Abstract
Durum wheat (Triticum turgidum L. var durum) is tetraploid wheat (AABB); it is the main source of semolina and other pasta products. Grain yield in wheat is quantitatively inherited and influenced by the environment. The genetic map construction constitutes the essential step in identifying quantitative trait loci (QTLs) linked to complex traits, such as grain yield. The study aimed to construct a genetic linkage map of two parents that are widely grown durum cultivars (Lahn and Cham1) in the Mediterranean basin, which is characterized by varying climate changes. The genetic linkage map of Lahn/Cham1 population consisted of 112 recombinant inbred lines (RILs) and was used to determine QTLs linked to the grain yield in 11 contrasting environments (favorable, cold, dry, and hot). Simple sequence repeat (SSR) molecular markers were used to construct an anchor map, which was later enriched with single nucleotide polymorphisms (SNPs). The map was constructed with 247 SSRs and enriched with 1425 SNPs. The map covered 6122.22 cM. One hundred and twenty-six QTLs were detected on different chromosomes. Chromosomes 2A and 4B harbored the most significant grain yield QTLs. Furthermore, by comparison with several wheat mapping populations, all the A and B chromosomes of Lahn/Cham1 QTLs contributed to grain yield. The results showed that the detected QTLs can be used as a potential candidate for marker-assisted selection in durum breeding programs.
Keywords: Molecular markers, grain yield, genetic linkage map, SNP, QTL
1. Introduction
Durum wheat originated through merging of two genomes (AABB) through natural interspecific hybridization and genome duplication (Zhang et al., 2012). Durum wheat (Triticum turgidum L. var durum) is the primary source of semolina for the production of pasta and couscous in the Mediterranean basin, and its cracked grain for burghul in the Middle East (Elouafi and Nachit, 2004). Moreover, durum wheat is grown mainly in drylands and is affected mainly by year-to-year environmental variation, drought, cold, and heat stresses (Nachit, 1992; El Hassouni et al., 2019).
Grain yield is quantitatively inherited and linked to multiple quantitative trait loci (QTLs) and their interactions with the environment. QTLs are DNA regions associated with a specific trait (Oduola et al., 2003). The phenotyping of grain yield and the use of molecular markers for the genotyping and the construction of a genetic map constitute a crucial step in QTL detection (Heidari et al., 2011). Molecular linkage maps have been constructed for durum wheat by several geneticists (Blanco et al., 1996, 1998; Korzun et al., 1999; Nachit et al., 2001; Elouafi and Nachit, 2004; Hail et al., 2012; Nagel et al., 2014; Alsaleh et al., 2015). The first durum map was made by the interspecific cross and was based on 65 recombinant inbred lines (RILs) and restriction fragment length polymorphism (RFLP) markers (Blanco et al., 1996, 1998), and later simple sequence repeats (SSR) were integrated into this genetic map (Korzun et al., 1999). The second durum map was based on an intraspecific cross Jennah Khetifa/Cham1 with 110 RILS using RFLP, SSR, amplified fragment length polymorphism (AFLP), seed storage proteins, and genes (Nachit et al., 2001). Several other studies on durum genetic mappings were conducted (Maccaferri et al., 2008, 2010; Haile et al., 2012; Dura et al., 2013). Moreover, single nucleotide polymorphisms (SNPs) have been included in durum wheat genetic maps (Poecke et al., 2013). Some researchers have integrated a significant number of SNP in bread and durum wheat genetic linkage maps (Maccaferri et al., 2015; Su et al., 2018; Zeng et al., 2019). Many QTL studies for different traits have been conducted for wheat (Tixier et al., 1998; Varshney et al., 2000; Sourdille et al., 2003; Elouafi and Nachit, 2004; Li et al., 2007; Pozniak et al., 2007; Maccaferri et al., 2008; Zhang et al., 2008; Bennett et al., 2012; Haile et al., 2012; Zhang et al., 2016; Tura et al., 2019; Xu et al., 2019; Zaïm et al., 2020).
In this study, the Lahn/Cham1 population was phenotyped for grain yield across 11 contrasting environments and was genotyped with mainly SSR and SNP markers to construct the genetic linkage map and to identify the QTLs linked to grain yield. The choice of Lahn and Cham1 as parents was due to their wide cultivation in the Mediterranean basin. Moreover, Lahn is highly productive in favorable environments and Cham1 is widely adapted, yield-stable, and drought-tolerant (Nachit et al., 2001; Kehel et al., 2010; Habash et al., 2014). We have provided the Lahn/Cham1 population to Barcelona University and Tunisia to be phenotyped for stable carbon isotope discrimination and other physiological traits (Bort et al., 2014). Moreover, the current study is the first on the genetic linkage mapping and QTL detection for Lahn/Cham1. (Lahn “Improved durum variety” was wrongly quoted as Jennah Khetifa, landrace in the paper published by Bort et al. (2014)).
The study’s aims were: 1) to construct and enrich the durum genetic linkage map of the Lahn/Cham1 population with SNP markers, and 2) to determine the QTLs linked to the grain yield in different contrasting environments.
2. Materials and methods
2.1. Plant materials
The studied durum population consisted of 112 F12 lines derived from a single-seed descent selection from the cross ICD-MN91-015 between the varieties Lahn and Cham1. The cross was made at CIMMYT/ICARDA durum breeding program for Mediterranean drylands. Lahn is an improved durum wheat variety developed for favorable and high-input environments with cold tolerance. It is also resistant to yellow rust (Puccinia striiformis) and moderately resistant to leaf rust (Puccinia recondita), powdery mildew (Erysiphe graminis), common bunt (Tilletia caries), and to Septoria leaf blotch (Mycosphaerella graminicola). Furthermore, Lahn has a large kernel size with superior hectoliter weight, milling extraction index, and gluten strength values. As for Cham1, the second parent of the population, it is also originated from the CIMMYT/ICARDA durum program, adapted to the Mediterranean climate, and has high yield stability. It exhibits an excellent resistance to drought and yellow rust with some tolerance to Russian wheat aphid. Cham1 also has high values for osmotic adjustment and high yellow pigment content, but its gluten strength is weak. However, Cham1 is susceptible to leaf rust, stem rust, Septoria tritici, and powdery mildew. This variety has a wide adaptation; it has been released in several countries in the Mediterranean basin and outside (Turkey, Syria, Cyprus, Iraq, Algeria, Portugal, Sudan, etc.) under different names.
2.2. Experimental field design
The Lahn/Cham1 population trial consisted of 112 recombinant inbred lines (RILs) and the two parents. For the experimental design, we used the augmented design (AD), according to Federer (1956) and Kehel et al. (2010), with 5 checks (Omrabi5, Haurani, Korifla, Waha, and Gidara2). The AD is based on randomized complete block design (RCBD) with 6 blocks. Each trial had 144 plots arranged as a grid layout of 12 rows by 12 columns. The Lahn/Cham1 population was grown in the field for grain yield (GY) evaluation in contrasting environments (Nachit and Elouafi, 2004; Nachit et al., 2016). The GY is harvested from 5 m2 plot and estimated as the harvested grain’s weight in kilogram per hectare. The testing sites description is shown in Table 1 with their locations, growing seasons, crop rotations, supplementary irrigation, and temperatures during the trial’s tillering and flowering stages.
Table 1.
Testing sites, environments (crop rotation) with growing seasons, sowing dates, annual rainfall, added irrigations, and mean temperatures at tillering and flowering stages for the field trials of Lahn/Cham1 population.
| Sites | Environment | Season | Sowing date | Rainfall (mm) | Irrigation (mm) | Mean temperature (°C) at | |
|---|---|---|---|---|---|---|---|
| Tillering(Zadoks scale 29) | Flowering(Zadoks scale 69) | ||||||
| Tel Hadya/Syria (ICARDA main station) (36°0.1′Nl 36°56′Em, 284m) | 02EPa (lentil) | 01/02 | Mid-October | 330 | 70 at sowing | 2 | 20 |
| 01IRb (vetch above biomass harvested) | 00/01 | Mid-November | 330 | 100 (50 at early tillering & 50 at booting) | 8 | 24 | |
| 01INCc (vetch biomass incorporated in soil) | 00/01 | Idem | 330 | None | 8 | 24 | |
| 02INCd (vetch biomass incorporated in soil) | 01/02 | Idem | 330 | None | 8 | 24 | |
| 02RFe (vetch above biomass harvested) | 01/02 | Idem | 330 | None | 8 | 24 | |
| Breda/Syria (dry) (35°0′Nl 38°0′Em, 300m) | 02BRf (fallow) | 01/02 | Idem | 260 | None | 6 | 27 |
| Ghab/Syria (high rainfall)(35°38′N 36°14′Em, 280m) | 01GHg (faba beans) | 00/01 | Idem | 650 | None | 5 | 20 |
| 02GHh (faba beans) | 01/02 | Idem | 614 | None | 5 | 20 | |
| Raqqa/Syria (irrigated) (35°57′ Nl 39°0′Em, 295m) | 01RQi (chickpea) | 00/01 | Idem | 150 | 450 (150 at sowing, 150 at tillering & 150 at flowering) | 10 | 30 |
| 02RQj (chickpea) | 01/02 | Idem | 150 | Idem | 10 | 30 | |
| Terbol/Lebanon (high & favorable rainfall) (33°49′Nl 35°58′Em, 890m) | 01TRk (faba beans) | 00/01 | Idem | 550 | None | 2 | 20 |
2.3. Population genotyping
The genotyping of the population was conducted at the ICARDA durum molecular breeding laboratory. The Lahn/Cham1 population was genotyped with SSR markers and 1500 polymorphic SNPs selected from 9400 SNP platform. The protocol for DNA extraction, molecular assay for microsatellites (SSR), AFLP, and seed storage proteins was conducted as described by Nachit et al. (2001). The genotyping by sequencing of RILs was performed using a whole-genome profiling service for SNP and diversity array technology sequencing (DarTseq markers). One hundred microliters of 50 ng µL-1 was analyzed using Diversity Array Technology, as described by Akbari et al. (2006).
