Abstract
Background
Cannabis is often used by patients with ulcerative colitis, but controlled studies are few. We aimed to assess the effect of cannabis in improving clinical and inflammatory outcomes in ulcerative colitis patients.
Methods
In a double-blind, randomized, placebo-controlled trial, patients received either cigarettes containing 0.5 g of dried cannabis flowers with80mgTetrahydrocannabinol (THC)or placebo cigarettes for 8 weeks. Parameters of disease including Lichtiger disease activity index, C reactive protein (CRP), calprotectin, Mayo endoscopic score and quality of life (QOL) were assessed before, during and after treatment.
Results
The study included 32 patients. Mean age was 30 years, 14 (43%) females. Lichtiger index improved in the cannabis group from 10.9 (IQR 9–14) to5 (IQR 1–7), (p<0.000), and in the placebo group from 11 (IQR 9–13) to 8 (IQR 7–10)(p = 0.15, p between groups 0.001). QOL improved in the cannabis group from 77±4 to 98±20 (p = 0.000) but not in the placebo group (78±3 at week 0 and 78±17 at week 8;p = 0.459; p between groups 0.007). Mayo endoscopic score changed in the cannabis group from 2.13±1 to 1.25±2 (p = 0.015) and in the placebo group from 2.15±1to 1.69±1 (p = 0.367, p between groups 0.17).
Conclusion
Short term treatment with THC rich cannabis induced clinical remission and improved quality of life in patients with mild to moderately active ulcerative colitis. However, these beneficial clinical effects were not associated with significant anti-inflammatory improvement in the Mayo endoscopic score or laboratory markers for inflammation.(clinicaltrials.gov NCT01040910).
Introduction
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) characterized by inflammation of the large intestine. The incidence of UC has increased over the past few years with a higher prevalence in the developed world [1, 2]. The disease poses a significant personal and socioeconomic burden due to its effects on patients’ quality of life, daily functioning and use of healthcare system. The overall response to currently available treatments is limited to 40–60% [3, 4], and secondary loss of response occurs in about 50% of the patients [5]. Moreover, the current treatment carries many long-term risks including malignancies, infections, and decreased bone density. Therefore, it is not surprising that many patients with IBD seek alternative treatments for their illnesses. A common such alternative treatment is the use of cannabis. Indeed, epidemiological data indicate that as many as 15% of patients with IBD use cannabis [6, 7].
Cannabinoids have been shown to decrease motility and secretions in the gastrointestinal tract [8, 9]. They also have an important role in the regulation of inflammatory response in the colon [10]. In several models of murine colitis Cannabinoids were also shown to improve inflammation [11].
However, despite the growing number of IBD patients using medical cannabis, data about its clinical therapeutic efficacy is limited. Several studies reported the prevalence of cannabis use among IBD patients and suggested clinical benefit, but they were not randomized controlled studies and did not include information about the doses, extent of endoscopic disease and the effect of the treatment on disease activity and inflammatory markers [6, 7].
We have previously conducted several studies to look at the effect of medical cannabis in patients with IBD. In an observational prospective open label study on30 patients with Crohn’s disease we found a significant clinical improvement with an average decrease in Harvey Bradshaw index from 14 ± 6.7 to 7 ± 4.7 (P < 0.001). We also found that the improvement was sustained over an average period of 2 years (ranging from 3 months to 9 years) [12]. In a double-blind placebo-controlled study of 21 patients with Crohn’s disease who were treated with cannabis over a period of 8 weeks, we found a significant improvement in Crohn’s disease activity index (CDAI) in the cannabis active group compared to the placebo group (152±109 vs. 306 ±143, P <0.05) [13]. However, the results of studies investigating the effect of cannabis in IBD are not always consistent. For example, in a study on 20 patients with Crohn’s disease who were treated with cannabidiol vs. placebo over 8 weeks, we did not find significant improvement in CDAI compared to placebo, [14]. Similarly, a recent study by Irving PM et al. [15] failed to show significant difference in remission rate in UC patients who were treated with cannabidiol(n = 29) vs. placebo (n = 31)over a period of 10 weeks. Taken together, the current data on the beneficial effect of cannabis in patients with IBD is limited due to the small number of prospective placebo-controlled studies and the focus on clinical outcome without comprehensive assessment of the effect of this treatment on objective disease parameters including mucosal inflammation and inflammatory markers. Thus, the key question of whether the reported beneficial clinical effect of cannabis in patients with IBD relates to relief of symptoms or improvement in patients’ ability to tolerate their symptoms, or to the anti-inflammatory effects of cannabis remained unanswered.
The aim of the current study was to investigate the clinical, laboratory and endoscopic effects of medical cannabis in patients with mild to moderate UC.
We hypothesized that the use of cannabis as an adjunct therapy in patients with mild to moderate UC will be associated with better clinical outcomes compared to placebo and that this beneficial effect of treatment will be associated with improvement in objective inflammatory disease parameters including laboratory and colonic mucosal markers for inflammation.
