Abstract
Background
Brucellosis occurs globally with highly variable incidence in humans from very low in North America and Western Europe to high in the Middle East and Asia. There are few data in Sub-Saharan Africa. This study estimated the incidence of human brucellosis in a pastoralist community in Kenya.
Methods
Between February 2015 and January 2016, we enrolled persons living in randomly selected households in Kajiado County. Free health care was offered at three facilities in the study area. Those who met the study clinical case definition completed a standardized questionnaire on demographics, clinical history and presentation. A blood sample was collected and tested by Rose Bengal test (RBT), then later tested at the Kenya Medical Research Institute laboratory for Brucella IgG and IgM by ELISA. Those who tested positive by both RBT and ELISA (IgG or IgM antibodies) were classified as confirmed while those that only tested positive for IgG or IgM antibodies were classified as probable. Further, sera were tested by polymerase chain reaction using a TaqMan Array Card (TAC) for a panel of pathogens causing AFI including Brucella spp. Annual incidence of brucellosis was calculated as the number of confirmed cases in one year/total number in the study population.
Results
We enrolled a cohort of 4746 persons in 804 households. Over half (52.3%) were males and the median age was 18 years (Interquartile range (IQR) 9 months– 32 years). A total of 236 patients were enrolled at three health facilities; 64% were females and the median age was 40.5 years (IQR 28–53 years). Thirty-nine (16.5%) were positive for Brucella antibodies by IgG ELISA, 5/236 (2.1%) by IgM ELISA and 4/236 (1.7%) by RBT. Ten percent (22/217) were positive by TAC. We confirmed four (1.7%) brucellosis cases giving an annual incidence of 84/100,000 persons/year (95% CI 82, 87). The incidence did not significantly vary by gender, age and location of residence.
Conclusion
We report a high incidence of brucellosis in humans among members of this pastoralist community. Brucellosis was the most common cause of febrile illness in this community.
Author summary
Brucellosis is a bacterial disease that affects both humans and animals. Humans get infected via ingestion of unpasteurized animal products from infected animals and direct contact during animal abortions and deliveries. Infected animals shed bacteria for life through milk and during deliveries posing a risk to those with occupational exposure to infected animals. As such, human disease is disproportionately high in regions with high prevalence of animal brucellosis. While human brucellosis is distributed globally, incidence is low in North America and Western Europe and high in Asia and the Middle East where the disease is endemic. Data from Africa are scarce. We set out to estimate the incidence of brucellosis in a pastoralist community with documented high Brucella sero-prevalence in humans and livestock. We followed up a cohort of 804 households for one year and tested household members who became ill in three designated health facilities. We estimated an incidence of 84 cases per 100,000 persons per year in this community. We also found that Brucella was the most common pathogen among persons who had febrile illness highlighting the importance of this pathogen in this rural pastoralist community. No brucellosis intervention measures were being implemented.
Introduction
Brucellosis is a common bacterial zoonosis caused by multiple Brucella spp, endemic in domestic and wild animals where it causes abortions, reduced fertility, poor weight gain and reduced milk production resulting in substantial productivity and economic losses [1]. Human transmission occurs via ingestion of unpasteurized animal products and direct contact during animal abortions and deliveries. Human infection is characterized by an acute or chronic debilitating illness characterized by fever, joint pains, night sweats, fatigue, headache, and weight loss persisting for weeks to months [2].
While human brucellosis is distributed globally, incidence is variable across regions [3,4]. Brucellosis incidence or rates ranges from low in North America and Western Europe (≤ 0.1 cases per 100,000 population), moderate in Central and Southern Latin America and parts of South Eastern Europe (3.5–35 cases per 100,000 population) and endemic in Asia and the Middle East where some of the highest estimates (>250 cases per 100,000 population) have been reported [4–7].
There are scarce data on incidence of human brucellosis in Africa, with typically only subnational data available from a few countries. Variable annual human brucellosis incidence has been reported, ranging from 3.5 per 100,000 population in Tunisia to 8.4 per 100,000 population in Algeria and 35 per 100,000 population in Chad and Tanzania [3,4,8]. Notably, most studies in Sub-Saharan Africa focused on Brucella antibody sero-prevalence in humans and livestock, data that provide an insight into the inferred high burden of brucellosis particularly among rural populations who heavily rely on livestock for their livelihood [9–11].
Data on incidence of human brucellosis in Kenya are not available. However, Brucella antibody sero-prevalence data suggest widespread exposure to Brucella spp in human and animal populations in Kenya. A recent review of occurrence of human brucellosis reported low sero-prevalence (<1%) in Western Kenya and Nairobi, to high sero-prevalence (up to 46%) in most regions under nomadic pastoralism in Kenya [11]. High Brucella sero-prevalence was reported in camels (10–38%), cattle (3–15%) and goats (4–17%) raised under pastoral and agro-pastoral systems and low seroprevalence reported in cattle (<1–9%) and goats (<2%) raised in small holder intensive farms [11–14]. These data on sero-prevalence taken together with the intricate relationships pastoralists have with their livestock and cultural practices around consumption of dairy products suggest high transmission rates in pastoralist communities [10,12,13,15].
The aim of this study was to estimate the incidence of and risk factors for brucellosis in a pastoralist community with documented high Brucella sero-prevalence in humans and livestock.
Methods
Ethics statement
The study received ethical approval by the Kenya Medical Research Institute Scientific Ethical Review Committee and Centers for Disease Control and Prevention Institutional Review Board. Approval was also obtained from the Kenya Ministry of Health, the Ministry of Agriculture Livestock and Fisheries and the County Government of Kajiado. Written informed consent was obtained from all enrolled participants.
