Abstract
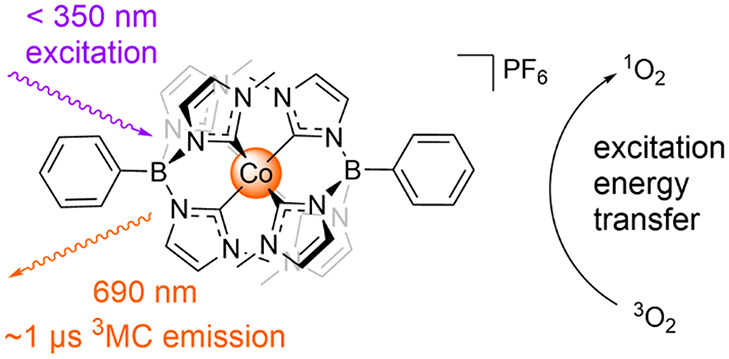
The photofunctionality of the cobalt–hexacarbene complex [Co(III)(PhB(MeIm)3)2]+ (PhB(MeIm)3 = tris(3-methylimidazolin-2-ylidene)(phenyl)borate) has been investigated by time-resolved optical spectroscopy. The complex displays a weak (Φ ∼ 10–4) but remarkably long-lived (τ ∼ 1 μs) orange photoluminescence at 690 nm in solution at room temperature following excitation with wavelengths shorter than 350 nm. The strongly red-shifted emission is assigned from the spectroscopic evidence and quantum chemical calculations as a rare case of luminescence from a metal-centered state in a 3d6 complex. Singlet oxygen quenching supports the assignment of the emitting state as a triplet metal-centered state and underlines its capability of driving excitation energy transfer processes.
Earth-abundant transition metal complexes have received increasing attention in recent years as photoactive components in prospective large-scale approaches for solar energy conversion and photocatalysis.1−3 Several first-row transition metals are interesting in this context, but unfortunately many 3d metal complexes suffer from short excited-state lifetimes compared to their 4d and 5d congeners.4,5 This is due to the presence of low-lying metal-centered (MC) states that facilitate fast, radiationless deactivation which limits their use in light-driven applications.6−9 Nevertheless, significant progress has recently been made to extend the excited-state lifetimes of 3d metal complexes, for example, by innovative ligand design to destabilize MC states by imposing a strong ligand field10,11 or by expanding investigations to a range of unconventional excited-state schemes beyond the common triplet metal-to-ligand charge transfer (3MLCT) state in d6 complexes.12,13 Though MC states are interesting for spin crossover (SCO) and light-induced excited-state spin trapping (LIESST) applications,14,15 in contrast to charge transfer (CT) states they are typically too low in energy to be interesting for photochemical applications or to display visible emission.8,16 As a rare case among the d6 complexes, very weak emission was reported from the 3MC state in [Co(CN)6]3–,17,18 while such states have only rarely been possible to observe in Ru(II) and Fe(II) complexes.19,20 Recently, remarkable photoproperties for Co(III) complexes were reported by Hannan and Zysman-Coleman and co-workers.21 Excited states of mixed triplet ligand-to-metal charge transfer/ligand centered (3LMCT/LC) character showed up to 8.7 ns blue emission in solution at room temperature. The photophysical properties of these complexes were related to the strong σ-donor ligands they contain.6,22 Tris(carbene)borate-based scorpionate ligands incorporating a negatively charged boron atom in their backbone are very strong σ-donors, and among these the tris(3-methylimidazolin-2-ylidene)(phenyl)borate anion ([PhB(MeIm)3]-) is one of the strongest.23,24 Hexacarbene complexes of Mn(IV) and Fe(III) featuring this ligand have been reported to exhibit extraordinary photophysical properties including photoluminescence.25,26 These findings spurred us to investigate the photophysics of [Co(III)(PhB(MeIm)3)2]+ (Figure 1) which was first reported in 2019 by Nishiura et al.27 Even though the complex showed no apparent absorption in the visible part of the spectrum, we nevertheless considered it interesting to obtain a new perspective on the excited-state properties of 3d6 complexes with strong σ-donor ligands.28,29
Figure 1.
