, Gastrointestinal reaction, Hypertrichosis |
| Herrera/2018 [16] | USA | 56.3 | 8−161 D | 2350−3700 | 10−15 | 295 | Retrospective cohort | NA | Neuthropenia, Pulmonary hypertension, Edema, Thrombocytopenia |
| Thornton/2019 [17] | USA | 58.8 | 1 D−17 Y | 580−6600 | 2−12 | 165 | Retrospective cohort | NA | Respond to diazoxide, Neuthropenia, Pulmonary hypertension |
D: Day; Y: Year; NA: Not available.
Table 2. Methodological quality of observational studies included in the meta-analysis1.
| First author | Representativeness of the exposed cohort | Selection of the unexposed cohort | Ascertainment of exposure | Outcome of interest not present at the start of the study | Control for important factor or additional factor | Outcome assessment | Follow-up long enough for outcomes to occur | Adequacy of the follow-up of cohorts | Total quality scores |
|---|---|---|---|---|---|---|---|---|---|
| Meissner/2003 [12] | ☆ | — | ☆ | ☆ | — | ☆ | ☆ | ☆ | 6 |
| Hu/2012 [13] | ☆ | — | ☆ | ☆ | — | ☆ | ☆ | ☆ | 6 |
| Wang/2017 [14] | ☆ | — | ☆ | — | — | ☆ | ☆ | ☆ | 5 |
| Fukutomi/2018 [15] | ☆ | ☆ | ☆ | ☆ | — | ☆ | ☆ | ☆ | 7 |
| Herrera/2018 [16] | ☆ | — | ☆ | ☆ | — | ☆ | ☆ | ☆ | 7 |
| Thornton/2019 [17] | ☆ | — | ☆ | ☆ | — | ☆ | ☆ | ☆ | 6 |
1 A study could be awarded a maximum of one star for each item except for the item Control for important factor or additional factor.
Quantitative synthesis
Response to diazoxide
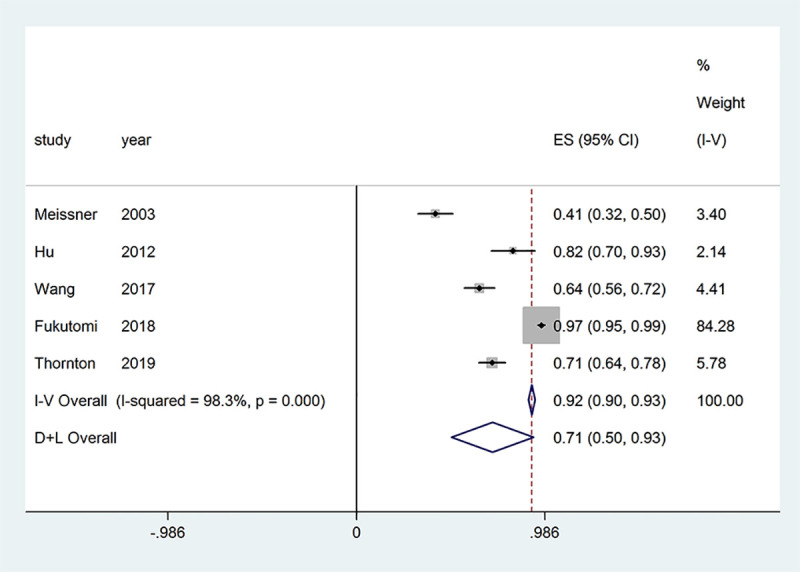
The response to diazoxide in patients with congenital hyperinsulinism (CHI) depends on the mutational status. Typically patients with focal CHI or patients with homozygous or compound heterozygous mutations in the K-ATP channel do not respond to diazoxide. Five studies provided outcomes regarding the response to diazoxide in patients with HH and were included in the meta-analysis. Significant evidence of heterogeneity was found among the studies (Pheterogeneity < 0.001, I2 = 98.3%); therefore, a random-effects model of analysis was used. The pooled proportion of patients who were responsive to diazoxide was 71% (95% CI = 50%–93%, Peffect < 0.001) (Fig 2).