2.4. Genetic linkage map construction
The genetic linkage map was constructed with 247 molecular markers: 216 SSR, 14 expressed sequence repeats (EST-SSR), 10 AFLP, 6 seed storage proteins (gliadin (Gli) and glutenin (Glu)), and 1 Pseudogliadine gene. Furthermore, we enriched the SSR map with 1425 polymorphic SNPs (Su et al., 2018); the remaining 75 SNPs were unlinked. We used the QTL IciMapping software V 4.1.0.0 (Wang et al., 2016) for mapping. Kosambi mapping function (Kosambi, 1944) was used to transform the recombination frequencies into centiMorgan (cM) distances.
2.5. Grain yield QTL detection
QTL IciMapping software V 4.1.0.0 (Wang et al., 2016) was used to detect the QTLs; the scanning was performed by the model “Inclusive Composite Interval Mapping Additive (ICIM-ADD)” through stepwise regression according to Li et al. (2007). The walking speed for all QTLs was 1.0 cM, and the stepwise regression probability was 0.01. We used a LOD threshold of 3 with 1000 permutations and an error of 0.05. Combined QTL analysis across all environments was conducted to identify QTLs with additive-by-environment (A by E) interaction effects, using the same parameters and the threshold of LOD 3 (Li et al., 2007) for the ICIM method of QTL analysis.
3. Results
3.1. Trial performance across the contrasting environments
Table 2 shows RIL mean, maximum, and minimum yields along the population parents Lahn (parent 1) and Cham1 (parent 2). In the irrigated and favorable environments, the parent’s grain yield performance is above the mean grain yields of the RILs mean. In the average yielding environments of 4000–5000 kg/ha (02RF, 02INC) and low yielding environments of 2000–2500 kg/ha (02BR), the parent’s grain yield was approximately similar to that of the mean grain yields of the RILs. Table 2 shows statistical parameters, including the trial heritability values. Furthermore, in all environments, several RILs have outyielded both parents (Table 2) significantly.
Table .
Table 2. Statistical parameters of grain yield (kg/ha) for RIL mean, maximum, and minimum yielders (kg/ha), and parents across testing environment.
| Parameter | 02RF | 01IR | 02EP | 01INC | 02INC | 02BR | 01GH | 02GH | 01RQ | 02RQ | 01TR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RILa mean | 4301 | 8590 | 8014 | 6010 | 5033 | 2311 | 6669 | 7947 | 6644 | 7517 | 6221 |
| Maximum | 5257 | 11217 | 13753 | 8457 | 6563 | 3373 | 11033 | 11933 | 10133 | 11083 | 8667 |
| Minimum | 2697 | 5033 | 3827 | 3433 | 2865 | 1273 | 2333 | 3100 | 1557 | 4277 | 1333 |
| P1b (Lahn) | 2873 | 9825 | 8787 | 6363 | 4821 | 2470 | 7517 | 8600 | 7867 | 8100 | 6000 |
| P2c (Cham1) | 3137 | 9617 | 10340 | 6433 | 5385 | 2170 | 8317 | 9867 | 7667 | 8433 | 7333 |
| LSDd (5%) | 436 | 758 | 1263 | 600 | 424 | 324 | 1306 | 2254 | 1897 | 1273 | 1620 |
| CVe % | 8.28 | 8.15 | 14.22 | 8.69 | 7.2 | 11.15 | 16.9 | 22.85 | 20.87 | 13.69 | 23.54 |
| Trial H2f | 0.36 | 0.43 | 0.54 | 0.46 | 0.32 | 0.44 | 0.29 | 0 | 0.09 | 0.31 | 0 |
| EGAg (%) | 5.27 | 27.39 | 28.27 | 19.95 | 34.1 | 13.6 | 40.44 | 12.19 | -6.55 | 27.59 | 16.77 |
The highest RIL grain yield means were achieved in the irrigated environments (01IR), the early sowing environment with supplementary irrigation (02EP), and the favorable and high-input environment (02GH). The maximum yields were observed in the early sowing environment with 13,753 kg/ha (02EP) followed by the favorable/high-input environment with fertile soil (02GH) 11,933 kg/ha, the irrigated environment (01IR) with 11,217 kg/ha, the favorable and high-input environment with fertile soil (01GH) 11,033 kg/ha, and the hot irrigated environment with 11,083 and 10,133 kg/ha (02RQ and 01RQ). The Ghab site with high soil fertility is located in a valley in north-western Syria, where soil is loamy and consists of sand, clay, silt, and black topsoil. Its fertility provides consistent high crops yields across years.
3.2. Map construction
The SNPs were mapped to all 14 chromosomes of durum wheat. The A chromosomes had 2902.61 cM (47.41%), whereas the B chromosomes had 3219.61 cM (52.59%). Furthermore, the A chromosomes were covered by 117 SSR markers (47.36%) and 598 SNPs (41.96%) with an average distance between adjacent loci of 4.06 cM. B chromosomes were covered by 130 SSR markers (52.64%) and 827 SNPs (58.04%) with an average distance between adjacent loci of 3.36 cM. The highest number of SNPs mapped was on 2B with 205 SNPs, whereas the lowest number of molecular markers mapped was on 7B with 23 SNP markers (Table 3).
Table 3.
Assignment and distribution of molecular markers, %, cM size, and coverage across the 14 A and B genomes for SSR and SNP enriched maps in the durum Lahn/Cham1 population.
| Chromosome | Markers | Size (cMb) in SSRc/SNPa enriched map | cMb/marker in SSRc/SNPa enriched map | |||
|---|---|---|---|---|---|---|
| SSRc | SNPa | All markers | % | |||
| 1A | 16 | 85 | 101 | 6.04 | 184.29/320.6 | 11.52/3.17 |
| 2A | 19 | 133 | 152 | 9.09 | 227.73/502.84 | 11.98/3.31 |
| 3A | 6 | 94 | 100 | 5.98 | 85.75/327.11 | 14.29/3.27 |
| 4A | 18 | 110 | 128 | 7.66 | 158.80/474.76 | 8.22/3.71 |
| 5A | 13 | 24 | 37 | 2.21 | 65.18/151.31 | 5.01/4.09 |
| 6A | 32 | 64 | 96 | 5.74 | 481.43/746.24 | 13.07/7.77 |
| 7A | 13 | 88 | 101 | 6.04 | 224.69/379.53 | 17.28/3.76 |
| A chromosomes | 117 | 598 | 715 | 42.76 | 1427.87/2902.61 | 12.20/4.06 |
| 1B | 37 | 166 | 203 | 12.14 | 252.27/678.09 | 6.82/3.34 |
| 2B | 21 | 205 | 226 | 13.52 | 246.29/694.15 | 11.73/3.07 |
| 3B | 11 | 27 | 38 | 2.27 | 81.13/245.37 | 7.37/6.46 |
| 4B | 18 | 138 | 156 | 9.33 | 111.63/477.61 | 6.20/3.06 |
| 5B | 12 | 166 | 178 | 10.65 | 101.55/609.17 | 8.46/3.42 |
| 6B | 21 | 102 | 123 | 7.36 | 165.68/350.54 | 7.89/2.85 |
| 7B | 10 | 23 | 33 | 1.97 | 105.32/164.68 | 10.53/4.99 |
| B chromosomes | 130 | 827 | 957 | 57.24 | 1063.87/3219.61 | 8.18/3.36 |
| Total | 247 | 1425 | 1672 | 100 | 2491.74/6122.22 | 10.09/3.66 |
For the group of A chromosomes, 2A harbored the largest number of markers (152) with the highest SNP mapping number (133), followed by 4A (128) with 110 SNPs and by 1A and 7A (101 each) with 85 and 88 SNPs, respectively (Table 3). The 2A showed the highest markers enrichment in the group of A chromosomes with a size of 502.84 cM and a density of 3.31 cM per marker. However, the 6A was the largest chromosome with 746.24 cM, but it was covered only with 96 markers; consequently, it has the lowest cM density per marker (7.77 cM/marker). The least enriched chromosome in the A chromosomes with SNP markers was the chromosome 5A (24 SNP). The entire A chromosomes had 715 markers with a map size of 2902.61 cM and an average density of 4.06 cM/marker (Table 3).