Study site
Between February 2015 and January 2016, we established a prospective community cohort at Mashuru Sub-County of Kajiado County, with clinical follow up of participants at study health facilities. An earlier study in the county reported Brucella seroprevalence of 15% in humans and 3% in livestock (cattle, sheep and goats); >50% of households in areas surrounding Mashuru sub-county had at least one sero-positive animal [12].
The sub-county covers 2903km2 with an estimated total human population of 50,245 (density of 17.3/km2) based on the Kenya 2009 census. Most parts of Mashuru sub-county, which is inhabited by nomadic pastoralists, have semi-arid conditions and receive an average of 400–500 mm rainfall per annum. Livestock-keeping and subsistence crop farming are the main agricultural activities.
Selection of study locations and households
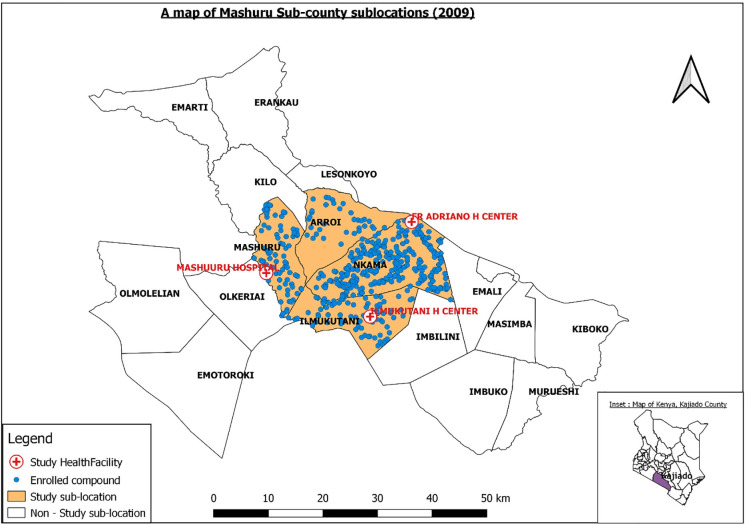
Four of 17 sub-locations (Arroi, Ilmukutani, Mashuru and Nkama), with a total human population of 15,036 living in 3210 households were selected in consideration of existing health centers. Total human population was 1321 in Arroi, 2266 in Ilmuktani, 2722 in Mashuru and 8727 in Nkama [16]. A sublocation is the smallest administrative unit in Kenya. We registered 500 compounds for longitudinal follow up (Fig 1). A compound comprised of a cluster of households of relatives or clanmates living in close proximity in an enclosure and who most often pooled and reared their livestock into one herd. The number of compounds targeted in each sub-location was weighted by population size based on the 2009 Kenya population census data [16]. In order to identify compounds to register, we generated 500 random geographical coordinates using ArcGIS (ESRI, Redlands, CA, USA) corresponding to number of compounds for each sublocation including 37 compounds in Arroi, 74 in Ilmuktani, 74 in Mashuru and 315 in Nkama sub-location. For each coordinate we selected the closest compound for enrollment. All households in a selected compound were invited to participate in the study. Each consenting household was assigned a unique household identifier.
Fig 1. Map of Mashuru sub-county showing the study sublocations and enrolled households.
Inset is a map of Kenya showing Kajiado county.
Recruitment of human cohort
All household members in a recruited household were eligible for enrollment into the study. Each household member was assigned a personal identification number and issued a card bearing their personal details for use during the health center visits. A baseline questionnaire was administered to the head of each household to collect demographic and health seeking behavior data on each household member and animal ownership data. Households were sensitized on clinical symptoms associated with brucellosis and asked to present at the only three health facilities distributed across the study area for care if they became ill with signs or symptoms compatible with brucellosis.
Longitudinal follow up in humans
A register of all household members was stored in all three study health facilities for use if any enrolled person presented for care. A study nurse and a laboratory technologist were placed in each health center to carry out study procedures. Enrollment into the study was performed Monday through Friday each week. A public health officer contacted all the household heads every two weeks to enquire of any illness and remind the household members to visit the study health facility for any illnesses.
Clinical case definition for enrollment at the health centers
For eligibility for enrollment, we used a non-specific clinical case definition that included a member of a registered household aged ≥ 1 year presenting at any of three study health facilities with temperature > 38°C at the time of clinic visit, or history of recurrent or continuous fever, and no identified cause of fever such as diarrhea and respiratory illness, and any two of: night sweats, joint pains, joint swelling, headache, fatigue, anorexia, muscle pain, or back pain.
Specimen and data collection
We enrolled into the study those who met the case definition and consented. We collected demographic data, history of illness and clinical presentation and contact with animals and animal products via a questionnaire on smartphones. A blood specimen was collected and centrifuged for serum separation at the health facility.
Diagnostic investigations
Initial serum testing was conducted at the health facility and two milliliters of serum were stored at -20°C at the health center and later shipped for storage and testing at the Center for Global Health Research, Kenya Medical Research Institute (KEMRI) laboratory in Kisumu.
Testing at health facility for case management
An aliquot of each serum sample was tested for brucellosis by Rose Bengal Plate Test (RBT) [VLA, UK] agglutination assay [17] and for malaria by a rapid diagnostic test (RDT) [Carestart] at the health facility to facilitate case management.
Testing at CGHR KEMRI laboratories in Kisumu
Brucella ELISA
All specimens were tested using IBL-America IgG and IgM enzyme-linked immunosorbent assay (ELISA) kits according to manufacturer’s recommendation as previously described [12].