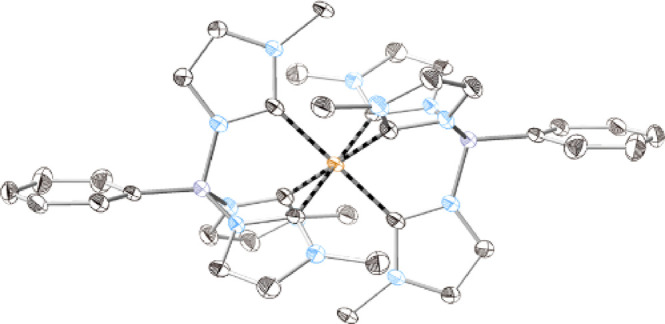
Crystal structure of [Co(PhB(MeIm)3)2]PF6. Ellipsoids drawn at 50% probability level. The counterion, solvent molecules, and hydrogen atoms omitted for clarity. Orange = Co; purple = B; blue = N; black = C.
We synthesized [Co(PhB(MeIm)3)2]PF6 following our procedure for the analogous Fe compound (see the Supporting Information). The crystal structure of [Co(PhB(MeIm)3)2]PF6 is almost identical with the one reported by Nishiura et al. of the same complex featuring triflate as a counterion.27 Compared to [Fe(PhB(MeIm)3)2]PF6, the C–M–C cis angles in the Co complex are closer to 90° (87.7° to 88.7° vs 86.4° to 87.5°), and the M–C bond lengths are on average 0.061 Å shorter (1.935 Å vs 1.996 Å) in the Co complex, which should lead to a stronger ligand field splitting.26 The same trend for bond lengths has earlier been found in structurally similar Fe and Co complexes with the tris(methylimidazolin-2-ylidene)hydroborate ligand.30
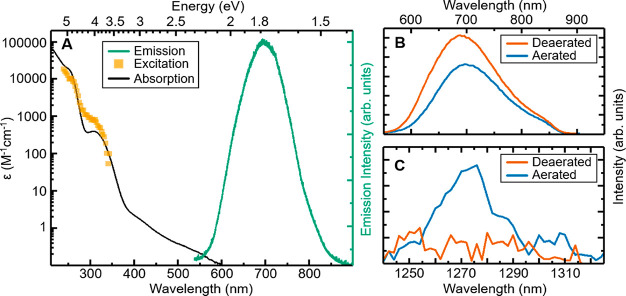
The absorption spectrum of [Co(PhB(MeIm)3)2]+ in acetonitrile is shown in Figure 2A. Very weak absorption features can be seen between 600 and 400 nm (ε < 3 M–1 cm–1; a more detailed discussion in the Supporting Information), followed by a slightly stronger absorption band around 310 nm (ε310 nm = 400 M–1 cm–1). A significantly stronger band is peaking around 250 nm (ε250 nm = 18000 M–1 cm–1) and further rising toward shorter wavelengths (ε212 nm = 63800 M–1 cm–1). These absorption features are similar to those of [Co(CN)6]3– incorporating strongly σ-donating cyanide ligands, which suggests MC character for the weak features >300 nm and transitions with more CT character for the more intense bands at lower wavelengths.31 Upon excitation of the complex an orange-red emission peaking ∼690 nm is observed (Figure 2A, green spectrum). The excitation spectrum corresponding to this emission (Figure 2A, orange spectrum) qualitatively follows the absorption spectrum, indicating the coupling of absorptive and emissive states. For excitation at 266 nm, we estimate a lower limit for the quantum yield of 0.01% (see the Supporting Information). The absorption at the 250 nm band is 45 times higher than at the 310 nm band. The excitation spectrum, however, yields only a factor of 17 ± 5 between those two bands, indicating that the quantum yield is about 2.5 times higher when exciting into the longer-wavelength absorption band.
Figure 2.
(A) Steady-state absorption (black) and excitation (orange, detected at 700 nm) spectrum of [Co(PhB(MeIm)3)2]+ in MeCN on a logarithmic scale. Normalized emission (green) after excitation at 266 nm. (B) Emission of [Co(PhB(MeIm)3)2]+ in deaerated MeCN (red) and after bubbling with oxygen (blue). (C) Emission of 1O2 from the same sample as in (B).
Experiments were also performed to check for sensitivity of the emissive state toward oxygen quenching. A comparison of the emission from aerated and deaerated samples (Figure 2B) showed additional quenching (∼30%) of the excited Co complex in the presence of oxygen with the concomitant appearance of a new emission peak at ∼1275 nm (Figure 2C). Although limited in yield, this observation is indicative of the excited state of the Co complex undergoing intersystem crossing (ISC) to a triplet excited state capable of excitation energy transfer (EET) forming singlet oxygen, which would not be possible from a quintet state.32
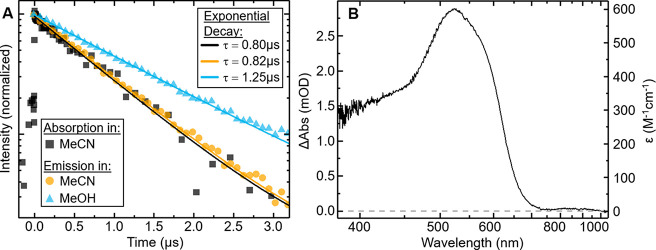
Time-resolved photoluminescence (TRPL) in several solvents was measured at room temperature by using 266 and 310 nm excitation. All combinations of solvent and excitation wavelength yield a single-exponential decay (see the Supporting Information). Figure 3A shows the observed PL kinetics in MeCN and MeOH for 266 nm excitation, yielding remarkable emission lifetimes of 0.82 and 1.25 μs, respectively.