As for the group of B chromosomes, 2B had the highest number of molecular markers (226). It also had the highest number of SNPs (205) mapped; 1B and 5B followed it with 203 and 178, respectively. In both 1B and 5B, 166 SNPs each were mapped (Table 3). The lowest coverage in B chromosomes was the 7B chromosome with SNPs (23). The total number of molecular markers harbored by the B chromosomes was 957.
In the B chromosomes, 2B covered 694.15 cM, and it was relatively saturated: 3.07 cM per marker. Moreover, it was the highest enriched genome with SNP markers of the entire genome. The 6B was the most densely saturated chromosome: 2.85 cM/marker and 350.54 cM size. Furthermore, the whole B chromosome was covered by 957 markers with a size of 32,19.61 cM and an average density of 3.36 cM per marker. There were more SNPs mapped in the B chromosomes than in the A chromosomes: 827 for B vs. 598 for A chromosomes; and for the total markers mapped: 957 for B vs. 715 for A chromosomes. The B chromosome map size was larger than that of the A chromosomes: 3219.61 vs. 2902.61 cM. Similarly, for marker density (Table 3), B chromosomes were denser (3.66 cM/marker) than A chromosomes (4.06 cM/marker).
3.3. QTL mapping for grain yield in specific environments
In the specific environments, three QTLs were detected in the rainfall-favorable environment (01GH and 02GH), and two in the irrigated and hot environment (02RQ). Furthermore, four QTLs were detected in the cold favorable environment (02EP) and the dry environments (02INC and 02RF).
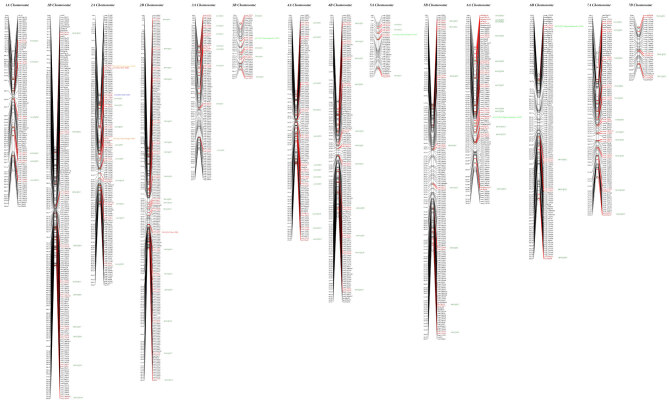
In 01GH, the first QTL was located at 5A chromosome and flanked on the left by ksm137bp420 and on the right by gwm154bp115 (Table 4). The second QTL was located at 6A and flanked on the left by McagEagc-bp170 and on the right by wsnp_1092345. The last QTL was detected at 6B chromosome, and its left flanking marker was wsnp_1708133, and the right flanking marker was gwm133bp125 (Table 4; Figure).
Table 4.
Significant QTL regions in contrasting environments for Lahn/Cham1 population with their chromosomal location, position, flanking markers, LOD scores, additive effect, and phenotypic variations.
| Environment | Chromosome | Position (cM) | Left marker | Right marker | LODh | PVEi (%) | Addj |
|---|---|---|---|---|---|---|---|
| 01GH | 5A | 54 | ksma137bp420 | gwmc154bp115 | 3.9248 | 11.4299 | 450.9197 |
| 01GH | 6A | 508 | McagEagcf-bp170 | wsnpg_1092345 | 3.4404 | 12.1341 | 520.5310 |
| 01GH | 6B | 14 | wsnpg_1708133 | gwmc133bp125 | 3.0045 | 8.8522 | 397.7209 |
| 02RQ | 2A | 63 | cnlb127bp435 | wsnpg_2276833 | 3.3358 | 9.435 | –518.7356 |
| 02RQ | 2B | 531 | wsnpg_1058086 | wmce175bp225 | 4.4356 | 13.0559 | –610.2649 |
| 02EP | 2A | 142 | wsnpg_2280363 | wmce177bp190 | 3.2202 | 12.5648 | 614.8357 |
| 02GH | 3B | 95 | wsnpg_1145565 | wsnpg_1005323 | 3.1525 | 11.4755 | –295.1018 |
| 02INC | 2A | 46 | wsnpg_2280371 | cnlb127bp435 | 3.9061 | 15.0246 | –180.9878 |
| 02RF | 2A | 270 | barcd183 | gwmc312bp189 | 3.6291 | 14.1871 | –159.9529 |
Figure.
Chromosomes and grain yield QTLs of Lahn/Cham1 genetic linkage map.
In the hot irrigated environment (02RQ), a QTL was detected at 2A with the flanking markers cnl127bp435 and wsnp_2276833 (Table 4; Figure). The analysis also showed another QTL at 2B chromosome, which was flanked by wsnp_1058086 and wmc175p225 (Table 4; Figure).
In addition, four other QTLs were detected, three of which at 2A chromosome in 02EP, 02INC, and in 02RF, whereas the fourth QTL was detected at 3B chromosome in 02GH environment (Table 4; Figure).
The expression of the QTLs occurred in all environments: moderately stressed (02INC and 02RF), favorable and cold (01GH, 02GH, and 02EP), and hot irrigated (02RQ). The differences of QTL detection between the high-input favorable sites (01GH and 02GH) are probably due to the different seasonal rainfall distribution: 02GH was affected by early drought.
3.4. Mapping in multienvironment trials
In multienvironmental mapping, 126 QTLs were detected for GY. The A chromosomes contained 53.17% of the total QTLs detected. The chromosomes 2A, 2B, and 6A harbored the highest QTL numbers: 13, 18, and 13, respectively. The GY-QTL distribution and their flanking markers are reported in Table 5 and Figure.
Table 5.
Significant QTL regions for multienvironment trials with their chromosomes, position, flanking markers, LOD scores,additive effect, and phenotypic variations.
| Chromosome | Position (cM) | Left marker | Right marker | LOD m | LOD (A) n | LOD (AbyE) o | PVE p | PVE (A) q | PVE (AbyE) r | Adds |
|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 75 | wsnp_993293l | gwm136bp300e | 4.25 | 1.01 | 3.24 | 1.91 | 0.78 | 1.13 | –61.09 |
| 1A | 105 | wsnp_1376461l | wsnp_1091468l | 3.45 | 0.10 | 3.35 | 0.91 | 0.08 | 0.83 | 19.37 |
| 1A | 127 | wsnp_1106684l | wsnp_1126484l | 5.27 | 0.36 | 4.91 | 2.11 | 0.29 | 1.82 | 37.24 |
| 1A | 203 | wsnp_1093499l | wsnp_2256752l | 5.39 | 0.41 | 4.98 | 2.89 | 0.31 | 2.57 | –38.66 |
| 1A | 228 | wsnp_3020949l | cfa2135bp175h | 3.72 | 0.40 | 3.32 | 3.63 | 0.31 | 3.33 | –38.29 |
| 1A | 281 | wsnp_2276661l | wsnp_1126203l | 3.10 | 0.39 | 2.71 | 1.04 | 0.31 | 0.74 | 38.28 |
| 1B | 26 | wsnp_1057112l | ksm112bp290a | 3.20 | 0.24 | 2.95 | 0.90 | 0.19 | 0.71 | 30.38 |
| 1B | 207 | wsnp_2266714l | wsnp_1718420l | 3.05 | 0.98 | 2.07 | 2.58 | 0.76 | 1.82 | –60.09 |
| 1B | 334 | wsnp_2280520l | wsnp_1090342l | 4.40 | 0.98 | 3.43 | 3.83 | 0.76 | 3.07 | 60.27 |
| 1B | 379 | wsnp_2278332l | wsnp_1207280l | 3.04 | 0.24 | 2.80 | 1.72 | 0.19 | 1.53 | 30.22 |
| 1B | 451 | wsnp_1240883l | wsnp_2265852l | 6.59 | 1.84 | 4.75 | 3.51 | 1.45 | 2.07 | 84.25 |