Multi-pathogen testing by TaqMan array cards for Acute Febrile Illness (AFI TAC)
Total nucleic acid was extracted from 166 μl of sera in a KingFisher ML extraction platform (Thermo Scientific, Waltham, MA) using a MagMAX nucleic acid isolation kit (Life Technologies, Carlsbad, CA). Briefly, 166 μl of sample was mixed with 433 μl of lysis-binding solution and was then washed once with 600 μl wash solution 1 and twice with 450 μl wash solution two and was eventually eluted in 200 μl elution buffer. Molecular testing was performed by polymerase chain reaction using acute febrile illness TaqMan Array Card (AFI TAC) diagnostic V2 as described previously [18]. The TAC cards used in this study detects 17 viruses, 8 bacteria and 3 protozoa (S1 Fig). We assessed detection of Brucella DNA and other etiologies of febrile illness among all enrolled participants [19–21].
Brucellosis case classification
We used a modified case definition to the World Health Organization for confirmed cases [22]. We defined confirmed cases as those who met the study clinical case definition and had confirmatory laboratory diagnosis by either (i) testing positive for anti-Brucella agglutinating antibodies by RBT and anti-Brucella IgG or IgM antibodies by ELISA. We defined probable cases as those who met the study clinical case definition and were sero-positive for anti-Brucella IgG antibodies by ELISA only, or IgM only but negative by RBT.
Data analysis
All analyses were done using STATA 12 (Stata Corporation, College Station, TX, USA). Descriptive statistics were conducted for socio-demographic and other characteristics for the study cohort and those enrolled with febrile illness. Cases were categorized as confirmed, probable, indeterminate and negative for brucellosis. Confirmed Brucella positivity was determined as the proportion of confirmed cases against all persons tested.
Calculating annual incidence of brucellosis in humans
Annual incidence was estimated as the number of brucellosis confirmed cases in the study year (February 2015 –January 2016) /total number at risk in the study population per 100,000 population. The total number at risk in the population was obtained from the baseline survey where all members in the registered households were listed, provided with a personal identification number and were eligible for enrollment into the study if they met the case definition for the brucellosis upon evaluation by the study nurse. We calculated the incidence by gender, age (categorized as <20, 21–40, 41–60 and >60 years) and location of residence and reported with 95% confidence interval estimates. The estimated incidences were compared, and incidence ratios were calculated across the gender, age categories and location of residence and 95% CI reported.
Results
Study cohort demographic information
In February 2015, a cohort of 504 compounds comprising a total of 804 households in the four locations were registered for follow up. The total number of household members was 4,746; 52.3% were males and the median age was 18 years (upper and lower bounds of 25th and 75th percentile 9 months, 32 years) (Table 1). The median number of persons in each household was 6 (25th and 75th percentile 1–19). At enrollment, majority of households 780 (97%) owned at least one type of livestock with 95% (n = 764), 84% (n = 674) and 81% (n = 654) owning goats, cattle, and sheep, respectively.
Table 1. Demographic characteristics and household livestock ownership of the study cohort and enrolled patients, Kajiado 2015–2016.
| Demographic characteristics | Cohort n (%) N = 4746 N = | Suspected brucellosis cases N = 236 |
|---|---|---|
| Age (median, IQR) Years | 18 (0.8, 32) | 40.5 (28, 53) |
| Gender | ||
| Male | 2,484 (52.3) | 85 (36.0) |
| Female | 2,262 (47.7) | 151 (64.0) |
| Education level completed | ||
| No formal education | 2,642 (55.7) | 124 (52.5) |
| Primary | 1,262 (26.6) | 72 (30.5) |
| Secondary | 5,49 (11.6) | 31 (13.1) |
| College | 293 (6.2) | 9 (3.8) |
| Employment status | ||
| Working on farm | 1527 (32.2) | 109 (46.2) |
| Non skilled | 185 (3.9) | 61 (25.8) |
| Skilled | 442 (9.3) | 31 (13.1) |
| Students and minors | 2591 (54.6) | 35 (14.8) |
| Total household population by Livestock ownership | ||
| Own any livestock | 4626 (97.5)%) | 154 (66.1) |
| Own goats | 4533 (95.5) | 151 (64.8)) |
| Own cattle | 3989 (84.0) | 142 (60.9) |
| Own sheep | 3897 (82.1) | 133 (57.1) |
IQR–Inter quartile range
Enrolled patients’ demographic information
Between February 2015 and January 2016, 236 persons from 178 (22%) registered households presented at one of three health care facilities and met the study clinical case definition and were enrolled. Of these, 64% were females and the median age was 40.5 years (25th and 75th percentile 28, 53 years) (Table 1). Over half of the participants (52%) had no formal education and 46% reported working full time on the farm (Table 1).
Among patient households, 66% owned at least one livestock type with 65%, 61% and 57% of the participants owning goats, cattle, and sheep, respectively, in their households in the three months prior to the health facility visit (Table 1). About 40% were referred to the health center by the study personnel during routine household visits. Almost all (99%) participants reported consuming animal milk, with 78% reporting drinking cow milk more than three times a week. Over 91% reported consuming only boiled cow milk. Over half of the participants reported abortions among their livestock in the previous year; 23% reported abortions in cattle, 42% in goats, and 23% in sheep.