Materials and methods
Study design
We conducted a single-center, prospective, randomized, double-blind, placebo-controlled, parallel-arm clinical study. The protocol included a two-week screening period to evaluate for baseline symptoms, an eight-week treatment period and a two-week follow-up period after the intervention was discontinued. Non-responders were offered to participate in an open arm eight-week treatment period.
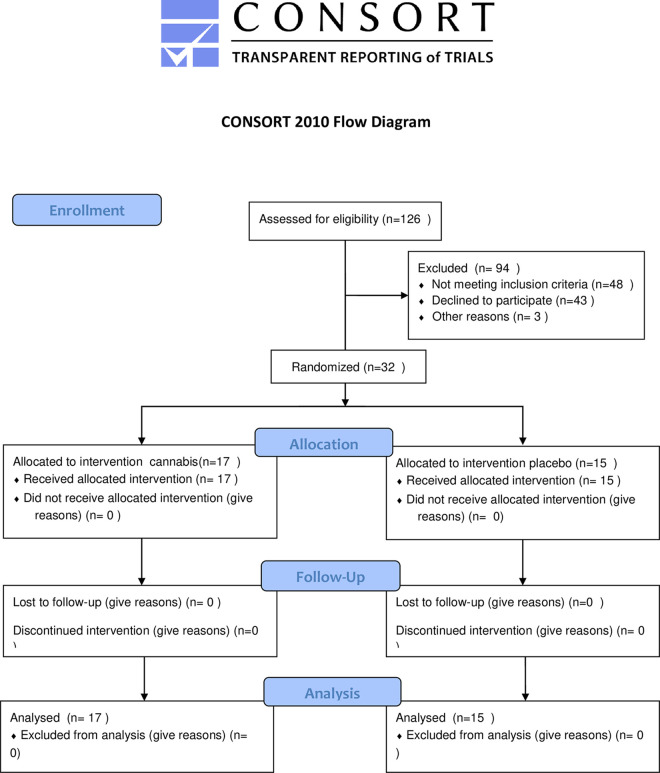
Patients were evaluated by medical interview, physical examination, blood, and stool tests at baseline (end of screening; week 0), after two weeks of study intervention (week 2), end of intervention (week 8), and end of the follow-up period (week 10). Colonoscopy was performed at screening (week 0) and after 8 weeks of treatment. (Fig 1, consort checklist).
Fig 1. CONSORT 2010 flow diagram.
Participant eligibility criteria
The study population included male and female patients age 20 to 80 years with mild to moderate UC diagnosed at least three months prior to enrollment. Mild to moderate disease severity was determined by Lichtiger Scoring Index of ≥4 and Mayo endoscopicsubscore≥1 [16]. Exclusion criteria included the use of cannabis, whether medical or recreational, pregnant or lactating, severe UC (Mayo score >10), proctitis (i.e. inflammatory segment of less than 15 cm), known psychiatric diagnosis or addiction traits based on self- reporting or noted in the patient’s electronic medical record. Patients were allowed to continue their chronic UC medications as long as they were on a stable dose; specifically, at least 4weeks for 5 ASA and at least 3 months immunomodulators and biologic treatments. Steroids were permitted if the patients were on a stable dose for at least 8 weeks prior to enrollment. Patients were specifically asked to avoid any change in their stable medications and study medication during participation in the study.
Study compounds
Treatment was provided in the form of cigarettes. The cigarettes were machine made to ensure they were identical and comprised of dried flowers of genetically identical plants of Cannabis sativa var. Indica "Erez" (courtesy of Tikun-Olam Ltd., Tel Aviv, Israel). Every batch used in the study was analyzed and the content was 16% THC (80mg THC), 0.5% CBG, 0.1% CBD and traces (less than 0.1%) of CBC, CBDV and Δ8THC. Terpenes content was: Myrcene, β-caryophyllene, Selina-3,7(11)-diene, γ-Selinene, 10-epi-γ-eudesmol, β-eudesmol, guaiol, α-pinene(analysis performed in the Lumirlab, Hebrew University Biotechnology Park Jerusalem, Israel. Tel: +972 (73) 733 0300).
The placebo cigarettes contained cannabis flowers from which THC had been extracted as previously described [13]. In short, dried flowers of Cannabis sativa var. Indica "Erez" (Tikun-Olam Ltd., Tel Aviv, Israel), known to contain 23% THC and <0.5% CBD and other cannabinoids weres oaked in 95% ethanol for two weeks. The procedure was repeated 3 times. Following this, the flowers were covered with a mixture of herbal spirits and 0.025% Saccharomyces cerevisiae var. "18" (Courtesy Rimontest Ltd., Haifa, Israel) for three more days and then allowed to dry in the ambient air with ventilation for 72 hours. The final product was tested for cannabinoids and shown to possess <0.4% THC with undetectable amounts of all other cannabinoids including CBD.
