Abstract
A critical review of the current state of knowledge of chemical emissions from indoor sources, partitioning among indoor compartments, and the ensuing indoor exposure leads to a proposal for a modular mechanistic framework for predicting human exposure to semivolatile organic compounds (SVOCs). Mechanistically consistent source emission categories include solid, soft, frequent contact, applied, sprayed, and high temperature sources. Environmental compartments are the gas phase, airborne particles, settled dust, indoor surfaces, and clothing. Identified research needs are the development of dynamic emission models for several of the source emission categories and of estimation strategies for critical model parameters. The modular structure of the framework facilitates subsequent inclusion of new knowledge, other chemical classes of indoor pollutants, and additional mechanistic processes relevant to human exposure indoors. The framework may serve as the foundation for developing an open-source community model to better support collaborative research and improve access for application by stakeholders. Combining exposure estimates derived using this framework with toxicity data for different end points and toxicokinetic mechanisms will accelerate chemical risk prioritization, advance effective chemical management decisions, and protect public health.
Graphical Abstract
INTRODUCTION
Rapid risk-based prioritization of large numbers of chemicals in numerous building materials, consumer products, and consumer articles used indoors (here collectively referred to as sources) has become an increasing focus of chemical management strategies to protect humans from potentially harmful exposures.1–5 Although these strategies emphasize the need for high-quality, representative exposure data and mechanistic (i.e., process-based) exposure models, approaches for implementation are highly fragmented among countries, authorities, and research institutions.5,6 We need to implement more efficiently the current understanding of fundamental mechanisms governing indoor exposure to chemicals released from sources. This is especially true for semivolatile organic compounds (SVOCs) due to their multimedia behavior. Unlike volatile organic compounds (VOCs) or most metals, SVOCs partition among multiple indoor compartments, including people, according to their physicochemical properties.2,7–9 As the number of source/chemical combinations increases steadily, traditional assessment approaches are challenged to keep pace given that key emission and exposure parameters have only been measured for very few combinations that do not necessarily span the full range of exposure conditions.8,10,11 Potential approaches to facilitate the transition from hazard-driven, single-chemical assessments toward rapid, risk-based, high-throughput (HT) prioritization are already being developed and include procedures to estimate exposure, toxicity, and toxicokinetics.3,12–16 For example, HT screening methods allow testing of large numbers of samples with highly automated instruments in combination with advanced data processing software.13,15,17,18 Substantial efforts have been made in generating HT hazard information and HT toxicokinetics, but essential information to predict human exposure to chemicals released from indoor sources is frequently missing.19,20 To fully realize the potential of new chemical risk assessment approaches, reliable chemical exposure models need to be coupled with quickly accessible toxicity data.2,21–23
This paper provides a critical review of mechanistic models for estimating chemical emissions from indoor sources, partitioning among indoor compartments, and exposure to humans indoors. The review develops a generic modular mechanistic framework that provides a roadmap for evaluating exposure scenarios associated with the use phase of materials, products, and articles, within a rapid risk screening and prioritization context. The specific objectives of this paper are thus to (1) define mechanistically consistent source emission categories, (2) review exposure pathways that are congruent with these source emission categories and the subsequent chemical distribution among indoor compartments, and (3) identify relevant knowledge gaps and needs for further research that will facilitate exposure estimates for indoor sources. The focus is on SVOCs, but the modular structure of the framework will make it possible to subsequently include other classes of chemicals released indoors.
Many of the underlying elements forming this framework have been described elsewhere.24–27 Here, we combine and connect these elements in a consistent and meaningful way to facilitate efficient implementation. To achieve this, we reviewed the existing research in the field, highlighted knowledge gaps, and then assembled a consensus regarding what is known about SVOC emission, transport, and exposure in indoor environments. The result is a framework for rapid exposure estimation that serves as a baseline among researchers and stakeholders regarding SVOC models and conditions of application. Exposure models and concepts underpinning the proposed framework will enable efficient exposure estimates for a wide range of source/chemical combinations, inform scientifically-based chemical management policy and public health decision-making, and provide new insights for manufacturers of building materials, consumer products, and consumer articles.