Clinical presentation of suspected brucellosis cases
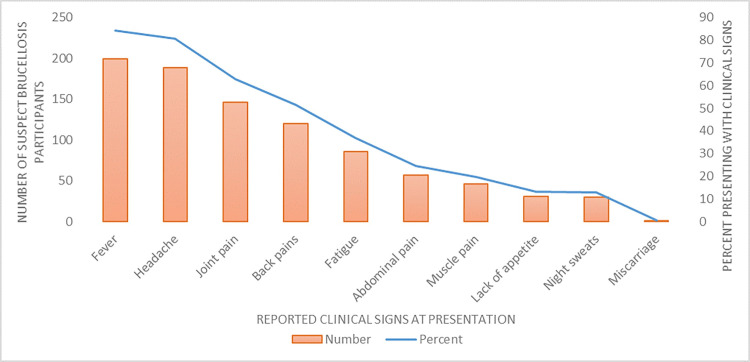
At the time of visiting the health facility, the most frequently reported clinical signs/symptoms among the 236 enrolled were: any fever in 199 (84%), headache in 188 (81%), joint pain in 146 (63%) and back pain in 120 (51%) [Fig 2]. The median time of presentation to the health facility from date of onset of symptoms was 7 days (25th = 4 and 75th percentile = 14 days), and this did not differ by sex or age (p = 0.05). In total, 102 (44%) of the patients reported having experienced similar symptoms in the last 12 months, with over half (59%) having experienced the symptoms two or more times. Most (89%) had previously sought medical care with only 36% reporting symptoms of previous illness having resolved.
Fig 2. Reported clinical signs of enrolled cases at presentation at health facilities (N = 236).
Brucella test results
Brucella serology: Of the 236 cases enrolled, 39 (16.5%) tested positive by Brucella IgG ELISA, five (2.1%) by IgM ELISA and four (1.7%) by RBT (Table 2). Four of 236 participants (1.7%) were classified as confirmed cases and 39 (16.5%) classified as probable cases.
Table 2. Distribution of laboratory test results by brucellosis test assay for febrile cases, Kajiado 2015–2016 (n = 236).
| Brucellosis Case classification | Laboratory test assay | |||
|---|---|---|---|---|
| Rose Bengal Test (RBT) N = 233 | Brucella IgM ELISA N = 236 | Brucella IgG ELISA N = 236 | Total Number by brucellosis case classification n(%) | |
|
2 | 2 | 0 | 2 |
| 1 | 1 | 1 | 1 | |
| 1 | 0 | 1 | 1 | |
| Total Confirmed | 4 | 3 | 2 | 4 (1.7) |
|
0 | 0 | 37 | 37 |
| 0 | 2 | 0 | 2 | |
| Total Probable | 0 | 2 | 37 | 39 (16.5) |
| Total Negative | 229 | 231 | 197 | 193 (81.8) |
| Overall Total no. positive by diagnostic assay (%) | 4 (1.7) | 5 (2.1) | 39 (16.5) | - |
ᵦ Confirmed cases tested positive by RBT and IgG or RBT and IgM ELISA.
† probable cases tested positive by ELISA (IgG) only, or IgM only but negative by RBT.
TAC testing for Brucella spp
AFI TAC was performed on 217 (91.9%) sera with sufficient volume and Brucella spp DNA was detected in 22 (10.6%) of the samples. The distribution of Brucella spp DNA detected by case classification is shown in S1 Table.
Test results for other etiologies of febrile illnesses
Fourteen (6%) of 233 febrile cases tested positive for malaria with the RDT and plasmodium DNA was detected in two of 217 sera tested by TAC for a total of 16/236 (7%) enrolled cases testing positive for malaria. From the TAC testing, six specimens had HIV-1 virus detected (Table 3). Of these, five had HIV virus alone detected and two were co-detection of Brucella spp and HIV virus. Two samples had two pathogens detected; Brucella spp and Plasmodium spp (n = 1), and Rickettsia spp DNA and hepatitis E virus (n = 1). There were single detections each of Rift Valley fever virus, Yersinia pestis, and Salmonella spp (Table 3). No pathogen was detected by TAC in the majority of cases (85%).
Table 3. Summary of etiologies of fever among patients by Acute Febrile Illness TacMann Array card (AFI TAC) testing in Kajiado, Kenya, 2015–2016 (n = 217)*.
| Pathogen(s) detected | AFI TAC test results n (%) |
|---|---|
| Brucella spp | 20 (9.2) |
| Plasmodium spp | 1 (0.5) |
| Plasmodium spp and Brucella spp | 1 (0.5) |
| HIV-1 | 5 (2.3) |
| HIV-1 & Brucella spp | 1 (0.5) |
| Rickettsia spp & Hepatitis E | 1 (0.5) |
| West Nile Virus | 1 (0.5) |
| Rift Valley Fever virus | 1 (0.5) |
| Salmonella spp | 1 (0.5) |
| Yersinia pestis | 1 (0.5) |
| Negative | 184 (84.8) |
*19 cases had insufficient sera for TAC testing.
Demographic and clinical characteristics of confirmed brucellosis cases
The median age of the four patients with confirmed brucellosis was 34 years (25th Percentile = 20 years and 75th percentile = 41.5 years) and 3 (75%) were females. Three of the confirmed cases reported not owning any livestock at household level. The median days of presentation to the health facility since symptom onset was 9 days (25th = 4 days and 75th = 21 days). All the patients presented with fever and headache. Other common presenting signs were joint pains, fatigue, night sweats and back pain (Table 4).
Table 4. Demographic, clinical characteristics and laboratory assessments of confirmed brucellosis cases in Kajiado, Kenya 2015–2016 (n = 4).
| Laboratory assessments | |||||||
|---|---|---|---|---|---|---|---|
| Patient # age[y]/sex | Occupation | Days since onset of symptoms | symptoms | RBT | ELISA IgM | ELISA IgG | TAC |
| Brucella spp* | |||||||
| 1. 10/M | Child | 4 | Fever, Headache, Joint pains | + | - | + | NT |
| 2. 38/F | Housewife | 4 | Fever, Headache, Joint pains, night sweats, lack of appetite, abdominal pains, fatigue | + | + | - | - |
| 3. 45/F | Housewife | 14 | Fever, Headache, Muscle pain, fatigue, back pains | + | + | - | + |
| 4. 30/F | Housewife | 28 | Fever, headache, joint pains, lack of appetite, back pains | + | + | + | + |
Demographic and clinical characteristics of probable brucellosis cases
The median age among the 39 probable cases was 50 years (25th = 35 and 75th percentile = 64) and 23 (59.0%) were females. The median days of presentation of probable cases to the health facility since symptoms onset was 7 days (25th = 3 and 75th percentile = 14). Seventy-seven percent presented with any fever, 79% with headache, 59% with joint pain, 59% with back pain, and 25.6% with muscle pain among others (S2 Table). Forty six percent (n = 18) of probable cases reported having had a similar illness in the previous 12 months with 12/18 (67%) reporting two or more episodes of similar illness. In 7/18 (39%) probable cases, illness symptoms had resolved.