Blinding and randomization
Before the study began cannabis and placebo cigarettes were prepared by the cannabis dispensary personnel that had no access to the patients, in packages that were numbered randomly. The code was kept outside the hospital in "Tikun-Olam" and was accessible only to people who had no access to the patients. Patients were randomly assigned using a block method in blocks of 5 [17] in a 1:1 ratio to receive either medical cannabis or placebo. Patients and investigators were blind to the treatment throughout the duration of the study and the data analysis.
Study intervention
Treatment was provided in the form of cigarettes. We chose this form because in "real life" it is reported by patients as the most effective form, with a rapid response and improvement of pain and general wellbeing. Therefore, despite the known hazards of smoking, we thought it should be the first form to be investigated [12]. Patients were required to start gradually, smoking half a cigarette (0.25gr) in the first day and increasing by 0.25 gr until a final dose of 0.5 gr twice daily was reached. To assess adherence, patients were required to bring the packages on each visit and the number of remaining cigarettes was counted.
Outcome assessment
The primary endpoint was statistically significant improvement of the Lichtiger score, Secondary end points were: statistically significant improvement of the bowel movements, abdominal pain and quality of life. Another secondary endpoint was statistically significant improvement of the Mayo endoscopic score.
Assessment of clinical effect
Patients were evaluated by medical interview, physical examination, blood and stool tests. Demographic data, smoking history, past medical history (including history of drug abuse and psychiatric co-morbidity), ulcerative colitis history, past and present medications, family history of IBD, results of recent blood tests, last endoscopic and imaging findings were collected from patients’ records.
For clinical assessment, we used the overall Lichtiger Score [18] as well as additional sub-analysis on Lichtiger Score specific variables of interest including the number of bowel movements per day, abdominal pain and rectal bleeding. The primary outcome was statistically significant reduction of the Lichtiger score after 8 weeks of intervention.
Quality of life (QOL) was assessed at baseline (week 0) and end of the intervention (week 8) using the Short Form (SF36)survey [19].
Patients were also asked to report their general satisfaction with the treatment on a 7 point Likert scale (1 = not at all satisfied to 7 = very satisfied) and overall improvement on specific symptoms including general health, appetite, libido and concentration on a 5 points Likert scale (1 = significant improvement to 5 = worsening).
Assessment of effect on inflammation
Inflammatory activity was assessed with laboratory blood tests, stool calprotectin, and endoscopic parameters. Blood tests included complete blood count, liver and kidney function and C-reactive protein (CRP). Colonoscopies were performed at baseline (week 0) and end of intervention (week 8) by physicians who were blinded to the patient’s study treatment. Endoscopic disease activity was assessed using the Mayo score [20].
All side effects, including symptoms of drug addiction as defined by the DSM- IV [21] were captured at week 2 and week 8 and rated for severity on a 0 to 7 scale.
Statistical analysis
Categorical variables were reported as number and percentage. Continuous variables were evaluated for normal distribution using histogram and QQ plot. Baseline characteristics at first visit evaluation and third visit were compared between groups using independent sample t-test or Mann-Whitney test for continuous and ordinal variables, while Chi-square test or Fisher exact test were used for categorical variables. In each group, differences between the first and third visits were tested using paired sample t-test or Wilcoxon test for continuous and ordinal variables, while McNemar test was used for categorical variables. Generalized estimating equations models were used to observe changes between the groups at two time points, the first week and the 8 weeks visits. This was evaluated using interaction between time and group.
Corrections for multiple comparisons were done using the False Discovery Rate method [22].
In order to identify a4 point difference in the Lichtiger score between the two groups after 8 weeks, we used a standard deviation of 2.5, [23] an alpha of 0.01and a power of 90%. The calculated sample size was 14 patients in each group. Taking into account the possibility of 10% dropout we aimed at 16 patients in each group.
All statistical tests were 2-sided, p<0.05 was considered statistically significant. SPSS software was used for statistical analysis (IBM SPSS statistics for windows, ver. 25, IBM Corp, Armonk, NY, USA).
Ethical considerations
The study was approved by the Ministry of Health cannabis authority ethics committee and the Meir Medical Center ethics committee. All participants provided informed consent before any study-related procedure was carried out. All methods were carried out in accordance with relevant guidelines and regulations. The study protocol and results are registered on the clinicaltrials.gov website. NCT01040910, first posted 30 December 2009, and modified on October 2013.
Results
A total of 126 patients were screened, among them,43 did not consent, 39 had inactive disease with a Lichtiger score ≤1, inclusion criteria were not met by 9 patients, and3 were already taking medical cannabis treatment. Thus, 32 patients were recruited and all completed the study.