Fig 2. Effect of diazoxide on patients with hyperinsulinemic hypoglycemia.
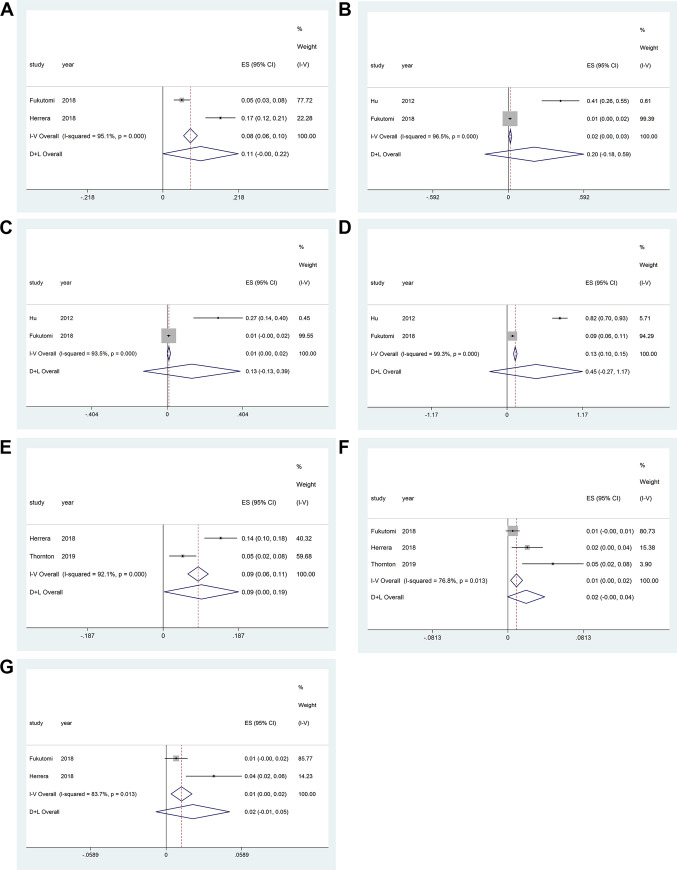
Edema
Two studies provided outcomes regarding edema in patients with HH and were included in the meta-analysis. Significant evidence of heterogeneity among the studies was found (Pheterogeneity < 0.001, I2 = 95.1%, Peffect < 0.001); therefore, a random-effects model of analysis was used. The pooled proportion of patients who had edema was 11% (95% CI = 0–22) (Fig 3A).
Fig 3. Safety of diazoxide in patients with hyperinsulinemic hypoglycemia.
(A) Edema; (B) fluid retention; (C) gastrointestinal reaction; (D) hypertrichosis; (E) neutropenia; (F) pulmonary hypertension; and (G) thrombocytopenia.
Fluid retention
Two studies provided outcomes regarding fluid retention in patients with HH and were included in the meta-analysis. Significant evidence of heterogeneity among the studies was found (Pheterogeneity < 0.001, I2 = 96.5%, Peffect = 0.008); therefore, a random-effects model of analysis was used. The pooled proportion of patients who had fluid retention was 20% (95% CI = –18 to 59) (Fig 3B).
Gastrointestinal reaction
Two studies provided outcomes regarding gastrointestinal reaction in patients with HH and were included in the meta-analysis. Significant evidence of heterogeneity among the studies was found (Pheterogeneity < 0.001, I2 = 93.5%, Peffect = 0.045); therefore, a random-effects model of analysis was used. The pooled proportion of patients who had gastrointestinal reaction was 13% (95% CI = –13 to 39) (Fig 3C).