Incidence of human brucellosis
The annual incidence of brucellosis in this population was estimated to be 84/100,000 persons/year (95% CI 82, 87). The annual incidence by gender, age and location is shown in Table 5. There were no confirmed cases among those living in the Arroi location (Fig 1). The risk of brucellosis did not vary significantly by age, gender or location of residence.
Table 5. Annual Incidence of brucellosis in humans by gender, age and location in Kajiado Kenya, 2015–2016.
| Characteristic | Population in the study cohort | Total enrolled febrile illness cases n (%) N = 236 | Number of confirmed Brucellosis cases N (%) (N = 7) | Point estimate annual incidence per 100,000 [95% CI) | Incidence ratio Estimate (95% CI) |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 2484 (52.3) | 85 (36.0) | 1 (1.2) | 40 [38,43] | Ref |
| Female | 2262 (47.7) | 151 (64.0) | 3 (2.0) | 133 [128,138] | 3.3 [0.2–173.0] |
| Age group | |||||
| ≤20 | 2542 (53.6) | 30 (12.7) | 1 (0.04) | 39 [37,42] | Ref |
| 21–40 | 1388 (29.2) | 83 (35.2) | 2 (4.8) | 144 [138,151] | 3.6 [0.19–216.1] |
| 41–60 | 570 (12.0) | 79 (33.5) | 1 (1.3) | 175 [165,187] | 4.4 [0.05–350.1] |
| >60 | 246 (5.2) | 44 (18.6) | 0 (0.0) | 0 | - |
| Location | |||||
| Arroi | 425 (8.9) | 9 (3.8) | 0 (0.0) | 0 | - |
| Ilmuktani Mashuru Nka | 831 (17.5) | 93 (39.4) | 1 (1.1) | 120 [17,853] | 3.2 [0.04–255.3] |
| Mashuru | 787 (16.6) | 54 (22.9) | 2 (3.7) | 254 [64,1014] | 6.8 [0.35–405.2] |
| Nkama | 2703 (56.9) | 80 (33.9) | 1 (1.2) | 37 [5,262] | Ref |
Discussion
We report a high annual incidence of human brucellosis of 84 per 100,000 persons in a pastoralist community in Kenya for which limited data had previously been available. Using a multi-pathogen molecular assay designed for detecting 26 pathogens associated with acute febrile illness, Brucella spp was the most common pathogen (detected in 10.1% of samples), highlighting its importance as a leading cause of febrile illness in this community. Other pathogens detected by TAC included Plasmodium (0.9%), HIV-1 (2.8%) and different combinations of co-detections of Brucella spp and Plasmodium. Of note, the majority (77%, n = 17) of those who had Brucella spp DNA detected did not have detectable antibodies by either serological assay. [23,24].
Diagnosis of brucellosis is complex and confirmatory diagnosis is made by Brucella culture or achieved through a series of serological tests. For these patients in a high sero-prevalence area who presented with a clinical syndrome compatible with brucellosis, we applied specific laboratory confirmation criteria for confirmed cases that included both detection of agglutinating and non-agglutinating antibodies. Based on these criteria, 16% of cases who were seropositive by IgG or IgM ELISA only were classified as probable cases. Non-agglutinating IgG antibodies are associated with prior exposure to Brucella, incomplete treatment or chronic brucellosis with focal complications [20,25]. We hypothesize that a proportion of these probable cases likely represent chronic brucellosis, relapsing brucellosis and/or occupational exposures in this endemic area in persons who may ultimately require brucellosis treatment. Hence, the incidence reported here (derived from confirmed cases only) may underestimate the true incidence in this community by more than threefold.
The reported incidence in this pastoralist area was 2.5 times higher than 33/100,000 population reported in 2008 and 35/100,000 persons in 2014 in the Kilimanjaro region in the neighboring country of Tanzania [8]. Variable incidence across regions and within a country is commonly demonstrated [4]. For example, Egypt reported human brucellosis incidence of 0.3–70/ 100,000 persons, Iraq 52–267/100,000 persons, Saudi Arabia 6–149/100,000 persons and Greece 4–32/100,000 persons variable by sub-national regions [4]. The higher rates are often reported among nomadic pastoralists and lower rates among urban populations with limited contact with infected livestock and unpasteurized dairy products [5,10,12,13,26,27]. Generally, the occurrence of human brucellosis is highly correlated with Brucella sero-prevalence in livestock, where infected animals are constantly shedding bacteria in milk and at parturition increasing the likelihood of infection among humans. However, the majority of participants (97.5%) in this pastoralist community owned at least one livestock type at household level; this limited the power to estimate incidence by livestock owned. A 2013 study conducted in three counties in Kenya, including Kajiado, reported six-fold increased odds of human seropositivity in households with a seropositive animal compared to those without [12]. Hence, this high incidence was not unexpected given the previously reported 3% and 15% seroprevalence in livestock and humans, respectively, in this county [12]. It is likely some areas in Kenya such as Marsabit with higher Brucella sero-prevalence in livestock (13%) and in humans (46%) could have higher incidence in humans and conversely areas with reported low brucellosis sero-prevalence in livestock in former provinces of Western, Nyanza and Nairobi could have lower incidence in humans [11,12,28]. Overall, this high incidence in pastoralist communities suggests significant impacts on socio-economic and livelihoods at household level and on public health systems at community level.