| 1B | 467 | wsnp_1215769l | wsnp_100004197l | 3.83 | 0.17 | 3.66 | 3.98 | 0.13 | 3.85 | 25.29 |
| 1B | 528 | wsnp_1089860l | wsnp_1138263l | 3.09 | 0.28 | 2.81 | 3.49 | 0.22 | 3.27 | 32.32 |
| 1B | 564 | wsnp_2265986l | wsnp_1164138l | 3.16 | 0.03 | 3.12 | 1.90 | 0.02 | 1.88 | 10.86 |
| 1B | 586 | wsnp_1006701l | wsnp_998041l | 5.27 | 0.18 | 5.09 | 3.36 | 0.14 | 3.23 | –26.09 |
| 1B | 677 | wsnp_10001692l | wsnp_1092947l | 3.46 | 0.93 | 2.54 | 2.59 | 0.71 | 1.88 | –58.38 |
| 2A | 46 | wsnp_2280371l | cnl127bp435b | 8.02 | 1.73 | 6.29 | 4.09 | 1.35 | 2.74 | –80.09 |
| 2A | 60 | cnl127bp435b | wsnp_2276833l | 7.08 | 4.27 | 2.81 | 7.08 | 2.99 | 4.09 | –119.35 |
| 2A | 142 | wsnp_2280363l | wmc177bp190g | 8.38 | 0.05 | 8.33 | 10.59 | 0.04 | 10.55 | 14.45 |
| 2A | 151 | wsnp_1122848l | wsnp_1092763l | 7.66 | 1.33 | 6.33 | 7.01 | 1.03 | 5.98 | –72.61 |
| 2A | 210 | wmc170bp225g | wsnp_1021220l | 3.60 | 1.32 | 2.28 | 3.14 | 1.02 | 2.12 | –69.75 |
| 2A | 253 | wsnp_2278093l | wsnp_1110051l | 6.17 | 2.16 | 4.02 | 5.16 | 1.60 | 3.56 | –87.19 |
| 2A | 269 | wsnp_1000185l | barc183f | 7.52 | 1.84 | 5.68 | 3.54 | 1.41 | 2.13 | –81.99 |
| 2A | 290 | gwm294bp135e | wsnp_1666851l | 5.02 | 1.61 | 3.40 | 4.05 | 1.25 | 2.81 | –77.04 |
| 2A | 335 | wsnp_979520l | wsnp_1128771l | 4.24 | 1.72 | 2.52 | 3.07 | 1.34 | 1.72 | –79.95 |
| 2A | 377 | wmc181bp255g | gwm311bp160e | 5.17 | 2.86 | 2.31 | 3.52 | 1.96 | 1.56 | –97.15 |
| 2A | 413 | wsnp_10017104l | wsnp_1010000l | 4.07 | 0.89 | 3.18 | 3.75 | 0.69 | 3.06 | –57.45 |
| 2A | 434 | wsnp_1128817l | wsnp_3029919l | 5.24 | 2.52 | 2.72 | 3.94 | 1.92 | 2.02 | –95.52 |
| 2A | 484 | wsnp_997103l | wsnp_2281375l | 5.24 | 2.56 | 2.67 | 4.83 | 2.00 | 2.84 | –97.50 |
| 2B | 4 | wsnp_1130302l | wsnp_998518l | 3.21 | 0.16 | 3.05 | 3.35 | 0.12 | 3.23 | 23.86 |
| 2B | 45 | wsnp_2257342l | wsnp_1199440l | 3.61 | 0.23 | 3.38 | 2.37 | 0.18 | 2.19 | 28.93 |
| 2B | 61 | wsnp_3025617l | wsnp_2278589l | 4.08 | 0.59 | 3.49 | 3.73 | 0.40 | 3.34 | 43.44 |
| 2B | 112 | wsnp_1021176l | wsnp_3022997l | 5.15 | 0.16 | 5.00 | 4.44 | 0.12 | 4.32 | 24.25 |
| 2B | 237 | wsnp_1204695l | wsnp_1164950l | 3.24 | 0.71 | 2.53 | 3.07 | 0.51 | 2.56 | 49.24 |
| 2B | 282 | wsnp_1205720l | wsnp_1160346l | 3.63 | 0.24 | 3.38 | 3.49 | 0.19 | 3.30 | 30.38 |
| 2B | 314 | wmc243bp175g | wsnp_1106597l | 3.20 | 0.52 | 2.68 | 1.56 | 0.41 | 1.16 | –45.31 |
| 2B | 335 | wsnp_100140l | wsnp_1082370l | 3.09 | 0.82 | 2.28 | 3.79 | 0.62 | 3.16 | 54.40 |
| 2B | 379 | wsnp_1109199l | gwm148bp175e | 4.72 | 0.12 | 4.59 | 1.87 | 0.09 | 1.78 | –21.16 |
| 2B | 406 | wsnp_3025955l | wsnp_1020753l | 4.66 | 0.17 | 4.49 | 2.66 | 0.13 | 2.53 | –24.79 |
| Chromosome | Position (cM) | Left marker | Right marker | LOD m | LOD (A) n | LOD (AbyE) o | PVE p | PVE (A) q | PVE (AbyE) r | Adds |
| 2B | 468 | wsnp_3027036l | wsnp_1218999l | 4.98 | 1.19 | 3.79 | 2.22 | 0.81 | 1.41 | –63.13 |
| 2B | 531 | wsnp_1058086l | wmc175bp225g | 8.08 | 2.15 | 5.94 | 8.17 | 1.67 | 6.50 | –89.23 |
| 2B | 552 | wsnp_1700082l | wsnp_1179226l | 4.80 | 0.20 | 4.59 | 2.27 | 0.15 | 2.12 | –26.95 |
| 2B | 575 | gwm47bp144e | wsnp_1386000l | 4.23 | 0.45 | 3.78 | 2.00 | 0.36 | 1.64 | –42.25 |
| 2B | 594 | wsnp_1133994l | wsnp_1228193l | 3.73 | 1.00 | 2.73 | 2.11 | 0.75 | 1.35 | –60.56 |
| 2B | 631 | wsnp_2282293l | wsnp_1009576l | 5.87 | 0.62 | 5.26 | 3.18 | 0.49 | 2.69 | –52.58 |
| 2B | 653 | wsnp_1122903l | wsnp_1227557l | 3.15 | 0.10 | 3.04 | 1.79 | 0.08 | 1.70 | –20.49 |
| 2B | 694 | wsnp_1393706l | barc159f | 3.63 | 0.01 | 3.62 | 3.17 | 0.01 | 3.16 | –4.94 |
| 3A | 0 | wsnp_1072225l | wsnp_1667169l | 6.41 | 0.00 | 6.41 | 5.50 | 0.00 | 5.50 | 1.63 |
| 3A | 20 | wsnp_1009844l | wsnp_1107433l | 3.79 | 0.21 | 3.58 | 3.18 | 0.16 | 3.02 | 27.88 |
| 3A | 46 | wsnp_1232627l | wsnp_3022183l | 3.72 | 0.13 | 3.60 | 3.37 | 0.10 | 3.28 | 21.62 |
| 3A | 67 | wsnp_1109262l | gwm5bp178e | 4.22 | 0.03 | 4.19 | 2.02 | 0.03 | 1.99 | –10.92 |
| 3A | 94 | wsnp_1218407l | gwm68bp190e | 4.65 | 1.85 | 2.79 | 3.80 | 1.42 | 2.38 | 82.33 |
| 3A | 151 | wsnp_2276600l | wsnp_1220348l | 4.13 | 0.67 | 3.46 | 3.09 | 0.52 | 2.57 | 49.95 |
| 3A | 196 | wsnp_1081242l | wsnp_3026254l | 5.35 | 1.26 | 4.10 | 4.79 | 0.97 | 3.82 | 69.19 |
| 3A | 291 | wsnp_1237635l | wsnp_1265491l | 3.10 | 0.60 | 2.50 | 2.97 | 0.47 | 2.50 | 47.71 |
| 3B | 1 | wsnp_1022121l | barc73bp205f | 3.69 | 0.04 | 3.65 | 3.61 | 0.03 | 3.58 | 11.79 |
| 3B | 92 | wsnp_1145565l | wsnp_1005323l | 4.48 | 0.31 | 4.17 | 2.56 | 0.23 | 2.33 | –33.34 |
| 3B | 164 | wsnp_1698409l | wsnp_1698619l | 5.16 | 1.63 | 3.53 | 2.49 | 1.17 | 1.31 | 76.47 |
| 3B | 213 | barc77bp220f | gwm547bp175e | 3.66 | 0.92 | 2.74 | 2.32 | 0.67 | 1.65 | 57.24 |
| 3B | 245 | gwm582bp200e | wsnp_1088815l | 4.15 | 0.87 | 3.28 | 3.07 | 0.67 | 2.40 | 56.77 |
| 4A | 21 | wsnp_1206766l | wsnp_1090457l | 3.30 | 0.00 | 3.29 | 2.35 | 0.00 | 2.35 | 4.06 |
| 4A | 59 | wsnp_1130454l | barc113bp140f | 7.38 | 1.17 | 6.21 | 3.27 | 0.86 | 2.41 | 66.30 |
| 4A | 150 | wsnp_979658l | wsnp_1218818l | 3.48 | 0.02 | 3.46 | 3.21 | 0.01 | 3.20 | –8.17 |
| 4A | 186 | wsnp_1064430l | wsnp_985258l | 3.25 | 0.21 | 3.04 | 4.00 | 0.16 | 3.83 | –28.01 |
| 4A | 204 | wsnp_1108161l | wsnp_1109226l | 3.33 | 0.45 | 2.87 | 1.84 | 0.33 | 1.51 | –40.10 |
| 4A | 266 | wsnp_1073246l | cfd31bp200i | 4.93 | 0.01 | 4.92 | 4.40 | 0.01 | 4.39 | 7.27 |
| 4A | 276 | wmc262bp150g | wmc232bp140g | 4.75 | 0.01 | 4.74 | 4.63 | 0.01 | 4.62 | –7.78 |
| 4A | 293 | wsnp_1309558l | wsnp_1766622l | 4.95 | 0.00 | 4.95 | 2.49 | 0.00 | 2.49 | 1.74 |
| 4A | 321 | wsnp_977411l | wsnp_3023881l | 4.99 | 0.00 | 4.99 | 6.62 | 0.00 | 6.62 | 3.57 |
| 4A | 442 | gwm160bp172e | wsnp_1086275l | 6.34 | 0.04 | 6.31 | 7.30 | 0.02 | 7.28 | 10.36 |