Hypertrichosis
Two studies provided outcomes regarding hypertrichosis in patients with HH and were included in the meta-analysis. Significant evidence of heterogeneity among the studies was found (Pheterogeneity<0.001, I2 = 99.3%, Peffect< 0.001); therefore, a random-effects model of analysis was used. The pooled proportion of patients who had hypertrichosis was 45% (95% CI = –27 to 117) (Fig 3D).
Neutropenia
Two studies provided outcomes regarding neutropenia in patients with HH and were included in the meta-analysis. Significant evidence of heterogeneity among the studies was found (Pheterogeneity < 0.001, I2 = 92.1%, Peffect = 0.005); therefore, a random-effects model of analysis was used. The pooled proportion of patients who had neutropenia was 9% (95% CI = 0–19) (Fig 3E).
Pulmonary hypertension
Three studies provided outcomes regarding pulmonary hypertension in patients with HH and were included in the meta-analysis. Significant evidence of heterogeneity among the studies was found (Pheterogeneity = 0.013, I2 = 76.8%, Peffect = 0.005); therefore, a random-effects model of analysis was used. The pooled proportion of patients who had pulmonary hypertension was 2% (95% CI = 0–4) (Fig 3F).
Thrombocytopenia
Two studies provided outcomes regarding thrombocytopenia in patients with HH and were included in the meta-analysis. Significant evidence of heterogeneity among the studies was found (Pheterogeneity = 0.013, I2 = 83.7%, Peffect < 0.008); therefore, a random-effects model of analysis was used. The pooled proportion of patients who had thrombocytopenia was 2% (95% CI = –1 to 5) (Fig 3G).
Publication bias
Funnel plot and Begg’s and Egger’s tests were performed to assess publication bias among the studies. The shapes of the funnel plots showed obvious evidence of asymmetry (Fig 4), and the P value of Egger’s test confirmed the existence of publication bias for the response to diazoxide (Begg’s test P = 0.462; Egger’s test P = 0.045). The trim-and-fill method showed no need for additional studies (Fig 5).
Fig 4. Funnel plot of diazoxide responsiveness for testing publication bias.
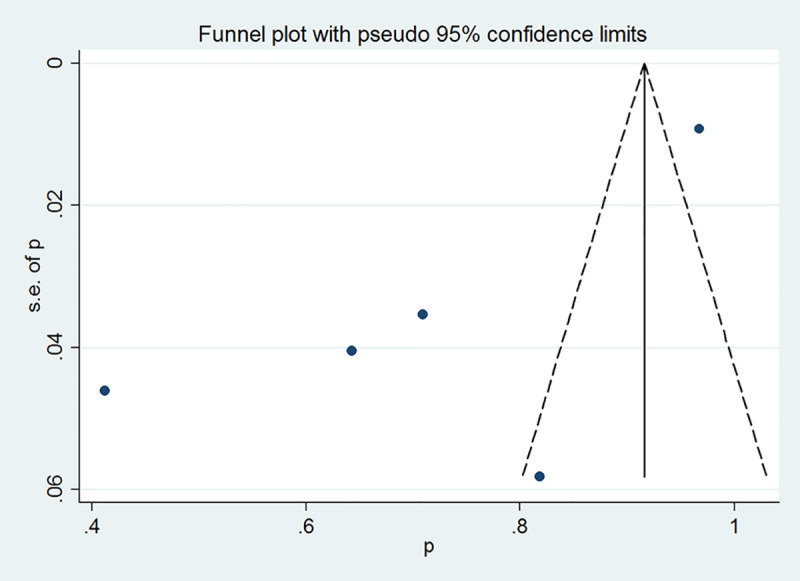
Each point represents a separate study for the indicated association.
Fig 5. Filled funnel plot of diazoxide responsiveness using the trim-and-fill method.