Figure 3.
(A) Normalized kinetics of transient absorption probed at 532 nm (black) and time-resolved photoluminescence (orange and blue) after 266 nm excitation in MeCN (squares and circles) and MeOH (triangles). Single-exponential fits (lines) as a guide to the eye. (B) Transient absorption spectrum of [Co(PhB(MeIm)3)2]+ in MeCN following 266 nm excitation at time delay of 7.2 ns.
The excited-state dynamics in MeCN was further investigated by using transient absorption (TA) optical spectroscopy (experimental details in the Supporting Information). Figure 3B shows the differential absorption spectrum recorded 7 ns after excitation at 266 nm. A peak at 515 nm is formed on the picoseconds time scale and does not change within the 10 ns accessible in this experiment. To explore the full decay process, single-wavelength nanosecond TA was measured. Probing the excited-state absorption (ESA) dynamics at 532 nm (Figure 3A) yields a very similar τESA ∼ 0.8 μs lifetime as observed in TRPL. As both the emission and ESA signals have the same lifetime they can be associated with the population of the same state. The radiative lifetime of the emissive transition, its oscillator strength f, and molar absorption coefficient can furthermore be evaluated from the experimental lifetime and quantum yield as summarized in Table 1 (calculations in the Supporting Information). The molar absorption coefficient at the maximum of the excited-state absorption (ESA) feature at 515 nm is calculated to have a lower limit of 600 M–1 cm–1, which indicates that the strength of ESA transition is much larger than that of the emissive transition (estimated in equivalent units to molar absorption coefficient: 17 M–1 cm–1). This explains the appearance of the differential spectrum with minor (if any) contribution of stimulated emission.
Table 1. Summary of Key Photophysical Properties of [Co(PhB(MeIm)3)2]+ in MeCNa.
| λ [nm] | E [eV] | ε [M–1 cm–1] | f | τ [μs] | Φ [%] | |
|---|---|---|---|---|---|---|
| abs | 250 | 4.96 | 18000 | 1.7 × 10–1 | ||
| 310 | 4.00 | 400 | 6.5 × 10–3 | |||
| ESA | 515 | 2.41 | 600 | 0.80 | ||
| em | 690 | 1.80 | 17 | 1 × 10–10 | 0.82 | >0.01 |
λ = wavelength of band, E = energy of band, ε = molar absorption coefficient, f = oscillator strength, τ = lifetime, Φ = quantum yield, abs = absorption, ESA = excited-state absorption, and em = emission.
Quantum chemical calculations were performed by using density functional theory (DFT) and time-dependent DFT (TD-DFT) to characterize key states and processes involved in the photophysical processes (methods detailed in the Supporting Information). Calculations of singlet and triplet excited states by means of DFT for the lowest state of each singlet/triplet/quintet spin type as well as TD-DFT for vertical excitations indicate that the lowest excited singlet and triplet states display predominant MC character (details in the Supporting Information). This is also reflected in the spin density plot for the lowest relaxed triplet state (Figure 4A) showing significant spin density on the metal center and some metal–ligand mixing. The TD-DFT calculations thus support an interpretation of the experimental electronic absorption spectrum in which the weak absorption band at 310 nm is dominated by excitations to states with large MC contributions, while the stronger band at 250 nm corresponds to excitations with more significant CT character. This is in good agreement with general expectations on the relative intensities of weak MC and stronger CT bands.33 The calculated excited-state energy landscape (Figure 4B) visualizes an explanation of the main spectroscopic observations. The initially excited high-energy states (>3.5 eV) relax rapidly and with a remarkably large drop in excited-state energy into a long-lived lower-energy emissive state with 3MC character. From an electronic structure perspective, the population of an antibonding eg orbital in the 3MC excited state leads to a significant structural rearrangement of the molecule, mainly characterized by increased metal–ligand bond lengths. This results in a drop of the 3MC state energy and a concomitant increase of the singlet ground-state (1GS) energy. Thus, the observed vertical deexcitation energy from the relaxed 3MC state is only about 1.8 eV (690 nm), yet the calculated energy difference to the relaxed GS corresponds to more than 2.1 eV.