SEMIVOLATILE ORGANIC COMPOUNDS IN INDOOR ENVIRONMENTS
There are several definitions of SVOCs.28 The World Health Organization (WHO) defines SVOCs as a group of chemicals with boiling points in the range of (240–260 °C) to (380–400 °C).29 According to International Standard ISO 16000–6:2011, SVOCs elute after n-hexadecane on a nonpolar gas chromatography capillary column.30 Other definitions refer to a liquid, pure compound vapor pressure range of 10−9 Pa to 10 Pa at room temperature31 or to a saturation mass concentration range of 0.3 μg m−3 to 300 μg m−3, corresponding to vapor pressure in the range of about 10−6 Pa to 10−3 PaPa, assuming an average molecular weight of 400 g mol−1 and standard conditions.32
SVOCs are present in many different consumer products, building materials, and other indoor articles where they are used as, for example, plasticizers, flame retardants, solvents, and pesticides. Often, they occur as additives that are not chemically bonded to the host polymer. Numerous SVOCs have been associated with adverse health effects, making them of particular concern to scientists, chemical managers, public health professionals, and policy makers.33–36 Their physicochemical properties make the characterization of SVOCs in indoor environments and exposure estimation challenging because they tend to partition among indoor compartments (e.g., gas phase, airborne particles, settled dust, and surfaces) at different rates.37
SVOC emissions can occur directly during use (e.g., spraying of indoor pesticides or application of varnish) or from the application itself, because the additive migrates over time, resulting in delayed exposure (e.g., migration from plastic products such as PVC shower curtains). Exposure to SVOCs is possible via inhalation, dermal uptake (i.e., transfer from a source to the skin surface) and ingestion (including hand-to-mouth transfer and mouthing).38 In 2004, Bennett and Furtaw39 introduced a fugacity-based model to describe exposure to SVOCs in indoor environments, while in 2006, Xu and Little40 extended a model originally developed for VOCs to predict emission rates of SVOCs from polymeric materials. As with SVOCs outdoors, Xu and Little showed that SVOC emission and transport are subject to external control, that is, partitioning, convective mass transfer and sorption to surfaces and airborne particles.40 In 2008, Weschler et al.41 investigated the distribution of phthalates among the gas phase, airborne particles, and settled dust, and provided a model for the gas/particle partition coefficient Kp, which allows an estimation of the SVOC concentration in airborne particles if the gas-phase concentration is known. Weschler and Nazaroff31 presented the first framework for characterizing equilibrium partitioning among indoor compartments for SVOCs. One key finding was that SVOCs might persist in indoor environments for years, depending on their vapor pressure, even after the original source has been removed.31,42 Several studies followed, presenting both measurements and models describing SVOC emission, transport, and subsequent exposure, often with a focus on phthalates such as di-2-ethylhexyl phthalate (DEHP).43–48
In 2012, Little et al.37 proposed a framework for rapid exposure estimates based on the source type in which the respective SVOC is present. Accounting for source composition and use (i.e., additive vs reactive use in the host polymer) as well as for emission characteristics, a simple method to estimate exposure to additives in sources used indoors and for sources sprayed or applied to interior surfaces was developed.37 The key parameters needed for these models have since been investigated, and reliable measurement methods of various levels of complexity are now available.49–57 Other aspects, such as the influence of clothing on dermal SVOC exposure,58,59 the dermal uptake of SVOCs from air,24,60,61 the impact of organic films on indoor surfaces on SVOC dynamics,48,62,63 and the influence of occupants on indoor exposure to SVOCs64,65 are being studied in increasing detail.
Li et al.66–68 developed a framework for describing the fate of chemicals at different product life-stages (Production-to-Exposure or PROTEX model). PROTEX is comprised of four modules, including a technosphere module, which is based on a flow analysis approach, a nested indoor-urban-rural fate module, a food-web bioaccumulation module, and a human toxicokinetic module, all based on the fugacity approach.68 PROTEX yields exposure estimates based on near- and far-field exposure pathways.68 Its modular structure and the consideration of different product life-stages make the PROTEX model a good example of how different aspects of chemical fate can be combined. However, the estimation of indoor emission rates in the technosphere module is based on empirical relationships to estimate emission factors68,69 or on mechanistic models that the framework presented here aims to expand and update.40,67 The modular structure of PROTEX allows the integration of consistent mechanistic models to describe the fate of SVOCs in indoor environments during the product use stage, thus making PROTEX highly complementary to the proposed framework.