A high proportion of participants presented with arthralgias (63%), back pains (52%), myalgias (20%) and fatigue (37%), all symptoms that might impact the ability to perform the strenuous daily physical activities required for livestock rearing. Further, looking at disease burden in global terms, brucellosis is an acute to chronic debilitating disease but its defined impact metric such as disability weights (a weight factor that reflects the severity of the disease) is not available. However, estimates of disability weights ranging from 0.15 to 0.22 (class II) have been used in a few studies to estimate disability-adjusted life years (DALYs), a measure of overall disease burden associated with human brucellosis [2,29,30]. For example, using brucellosis seropositivity of <2% in India, Singh et al. estimated moderate DALYs of 0.29 (95% uncertainty interval 0.08–0.7) per thousand person-years among adults with occupational exposures to livestock [30]. With higher seroprevalence, brucellosis-associated DALYs may be substantially higher in Kenyan pastoralist communities.
We could not determine the brucellosis infection status of 7% (n = 17) of cases who had Brucella spp DNA detected but had no detectable antibodies by either IgM, IgG ELISA or RBT assays. Detection of Brucella spp DNA has been demonstrated in some patients on brucellosis treatment for up to 6 months after effective treatment, among patients with relapsing brucellosis, and in asymptomatic persons with occupational exposures to Brucella in endemic regions [31–33] but these studies did not report the immunologic responses among the patients. None of the enrolled cases in this study reported being on brucellosis treatment. Whereas IgG antibody titers drop with onset of treatment absence of antibody detection among these patients could not be explained. There is the likelihood that some of these 17 sero-negative, Brucella PCR-positive individuals are infected with a rough Brucella species such as Brucella canis, that does not cross react with standard diagnostic agglutination and ELISA tests using capture antigen that only detects antibody from smooth Brucella strains [34]. Additionally, though rare, there have been case reports of sero-negative and culture positive or PCR-positive brucellosis cases [23,24,35]. In clinical settings, case management decisions surrounding such diagnostic results are challenging. Given the high proportion (17%) of Brucella IgG positives detected in this community, availability of effective diagnostic assays in health centers to inform management would improve timely brucellosis treatment and outcomes. Extensive validation of molecular diagnostic techniques for clinical management in endemic regions would be useful as these assays have increasingly become available in referral facilities.
As expected, malaria was the second most commonly detected pathogen. Other identified etiologies of febrile illness that are unlikely to be diagnosed by clinical presentation alone included West Nile Virus, Rift Valley fever, hepatitis E, Yersinia pestis and Rickettsia spp. Testing for these pathogens is not routinely conducted in peripheral health facilities. Similar to other studies focusing on etiology of febrile illnesses, the etiology for a large proportion of the patients (68%) was not identified. This was in part because we only collected sera from febrile patients (poorly sensitive for bacterial infections that cause no or low bacteremia) and no other specimens (e.g., stool, urine, nasopharyngeal swabs) that might offer higher sensitivity for detection of some pathogens [18]. Further, only pathogens included in the TAC panel could be detected.
It is important to note the diagnostic and spatio-temporal limitations of extrapolating data reported here. First, bacterial culture which is the confirmatory diagnostic assay for brucellosis was not done. This was due to logistical and biosafety requirements of culturing Brucella in the study area. Second, this study was conducted in a geographically limited region and over a one-year period, hence, data obtained may not be extrapolated to national estimates or regions with substantially different risk factors of Brucella infection in humans and could vary across years [8]. We also did not adjust the incidence estimated to account for those in the enrolled households who sought medical care elsewhere, or who did not seek care at all despite our biweekly visits. These results however provide insight into the likely high incidence in similar nomadic pastoralist communities living in arid and semi-arid regions in Kenya and the Eastern African region with comparable animal ownership, husbandry and cultural practices.
Globally, brucellosis is a neglected zoonosis, yet not recognized as such [36,37]. In Kenya, brucellosis has been designated a priority zoonotic disease targeted for control [38], but currently a national prevention and control strategy has not been developed and there are no systematic risk reduction activities being implemented. This could in part be due to lack of local disease burden estimates to highlight its public health importance for policy makers. Reducing the burden of human brucellosis can be achieved through strategies aimed at reducing brucellosis in animals mainly though animal vaccination and public health education to adopt risk reduction measures [13,39]. For example, in north-western Greece, five years after introduction of livestock vaccination and public health education, there was large decrease in incidence in humans from 1,100 to 30 cases per 100,000 population by 2002 [26,40]. Data generated here and elsewhere could be useful to advocate for recognition by national and international policy makers and partners in global health, of the need to control the high burden this prevalent endemic zoonosis impacts in rural poor populations in sub-Saharan Africa and other endemic regions.