Figure 4.
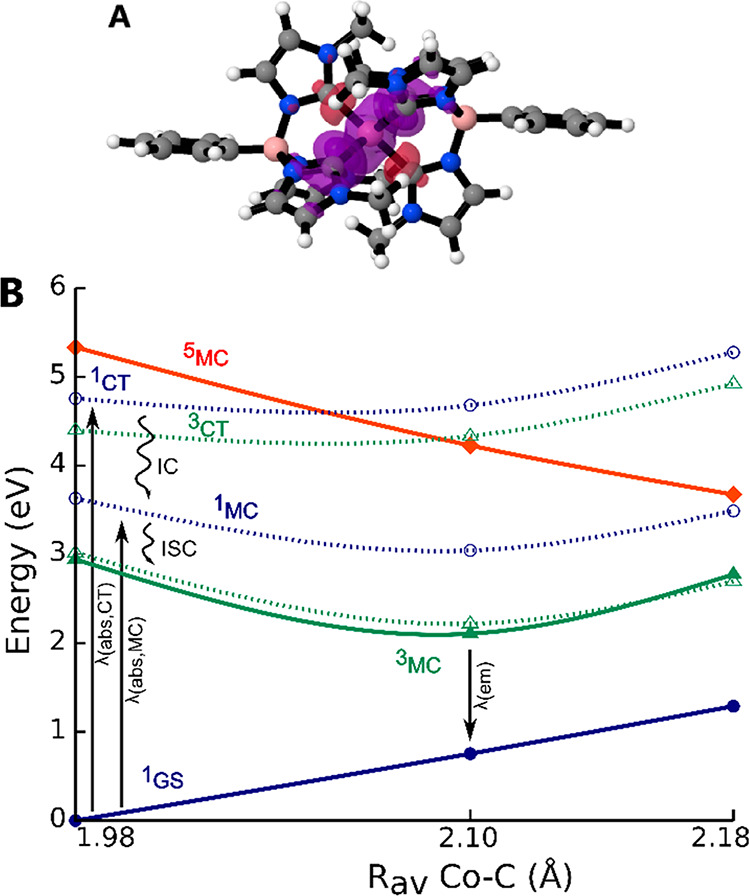
(A) Spin density plot for the lowest relaxed triplet state. Positive (excess α) and negative (excess β) spin density contributions are shown as purple and red isosurfaces, respectively, Contour of the plot 0.03. (B) Excited-state potential energy landscape, including calculated vertically excited singlet and triplet state energies from TD-DFT with sketched energy surfaces (dashed lines) for visual guidance, as well as singlet, triplet, and quintet unrestricted DFT energies at the lowest computational relaxed singlet, triplet, and quintet geometries with sketched energy surfaces (bold lines). Excitation and decay processes discussed in the text are marked by vertical arrows. IC = internal conversion, and ISC = intersystem crossing.
Key to achieving the experimentally observed, rare 3MC emission is that this state retains significantly higher energy than the 1GS also at its fully relaxed geometry. This is enabled by the very strong ligand field splitting (10 Dq = 38600 cm–1; more details in the Supporting Information) induced by the scorpionate carbene ligands. At the same time these ligands form a tight and rather rigid coordination environment around the metal center. This could be beneficial to slow down nonradiative decay pathways as recently found for other transition metal complexes.34,35 In our photophysical model, the ESA feature at 515 nm could correspond either to MC transitions in analogy to the suggestions by Viaene et al. for [Co(CN)6]3– or to CT transitions that are weakened due to the distorted geometry as suggested by Sun et al.36,37 The weak emission from the lowest excited state can be explained by the spin- and Laporte-forbidden nature of the transition to the 1GS, and its sensitivity to oxygen is indicative for triplet multiplicity. The photophysical properties of [Co(PhB(MeIm)3)2]+ with UV absorption and orange/red emission most closely parallel similar behavior previously established for [Co(CN)6]3– (at low temperatures) with the lowest energy excitation located at 396 nm and emission located at 714 nm.36 The excited-state lifetime of the 3T1 state of [Co(CN)6]3– was, however, found to be limited to <5 ns in aqueous solution at 22 °C.18 Furthermore, the excited-state lifetime of neither CT nor MC states in related Fe(II) complexes usually exceed a few nanoseconds under ambient conditions.38 It is worth noting that the photophysical behavior of [Co(PhB(MeIm)3)2]+ is fundamentally different from the blue-emitting Co complexes recently presented by Zysman-Colman, Hanan, and co-workers as well as from structurally related Fe carbene complexes.21,26,39 Instead of extending CT excited-state lifetimes by destabilizing the MC states, here the 3MC state itself is sufficiently high in energy above the 1GS energy surface to become a long-lived emissive state. This atypical behavior for d6 emitters more closely resembles the photophysics of transition metal complexes with MC states nested above the ground-state potential, such as some Cr(III) complexes, with long-lived emissive states.40
In summary, [Co(PhB(MeIm)3)2]+ shows microsecond emission from a 3MC state which is unique for 3d6 metal complexes. The capability of this complex to drive energy transfer reactions furthermore highlights the photofunctionality of this unconventional excited state. It will be important in further work to improve the light-harvesting capabilities through ligand design modifications while retaining the favorable 3MC excited-state properties. Fortunately, carbene ligands can be tuned in regards to their σ- and π-properties, in contrast to earlier used CN– ligands. It will also be interesting to explore other avenues to utilize the long-lived excited MC state for a broader range of photochemical applications, for example, similar to the MC states that were recently suggested to play a key role in photoredox catalysis.41,42
Acknowledgments
Nidhi Kaul and Reiner Lomoth are acknowledged for providing (spectro)electrochemical data. Sofia Essén is acknowledged for HRMS measurements. Villy Sundström, Jonathan Taylor Yarraton, and James McCusker are acknowledged for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c12151.