Dietary exposure to SVOC residues present in food and beverages is an important component of overall exposure to certain SVOCs, for example to DEHP, diethyl phthalate (DEP), and triphenyl phosphate (TPHP).70–73 This exposure route can occur due to “far field” effects of bioaccumulation through the food web or due to migration from food contact materials and appliances.74,75 To assess the far-field dietary exposure pathway, it would be necessary to expand the framework discussed here; however, a detailed review of the relevant mechanisms is beyond the scope of this critical review. One example of how to connect indoor exposure and dietary exposure as well as far-field exposures can be found in the PROTEX framework, which includes a food-web bioaccumulation module.66,76
A FRAMEWORK FOR PREDICTING EXPOSURE TO INDOOR SVOCS
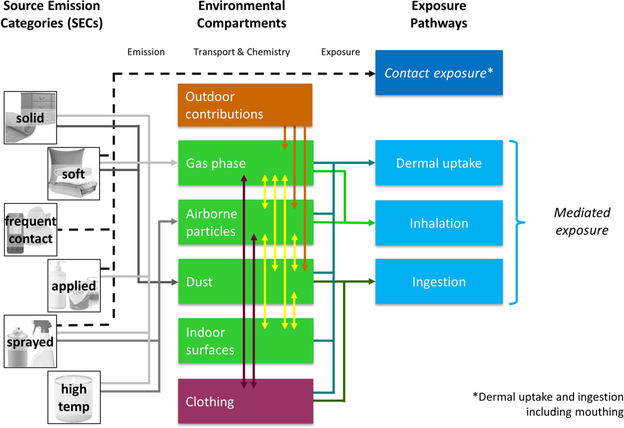
Figure 1 illustrates a mechanistic modeling framework for estimating exposure to SVOCs emitted from sources present or used in the indoor environment. The central elements of the framework are (1) the modeling of emissions based on mechanistically consistent source emission categories (SECs), (2) the modeling of transport, chemical transformations, and resulting concentrations in the respective indoor environ- mental compartments, and (3) the estimation of exposure via different pathways based on the concentrations in the compartments for different exposure scenarios. Based on this framework and with the SECs as starting points, we review the scientific background and underlying assumptions of the mechanistic models for emission, transport, chemistry, and exposure, as well as their main input parameters, assumptions, uncertainties, limitations, and conditions of model application.
Figure 1.
A modular framework for modeling indoor SVOC emission, transport, chemistry and exposure.
In the framework, the focus is on mechanistic modeling as the underlying principles are robust, and the model can be applied to multiple chemicals when predicting exposure.21,25,77,78 Mechanistic models, also referred to as process-based models,19 rely on well-established physicochemical processes such as diffusion or sorption. They are generalizable and can vary in complexity based on the needs of a given assessment context, in contrast to empirical models.27 However, if suitable mechanistic models are not available, other approaches such as machine-learning, expert opinions, statistical, or empirical models may serve in a preliminary role.78
Assumptions.
The following general assumptions are often made for SVOC emission, transport, and exposure models. They are valid in many cases, but there are exceptions that increase the uncertainty of the derived exposure estimates.
The indoor compartments considered in this framework are the gas phase, airborne particles, dust, and surfaces. We included clothing as an additional compartment because its role in exposure scenarios is particularly important.58,79 People are in constant, intimate contact with clothing. Clean clothing has been shown to protect against dermal uptake of SVOCs from air, while clothing that had been exposed to contaminated air can serve as an additional exposure source leading to potentially increased dermal uptake and inhalation exposure.58,80,81 These characteristics, together with other properties discussed below, distinguish clothing from other environmental compartments.
The relationship between the indoor compartments is fundamentally dynamic. In some cases, equilibrium conditions between gas phase, particle phase, dust, and surfaces can be assumed to simplify the models.52,82 However, the greater the capacity of an environmental compartment, the longer it will take for that compartment to reach equilibrium with other compartments, and the less applicable the equilibrium assumption will be. A detailed discussion of this issue can be found in Mackay et al.,83 including recommendations for identifying and quantifying kinetic delays.
SVOC emissions from source materials are mostly externally controlled, meaning that internal mass transfer (e.g., diffusion) is faster than external mass transfer.31,40,84 Furthermore, the material-phase SVOC concentration C0 can be considered constant as depletion usually happens at a very slow rate, as shown by Xu and Little,40 Xu et al.85 and Pei et al.86 However, these assumptions are only valid for sufficiently small external mass transfer coefficients and SVOCs with relatively low vapor pressures.87 Internal diffusion may become important when considering transfer in, for example, clothing.57,88
It is generally assumed for modeling purposes that the air in the indoor environment is well-mixed, but this may not be the case for many indoor environments, especially not for indoor air in poorly ventilated spaces, for example within cabinets and closets.89,90
In some cases, it may be reasonable to assume that background outdoor concentrations of SVOCs are negligible for exposure assessments of chemicals released from indoor sources. This assumption depends on the type of SVOC considered, as some are present both indoors and outdoors, for example, pesticides and polycyclic aromatic hydrocarbons (PAHs).91–93 If an SVOC has an outdoor source, its concentration in the infiltrating air and the possibility of transport into the indoor environment with outdoor dust should be taken into account.94
Emission.