Discussion
This systematic review and meta-analysis evaluated the efficacy and safety of diazoxide for patients with HH. Six cohort studies involving 1142 patients were included. This meta-analysis was novel in evaluating the efficacy and safety of diazoxide for patients with HH. The meta-analysis showed that diazoxide was potentially useful in HH management; however, it had some side effects needing careful monitoring. Better-designed randomized controlled trials are still required to confirm the findings.
Diazoxide is the first-line drug for hyperinsulinemia and the only drug approved by the Federal Drug Administration. Its use has increased over the years, including patients with various genetic forms of hyperinsulinemia or perinatal stress hyperinsulinemia as well as infants of mothers with diabetes who have received this treatment more than ever before [25]. In 1964, diazoxide was first reported as an effective treatment for leucine-sensitive hypoglycemia [26]. Subsequently, several studies reported its effectiveness in treating metastatic or inoperable insulinomas [27,28]. Diazoxide is usually effective in children with complete KATP channels, but it is ineffective if the KATP channels are malformed due to ABCC8/KCNJ11 mutation. However, the exception is that children with ABCC8 mutation respond to diazoxide, indicating that cellular adaptation and redundancy in the standard KATP channel model determine the pathophysiology of CHI [29]. In this study, 71% (646/831) of patients responded to diazoxide treatment. Besides, about 25% of children with CHI were partially or completely unresponsive to diazoxide [30]. Other drugs, such as octreotide, may be required for the second-line treatment in such children [31].
The most common side effect is mild-to-severe hypertrichosis, which is thought to depend on the dose for each patient [18]. In this study, the most common adverse drug reaction was hypertrichosis (45%). For older children, body hair can be troublesome, and they may choose to use other drugs to avoid this complication. In theory, local KATP channel blockers, such as toluene butylamine, may reduce hair growth, but this indication has not been systematically assessed [32]. The retention of sodium and fluorine is a common side effect of diazoxide (18%) [17]. All patients taking diazoxide should be carefully monitored for weight, electrolytes and edema, and cardiopulmonary function, especially when starting or increasing doses. The simultaneous use of diuretics, such as chlorothiazide, can reduce urinary retention. In 2015, the US Food and Drug Administration issued a drug safety statement because 11 infants treated with nitrous oxide developed pulmonary hypertension (PH) [33]. It is believed that about 2.4% of all children treated with diazoxide have PH [17,18,34]. These data were consistent with the results of the present study (2%). A recent study published by Herrera et al. [17] employed all patients with a formal diagnosis of hyperinsulinemia. Their study included perinatal stress−induced hyperinsulinemia and showed that 2.4% of patients developed PH after starting the use of sodium diazoxide. They believed that PH is more likely to occur in preterm and low-birth-weight infants.
At the same time, some limitations of this meta-analysis should be emphasized. First, meta-analyses may be biased when literature searches fail to identify all relevant trials or subjectively apply selection criteria for including trials. To minimize these risks, thorough searches in multiple bibliographic databases were conducted, and clear criteria were used for research selection, data abstraction, and data analysis. Second, all the studies included in the present meta-analysis were observational. Observational studies are susceptible to selection bias and confusion, leading to the underestimation or overestimation of the actual effects of the intervention. Finally, some studies had small sample sizes, thus reducing the statistical power.
Conclusions
In summary, the results demonstrated that diazoxide had a potential role in treating HH; however, it had some side effects needing careful monitoring. Furthermore, well-designed large-scale studies, such as randomized controlled trials, might be necessary in the future to obtain more evidence.