The Swedish Foundation for Strategic Research (SSF) and the Knut and Alice Wallenberg (KAW) Foundatations are acknowledged for financial support. S.K. acknowledges funding from Wenner-Gren Stiftelserna (The Wenner-Gren Foundations). N.W.R. gratefully acknowledges funding from the Alexander von Humboldt Foundation within the Feodor-Lynen Fellowship program. P.P. acknowledges support from the Swedish Research Council (VR), the Swedish Energy Agency (Energimyndigheten), eSSENCE, and the computing centers LUNARC and NSC through support via SNIC. K.W. acknowledges support from the Swedish Research Council (VR), the LMK foundation and the Swedish Energy Agency (Energimyndigheten).
The authors declare no competing financial interest.
Supplementary Material
References
- Wenger O. S. Photoactive Complexes with Earth-Abundant Metals. J. Am. Chem. Soc. 2018, 140 (42), 13522–13533. 10.1021/jacs.8b08822. [DOI] [PubMed] [Google Scholar]
- Hockin B. M.; Li C.; Robertson N.; Zysman-Colman E. Photoredox Catalysts Based on Earth-Abundant Metal Complexes. Catal. Sci. Technol. 2019, 9 (4), 889–915. 10.1039/C8CY02336K. [DOI] [Google Scholar]
- Bozic-Weber B.; Constable E. C.; Housecroft C. E. Light Harvesting with Earth Abundant d-Block Metals: Development of Sensitizers in Dye-Sensitized Solar Cells (DSCs). Coord. Chem. Rev. 2013, 257 (21–22), 3089–3106. 10.1016/j.ccr.2013.05.019. [DOI] [Google Scholar]
- Photochemistry and Photophysics of Coordination Compounds I; Balzani V., Campagna S., Eds.; Topics in Current Chemistry; Springer: Berlin, 2007; Vol. 280. [Google Scholar]
- Photochemistry and Photophysics of Coordination Compounds II; Balzani V., Campagna S., Eds.; Topics in Current Chemistry; Springer: Berlin, 2007; Vol. 281. [Google Scholar]
- McCusker J. K. Electronic Structure in the Transition Metal Block and Its Implications for Light Harvesting. Science (Washington, DC, U. S.) 2019, 363 (6426), 484–488. 10.1126/science.aav9104. [DOI] [PubMed] [Google Scholar]
- Förster C.; Heinze K. Photophysics and Photochemistry with Earth-Abundant Metals – Fundamentals and Concepts. Chem. Soc. Rev. 2020, 49 (4), 1057–1070. 10.1039/C9CS00573K. [DOI] [PubMed] [Google Scholar]
- Wagenknecht P. S.; Ford P. C. Metal Centered Ligand Field Excited States: Their Roles in the Design and Performance of Transition Metal Based Photochemical Molecular Devices. Coord. Chem. Rev. 2011, 255 (5–6), 591–616. 10.1016/j.ccr.2010.11.016. [DOI] [Google Scholar]
- Banziger S. D.; Li X.; Valdiviezo J.; Zeller M.; Zhang P.; Beratan D. N.; Rubtsov I. V.; Ren T. Unsymmetrical Bis-Alkynyl Complexes Based on Co(III)(Cyclam): Synthesis, Ultrafast Charge Separation, and Analysis. Inorg. Chem. 2019, 58 (22), 15487–15497. 10.1021/acs.inorgchem.9b02557. [DOI] [PubMed] [Google Scholar]
- Lindh L.; Chábera P.; Rosemann N. W.; Uhlig J.; Wärnmark K.; Yartsev A.; Sundström V.; Persson P. Photophysics and Photochemistry of Iron Carbene Complexes for Solar Energy Conversion and Photocatalysis. Catalysts 2020, 10 (3), 315. 10.3390/catal10030315. [DOI] [Google Scholar]