The framework is structured to distinguish between contact and mediated exposure (Figure 1). Contact exposure can occur immediately via direct dermal uptake or (inadvertent) ingestion of chemicals in the source, which includes mouthing or licking of a source. Mediated exposure occurs when emission from the source to environmental compartments happens first, followed by partitioning and chemical transformations, resulting in accumulation in one or more compartments where exposure may take place. Frequently, exposure pathways are categorized as “direct” and “indirect”, but these categories are used inconsistently in the literature, thus we chose different terms.
Chemical emission from a source depends largely on chemical characteristics, on the source characteristics, and on the properties of the environmental compartment into which the chemical is emitted.37 However, all indoor environmental compartments can serve as both sources and sinks, even simultaneously, depending on the chemical and the direction of the chemical fugacity (or chemical activity) gradient. The general direction for chemical mass transfer in this framework is the transport from sources with high fugacity to sinks (including humans) with lower fugacity, thus the arrows in Figure 1 point from the left (sources) to the right (human exposure). It should be noted that the direction of the chemical fugacity gradient may reverse.
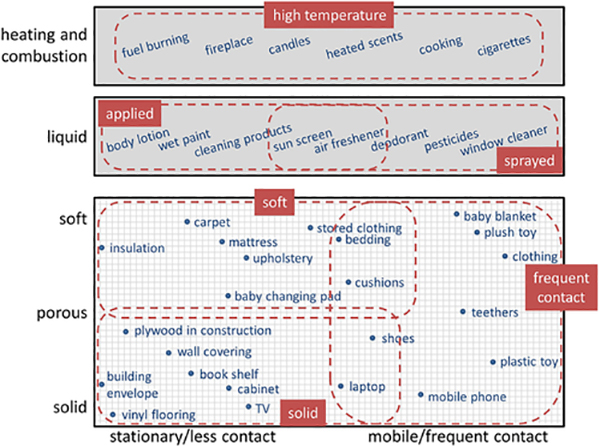
Traditionally characterized sources of emission are furniture, building materials including flooring and carpet, electrical and electronic products, personal care products, as well as combustion processes (e.g., burning a scented candle) and cooking (e.g., heating oil in a pan). For our modeling framework, we derived SECs from a continuum of possible sources (Figure 2). We recognize that there is some overlap between the categories and that some sources may not fit perfectly into one category. In such cases, multiple scenarios may have to be considered.
Figure 2.
Classification of source emission categories (SECs) from a continuum of possible sources, which range from solid to soft, from stationary to frequent contact, and including certain specific uses such as applied, sprayed, and heated/combusted.
For SVOCs, Little et al.37 differentiated between two main categories of sources: One included solid sources in which SVOCs are present as additives or surface coating components and the other category included sources containing SVOCs that are either sprayed or applied. Mechanistic emission models were presented for both categories.37 Here, we modify and extend the list of categories to include the following SECs: solid sources, soft sources, sources that humans frequently contact, liquid applied sources, liquid sprayed sources, and high temperature sources, resulting in a total of six mechanistically consistent SECs (Figure 2), which cover most sources of SVOCs.10,95
These SECs can be mapped to those defined by organizations such as the Organisation for Economic Co-operation and Development (OECD) and the U.S. EPA. The sources in the categories “solid”, “soft”, and “frequent contact” are usually “articles” as defined by the OECD, that is, solids, polymers, foams, metals or woods that are continuously present in the indoor environment during their useful life.96 “Applied”, “sprayed”, and “high temperature” sources correspond to “products” as defined by the OECD, which are consumable liquids, aerosols, or semisolids that are used a certain number of times until they are exhausted.96 Furthermore, the Level 1 and Level 2 product use categories (PUCs) listed in Isaacs et al.97 overlap to a large extent with the SECs “frequent contact”, “applied”, and “sprayed” and to some extent with the categories “solid” and “soft”. The PUCs were derived from the products listed in the U.S. EPA’s Chemicals and Product Database (CPDat).97 Although they do not align completely, it would be possible to map each PUC to an SEC and potentially to incorporate PUCs in this framework. Descriptors and schemes used in other instances for tracking and managing products and source/chemical combinations could potentially be adapted as well, given that the categories proposed here are intended to be relatively flexible, while still remaining mechanistically consistent.98
The SEC-based emission rates and chemical concentrations for each indoor compartment can be used in combination with the exposure estimation models presented as part of the framework or independently in different types of specific exposure and intake models to derive exposure estimates in the required format.