The mean age was 30, range 26–40, 14 (43%) women. Left-sided colitis was noted in 8 (25%) and extended or pancolitis in 24 (75%) patients. The mean length of the colonic involved segment was 46±20 cm. Twenty-four (75%) patients had never smoked tobacco, 6 (18%) smoked in the past and 2(6.3%) were still smoking during the study. Demographic data are presented in Table 1.
Table 1. Demographic data.
| Cannabis | Placebo | p value | |
|---|---|---|---|
| No. | 17 (53%) | 15 (47%) | |
| Age(years) | 30 (27–37) | 30 (26–40) | 0.882 |
| Gender (M/F) | 7/10 | 11/4 | 0.067 |
| Duration of disease (years) (median±IQR) | 8 (2–12) | 6 (2–10) | 0.970 |
| Current smoking | 0 (0%) | 2 (13%) | 0.411 |
| IBD in family | 6 | 6 | 0.7 |
IBD related treatments prior to enrollment included 5 (15%) patients using steroids, 5(15%)immunomodulators, and 6 (18%) biologics. Seven patients did not respond or had lost response to TNF inhibitors after at least a full induction dose (Table 2). No change in UC treatment was made during the study.
Table 2. Medications.
| Past | Present | |||||
|---|---|---|---|---|---|---|
| cannabis | placebo | p value | cannabis | Placebo | p | |
| 5 ASA | 17 (100%) | 14 (93%) | 0.615 | 7 (41%) | 10 (66%) | 0.596 |
| Antibiotics | 4 (44%) | 5 (55%) | 0.699 | 0 | 0 | 1 |
| Steroids | 9 (42%) | 12 (57%) | 0.54 | 2 (12%) | 3(20%) | 0.659 |
| Immunomodulators | 7 (41%) | 8 (53%) | 0.615 | 2 (12%) | 3 (20%) | 0.659 |
| Biologics | 7 (41%) | 4 (27%) | 0.615 | 4 (23%) | 2 (13%) | 0.659 |
Lichtiger disease activity index improved in the active arm group from 10.9 (IQR 9–14) to5 (IQR 1–7, p<0.001), and in the placebo group from 11 (IQR 9–13) to 8 (IQR 7–10, p = 0.37). (p between groups 0.006). When looking at the delta of the Lictiger score, the average change was 6.4 ±3.1 in the cannabis group and 2 ±2.5 in the placebo group (p<0.05), only two patients, both from the placebo treated group, had an increase in the Lichtiger score, but the change was less then 3 points, and thus not defined as a disease flare. The number of bowel movements per day decreased from 2.6 (IQR 2–4) to 1 (IQR 0–1, p<0.001)and from 2.6 (IQR 2–4) to 2 (IQR 2–3, p = 0.168) in the active arm and placebo groups respectively (p between groups 0.006). The number of patients who reported severity of abdominal pain of ≥ 2 decreased from 10 (59%) at baseline to 1 (6%) after 8 weeks of treatment(p = 0.006) in the cannabis group and from9 (60%) to8 (55%),(p = 0.429) in the placebo group, (p between groups = 0.04). The number of patients who reported blood in stool decreased from 13 (76%) to 5 (30%) in the cannabis group (p = 0.015). and from 9 (60%) to 6 (40%) in the placebo group (p = 0.589)(p between groups = 0.64) (Table 3).
Table 3. Effect of cannabis on disease activity.
| Cannabis | Placebo | p Cannabis vs placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| visit 1 | visit 3 | p | visit 1 | visit 3 | p | visit 1 | visit 3 | |
| Lichtiger score | 10.9 (IQR 9–14) | 5 (IQR 1–7) | 0.001 | 11 (IQR 9–13) |
8 (IQR 7–10) | 0.37 | 0.914 | 0.006 |
| Bowel movements (median IQR) | 2.6 (IQR 2–4) | 1 (IQR 0–1) | 0.001 | 2.6 (IQR 2–4) | 2 (IQR 2–3) | 0.168 | 0.914 | 0.006 |
| Abdominal pain≥2 | 10 (59%) | 1 (6%) | 0.006 | 9 (60%) | 8 (55%) | 0.429 | 0.914 | 0.04 |
| Blood in stool | 13 (76%) | 5 (30%) | 0.015 | 9 (60%) | 6 (40%) | 0.589 | 0.73 | 0.645 |
| Endoscopic Mayo score | 2.13±1 | 1.25±2 | 0.015 | 2.15±1 | 1.69±1 | 0.367 | 0.914 | 0.374 |
| QOL | 77±4 | 98±20 | 0.001 | 78±3 | 78±17 | 0.631 | 0.914 | 0.026 |
| WBC | 6.7±0.4 | 6.8±0.4 | 0.044 | 8.9±0.7 | 7.9±0.8 | 0.37 | 0.587 | 0.393 |
| Hb | 12.9±0.6 | 13.1±0.5 | 0.776 | 13.6±0.5 | 13.1±0.5 | 0.828 | 0.733 | 0.911 |
| CRP | 1.8±0.2 | 2.8±1.9 | 0.652 | 0.8±0.4 | 1.1±0.3 | 0.828 | 0.578 | 0.843 |
| Calprotectin | 170±33 | 134±33 | 0.072 | 226±34 | 218±67 | 0.9 | 0.688 | 0.393 |
| Weight | 68±4.7 | 66±5.2 | 0.24 | 60±1.8 | 58±2.3 | 0.367 | 0.578 | 0.286 |
QOL improved in the cannabis group from 77±4 to 98±20 (p = 0.001) but not in the placebo group (78±3 at week 0 and 78±17 at week 8;p = 0.631; p between groups 0.026) (Table 3).