- Braun J. D.; Lozada I. B.; Kolodziej C.; Burda C.; Newman K. M. E.; van Lierop J.; Davis R. L.; Herbert D. E. Iron(II) Coordination Complexes with Panchromatic Absorption and Nanosecond Charge-Transfer Excited State Lifetimes. Nat. Chem. 2019, 11 (12), 1144–1150. 10.1038/s41557-019-0357-z. [DOI] [PubMed] [Google Scholar]
- Dorn M.; Kalmbach J.; Boden P.; Päpcke A.; Gómez S.; Förster C.; Kuczelinis F.; Carrella L. M.; Büldt L. A.; Bings N. H.; Rentschler E.; Lochbrunner S.; González L.; Gerhards M.; Seitz M.; Heinze K. A Vanadium(III) Complex with Blue and NIR-II Spin-Flip Luminescence in Solution. J. Am. Chem. Soc. 2020, 142 (17), 7947–7955. 10.1021/jacs.0c02122. [DOI] [PubMed] [Google Scholar]
- Chábera P.; Lindh L.; Rosemann N. W.; Prakash O.; Uhlig J.; Yartsev A.; Wärnmark K.; Sundström V.; Persson P. Photofunctionality of Iron(III) N-Heterocyclic Carbenes and Related d Transition Metal Complexes. Coord. Chem. Rev. 2021, 426, 213517. 10.1016/j.ccr.2020.213517. [DOI] [Google Scholar]
- Gütlich P.; Hauser A. Thermal and Light-Induced Spin Crossover in Iron(II) Complexes. Coord. Chem. Rev. 1990, 97 (C), 1–22. 10.1016/0010-8545(90)80076-6. [DOI] [Google Scholar]
- Fatur S. M.; Shepard S. G.; Higgins R. F.; Shores M. P.; Damrauer N. H. A Synthetically Tunable System to Control MLCT Excited-State Lifetimes and Spin States in Iron(II) Polypyridines. J. Am. Chem. Soc. 2017, 139 (12), 4493–4505. 10.1021/jacs.7b00700. [DOI] [PubMed] [Google Scholar]
- McCusker J. K.; Walda K. N.; Magde D.; Hendrickson D. N. Picosecond Excited-State Dynamics in Octahedral Cobalt(III) Complexes: Intersystem Crossing versus Internal Conversion. Inorg. Chem. 1993, 32 (4), 394–399. 10.1021/ic00056a010. [DOI] [Google Scholar]
- Viaene L.; D’Olieslager J. Luminescence from and Absorption by the 3T1g Level of the Hexacyanocobaltate(III) Ion. Inorg. Chem. 1987, 26 (6), 960–962. 10.1021/ic00253a039. [DOI] [Google Scholar]
- Conti C.; Castelli F.; Forster L. S. Photophysics of Hexakis(Cyano)Chromate(3-) and Hexakis(Cyano)Cobaltate(3-) in Polyalcohol-Water Solutions at Room Temperature. J. Phys. Chem. 1979, 83 (18), 2371–2376. 10.1021/j100481a013. [DOI] [Google Scholar]
- Sun Q.; Mosquera-Vazquez S.; Suffren Y.; Hankache J.; Amstutz N.; Lawson Daku L. M.; Vauthey E.; Hauser A. On the Role of Ligand-Field States for the Photophysical Properties of Ruthenium(II) Polypyridyl Complexes. Coord. Chem. Rev. 2015, 282–283, 87–99. 10.1016/j.ccr.2014.07.004. [DOI] [Google Scholar]
- Britz A.; Gawelda W.; Assefa T. A.; Jamula L. L.; Yarranton J. T.; Galler A.; Khakhulin D.; Diez M.; Harder M.; Doumy G.; March A. M.; Bajnóczi É.; Németh Z.; Pápai M.; Rozsályi E.; Sárosiné Szemes D.; Cho H.; Mukherjee S.; Liu C.; Kim T. K.; Schoenlein R. W.; Southworth S. H.; Young L.; Jakubikova E.; Huse N.; Vankó G.; Bressler C.; McCusker J. K. Using Ultrafast X-Ray Spectroscopy To Address Questions in Ligand-Field Theory: The Excited State Spin and Structure of [Fe(Dcpp)2]2+. Inorg. Chem. 2019, 58 (14), 9341–9350. 10.1021/acs.inorgchem.9b01063. [DOI] [PubMed] [Google Scholar]