Solid Sources.
Solid sources containing SVOCs include, for example, PVC flooring, electronic devices such as TVs, plywood furniture, and previously painted walls. They have relatively large emitting surface areas relative to the volume of most indoor environments or to the area that may come in contact with an exposed person. Exposure to SVOCs present in these solid sources is predominantly mediated. Contact exposure contributes much less to overall exposure.76,99
As discussed above, depletion of the SVOC in the source can often be assumed to be negligible.40,85,86,100 Including the variability of source strength over time in the model increases its complexity, but the mechanisms governing emission from solid sources remain the same. A prerequisite for calculating the depletion rate is that C0 is known.
From a solid source, emission may occur either into the gas phase or by migration into dust in direct contact with the solid source material.37,101 Governing parameters for emission into the gas phase are the SVOC gas-phase concentration immediately adjacent to the source material y0 and the mass transfer coefficient hm (Section 1a in the Supporting Information (SI)).25,37,102 y0 is itself a function of C0 (SI Section III)53,56 and can also be measured49 or derived using the material/air partition coefficient, Kma.103,104 Addington et al.105 developed a quantitative structure−activity relationship (QSAR)-based method for estimating activity coefficients and y0 for plasticizers based on chemical structure, polymer characteristics, and physicochemical properties. An expansion of this approach to other types of SVOCs and chemicals in general would greatly facilitate our ability to estimate indoor emissions.
If it is assumed that a linear equilibrium relationship exists between the settled dust and the gas layer directly adjacent to the source material (SI Section 3), the critical parameters for modeling emission into dust are y0, hm (SI Section IV), the dust/air partition coefficient Kdust (SI Section VI), the particle deposition velocity vd, and the concentration of airborne particles, which is usually given as total suspended particles, TSP.25,43,106,107 Instead of TSP, other particle concentration ranges such as PM2.5 can be used, depending on the research question.9,41
Soft Sources.
Exposure to soft sources can occur both via contact exposure due to frequent close contact of a person with these sources and via mediated exposure due to their often large emitting surface areas.108–110 Soft sources include cushions, mattresses, foams, carpets, upholstery, draperies, and clothing. Note that this SEC includes clothing as a source, while clothing as environmental compartment is discussed in the Transport section below. New clothing may contain SVOCs introduced during manufacture, transport, storage, or from contact with packaging materials, or because they have been added deliberately for product enhancement, for example water-resistant/repellent coatings.79 These SVOCs may be emitted into the indoor environment after the clothing has been brought into a building and even after laundering.79 Stored clothing may accumulate SVOCs from the air and reactions of oxidants with soiled clothing can be a source of SVOCs; thus they serve as an additional environmental compartment.58,111 Emission from soft sources into the gas phase or into settled dust can be modeled using the equations in SI Sections 1a and 3, respectively.
Frequent Contact Sources.
Exposure to certain SVOCs in solid sources that are frequently handled (e.g., smartphones, smartwatches, toys) or mouthed by children (e.g., teethers and pacifiers) is more likely to occur via contact exposure.112 These sources are not stationary, usually irregularly shaped and their emitting surface area is comparatively small and thus contributes less to the SVOC concentrations in the environmental compartments; however, their frequent handling can increase their importance as an exposure source. Emission may be modeled as for solid sources.79
Applied Sources.