| 4A | 460 | wsnp_1111699l | wsnp_1053664l | 5.07 | 0.00 | 5.07 | 5.04 | 0.00 | 5.04 | 0.30 |
| 4A | 474 | wsnp_1073442l | barc327bp245f | 5.62 | 0.19 | 5.43 | 7.89 | 0.13 | 7.75 | 25.31 |
| 4B | 32 | barc193bp260 | wsnp_1033286 | 6.48 | 1.68 | 4.80 | 2.70 | 1.32 | 1.39 | 79.35 |
| 4B | 58 | wsnp_1009219 | wsnp_1201110 | 6.33 | 1.75 | 4.58 | 3.99 | 1.34 | 2.65 | 80.30 |
| 4B | 113 | wsnp_2281754 | wsnp_1260076 | 5.22 | 1.00 | 4.22 | 2.03 | 0.74 | 1.29 | 59.50 |
| 4B | 126 | gwm368bp287 | wsnp_2254072 | 6.04 | 0.15 | 5.89 | 3.63 | 0.12 | 3.51 | 23.89 |
| 4B | 182 | gwm513bp190 | dp23bp235 | 4.86 | 0.11 | 4.75 | 2.51 | 0.08 | 2.43 | 20.00 |
| 4B | 196 | gwm513bp150 | barc340bp210 | 4.05 | 0.06 | 3.99 | 2.81 | 0.05 | 2.76 | 15.13 |
| 4B | 225 | wsnp_1264915 | wsnp_1090257 | 3.09 | 0.02 | 3.06 | 1.93 | 0.02 | 1.91 | –9.34 |
| Chromosome | Position (cM) | Left marker | Right marker | LOD m | LOD (A) n | LOD (AbyE) o | PVE p | PVE (A) q | PVE (AbyE) r | Adds |
| 4B | 290 | wsnp_1036270 | wsnp_1220743 | 3.85 | 0.24 | 3.61 | 3.13 | 0.17 | 2.96 | –28.40 |
| 4B | 336 | wsnp_992780 | wsnp_1090893 | 3.80 | 0.15 | 3.65 | 1.68 | 0.11 | 1.57 | 22.84 |
| 4B | 375 | wsnp_1050158 | wsnp_1010596 | 3.01 | 1.45 | 1.55 | 1.67 | 1.10 | 0.57 | 72.61 |
| 4B | 449 | wsnp_1206903 | wsnp_1204109 | 3.21 | 0.82 | 2.40 | 2.25 | 0.62 | 1.62 | –55.05 |
| 5A | 12 | wsnp_2283193l | wsnp_1071495l | 3.80 | 0.02 | 3.78 | 2.38 | 0.02 | 2.36 | 9.03 |
| 5A | 35 | wsnp_3022906l | wsnp_981078l | 4.25 | 0.00 | 4.25 | 1.48 | 0.00 | 1.48 | 1.08 |
| 5A | 55 | ksm137bp420a | gwm154bp115e | 8.60 | 3.33 | 5.27 | 6.37 | 2.62 | 3.75 | 111.84 |
| 5A | 148 | barc100bp110f | wsnp_2257379l | 3.70 | 2.10 | 1.60 | 3.42 | 1.56 | 1.87 | 86.39 |
| 5B | 3 | wsnp_2280241l | wsnp_1095057l | 5.21 | 2.65 | 2.56 | 3.63 | 2.06 | 1.57 | –99.43 |
| 5B | 10 | wsnp_1079002l | wsnp_2279190l | 6.93 | 3.72 | 3.21 | 4.00 | 2.83 | 1.16 | –116.00 |
| 5B | 89 | wsnp_1111097l | wsnp_1094116l | 3.42 | 0.03 | 3.40 | 3.50 | 0.02 | 3.48 | –9.82 |
| 5B | 187 | wsnp_1695891l | wsnp_1078695l | 4.23 | 0.19 | 4.04 | 4.90 | 0.14 | 4.76 | –26.03 |
| 5B | 332 | wsnp_1091357l | wsnp_3024240l | 3.41 | 0.08 | 3.33 | 2.83 | 0.06 | 2.77 | –17.13 |
| 5B | 434 | wsnp_2278459l | wsnp_1081891l | 3.39 | 0.59 | 2.79 | 3.61 | 0.47 | 3.14 | –47.05 |
| 5B | 521 | barc74bp120f | gwm371bp317e | 3.03 | 0.58 | 2.45 | 2.12 | 0.44 | 1.68 | –45.84 |
| 5B | 584 | wsnp_2277122l | wsnp_1126527l | 3.67 | 0.54 | 3.13 | 3.41 | 0.42 | 3.00 | 44.54 |
| 6A | 35 | cfd190bp160i | wmc123bp135g | 3.70 | 0.39 | 3.30 | 2.58 | 0.29 | 2.29 | 45.50 |
| 6A | 56 | McacEaac-bp225d | pk7bp190k | 4.66 | 2.51 | 2.15 | 4.28 | 1.93 | 2.35 | 108.11 |
| 6A | 97 | pk7bp190k | gwm497bp118e | 3.05 | 0.76 | 2.29 | 1.42 | 0.58 | 0.84 | –58.32 |
| 6A | 185 | wsnp_1002536l | wsnp_2331540l | 3.65 | 0.30 | 3.35 | 3.00 | 0.23 | 2.77 | 34.26 |
| 6A | 345 | wmc201bp250g | gwm570bp140e | 4.32 | 0.50 | 3.82 | 1.75 | 0.40 | 1.35 | 43.80 |
| 6A | 387 | wsnp_1126162l | wsnp_30272001l | 3.98 | 0.24 | 3.74 | 3.84 | 0.18 | 3.66 | 29.25 |
| 6A | 401 | wsnp_1202412l | wsnp_1202955l | 3.81 | 0.02 | 3.79 | 4.07 | 0.02 | 4.05 | –9.48 |
| 6A | 443 | gwm169bp190e | wsnp_1231938l | 5.39 | 0.67 | 4.72 | 3.29 | 0.49 | 2.80 | 48.34 |
| 6A | 477 | wsnp_1231938l | wsnp_1125460l | 4.09 | 0.06 | 4.03 | 2.25 | 0.05 | 2.20 | –14.68 |
| 6A | 507 | McagEagc-bp170c | wsnp_1092345l | 6.00 | 0.23 | 5.76 | 4.33 | 0.18 | 4.15 | 33.96 |
| 6A | 566 | wmc41bp155g | gwm617bp123e | 5.25 | 2.01 | 3.23 | 2.55 | 1.40 | 1.15 | –112.97 |
| 6A | 578 | wmc173bp235g | wmc175bp260g | 4.80 | 1.26 | 3.54 | 3.75 | 0.90 | 2.85 | –122.39 |
| 6A | 700 | McagEagc-bp117c | pk19bp160k | 3.66 | 0.05 | 3.61 | 2.66 | 0.04 | 2.62 | 14.80 |
| 6B | 15 | wsnp_1708133l | gwm133bp125e | 7.26 | 0.69 | 6.57 | 5.59 | 0.49 | 5.09 | 48.87 |
| 6B | 175 | wsnp_2329478l | wsnp_2261831l | 4.85 | 0.00 | 4.84 | 1.95 | 0.00 | 1.95 | 4.27 |
| 6B | 201 | wsnp_2266428l | wsnp_1178437l | 3.84 | 0.19 | 3.66 | 2.45 | 0.14 | 2.31 | 25.77 |
| 6B | 350 | gwm219bp255e | barc24bp190f | 3.35 | 0.43 | 2.93 | 1.94 | 0.32 | 1.62 | 39.68 |
| 7A | 43 | wsnp_1070109l | wsnp_3027359l | 4.09 | 0.37 | 3.72 | 3.69 | 0.28 | 3.41 | 36.34 |
| 7A | 88 | wsnp_1225184l | wsnp_1210139l | 3.81 | 0.20 | 3.61 | 2.69 | 0.15 | 2.53 | 27.06 |
| 7A | 150 | wsnp_1041994l | wsnp_1128093l | 5.42 | 0.54 | 4.88 | 4.18 | 0.34 | 3.84 | 40.19 |
| 7A | 177 | wsnp_1221352l | wsnp_3027187l | 6.82 | 1.03 | 5.79 | 6.02 | 0.76 | 5.25 | 60.38 |
| 7A | 202 | wsnp_1197565l | wsnp_1125508l | 3.78 | 1.48 | 2.30 | 2.73 | 1.13 | 1.60 | 73.22 |
| 7A | 223 | wsnp_996562l | wsnp_1080709l | 4.23 | 1.35 | 2.88 | 4.23 | 1.06 | 3.17 | 71.05 |
| 7A | 244 | wsnp_100078174l | wsnp_1130808l | 3.31 | 1.13 | 2.18 | 3.55 | 0.88 | 2.67 | 64.83 |
| Chromosome | Position (cM) | Left marker | Right marker | LOD m | LOD (A) n | LOD (AbyE) o | PVE p | PVE (A) q | PVE (AbyE) r | Adds |
| 7A | 268 | wsnp_1004422l | wsnp_2280119l | 4.34 | 0.03 | 4.31 | 5.06 | 0.02 | 5.04 | –10.06 |
| 7A | 289 | wsnp_1262886l | wsnp_1001581l | 3.80 | 0.00 | 3.80 | 2.17 | 0.01 | 2.16 | 7.00 |
| 7A | 326 | wsnp_1235900l | cfd020bp305i | 3.14 | 0.01 | 3.13 | 2.68 | 0.01 | 2.68 | 5.71 |
| 7A | 379 | gwm344bp135e | cfa2040bp325h | 4.90 | 0.23 | 4.67 | 5.82 | 0.17 | 5.65 | –28.56 |
| 7B | 5 | wmc606bp200g | barc279bp205f | 4.34 | 0.32 | 4.02 | 3.13 | 0.20 | 2.94 | 30.70 |
| 7B | 93 | wsnp_3021533l | gwm400bp148e | 4.40 | 0.03 | 4.37 | 2.80 | 0.02 | 2.77 | 10.66 |
| 7B | 150 | gwm573bp220e | wmc426bp200g | 5.56 | 0.96 | 4.60 | 3.03 | 0.69 | 2.35 | 57.47 |