Colonoscopy at baseline and at the end of treatment was performed in 29 out of 32 (90%) patients, Mayo endoscopic score improved in the cannabis-treated group from an average of 2.13±1 to 1.25±2 (p = 0.015) and in the placebo group from 2.15±1to 1.69±1 (p = 0.367). However, pre- to post-intervention differences between the groups (delta between pre intervention and post intervention score)did not reach statistical significance(1.25±2and 1.69±1 in the study and placebo groups, respectively, p = 0.374).
Baseline to end of 8 weeks treatment laboratory parameters of inflammation, including blood count, CRP, and fecal calprotectin did not change in both groups (Table 3).
When asked about the effect of treatment on specific symptoms, patients in the cannabis group reported improvement in their general health, appetite, libido, concentration, and pain. The placebo group did not report similar changes. General satisfaction with treatment was high among the cannabis treated group. Interestingly, the improvement was noted within one week (Table 4).
Table 4. Clinical effect of cannabis (assessed by patient questioning*).
| Parameter: | Study group | Placebo group | p |
|---|---|---|---|
| General health (yes/no) | 14/1 (82%) | 1/14 (6.7%) | 0.003 |
| Mood | 3.3±1.1 | 3.6 ±1.1 | 0.384 |
| Memory | 4.5±0.7 | 4.07±2.5 | 0.168 |
| Appetite | 2.5±1.2 | 3.7±0.8 | 0.019 |
| Concentration | 2.0±1.1 | 3.9±0.5 | 0.002 |
| Sleep | 4.47±0.7 | 4.07±0.4 | 0.178 |
| Daily function | 3.47±0.7 | 4±0 | 0.099 |
| Alertness | 3.85±0.9 | 3.9±0.2 | 0.852 |
| Libido | 1.93±0.7 | 4±0 | 0.001 |
| Pain | 2.7±1.3 | 3.9±0.25 | 0.013 |
| Abdominal swelling | 3.7±1.1 | 4±2.5 | 0.446 |
| General satisfaction from treatment | 2.4±1.5 | 5.6±1.6 | 0.001 |
| How long did it take to feel the change (1 = Immediately, 2 = within 1 week, 3 = within 2 weeks, 4 = no change) | 0.9±0.7 | 3.8±0.4 | 0.001 |
* On a grade from 1 to 7, 1 = improved, 4 = no change, 7 = deteriorated
The reported side effects were minor and did not lead to cessation of treatment in any patients (Table 5)
Table 5. Reported side effects.
| Cannabis | placebo | p | |
|---|---|---|---|
| Cough | 7 (41%) | 3 (20%) | 0.272 |
| Dizziness | 6 (35%) | 1 (6%) | 0.272 |
| confusion | 5 (29%) | 1(6%) | 0.304 |
| Difficulty to stop use | 5 (29%) | 2 (12%) | 0.543 |
| Behavioral change | 4 (23%) | 0 (0%) | 0.27 |
| Restlessness | 2 (11%) | 0 (0%) | 0.543 |
| Shortness of breath | 1 (6%) | 0 (0%) | 0.543 |
| Decreased memory | 0 (0%) | 6 (40%) | 0.153 |
| Hallucinations | 0 (0%) | 0 (0%) | 0.883 |
Of the 32 patients who completed the study, 17 patients continued active cannabis use and follow up in our clinic for an additional period of one year. Eight were from the cannabis study group and 9 from the placebo group. The majority (n = 14) of these patients did not need any medical intervention throughout this one-year follow-up. Two patients needed a course of steroids and one patient started treatment with Vedolizumab. At the end of this year, 11 patients underwent a colonoscopy and 10 of them had a Mayo score of 1–0, whereas before initiation of cannabis 2 had a score of 3 and 8 had a score of 2.
Reasons for not continuing follow up (n = 15 patients) included: lost contact (n = 5), change of residence (n = 3) and wish to stop cannabis (n = 7). The reasons for discontinuing cannabis treatments were intolerance (n = 4), clinical deterioration (n = 2) and planning to become pregnant (n = 1).