A liquid source that is directly applied to the body (e.g., lotion, shampoo, sunscreen) is mainly linked to contact exposure. With a liquid source applied to the body, aside from the direct dermal exposure, emission into the gas phase and further partitioning occurs, which may contribute to total exposure to a lesser extent, depending on the SVOC and individual occupant behavior.113,114
If the liquid source is applied to a surface or remains open to the indoor air, mediated exposure via inhalation of the gas phase and aerosols dominates for more volatile SVOCs.9,115,116 Examples are floor care products, other cleaning products, detergents, or wet paint. Emission from an applied source is usually confined to a relatively short period of time during and shortly after application. For certain cases, for example the application of paint (and depending on which constituents in paint are being considered, for example, solvents or PCB-11 as a degradation product of pigments117), it might be necessary to consider the application exposure scenario first, and then exposure from the essentially solid source after the paint has dried.118,119
After initial release, SVOCs partition onto indoor surfaces (including exposed skin, hair, and clothing), airborne particles and dust, from where long-term exposure may occur even if the initial short-term source has evaporated or been depleted. In the case of dried paint or other dried sources, emission into the gas phase can be modeled using the same approach as for emission into the gas phase from a solid source (SI Section 1a).40
Sprayed Sources.
A liquid source that is sprayed toward the body can result in both contact and mediated exposure. Dermal uptake by direct contact with the source occurs together with exposure by dermal uptake from air and inhalation of both gas and particle phases. Examples are deodorants, perfumes, or sprayed sunscreens. If the source is sprayed away from a person, for example, air freshener or window cleaner, mediated exposure may contribute more.
Sprayed sources are pulse emission sources with constant or variable time patterns. The emission mechanism of sprayed sources differs from that of applied liquid sources because of the force with which the product is released within a relatively small volume of air, resulting in high concentrations for a short period of time. The formation of aerosol droplets that interact with airborne particles, dust, and indoor surfaces enhances the potential for exposure that may occur long after the initial release. Additionally, powdery sources may fall into this category, as their application can also result in high concentrations in a short period of time and the release of particles into indoor air. Further research is needed to develop mechanistically consistent models describing the mechanism for this SEC.
High Temperature Sources.
High-temperature events like candle burning, cooking, or using a fireplace are pulse emission sources that can release both gas-phase and particle-phase SVOCs over a longer period of time compared to sprayed sources.9,120 Appropriate mechanistic modeling approaches are also needed for this SEC. In addition, transformations and reactions (including oxidation) possibly happening during and shortly after emission must be taken into account. Exposure to these types of sources is primarily mediated via inhalation. Although all sources may undergo temperature variations that influence their chemical activity and thus emission behavior, this category targets sources that participate in intentional events and experience high temperatures for a certain period of time.
Transport.
SVOC transport and partitioning occur among the compartments as shown in Figure 1. The lower their saturation vapor pressure ps (SI Section I) and the higher their octanol/air partition coefficient Koa (SI Section II), the more SVOCs tend to partition to particles, dust or surfaces.37,41
Airborne Particles.
Particles enter either from outdoors (via unintentional infiltration or intentional ventilation) or are emitted by occupants, for example, as exhaled aerosols, and from other occupant activities.84,120–122 Gas/particle partitioning is described in SI Section 4. The equation in SI Section 7a shows the mass balance for particle transport, taking into account particle infiltration, dust resuspension, particle generation, and removal via ventilation and deposition. Particle residence time and air change rate are directly correlated.82 For less volatile SVOCs, instantaneous equilibrium between the gas and particle phases cannot be assumed, and dynamic models exist that account for particle residence times.82,123,124 Particle composition also plays an important role for both the dominant partitioning mechanism (ad- or absorption) and the amount of an SVOC that a particle can accumulate.52,125,126 The gas/particle partition coefficient Kp is a lumped parameter reflecting these considerations. Estimation strategies for Kp are described in SI Section V.
Dust.
Airborne particles contribute to settled dust when they are deposited on surfaces, while settled dust can be resuspended and become airborne.127 Occupants can also contribute to settled dust, e.g. by shedding of skin flakes.25,121 SVOC partitioning may occur between the gas phase and dust settled on source surfaces (SI Section 5a) and on indoor surfaces (SI Section 5b). A mass balance for dust settled on indoor surfaces can be found in SI Section 8.
SI Section VI shows strategies for the estimation of the dust/gas partition coefficient Kdust. Clausen et al.,128 Schripp et al.129 and Liu et al.101 focus on the kinetic process of SVOC uptake by dust particles and explore the relationship between the source surface and layers of settled dust. Dust may also contribute to SVOC losses due to a combination of abiotic and microbial degradation, if the relative humidity is elevated.130 Cleaning can have a short-term removal effect on the SVOC mass balance, but the long-term effect depends on the specific scenario and on the volatility of the SVOC.65,127 However, the possibility of enhanced SVOC removal should be considered when exploring unusual exposure scenarios.
Indoor Surfaces.