aksm: Kansas State University (EST-SSR); bcnl: Cornell University (EST-SSR); cMcagEagc: MseI+cag and EcoRI+agc (AFLP); dMcacEaac: MseI+cac and EcoRI+aac (AFLP); egwm: Gatersleben Wheat Microsatellite (SSR); fbarc: Beltsville Agriculture Research Center (SSR); gwmc: Wheat Microsatellite Consortium ; hcfa: Clermont-Ferrand A-genome (SSR); icfd: Clermont-Ferrand D-genome (SSR); jdp: Dehydrin primers “functional genes” (SSR); kpk: PK Gupta /india (SSR); lwsnp: Wheat SNP (SNP); mLOD: Logarithm of odds; nLOD(A): LOD score for additive and dominance effects; oLOD(AbyE): LOD score for additive and dominance by environment effects; pPVE: Phenotypic variation explained by QTL at the current scanning position; qPVE(A): Phenotypic variation explained by additive and dominance effect at the current scanning position; rPVE(AbyE): Phenotypic variation explained by additive and dominance by environment effect at the current scanning position; sAdd: Additive effect at the current scanning position; positive numbers shows allelic effect from Lahn, negative numbers from Cham1.
The number of QTL contributions to grain yield was 126. The QTL detected covered all chromosomes. Furthermore, the A chromosomes QTL contributions were higher than in B chromosomes 67 vs. 59. The chromosomes with the highest number of QTL were 2B (18) followed by 2A and 6A (13) for each, whereas the lowest chromosome with QTL was 7B (Table 5; Figure). The comparison with several published wheat mapping populations (Table 6) showed that Lahn/Cham1 population harbored more QTLs contributing to grain yield and in all A and B chromosomes (LOD used for QTL detection was ≥3). Some chromosomes (Table 5; Figure) were harboring a large number of QTLs, as in the case of 1A, 1B, 2A, 2B, 4A, 6A, and 7A.
Moreover, the QTLs with an important QTL*Environment effects were on the chromosomes 4A, where the additive effects ranged from 25.31 to 66.3 and from –40.1 to –28.0. On 5B, the additive effect ranged from –116 to –26.03, and one QTL with an additive effect of 44.54. On 7A, several QTLs with a positive additive effect ranged from 27.06 to 73.22 and one QTL with a negative additive effect (–28.56). The contribution of the Lahn parent was 74 QTLs, of which 40 originated from A chromosomes and 34 from B chromosomes, while Cham1 contributed with 52 QTLs, 29 of which were originated from A chromosomes and 23 from B chromosomes.
3. Discussion
The Lahn/Cham1 genetic linkage map has been shown to share several SSR markers with other linkage maps (Nachit et al., 2001; Elouafi and Nachit, 2004; Haile et al., 2012; Zhang et al., 2012; Zhang et al., 2012; Nagel et al., 2014 Alsaleh et al., 2015). It shared an important number of SSR markers with the high-density microsatellite consensus maps for bread wheat (Somers et al., 2004) and durum wheat (Maccaferri et al., 2015). Moreover, Lahn/Cham1 map was concordant with the marker orders of the consensus maps.
Su et al. (2018) adopted a similar approach to include SNP markers. Similarly, Zeng et al. (2019) constructed a bread wheat linkage map with SNP markers using the Wheat 35K SNP array for genotyping the RILs of the population MX169 X CW86.
Kirigwi et al. (2007) reported that molecular markers on chromosome 4A linked to grain yield, explaining 20% of the phenotypic variation of grain yield. Concerning QTL detection, grain yield QTLs in wheat have been described in various studies (Maccaferri et al., 2010; McIntyre et al., 2010; Soriano et al., 2017). In Kuchel et al. (2007), bread wheat linkage map, which was mainly based on microsatellite molecular markers, the QTLs linked with grain yield were detected on chromosomes1B, 2D, 3B, 5A, 6B, 7A, 7B, 7D. Multiple QTL detection on similar chromosomes were also reported by Bogard et al. (2011). Additionally, Maccaferri et al. (2008) also reported among the 16 QTLs that affected grain yield, two major QTLs on chromosomes 2BL and 3BS showed significant effects across several environments. Kato et al. (2000) also recovered five regions in wheat chromosome 5A that contributed to grain yield; and according to Heidari et al. (2011), QTLs located on chromosomes 6A, 6B, and 6D accounted mostly for total grain yield variation in wheat. Furthermore, McIntyre et al. (2010) established that several chromosomes harbored QTLs for grain yield on chromosomes 1B, 1D, 4A, 4D, 6A-a, 6B, and 7A. In addition, Quarrie et al. (2005) found that the strongest grain yield QTL effects were located on chromosomes 7AL and 7BL. Under drought conditions, the microsatellite wmc89 located on 4A chromosome was suggested as a marker to enhance drought tolerance in wheat (Kirigwi et al., 2007). Furthermore, Bennett et al. (2012) found that two of the detected QTLs linked to heat tolerance were located on chromosome 3B.
Marza et al. (2006) showed that the QTL contribution effects ranged from 7% to 23% in Ning7840xClark wheat population, where the marker alleles from the parent Clark were associated with a positive effect for the majority of QTLs for yield and its components on 1AL, 1B, 4B, 5A, 6A, and 7A, and specific QTLs on 2BL, 2BS, 2DL, and 6B. Tura et al. (2019) identified 38 grain yield QTLs spread over the whole genome of the Excalibur/Kuri population of bread wheat with the exception of 5D and 6A chromosomes. The grain yield QTLs with the highest QTL*Environment effects were observed on chromosomes 4A, 5B, and 7A. In the Lahn/Chm1 population, the QTLs linked to grain yield were harbored in all 14 chromosomes. These results agree with those of other studies (Heidari et al., 2011; Zhang et al., 2012; Farré et al., 2016). These results were also similar to those of the recently published work by Tura et al. (2019). In contrast, most of the populations showed a few QTLs (Table 6). It appears that the choice of genetically diverse population parents is of paramount importance to detect all involved QTLs linked with wheat grain yield (Table 6).
Table 6.