Discussion
Epidemiological studies indicate that between 15–45% of patients with IBD use cannabis [6, 7] and anecdotal clinical reports suggest improvement in patient’s wellbeing and IBD-related symptoms [7, 12, 24]. In addition, preclinical animal and laboratory investigational models have demonstrated anti-inflammatory effects of cannabis, thus further supporting a potential benefit of using cannabis in patients with IBD [7, 10, 11].
The endocannabinoid system has an important role in the regulation of inflammatory response in the colon [10]. Cannabinoids were shown to ameliorate colitis in various murine models of colitis, with an anti-inflammatory effect mediated thorough activation of the cannabinoid receptors CB1 and CB2, inhibition of the endocannabinoid degrading enzymes Monoacylglycerol lipase(MAGL) and fatty acid amid hydrolase (FAAH), and activation of the G protein-coupled receptor 55 (GPR55) and Transient receptor potential vanilloid 1 (TRPV1) receptors [25, 26].
However, despite the increasing anecdotal reports suggesting a clinical benefit of cannabis in patients with IBD and the accumulating data on its intestinal, and specifically colonic anti-inflammatory effects in animal models of IBD, only a few prospective, placebo-controlled studies have been conducted. Furthermore, most of the studies focused on clinical outcomes and did not include investigation of objective anti-inflammatory effects [6, 12, 24]. Therefore, the question whether the observed effect is limited to symptomatic improvement or due to a reduction in inflammation remains open.
In the current study, we investigated clinical as well as endoscopic and laboratory responses to cannabis treatment in patients with UC in a randomized placebo-controlled study. Unlike previous studies we were specifically interested to see if the clinical effects of cannabis treatment will be associated with a reduction of inflammation.
From a clinical perspective, we found that treatment with cannabis led to a significant reduction in the Lichtiger Disease Activity Index and improvement in major IBD-related clinical symptoms including abdominal pain and number of bowel movements per day. We also observed a significant improvement in quality of life, general health, appetite, libido, concentration, and patient satisfaction with the treatment.
Regarding the effect on inflammation, we found a significant pre- to post-intervention improvement in the Mayo endoscopic score in both study groups, This effect was greater in the cannabis than in the placebo group, however it did not reach statistical significance in between groups’ analysis. In addition, we could not find significant pre- to post-intervention changes in laboratory markers of inflammation including blood count, CRP and fecal calprotectin within the cannabis and the placebo groups, nor in between groups analysis.
In a study from our group using THC rich cannabis in patients with Crohn’s disease, we found significant clinical improvement, reduction of CDAI and improved quality of life, but no change in CRP [12, 13]. Similarly, Irving et al, who gave Cannabidiol (CBD)to patients with UC showed clinical improvement in partial Mayo score without improvement in inflammatory markers including endoscopic Mayo score [15]. The lack of association between clinical beneficial observation and anti-inflammatory effects could result from differences in the effect of various chemical components of cannabis. The two major active components of cannabis are cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC). While CBD works mainly peripherally without a central effect, THC works mainly centrally and is responsible for the dominant psychoactive effects of cannabis [25]. These two components seem to act synergistically onCB1 and CB2 at the level of the enteric nervous system [26].
In the current study, we used THC rich cannabis., Thus it is quite likely that the observed effect was rather central than peripheral and therefore resulted in a weaker anti-inflammatory effect. Another possibility is that the onset of the central clinical effect is faster while the anti-inflammatory effect may take longer and therefore we could not detect an accompanying effect on peripheral inflammatory markers in this relatively short, 8 weeks study. Lastly, through its effect on CB1 and CB2 receptors in the gut, cannabis also affects GI physiology including reducing intestinal motility, increasing fluid absorption and inducing analgesia [8, 24]. Therefore, it is possible that the symptomatic improvement observed in our study reflects the effect on intestinal physiology without a significant effect on inflammation.
Smoking tobacco is known to have a positive effect in UC. We chose smoking as a mode of cannabis consumption because this is the most common form used by patients in "real life". However, this may have lead to the high rate of response in the placebo group.
Regardless of the mechanism by which cannabis exerts its clinical effect, the endpoint of patient wellbeing, quality of life and daily functioning is of no lesser value than improvement in inflammation.
Overall, cannabis was well tolerated in our study. Patients reported only minor side effects, mostly dizziness (n = 6, 35%) and confusion (n = 5,29%) and none of our patients dropped out of the study due to side effects. A study among 3,341 patients using cannabis reported the most common side effects of dry mouth (26%) and feeling foggy (23%). These side effects were associated with THC and much less with CBD [27, 28]. In the study by Irving et al [15], doses of up to 500mg/day of CBD produced a high rate of side effects which led to violation of protocol and/or dropouts by 41% of the participants. The low level of side effects and lack of drop out in our study could be explained by our treatment protocol which started cannabis treatment at a low dose and increased the dose gradually, hence enabling the patients time to develop tolerance to the treatment.