From the gas phase, SVOCs partition to indoor surfaces, which form substantial reservoirs that act as both sinks and sources.37,131 Here, surfaces are defined as the readily accessible interfacial region (air–substrate interface and bulk substrate near the interface) of materials such as wood, painted walls, furnishings, glass, nonclothing fabrics, and upholstery. The sink surface/gas partition coefficient Ks depends on SVOC and surface material characteristics, and has been measured for some SVOC/surface material combinations (SI Section VII).51,56,132–135 SI Section 6a describes surface/gas partitioning for solid indoor surfaces, while SI Section 6b describes surface/gas partitioning for soft indoor surfaces with dust settled on those surfaces.
Modeling the uptake of SVOCs by indoor surfaces is strongly influenced by thin organic films that are likely present on most indoor surfaces (SI Section 6c).39,62,133,136 Their presence affects the SVOC dynamics and is thus also relevant for modeling of partitioning onto surfaces, because the presence of organic materials on surfaces and their properties (i.e., chemical composition, phase state) change partitioning parameters, especially Ks, and potentially simplify the model.51 Weschler and Nazaroff62 developed a model of the growth of organic films on impermeable surfaces and its effect on partitioning of SVOCs with different octanol/air partition coefficients Koa and predicted that SVOCs with a logKoa between 10 and 13 would be predominantly present in the film. Furthermore, the growth rate of the film was predicted to be initially high and to decrease over time. Low molecular weight SVOCs reach their equilibrium surface concentration relatively quickly, compared to higher molecular weight SVOCs.24,62 Eichler et al.63 added a mass-transfer model that describes the initial formation of an organic film on clean impermeable surfaces, identifying a two-stage process that requires the film to reach a critical thickness before the growth model becomes applicable. This approach is supported by Liang et al.137 Coarse particle deposition via gravitational settling on upward-oriented surfaces is also likely to influence the growth of organic surface films, depending on the gas-phase concentration of SVOCs with higher logKoa values, the concentration of airborne particles and their organic matter fraction.62
Finally, the equation in SI Section 9 shows the general mass balance for indoor SVOCs, combining the previously listed elements. The mass balance includes entry of gas- and particle-phase SVOCs into the indoor environment from outdoors, removal of gas- and particle-phase SVOCs by ventilation, SVOC emission from source surfaces, mass transfer to surfaces, deposition of particles on surfaces, and resuspension of dust from surfaces. It does not include clothing-mediated sink or source effects or chemical elimination by humans, but these processes may be relevant in some scenarios. For lower volatility SVOCs, it has been shown in models that humans may affect the indoor mass balance significantly, if no degradation in the particle phase occurs and if large reservoirs are present.37,64,138 Potential removal pathways by humans include hand washing, bathing, skin peeling and skin renewal, and chemical elimination in the body (exhalation, biotransformation, and excretion).138 To better quantify the effect of humans on the SVOC mass balance, further research on human activity patterns as well as on chemical and biological removal rates is warranted.
Clothing.
Clothing has been identified in recent years as an important mediator of human exposure to chemicals and particles because of its close proximity to people at almost all times, potentially acting as a barrier to exposure or having a prolonging effect.59,79 Newly purchased clothing as a source of emission has been discussed above. Postpurchase, clothing contains a mix of SVOCs (among other chemicals) present at the time of purchase and those sorbed while stored or in use.79,111,139 Laundering clothing can remove SVOCs to some extent.140 Several studies have shown that SVOCs present in indoor air can accumulate in clothing.58,59,88,141,142 Partitioning between clothing and air can be modeled using the equation in SI Section 10a. For very thin clothing, diffusion inside the material can be ignored, and a simplified model can be applied (SI Section 10b).57,88 The simplified model includes the clothing/air partition coefficient Kca, which can be approximated for cotton based on Koa.111,142 Partitioning between multiple layers of clothing can be derived from the respective Kca values of each layer. Morrison et al.,141,142 Cao et al.,57 and Saini et al.111 reported values for Kca for polychlorinated biphenyls (PCBs) and phthalates in different types of clothing material (SI Section VIII). Additionally, clothing may take up SVOCs from personal care products applied to skin, from laundering detergents and dry-cleaning additives, and also from cross-contamination with other fabrics during laundering and storage.79,111,140 Partitioning between clothing and particles has only been studied to a limited extent, but clothing can serve as a source of biotic and abiotic particles that may contribute to the particle mass balance indoors and thus to human exposure. Potential strategies to determine emission rates of particles from clothing can be found in Licina et al.79
Indoor Chemistry.