Comparison of detected grain yield QTLs harbored by A and B chromosomes in different wheat mapping populations.
| Kuchel et al. (2007) | Maccaferri et al. (2008) | Bennett et al. (2012) | Kirigwi et al. (2007) | Heidari et al. (2011) | Quarrie et al. (2005) | Bogard et al. (2011) | McIntyre et al. (2010) | Marza et al. (2006) | Tura et al. (2019) | Lahn/Cham1 population | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent QTLs contribution per chromosome | ||||||||||||||
| RIL/Marker | 182/251 | 249/254 | 250/456 | 127/ 136 | 107/371 | 96/567 | 140/499 | 192/587 | 132/410 | 155/3502 | 112/1672 | TotalQTL | Lahn | Cham1 |
| 1A | + | + | + | 6 | 3 | 3 | ||||||||
| 1B | + | + | + | + | + | 10 | 7 | 3 | ||||||
| 2A | + | + | 13 | 1 | 12 | |||||||||
| 2B | + | + | + | + | 18 | 7 | 11 | |||||||
| 3A | + | + | + | 8 | 7 | 1 | ||||||||
| 3B | + | + | + | + | + | 5 | 4 | 1 | ||||||
| 4A | + | + | + | + | + | 12 | 8 | 4 | ||||||
| 4B | + | + | + | 11 | 8 | 3 | ||||||||
| 5A | + | + | + | + | 4 | 4 | 0 | |||||||
| 5B | + | + | + | 8 | 1 | 7 | ||||||||
| 6A | + | + | + | + | 13 | 8 | 5 | |||||||
| 6B | + | + | + | + | + | + | 4 | 4 | 0 | |||||
| 7A | + | + | + | + | + | + | + | 11 | 9 | 2 | ||||
| 7B | + | + | + | + | 3 | 3 | 0 | |||||||
Furthermore, the analysis of the multienvironment trial showed the overall QTLs present on the 4B chromosome has an important additive effect with parent Lahn contribution ranging from 15.13 to 80.30 and the parent Cham1 contribution from –55.05 to –9.34. In light of this, it appears that the 4B chromosome contributes largely to grain yield in the dry areas.
Our results under stressed environments showed agreement with those of Ribaut et al. (1997) and Almeida et al. (2013). It is expected that using marker-assisted selection for grain yield under drought and heat stresses will improve breeding under stress conditions.
Several studies have indicated the presence of QTLs and candidate genes involved in response to drought in the 4B chromosome (Nachit and Elouafi, 2004; Habash et al., 2009). In addition, in Mediterranean terminal stress, drought is usually combined with heat stress; both stresses are a major factor limiting grain yield in the Mediterranean region (Nachit, 1992; Nachit and Elouafi, 2004). Other findings on 4B have revealed the presence of QTLs linked to total biomass yield, grain yield, and straw yield (Li et al., 2014). Recently, the International Wheat Genome Sequence (IWGSC) released a wheat genome reference sequence along with annotated genes (called RefSeq v.1.0, IWGSC 2018)Wheat@URGI. Seq Repository [Online]. Website http://wheaturgi.versailles.inra.fr/Seq-Repository [Accessed 22 Jan 2021]. This information, along the whole genome sequence dataset of 16 wheat varieties (Edwards et al., 2012), allows identifying new SNP markers for QTL fine-mapping and identifying genes responsible for abiotic and biotic stress tolerance in wheat; as consequence, SNP markers linked to detected QTLs need to be annotated for genes involved in traits tolerance/resistance and grain yield performance in diverse and contrasting environments.
Acknowledgments
We would like to thank the International Center for Agricultural Research in Dry Area (ICARDA) for the financial support of this study and the support staff of the CIMMYT/ICARDA durum breeding program.
Footnotes
http://www.diversityarrays.com
References
- Akbari M Wenz P Craig V Carling J Xia L Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theoretical and Applied Genetics. 2006;113:1409–1420. doi: 10.1007/s00122-006-0365-4. [DOI] [PubMed] [Google Scholar]
- Almeida G Dan Macomb D Moroboshi C Nair S Bore A QTL mapping in three tropical maize populations reveals a set of constitutive and adaptive genomic regions for drought tolerance. Theoretical and Applied Genetics. 2013;126:583–600. doi: 10.1007/s00122-012-2003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaleh A Baloch FS Derma M Zara M Kilian B Genetic linkage map of anatolian durum wheat derived from a cross of Kunduru-1149× Cham1. Plant Molecular Biology Reporter. 2015;33:209–220. [Google Scholar]
- Bennett D Reynolds M Mullan D Izanloo A Kuchel H Detection of two major grain yield QTL in bread wheat (Triticum aestivum L.) under heat, drought and high yield potential. Theoretical and Applied Genetics. 2012;125:1473–1485. doi: 10.1007/s00122-012-1927-2. [DOI] [PubMed] [Google Scholar]
- Blanco A Bello MP Cenci A De Giovanni C D’Ovidien R A genetic linkage map of durum wheat. Theoretical and Applied Genetics. 1998;97:721–728. [Google Scholar]
- Blanco A De Giovanni C Laddomada B Sciancalepore A Simeone R Quantitative trait loci influencing grain protein content in tetraploid wheats. Plant Breeding. 1996;115:310–316. [Google Scholar]
- Bogard M Jourdan M Jourdan M Allard V Maître P Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield, and grain protein concentration in a winter wheat population segregating for flowering time QTLs. Journal of Experimental Botany. 2011;62:3621–3636. doi: 10.1093/jxb/err061. [DOI] [PubMed] [Google Scholar]
- Bort J Belhaj M Latiri K Kehel Z Araus JL Comparative performance of the stable isotope signatures of carbon, nitrogen and oxygen in assessing early vigour and grain yield in durum wheat. The Journal of Agricultural Science. 2014;152:408–426. [Google Scholar]
- Dura S Dower M Nachit MM Al Shehab F Detection of molecular markers associated with yield and yield components in durum wheat (Triticum turgidum L. var. durum) under saline conditions. Crop and Pasture Science. 2013;64:957–964. [Google Scholar]
- Edwards D Wilcox S Barrera RA Fleury D Cavanagh CR Bread matters: a national initiative to profile the genetic diversity of Australian wheat. Plant Biotechnology Journal. 2012;10:703–708. doi: 10.1111/j.1467-7652.2012.00717.x. [DOI] [PubMed] [Google Scholar]
- El Hassouni K Berkadia B Filali-Maltouf A Tidiane-Sall A Al-Abdellat A Loci Controlling Adaptation to Heat Stress Occurring at the Reproductive Stage in Durum Wheat. Agronomy. 2019;9:414–414. [Google Scholar]
- Elouafi I Nachit MM A genetic linkage map of the Durum x Triticum dicoccoides backcross population based on SSRs and AFLP markers, and QTL analysis for milling traits. Theoretical and Applied Genetics. 2004;108:401–413. doi: 10.1007/s00122-003-1440-8. [DOI] [PubMed] [Google Scholar]
- Farré A Sayers L Leverington-Waite M Goram R Orford S Application of a library of near isogenic lines to understand context dependent expression of QTL for grain yield and adaptive traits in bread wheat. BMC Plant Biology. 2016;16:161–161. doi: 10.1186/s12870-016-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federer WT Augmented (or hoonuiaku) designs. Hawaiian Planters. Record. 1956;55:191–208. [Google Scholar]
- Habash D Kehel Z Nachit MM Genomic approaches for designing durum wheat ready for climate change with a focus on drought. Journal of Experimental Botany. 2009;60:2805–2815. doi: 10.1093/jxb/erp211. [DOI] [PubMed] [Google Scholar]
- Habash DZ Baudo M Hindle M Powers SJ Defoin-Platel M Systems responses to progressive water stress in durum wheat. PLOS ONE 9: e108431. doi: 10. 2014;0108431 doi: 10.1371/journal.pone.0108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile K J Nachit MM Hammer K Badebo A Röder M S QTL mapping of resistance to race Ug99 of Puccinia graminis f. sp. tritici in durum wheat (Triticum durum Desf. Molecular Breeding. 2012;30:1479–1493. [Google Scholar]
- Heidari B Sayed-Tabatabaei BE Saeidi G Kearsey M Suenaga K Mapping QTL for grain yield, yield components, and spike features in a doubled haploid population of bread wheat. Genome. 2011;54:517–527. doi: 10.1139/g11-017. [DOI] [PubMed] [Google Scholar]
- Kato K Miura H Sawada S Mapping QTLs controlling grain yield and its components on chromosome 5A of wheat. Theoretical and Applied Genetics. 2000;101:1114–1121. [Google Scholar]
- Kehel Z Habash DZ Gezan SA Welham SJ Nachit MM Estimation of spatial trend and automatic model selection in augmented designs. Agronomy Journal. 2010;102:1542–155. [Google Scholar]
- Kirigwi FM Van Ginkel M Brown-Guedira G Gill BS Paulsen GM Markers associated with a QTL for grain yield in wheat under drought. Molecular Breeding. 2007;20:401–413. [Google Scholar]
- Korzun V Röder MS Wendehake K Pasqualone A Lotti C Integration of dinucleotide microsatellites from hexaploid bread wheat into a genetic linkage map of durum wheat. Theoretical and Applied Genetics. 1999;98:1202–1207. [Google Scholar]
- Kosambi DD The estimation of map distances from recombination values. Annual Eugenics. 1944;12:172–175. [Google Scholar]
- Kuchel H Williams KJ Langridge P Eagles HA Jefferies SP Genetic dissection of grain yield in bread wheat. I. QTL analysis. Theoretical and Applied Genetics. 2007;115:1029–1041. doi: 10.1007/s00122-007-0629-7. [DOI] [PubMed] [Google Scholar]
- Li H Ye G Wang J A modified algorithm for the improvement of composite interval mapping. Genetics. 2007;175:361–374. doi: 10.1534/genetics.106.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S Jia J Wei X Zhang X Li L A intervarietal genetic map and QTL analysis for yield traits in wheat. Molecular Breeding. 2007;20:167–178. [Google Scholar]
- Li ZK Jiang XL Peng T Shi CL Han SX Mapping quantitative trait loci with additive effects and additive x additive epistatic interactions for biomass yield, grain yield, and straw yield using a doubled haploid population of wheat (Triticum aestivum L.) Genetics and Molecular Research. 2014;13:1412–1424. doi: 10.4238/2014.February.28.14. [DOI] [PubMed] [Google Scholar]
- Maccaferri M Ricci A Salvi S Milner SG Noli E High-density, SNP-based consensus map of tetraploid wheat as a bridge to integrate durum and bread wheat genomics and breeding. Plant Biotechnology Journal. 2015;13:648–663. doi: 10.1111/pbi.12288. [DOI] [PubMed] [Google Scholar]
- Maccaferri M Sanguineti MC Corneti S Araus O Ben Salem M Quantitative trait loci for grain yield and adaptation of durum wheat (Triticum durum Desf.). across a wide range of water availability. Genetics. 2008;178:489–511. doi: 10.1534/genetics.107.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri M Sanguineti MC Demontis El-Ahmed A Del Moral LFG Association mapping in durum wheat grown across a broad range of water regimes. Journal of Experimental Botany. 2010;62:409–438. doi: 10.1093/jxb/erq287. [DOI] [PubMed] [Google Scholar]
- Marza F Bai GH Carver FB Zhou WC Quantitative trait loci for yield and related traits in the wheat population Ning7840× Clark. Theoretical and Applied Genetics. 2006;112:688–698. doi: 10.1007/s00122-005-0172-3. [DOI] [PubMed] [Google Scholar]
- McIntyre CL Mathews KL Rattey A Chapman SC Drenth J Molecular detection of genomic regions associated with grain yield and yield-related components in an elite bread wheat cross evaluated under irrigated and rainfed conditions. Theoretical and Applied Genetics. 2010;120:527–541. doi: 10.1007/s00122-009-1173-4. [DOI] [PubMed] [Google Scholar]
- Durum wheat breeding for Mediterranean dryland of North Africa and West Asia. 1992. pp. 14–27.