Our study has several strengths including the stable dose of cannabis used, the placebo-controlled design and the examination of inflammatory parameters, including endoscopic and laboratory markers for disease activity, in addition to clinical parameters. The weaknesses of the study are the small sample size, short duration of the study, lack of histological data and the inherent difficulty of blinding cannabis use. Future studies are needed with higher sample sizes, and combining other populations. Another weakness is the consumption of cannabis as cigarettes. Although in "real-life" most patients who report beneficial effects of cannabis consume it by smoking, this mode of delivery is not advisable and could not be acceptable for medical treatment. Other healthier modes of consumption should be investigated. Vaping could be an option since vaporizers do not produce toxic compounds formed by pyrolysis and the pharmacokinetics of vaporized and smoked cannabinoids is comparable. Oral consumption is another possibility, but oral THC formulations exhibit variable absorption and undergo extensive hepatic first-pass metabolism, producing lower peak plasma concentrations relative to inhalation. Further studies are needed to evaluate the various modes of cannabis consumption and select those that safest and most efficient [29–31].
Placebo controlled studies are particularly challenging when using psychoactive substances. We tried to overcome this difficulty by recruiting only patients who did not experience previous cannabis use. Indeed, at least 3 patients receiving placebo were convinced they were receiving cannabis, but we do not have this data on all the study participants.
Our study was designed as a short (8 weeks) intervention study. However, we had the opportunity to follow a third of the patients for another year and found that endoscopic remission was retained (with a Mayo score of 0–1) in 10/11 patients. This long-term remission suggests a possible durable beneficial effect of cannabis. Larger, long-term studies are warranted to investigate this finding.
Conclusion
This study demonstrates that treatment with THC-rich cannabis in patients with mild to moderate UC is associated with clinical improvement. Our findings indicate that the reported cannabis-induced clinical effect is not directly linked to an anti-inflammatory effect of cannabis. However, the results demonstrate a signal for associated reduction in mucosal inflammation in patients with UC. This preliminary observation requires additional investigation in larger and longer intervention clinical studies. Such studies will enable us to determine whether cannabis has mainly a symptom relieving role or a more specific anti-inflammatory therapeutic effect. Future research should focus on alternative ways of providing cannabis (other than smoking), and explore various cannabinoid compounds in order to reveal the most effective and safe mode of cannabis use by patients with IBD.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Data Availability
Data cannot be shared publicly because of privacy of the patients. Data are available from the Meir medical center Institutional Data Access / Ethics Committee (contact via the author, Dr Timna Naftali, timna.naftali@clalit.org.il) for researchers who meet the criteria for access to confidential data. You can also contact Dr Dan Feldman, who is not an author, Tel: 97297472580, E mail:dan.feldman@clalit.org.il.
Funding Statement
Funding: the cannabis used in the study was supplied by Tikun Olam, Israel. otherwise there was no funding.
References
- 1.Da Silva BC1, Lyra AC1, Rocha R1, et al. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol. 2014: 28;20(28):9458–67. 10.3748/wjg.v20.i28.9458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asakura K, Nishiwaki Y, Inoue N, et al. Prevalence of ulcerative colitis and Crohn’ s disease in Japan. J Gastroenterol 2009; 44: 659–65 10.1007/s00535-009-0057-3 [DOI] [PubMed] [Google Scholar]
- 3.Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018. 53(3):305–53 10.1007/s00535-018-1439-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungar B., Kopylov U. (2016). Advances in the development of new biologics in inflammatory bowel disease, Ann Gastroenterol 2016; 29 (3): 243–48. 10.20524/aog.2016.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma C1, Huang V, Fedorak DK, et al. Outpatient Ulcerative Colitis Primary Anti-TNF Responders Receiving Adalimumab or Infliximab Maintenance Therapy Have Similar Rates of Secondary Loss of Response. J Clin Gastroenterol. 2015; 49(8):675–82. 10.1097/MCG.0000000000000265 [DOI] [PubMed] [Google Scholar]
- 6.Weiss A, Friedenberg F. Patterns of cannabis use in patients with Inflammatory Bowel Disease: A population-based analysis. Drug Alcohol Depend. 2015;1(156):6–11. 10.1016/j.drugalcdep.2015.08.035 [DOI] [PubMed] [Google Scholar]
- 7.Waseem A, Katz S. Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterology & Hepatology, 2016, 12(11), 668–79. [PMC free article] [PubMed] [Google Scholar]