Supporting information
(DOC)
(DOC)
(ZIP)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hasegawa T, Tanaka T, Kanzaki S, Sugihara S, Yakotani S, Tanaka H. Diagnosis and therapeutic guideline of hyperinsulinemic hypoglycemia. J Japan Pediatr Soc. 2006;110:1472–4. [Google Scholar]
- 2.Yorifuji T, Masue M, Nishibori H. Congenital hyperinsulinism: Global and J apanese perspectives. Pediatrics International. 2014;56(4):467–76. 10.1111/ped.12390 [DOI] [PubMed] [Google Scholar]
- 3.Flanagan SE, Kapoor RR, Hussain K, editors. Genetics of congenital hyperinsulinemic hypoglycemia Seminars in pediatric surgery; 2011: Elsevier. [DOI] [PubMed] [Google Scholar]
- 4.Cabezas OR, Flanagan SE, Stanescu H, García-Martínez E, Caswell R, Lango-Allen H, et al. Polycystic kidney disease with hyperinsulinemic hypoglycemia caused by a promoter mutation in phosphomannomutase 2. Journal of the American Society of Nephrology. 2017;28(8):2529–39. 10.1681/ASN.2016121312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tegtmeyer LC, Rust S, van Scherpenzeel M, Ng BG, Losfeld M-E, Timal S, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. New England Journal of Medicine. 2014;370(6):533–42. 10.1056/NEJMoa1206605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinney SE, Ganapathy K, Bradfield J, Stokes D, Sasson A, Mackiewicz K, et al. Dominant form of congenital hyperinsulinism maps to HK1 region on 10q. Horm Res Paediatr. 2013;80(1):18–27. Epub 2013/07/19. 10.1159/000351943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sempoux C, Capito C, Bellanne-Chantelot C, Verkarre V, De Lonlay P, Aigrain Y, et al. Morphological mosaicism of the pancreatic islets: a novel anatomopathological form of persistent hyperinsulinemic hypoglycemia of infancy. The Journal of Clinical Endocrinology & Metabolism. 2011;96(12):3785–93. 10.1210/jc.2010-3032 [DOI] [PubMed] [Google Scholar]
- 8.Demirbilek H, Hussain K. Congenital Hyperinsulinism: Diagnosis and Treatment Update. J Clin Res Pediatr Endocrinol. 2017;9(Suppl 2):69–87. Epub 2017/12/28. 10.4274/jcrpe.2017.S007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Cosio AP, Thornton P. Current and Emerging Agents for the Treatment of Hypoglycemia in Patients with Congenital Hyperinsulinism. Pediatric Drugs. 2019:1–14. 10.1007/s40272-018-0323-z [DOI] [PubMed] [Google Scholar]
- 10.Drash A, Kenny F, Field J, Blizzard R, Langs H, Wolff F. The therapeutic application of diazoxide in pediatric hypoglycemic states. Annals of the New York Academy of Sciences. 1968;150(2):337–55. 10.1111/j.1749-6632.1968.tb19059.x [DOI] [PubMed] [Google Scholar]
- 11.von Oettingen J, Armstrong K, Raza J, Raskin J, Zacharin M, Chanoine J-P. Application for inclusion of Diazoxide in the WHO Model List of Essential Medicines for Children (March 2019). [Google Scholar]
- 12.Hansen JB. Towards selective Kir6. 2/SUR1 potassium channel openers, medicinal chemistry and therapeutic perspectives. Current medicinal chemistry. 2006;13(4):361–76. 10.2174/092986706775527947 [DOI] [PubMed] [Google Scholar]
- 13.Meissner T, Wendel U, Burgard P, Schaetzle S, Mayatepek E. Long-term follow-up of 114 patients with congenital hyperinsulinism. European journal of endocrinology. 2003;149(1):43–51. 10.1530/eje.0.1490043 [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Xu Z, Yan J, Liu M, Sun B, Li W, et al. The treatment effect of diazoxide on 44 patients with congenital hyperinsulinism. Journal of Pediatric Endocrinology and Metabolism. 2012;25(11–12):1119–22. 10.1515/jpem-2012-0224 [DOI] [PubMed] [Google Scholar]