Supporting information
(DOCX)
(DOCX)
(TIF)
Acknowledgments
We thank the study personnel, the study participants and community, and the Government of Kenya sectors including Ministry of Health and Ministry of Agriculture, Livestock, Fisheries and Irrigation, and the Kajiado County governments for their support during the study.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States’ Centers for Disease Control and Prevention or Department of Defense.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by funding from the Defense Threat Reduction Agency of the U.S. Department of Defense and the U. S. Centers for Disease Control and Prevention. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Corbel MJ. Recent advances in brucellosis. J Med Microbiol 1997;46(2):101–3. Epub 1997/02/01. 10.1099/00222615-46-2-101 . [DOI] [PubMed] [Google Scholar]
- 2.Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6(12):e1929 Epub 2012/12/14. 10.1371/journal.pntd.0001929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis 2006;6(2):91–9. Epub 2006/01/28. 10.1016/S1473-3099(06)70382-6 . [DOI] [PubMed] [Google Scholar]
- 4.Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis. 2012;6(10):e1865 Epub 2012/11/13. 10.1371/journal.pntd.0001865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shevtsova E, Shevtsov A, Mukanov K, Filipenko M, Kamalova D, Sytnik I, et al. Epidemiology of Brucellosis and Genetic Diversity of Brucella abortus in Kazakhstan. PLoS One. 2016;11(12):e0167496 Epub 2016/12/03. 10.1371/journal.pone.0167496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ECDC. European Centre for Disease Prevention and Control. Brucellosis. In: ECDC. Annual epidemiological report for 2016. Stockholm, August 2018: European Centre for Disease Prevention and Control, 2018 7, March, 2019. Report No.
- 7.Minas M, Minas A, Gourgulianis K, Stournara A. Epidemiological and clinical aspects of human brucellosis in Central Greece. Jpn J Infect Dis 2007;60(6):362–6. Epub 2007/11/23. . [PubMed] [Google Scholar]
- 8.Carugati M, Biggs HM, Maze MJ, Stoddard RA, Cash-Goldwasser S, Hertz JT, et al. Incidence of human brucellosis in the Kilimanjaro Region of Tanzania in the periods 2007–2008 and 2012–2014. Trans R Soc Trop Med Hyg. 2018;112(3):136–43. Epub 2018/04/27. 10.1093/trstmh/try033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ducrotoy MJ, Bertu WJ, Ocholi RA, Gusi AM, Bryssinckx W, Welburn S, et al. Brucellosis as an emerging threat in developing economies: lessons from Nigeria. PLoS Negl Trop Dis. 2014;8(7):e3008 Epub 2014/07/25. 10.1371/journal.pntd.0003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P, Tanner M, et al. Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev Vet Med. 2003;61(4):279–93. Epub 2003/11/19. 10.1016/j.prevetmed.2003.08.004 . [DOI] [PubMed] [Google Scholar]
- 11.Njeru J, Wareth G, Melzer F, Henning K, Pletz MW, Heller R, et al. Systematic review of brucellosis in Kenya: disease frequency in humans and animals and risk factors for human infection. BMC Public Health. 2016;16(1):853 Epub 2016/08/24. 10.1186/s12889-016-3532-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osoro EM, Munyua P, Omulo S, Ogola E, Ade F, Mbatha P, et al. Strong Association Between Human and Animal Brucella Seropositivity in a Linked Study in Kenya, 2012–2013. Am J Trop Med Hyg. 2015;93(2):224–31. Epub 2015/06/24. 10.4269/ajtmh.15-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDermott JJ, Arimi SM. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol 2002;90(1–4):111–34. Epub 2002/11/05. 10.1016/s0378-1135(02)00249-3 . [DOI] [PubMed] [Google Scholar]
- 14.Njuguna JN, Gicheru MM, Kamau LM, Mbatha PM. Incidence and knowledge of bovine brucellosis in Kahuro district, Murang’a County, Kenya Trop Anim Health Prod 2017;49(5):1035–40. 10.1007/s11250-017-1296-6 . [DOI] [PubMed] [Google Scholar]
- 15.Njeru J, Melzer F, Wareth G, El-Adawy H, Henning K, Pletz MW, et al. Human Brucellosis in Febrile Patients Seeking Treatment at Remote Hospitals, Northeastern Kenya, 2014–2015. Emerg Infect Dis. 2016;22(12):2160–4. Epub 2016/09/24. 10.3201/eid2212.160285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The 2009 Kenya Population and Housing Census volume- 1b Summary Population Distribution By District [Internet]. 2009 [cited March 20, 2019]. Available from: https://www.opendata.go.ke/datasets/summary-population-distribution-by-district/data.