- 8.Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med (Berl). 2005; 83:944–54 10.1007/s00109-005-0698-5 [DOI] [PubMed] [Google Scholar]
- 9.Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57:1140–55 10.1136/gut.2008.148791 [DOI] [PubMed] [Google Scholar]
- 10.Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113: 1202–09. 10.1172/JCI19465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacher P, Bátkai S. (2006). The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacological Reviews, 58(3), 389–462. 10.1124/pr.58.3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naftali T, Lev LB, Yablecovitch D, et al. Treatment of Crohn’s disease with cannabis: an observational study. Isr Med Assoc J. 2011;13(8):455–8. [PubMed] [Google Scholar]
- 13.Naftali T., Bar-Lev L., Dotan I., et al. Cannabis Induces a Clinical Response in Patients with Crohn’s Disease: a Prospective Placebo-Controlled Study. Clinical Gastroenterology and Hepatology,2013:11(10), 1276–1280. 10.1016/j.cgh.2013.04.034 [DOI] [PubMed] [Google Scholar]
- 14.Naftali T, Mechulam R, Marii A, et al. Low-Dose Cannabidiol is safe but not effective in the treatment for Crohn’s Disease, a Randomized Controlled Trial. Dig Dis Sci. 2017;62(6):1615–20. 10.1007/s10620-017-4540-z [DOI] [PubMed] [Google Scholar]
- 15.Irving PM, Iqbal T, Nwokolo C, et al. A Randomized, Double-blind, Placebo-controlled, Parallel-group, Pilot Study of Cannabidiol-rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm Bowel Dis. 2018:19;24(4):714–24 10.1093/ibd/izy002 [DOI] [PubMed] [Google Scholar]
- 16.Lobatón T., Bessissow T., De Hertogh G., et al. The Modified Mayo Endoscopic Score (MMES): A New Index for the Assessment of Extension and Severity of Endoscopic Activity in Ulcerative Colitis Patients. Journal of Crohn’s& Colitis, 2015: 9(10), 846–52. 10.1093/ecco-jcc/jjv111 [DOI] [PubMed] [Google Scholar]
- 17.Altman DG, Bland JM. How to randomize. BMJ. 1999;319(7211): 703–04. 10.1136/bmj.319.7211.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtiger S1, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994. 30;330(26):1841–5. 10.1056/NEJM199406303302601 [DOI] [PubMed] [Google Scholar]
- 19.Knowles SR, Graff LA, Wilding H, et al. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses-Part I. Inflamm Bowel Dis. 2018. 19;24(4):742–51 10.1093/ibd/izx100 [DOI] [PubMed] [Google Scholar]
- 20.Vashist N, Samaan M, Mosli MH, et al. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2017:25:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed Washington, DC: American Psychiatric Association, 1994:215–223. [Google Scholar]
- 22.Benjamini Y; Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B.1995, 57 (1): 289–300. [Google Scholar]
- 23.Ashida T, Kohgo Y, Munakata A, Noguchi M, Iizuka B, Endo Y, et al. A multicenter study of the efficacy and safety of leukocytapheresis therapy without concomitant systemic steroid treatment in patients with active ulcerative colitis. TransfusApher Sci. 2011; 44(2):113–7. 10.1016/j.transci.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 24.Pi S, Rosenfeld G, Enns R, et al. Patterns and Motivations of Cannabis Use Amongst Patients with Inflammatory Bowel Disease. GastroHep.2019: 100–107.) [Google Scholar]
- 25.Lohar V, Rathore A. S. Cannabinoids: Pharmacological profile of promising molecules, Phytopharmacology 2013, 4(1), 41–52 [Google Scholar]
- 26.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins LeukotEssent Fatty Acids. 2002;66:101–21. 10.1054/plef.2001.0341 [DOI] [PubMed] [Google Scholar]
- 27.Stith SS, Vigil JM, Brockelman F, et al. The Association between Cannabis Product Characteristics and Symptom Relief. Sci Rep. 2019:25;9(1):2712 10.1038/s41598-019-39462-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaston TE, Szaflarski JP, Cannabis for the Treatment of Epilepsy: an Update. Curr Neurol Neurosci Rep. 2018: 8;18(11):73 10.1007/s11910-018-0882-y [DOI] [PubMed] [Google Scholar]
- 29.Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, et al. Acute Pharmacokinetic Profile of Smoked and Vaporized Cannabis in Human Blood and Oral Fluid. Anal Toxicol. 2019. May 1;43(4):233–258. 10.1093/jat/bky104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84(11):2477–2482. 10.1111/bcp.13710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newmeyer MN, Swortwood MJ, Barnes AJ, Abulseoud OA, Scheidweiler KB, Huestis MA. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: identification of recent cannabis intake. Clin Chem 2016; 62: 1579–1592. 10.1373/clinchem.2016.263475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data cannot be shared publicly because of privacy of the patients. Data are available from the Meir medical center Institutional Data Access / Ethics Committee (contact via the author, Dr Timna Naftali, timna.naftali@clalit.org.il) for researchers who meet the criteria for access to confidential data. You can also contact Dr Dan Feldman, who is not an author, Tel: 97297472580, E mail:dan.feldman@clalit.org.il.