- 15.Wang W-Y, Sun Y, Zhao W-T, Wu T, Wang L, Yuan T-M, et al. Congenital hyperinsulinism in China: a review of Chinese literature over the past 15 years. Journal of clinical research in pediatric endocrinology. 2017;9(3):194 10.4274/jcrpe.3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukutomi M, Shimodera M, Maeda Y, Iwakura M, Hara M. Safety and effectiveness, including intelligence prognosis, of diazoxide in pediatric patients with hyperinsulinemic hypoglycemia: special survey in Japan (long-term, all-case survey). Clinical Pediatric Endocrinology. 2018;27(3):131–43. 10.1297/cpe.27.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera A, Vajravelu ME, Givler S, Mitteer L, Avitabile CM, Lord K, et al. Prevalence of adverse events in children with congenital hyperinsulinism treated with diazoxide. The Journal of Clinical Endocrinology & Metabolism. 2018;103(12):4365–72. 10.1210/jc.2018-01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornton P, Truong L, Reynolds C, Hamby T, Nedrelow J. Rate of serious adverse events associated with diazoxide treatment of patients with hyperinsulinism. Hormone research in paediatrics. 2019;91(1):25–32. 10.1159/000497458 [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 21.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Annals of internal medicine. 1997;127(9):820–6. 10.7326/0003-4819-127-9-199711010-00008 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, López-López JA, Becker BJ, Davies SR, Dawson S, Grimshaw JM, et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ global health. 2019;4(Suppl 1):e000858 Epub 2019/02/19. 10.1136/bmjgh-2018-000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455–63. 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 25.Welters A, Lerch C, Kummer S, Marquard J, Salgin B, Mayatepek E, et al. Long-term medical treatment in congenital hyperinsulinism: a descriptive analysis in a large cohort of patients from different clinical centers. Orphanet journal of rare diseases. 2015;10(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drash A, Wolff F. Drug therapy in leucine-sensitive hypoglycemia. Metabolism-Clinical and Experimental. 1964;13(6):487–92. 10.1016/0026-0495(64)90133-7 [DOI] [PubMed] [Google Scholar]
- 27.Marks V, Rose FC, Samols E. Hyperinsulinism due to metastasizing insulinoma: treatment with diazoxide. Proceedings of the Royal Society of Medicine. 1965;58(8):577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill G, Rauf O, MacFarlane I. Diazoxide treatment for insulinoma: a national UK survey. Postgraduate medical journal. 1997;73(864):640–1. 10.1136/pgmj.73.864.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henquin J-C, Nenquin M, Sempoux C, Guiot Y, Bellanné-Chantelot C, Otonkoski T, et al. In vitro insulin secretion by pancreatic tissue from infants with diazoxide-resistant congenital hyperinsulinism deviates from model predictions. The Journal of clinical investigation. 2011;121(10). 10.1172/JCI58400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee I, Skae M, Flanagan S, Rigby L, Patel L, Didi M, et al. The contribution of rapid KATP channel gene mutation analysis to the clinical management of children with congenital hyperinsulinism. European journal of endocrinology. 2011;164(5):733–40. 10.1530/EJE-10-1136 [DOI] [PubMed] [Google Scholar]
- 31.De León DD, Stanley CA. Mechanisms of disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nature Reviews Endocrinology. 2007;3(1):57 10.1038/ncpendmet0368 [DOI] [PubMed] [Google Scholar]
- 32.Shorter K, Farjo NP, Picksley SM, Randall VA. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. The FASEB Journal. 2008;22(6):1725–36. 10.1096/fj.07-099424 [DOI] [PubMed] [Google Scholar]
- 33.Food U, Administration D, Podcast FDS. FDA warns about a serious lung condition in infants and newborns treated with Proglycem (diazoxide). 2015. July 16. [Google Scholar]
- 34.Gray KD, Dudash K, Escobar C, Freel C, Harrison T, McMillan C, et al. Prevalence and safety of diazoxide in the neonatal intensive care unit. Journal of Perinatology. 2018;38(11):1496 10.1038/s41372-018-0218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]