- Pal A. K.; Li C.; Hanan G. S.; Zysman-Colman E. Blue-Emissive Cobalt(III) Complexes and Their Use in the Photocatalytic Trifluoromethylation of Polycyclic Aromatic Hydrocarbons. Angew. Chem. 2018, 130 (27), 8159–8163. 10.1002/ange.201802532. [DOI] [PubMed] [Google Scholar]
- Wenger O. S. Is Iron the New Ruthenium?. Chem. - Eur. J. 2019, 25 (24), 6043–6052. 10.1002/chem.201806148. [DOI] [PubMed] [Google Scholar]
- Forshaw A. P.; Bontchev R. P.; Smith J. M. Oxidation of the Tris(Carbene)Borate Complex PhB(MeIm)3MnI(CO)3 to MnIV[PhB(MeIm)3]2(OTf)2. Inorg. Chem. 2007, 46 (10), 3792–3794. 10.1021/ic070187w. [DOI] [PubMed] [Google Scholar]
- Muñoz S. B.; Foster W. K.; Lin H.-J.; Margarit C. G.; Dickie D. A.; Smith J. M. Tris(Carbene)Borate Ligands Featuring Imidazole-2-Ylidene, Benzimidazol-2-Ylidene, and 1,3,4-Triazol-2-Ylidene Donors. Evaluation of Donor Properties in Four-Coordinate {NiNO}10 Complexes. Inorg. Chem. 2012, 51 (23), 12660–12668. 10.1021/ic301204b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. P.; Reber C.; Colmer H. E.; Jackson T. A.; Forshaw A. P.; Smith J. M.; Kinney R. A.; Telser J. Near-Infrared 2Eg → 4A2g and Visible LMCT Luminescence from a Molecular Bis-(Tris(Carbene)Borate)Manganese(IV) Complex. Can. J. Chem. 2017, 95 (5), 547–552. 10.1139/cjc-2016-0607. [DOI] [Google Scholar]
- Kjær K. S.; Kaul N.; Prakash O.; Chábera P.; Rosemann N. W.; Honarfar A.; Gordivska O.; Fredin L. A.; Bergquist K.; Häggström L.; Ericsson T.; Lindh L.; Yartsev A.; Styring S.; Huang P.; Uhlig J.; Bendix J.; Strand D.; Sundström V.; Persson P.; Lomoth R.; Wärnmark K. Luminescence and Reactivity of a Charge-Transfer Excited Iron Complex with Nanosecond Lifetime. Science (Washington, DC, U. S.) 2019, 363 (6424), 249–253. 10.1126/science.aau7160. [DOI] [PubMed] [Google Scholar]
- Nishiura T.; Takabatake A.; Okutsu M.; Nakazawa J.; Hikichi S. Heteroleptic Cobalt(III) Acetylacetonato Complexes with N-Heterocyclic Carbine-Donating Scorpionate Ligands: Synthesis, Structural Characterization and Catalysis. Dalton Trans. 2019, 48 (8), 2564–2568. 10.1039/C8DT04469D. [DOI] [PubMed] [Google Scholar]
- Ericson F.; Honarfar A.; Prakash O.; Tatsuno H.; Fredin L. A.; Handrup K.; Chabera P.; Gordivska O.; Kjær K. S.; Liu Y.; Schnadt J.; Wärnmark K.; Sundström V.; Persson P.; Uhlig J. Electronic Structure and Excited State Properties of Iron Carbene Photosensitizers - A Combined X-Ray Absorption and Quantum Chemical Investigation. Chem. Phys. Lett. 2017, 683, 559–566. 10.1016/j.cplett.2017.03.085. [DOI] [Google Scholar]
- Ponseca C. S.; Chábera P.; Uhlig J.; Persson P.; Sundström V. Ultrafast Electron Dynamics in Solar Energy Conversion. Chem. Rev. 2017, 117 (16), 10940–11024. 10.1021/acs.chemrev.6b00807. [DOI] [PubMed] [Google Scholar]
- Fränkel R.; Kernbach U.; Bakola-Christianopoulou M.; Plaia U.; Suter M.; Ponikwar W.; Nöth H.; Moinet C.; Fehlhammer W. P. Homoleptic Carbene Complexes. J. Organomet. Chem. 2001, 617–618, 530–545. 10.1016/S0022-328X(00)00713-0. [DOI] [Google Scholar]
- Gray H. B.; Beach N. A. The Electronic Structures of Octahedral Metal Complexes. I. Metal Hexacarbonyls and Hexacyanides. J. Am. Chem. Soc. 1963, 85 (19), 2922–2927. 10.1021/ja00902a014. [DOI] [Google Scholar]