- 17.Diaz R, Casanova A, Ariza J, Moriyon I. The Rose Bengal Test in human brucellosis: a neglected test for the diagnosis of a neglected disease. PLoS Negl Trop Dis. 2011;5(4):e950 Epub 2011/04/29. 10.1371/journal.pntd.0000950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Ochieng C, Wiersma S, Stroher U, Towner JS, Whitmer S, et al. Development of a TaqMan Array Card for Acute-Febrile-Illness Outbreak Investigation and Surveillance of Emerging Pathogens, Including Ebola Virus. J Clin Microbiol. 2016;54(1):49–58. Epub 2015/10/23. 10.1128/JCM.02257-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez MC, Nieto JA, Rosa C, Geijo P, Escribano MA, Munoz A, et al. Evaluation of seven tests for diagnosis of human brucellosis in an area where the disease is endemic. Clin Vaccine Immunol. 2008;15(6):1031–3. Epub 2008/05/02. 10.1128/CVI.00424-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avijgan M, Rostamnezhad M, Jahanbani-Ardakani H. Clinical and serological approach to patients with brucellosis: A common diagnostic dilemma and a worldwide perspective. Microb Pathog 2019;129:125–30. Epub 2019/02/12. 10.1016/j.micpath.2019.02.011 . [DOI] [PubMed] [Google Scholar]
- 21.Vrioni G, Pappas G, Priavali E, Gartzonika C, Levidiotou S. An eternal microbe: Brucella DNA load persists for years after clinical cure. Clin Infect Dis 2008;46(12):e131–6. Epub 2008/05/09. 10.1086/588482 . [DOI] [PubMed] [Google Scholar]
- 22.WHO. WHO Recommended Surveillance Standards. Second edition In: WHO, editor. Geneva: WHO. [Google Scholar]
- 23.Naha K, Dasari S, Pandit V, Seshadri S. A rare case of seronegative culture—proven infection with Brucella suis. Australas Med J. 2012;5(7):340–3. Epub 2012/08/21. 10.4066/AMJ.2012.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharathan B, Backhouse L, Rawat D, Naik S, Millar M. An unusual case of seronegative, 16S PCR positive Brucella infection. JMM Case Rep. 2016;3(5):e005050 Epub 2017/03/30. 10.1099/jmmcr.0.005050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantur BG, Biradar MS, Bidri RC, Mulimani MS, Veerappa, Kariholu P, et al. Protean clinical manifestations and diagnostic challenges of human brucellosis in adults: 16 years’ experience in an endemic area. J Med Microbiol 2006;55(Pt 7):897–903. Epub 2006/06/15. 10.1099/jmm.0.46097-0 . [DOI] [PubMed] [Google Scholar]
- 26.Bikas C, Jelastopulu E, Leotsinidis M, Kondakis X. Epidemiology of human brucellosis in a rural area of north-western Peloponnese in Greece. Eur J Epidemiol 2003;18(3):267–74. Epub 2003/06/13. 10.1023/A:1023368420840 . [DOI] [PubMed] [Google Scholar]
- 27.Kansiime C, Rutebemberwa E, Asiimwe BB, Makumbi F, Bazira J, Mugisha A. Annual trends of human brucellosis in pastoralist communities of south-western Uganda: a retrospective ten-year study. Infect Dis Poverty. 2015;4:39 Epub 2015/09/05. 10.1186/s40249-015-0072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omballa VO, Musyoka RN, Vittor AY, Wamburu KB, Wachira CM, Waiboci LW, et al. Serologic Evidence of the Geographic Distribution of Bacterial Zoonotic Agents in Kenya, 2007. Am J Trop Med Hyg. 2016;94(1):43–51. Epub 2015/11/26. 10.4269/ajtmh.15-0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roth F, Zinsstag J, Orkhon D, Chimed-Ochir G, Hutton G, Cosivi O, et al. Human health benefits from livestock vaccination for brucellosis: case study. Bull World Health Organ. 2003;81(12):867–76. Epub 2004/03/05. [PMC free article] [PubMed] [Google Scholar]
- 30.Singh BB, Khatkar MS, Aulakh RS, Gill JPS, Dhand NK. Estimation of the health and economic burden of human brucellosis in India. Prev Vet Med 2018;154:148–55. Epub 2018/04/25. 10.1016/j.prevetmed.2018.03.023 . [DOI] [PubMed] [Google Scholar]
- 31.Navarro E, Segura JC, Castano MJ, Solera J. Use of real-time quantitative polymerase chain reaction to monitor the evolution of Brucella melitensis DNA load during therapy and post-therapy follow-up in patients with brucellosis. Clin Infect Dis 2006;42(9):1266–73. Epub 2006/04/06. 10.1086/503035 . [DOI] [PubMed] [Google Scholar]
- 32.Morata P, Queipo-Ortuno MI, Reguera JM, Garcia-Ordonez MA, Pichardo C, Colmenero JD. Posttreatment follow-Up of brucellosis by PCR assay. J Clin Microbiol. 1999;37(12):4163–6. Epub 1999/11/24. 10.1128/JCM.37.12.4163-4166.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maas KS, Mendez M, Zavaleta M, Manrique J, Franco MP, Mulder M, et al. Evaluation of brucellosis by PCR and persistence after treatment in patients returning to the hospital for follow-up. Am J Trop Med Hyg. 2007;76(4):698–702. Epub 2007/04/12. . [PubMed] [Google Scholar]
- 34.Lucero NE, Escobar GI, Ayala SM, Jacob N. Diagnosis of human brucellosis caused by Brucella canis. J Med Microbiol 2005;54(Pt 5):457–61. Epub 2005/04/13. 10.1099/jmm.0.45927-0 . [DOI] [PubMed] [Google Scholar]
- 35.Ortega M, Pérez M, Rodríguez M, Lara A, Díaz V, Cantero A. Bacteriemia por Brucella sp. con serología convencional negativa Enfermedades Infecciosas y Microbiología Clínica. 2001. 19:34 10.1016/S0213-005X(01)72547-5 [DOI] [PubMed] [Google Scholar]
- 36.Franc KA, Krecek RC, Hasler BN, Arenas-Gamboa AM. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health. 2018;18(1):125 Epub 2018/01/13. 10.1186/s12889-017-5016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubach MP, Halliday JE, Cleaveland S, Crump JA. Brucellosis in low-income and middle-income countries. Curr Opin Infect Dis. 2013;26(5):404–12. Epub 2013/08/22. 10.1097/QCO.0b013e3283638104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munyua P, Bitek A, Osoro E, Pieracci EG, Muema J, Mwatondo A, et al. Prioritization of Zoonotic Diseases in Kenya, 2015. PLoS One. 2016;11(8):e0161576 Epub 2016/08/25. 10.1371/journal.pone.0161576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDermott J, Grace D, Zinsstag J. Economics of brucellosis impact and control in low-income countries. Rev Sci Tech 2013;32(1):249–61. Epub 2013/07/11. 10.20506/rst.32.1.2197 . [DOI] [PubMed] [Google Scholar]
- 40.Jelastopulu E, Bikas C, Petropoulos C, Leotsinidis M. Incidence of human brucellosis in a rural area in Western Greece after the implementation of a vaccination programme against animal brucellosis. BMC Public Health. 2008;8:241 Epub 2008/07/19. 10.1186/1471-2458-8-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.