- Nachit MM Elouafi I Rao SC Ryan J Durum wheat adaptation in the Mediterranean dryland: Breeding, stress physiology, and molecular markers. 2004;10:203–218. [Google Scholar]
- Nachit MM Elouafi I Pagnotta A El Saleh A Iacono E Molecular linkage map for an intraspecific recombinant inbred population of durum wheat (Triticum turgidum L. var. Theoretical and Applied Genetics. 2001;102:177–186. [Google Scholar]
- Gene flow as a source of adaptation of durum wheat to changing climate conditions: double gradient selection technique. Applied Mathematics. 2016. pp. 259–268.
- Nagel M Navakode S Scheibal V Baum M Nachit MM The genetic basis of durum wheat germination and seedling growth under osmotic stress. Biologia Plantarum. 2014;58:681–688. [Google Scholar]
- Oduola A Joe M Philip A Alexander AB John K The nature and identification of quantitative trait loci: a community’s view. Nature Reviews Genetics. 2003;4:911–916. doi: 10.1038/nrg1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poecke RM Maccaferri M Tang J Truong HT Janssen Sequence-based SNP genotyping in durum wheat. Plant Biotechnology Journal. 2013;11:809–817. doi: 10.1111/pbi.12072. [DOI] [PubMed] [Google Scholar]
- Pozniak CJ Knox RE Clarke FR Clarke JM Identification of QTL and association of a phytoene synthase gene with endosperm colour in durum wheat. Theoretical and Applied Genetics. 2007;114:525–537. doi: 10.1007/s00122-006-0453-5. [DOI] [PubMed] [Google Scholar]
- Quarrie SA Steed A Calestani C Semikhodskii A Lebreton C A high-density genetic map of hexaploid wheat (Triticum aestivum L.). from the cross Chinese Spring × SQ1 and its use to compare QTLs for grain yield across a range of environments. Theoretical and Applied Genetics. 2005;110:865–880. doi: 10.1007/s00122-004-1902-7. [DOI] [PubMed] [Google Scholar]
- Ribaut JM Jiang C Gonzalez-de-Leon D Edmeades GO Hoisington D A Identification of quantitative trait loci under drought conditions in tropical maize. 2. Yield components and marker-assisted selection strategies. Theoretical and Applied Genetics. 1997;94:887–896. [Google Scholar]
- Röder MS Korzun V Wendehake K Plaschke MHT Leroy P A microsatellite map of wheat. Genetics. 1998;149:2007–2023. doi: 10.1093/genetics/149.4.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DJ Isaac P Edwards K A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.) Theoretical and Applied Genetics. 2004;109:1105–1114. doi: 10.1007/s00122-004-1740-7. [DOI] [PubMed] [Google Scholar]
- Soriano JM Malosetti M Roselló M Sorrells ME Royo C Dissecting the old Mediterranean durum wheat genetic architecture for phenology, biomass and yield formation by association mapping and QTL meta-analysis. PLoS ONE 12: e0178290. doi: 10. 2017;0178290 doi: 10.1371/journal.pone.0178290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourdille P Cadalen T Guyomarch H Snape JW Perretant MR An update of the courtot x Chinese spring intervarietal molecular marker linkage map for the QTL detection of agronomic traits in wheat. Theoretical and Applied Genetics. 2003;106:530–538. doi: 10.1007/s00122-002-1044-8. [DOI] [PubMed] [Google Scholar]
- Su Q Zhang X Zhang W Zhang N Song L QTL detection for kernel size and weight in bread wheat (Triticum aestivum L.) using a high-density SNP and SSR-Based linkage map. Frontiers in Plant Science. 2018;9:1484–1484. doi: 10.3389/fpls.2018.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tixier MH Sourdille P Charmet G Gay G Jaby C Detection of QTLs for crossability in wheat using a doubled-haploid population. Theoretical and Applied Genetics. 1998;97:1076–1082. [Google Scholar]
- Tura H Edwards J Gahlaut V Garcia M Sznajder B QTL analysis and fine mapping of a QTL for yield-related traits in wheat grown in dry and hot environments. Theoretical and Applied Genetics. 2019;133:239–257. doi: 10.1007/s00122-019-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney RK Prasad M Roy JK Kumar N Harjit-Singh Dhaiwal HS Identification of eight chromosomes and a microsatellite marker on 1AS associated with QTL for grain weight in bread wheat. Theoretical and Applied Genetics. 2000;100:1290–1294. [Google Scholar]
- QTL IciMapping software. 4.1.0. 2016.
- Xu D Wen W Fu L Li F Li J Genetic dissection of a major QTL for kernel weight spanning the Rht-B1 locus in bread wheat. Theoretical and Applied Genetics. 2019;132:3191–3200. doi: 10.1007/s00122-019-03418-w. [DOI] [PubMed] [Google Scholar]
- Zaïm M Kabbaj H Kehel Z Gorjanc G Filali-Maltouf A Combining QTL analysis and genomic predictions for four durum wheat populations under drought conditions. Frontiers in Genetics. 2020;11:316–330. doi: 10.3389/fgene.2020.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q Wu J Huang S Yuan F Liu S SNP-based linkage mapping for validation of adult plant stripe rust resistance QTL in common wheat cultivar Chakwal 86. The Crop Journal. 2019;7:176–186. [Google Scholar]
- Zhang D Hao C Wang L Zhang X Identifying loci influencing grain number by microsatellite screening in bread wheat (Triticum aestivum L.) Planta. 2012;236:1507–1517. doi: 10.1007/s00425-012-1708-9. [DOI] [PubMed] [Google Scholar]
- Zhang H Chen J Li R Deng Z Zhang K Conditional QTL mapping of three yield components in common wheat (Triticum aestivum L.) The Crop Journal. 2016;4:220–228. [Google Scholar]
- Zhang L Luo JT Hao M Zhang LQ Yuan ZW Genetic map of Triticum turgidum based on a hexaploid wheat population without genetic recombination for D genome. BMC Genetics. 2012;13:69–69. doi: 10.1186/1471-2156-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W Chao S Manthey F Chicaiza O Brevis JC QTL analysis of pasta quality using a composite microsatellite and SNP map of durum wheat. Theoretical and Applied Genetics. 2008;117:1361–1377. doi: 10.1007/s00122-008-0869-1. [DOI] [PubMed] [Google Scholar]