- Guo D.; Knight T. E.; McCusker J. K. Angular Momentum Conservation in Dipolar Energy Transfer. Science (Washington, DC, U. S.) 2011, 334 (6063), 1684–1687. 10.1126/science.1211459. [DOI] [PubMed] [Google Scholar]
- Balzani V.; Bergamini G.; Campagna S.; Puntoriero F.. Photochemistry and Photophysics of Coordination Compounds: Overview and General Concepts. In Photochemistry and Photophysics of Coordination Compounds I; Balzani V., Campagna S., Eds.; Springer: Berlin, 2007; pp 1–36. [Google Scholar]
- Herr P.; Glaser F.; Büldt L. A.; Larsen C. B.; Wenger O. S. Long-Lived, Strongly Emissive, and Highly Reducing Excited States in Mo(0) Complexes with Chelating Isocyanides. J. Am. Chem. Soc. 2019, 141 (36), 14394–14402. 10.1021/jacs.9b07373. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lee T. S.; Favale J. M.; Leary D. C.; Petersen J. L.; Scholes G. D.; Castellano F. N.; Milsmann C. Delayed Fluorescence from a Zirconium(IV) Photosensitizer with Ligand-to-Metal Charge-Transfer Excited States. Nat. Chem. 2020, 12 (4), 345–352. 10.1038/s41557-020-0430-7. [DOI] [PubMed] [Google Scholar]
- Viaene L.; D’Olieslager J.; Ceulemans A.; Vanquickenborne L. G. Excited-State Spectroscopy of Hexacyanocobaltate(III). J. Am. Chem. Soc. 1979, 101 (6), 1405–1409. 10.1021/ja00500a009. [DOI] [Google Scholar]
- Sun Q.; Mosquera-Vazquez S.; Lawson Daku L. M.; Guénée L.; Goodwin H. A.; Vauthey E.; Hauser A. Experimental Evidence of Ultrafast Quenching of the 3MLCT Luminescence in Ruthenium(II)Tris-Bipyridyl Complexes via a 3dd State. J. Am. Chem. Soc. 2013, 135 (37), 13660–13663. 10.1021/ja407225t. [DOI] [PubMed] [Google Scholar]
- Rosemann N. W.; Chábera P.; Prakash O.; Kaufhold S.; Wärnmark K.; Yartsev A.; Persson P. Tracing the Full Bimolecular Photocycle of Iron(III)–Carbene Light Harvesters in Electron-Donating Solvents. J. Am. Chem. Soc. 2020, 142 (19), 8565–8569. 10.1021/jacs.0c00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chábera P.; Kjaer K. S.; Prakash O.; Honarfar A.; Liu Y.; Fredin L. A.; Harlang T. C. B.; Lidin S.; Uhlig J.; Sundström V.; Lomoth R.; Persson P.; Wärnmark K. FeII Hexa N-Heterocyclic Carbene Complex with a 528 ps Metal-to-Ligand Charge-Transfer Excited-State Lifetime. J. Phys. Chem. Lett. 2018, 9 (3), 459–463. 10.1021/acs.jpclett.7b02962. [DOI] [PubMed] [Google Scholar]
- Otto S.; Dorn M.; Förster C.; Bauer M.; Seitz M.; Heinze K. Understanding and Exploiting Long-Lived near-Infrared Emission of a Molecular Ruby. Coord. Chem. Rev. 2018, 359, 102–111. 10.1016/j.ccr.2018.01.004. [DOI] [Google Scholar]
- Ting S. I.; Garakyaraghi S.; Taliaferro C. M.; Shields B. J.; Scholes G. D.; Castellano F. N.; Doyle A. G. 3d-d Excited States of Ni(II) Complexes Relevant to Photoredox Catalysis: Spectroscopic Identification and Mechanistic Implications. J. Am. Chem. Soc. 2020, 142 (12), 5800–5810. 10.1021/jacs.0c00781. [DOI] [PubMed] [Google Scholar]
- Woodhouse M. D.; McCusker J. K. Mechanistic Origin of Photoredox Catalysis Involving Iron(II) Polypyridyl Chromophores. J. Am. Chem. Soc. 2020, 142 (38), 16229–16233. 10.1021/jacs.0c08389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.