Indoor chemistry involves thousands of species that undergo transformations, resulting in changes in the composition of the gas and particle phases, and countless different indoor materials serve as sinks, sources, and reaction sites with at times very different properties.24,143–148 In the context of exposure assessments, the focus is usually on one specific chemical; however, the effect of chemical mixtures on partitioning behavior, reactivity, and subsequent exposure, and the potential relevance of additive exposures may affect the outcome of the assessment. Occupants influence indoor chemistry as well, serving as sinks and sources and providing surfaces for chemical transformations.138,149,150 The formation of secondary organic aerosols (SOA), that is, the generation of particles as a result of the oxidation of some reactive organic species in the gas phase by indoor oxidants, including ozone and the OH radical, is also important when evaluating gas/particle partitioning of SVOCs.144,150–152 Because of their reactivity and composition, SOA emission rates and their partitioning can only be assessed using detailed chemical models.153 Efforts have been made to develop thermodynamic models that consider oxidative aging of SVOCs154 and nonideal thermodynamic mixing on SOA partitioning.155 Although still at a rudimentary stage, the possibility of the formation of SVOCs should be addressed at least qualitatively as part of any exposure assessment.
Finally, ozone and other oxidants react with organic species sorbed to surfaces, including human skin.144,149,151,156–158 For example, a recent study by Zhou et al.159 found that the chemical fate of PAHs embedded in organic oil is affected by the phase state and morphology of the film. The reactivity of SVOCs with oxidizing agents varies greatly and thus indoor chemistry may not be relevant for all applications.160 For chemical reactions in the gas phase to affect indoor environments, their reaction rate has to be shorter than or close to the air change rate.121 This time constraint does not apply to reactions on indoor surfaces, making those reactions particularly important.121 Indoor chemistry modeling approaches are available, but still limited regarding their parametrization, especially for SVOCs, and are a focus of ongoing research.121,151,152
Exposure.
Based on the equations discussed in the previous sections, SVOC concentrations in specific indoor compartments can be predicted and then used to estimate exposure on an individual level. The following general exposure routes are considered: dermal exposure, inhalation exposure, and nondietary ingestion. Dermal exposure can be regarded as a two-step process: Skin-surface lipid uptake refers to the transfer of a chemical to the skin surface, whereas transdermal uptake describes transfer through the skin into body tissues and blood. For transdermal uptake, the permeability of the skin becomes important.150,161 The equations in the SI yield exposure rates (μg h−1) for each exposure route. The details of the equations are discussed below. The exposure rates can be used to express exposure (or intake) in the metrics necessary for a specific research question or risk assessment approach, for example as intake fractions (unitless) or steady-state whole-body concentrations (ng kg−1 BW).
As shown in Figure 1, contact exposure can occur via dermal uptake by direct contact with the source and by ingestion of a source and mouthing of the source (source-to-mouth behavior). Mediated exposure pathways are (1) inhalation of air (gas phase and airborne particles), (2) dermal uptake from the gas phase, from airborne particles, and from contact with dust, surfaces or clothing, and (3) nondietary ingestion, that is, ingestion of dust and mouthing of nonsource objects such as clothing and hands.
Dermal Uptake by Direct Contact with the Source.
When the skin is in direct contact with a source (products and articles), partitioning of SVOCs between the source and the skin-surface lipids takes place (SI Section 12a). A simple approach to describe the flux from a solid source surface to the skin surface has been used by the U.S. Consumer Product Safety Commission (U.S. CPSC). It incorporates a migration rate and the skin surface area in contact with the source.162 Other approaches assume that the transferred amount of SVOC depends on the SVOC concentration in the source and relate this property to either a thin layer of SVOC present on the source surface or to the diffusive flux of SVOC from within the source to its exterior.163,164 A summary of these equations can be found in Huang et al.26 However, further research to develop a full mechanistic model is needed. For describing the transfer from liquid sources applied to the skin, a simplified form based on the approaches used by Wormuth et al.71 and Giovanoulis et al.165 can be used.
Following the transfer from the source to the skin-surface lipids, the subsequent transdermal uptake from the skin-surface lipids to the dermal capillaries can be described by multiplying the concentration of SVOCs on the skin surface Cssl with the dermal permeability coefficient kp_ssl.24,150 Huang and Jolliet166 proposed calculating the dermal uptake by direct contact with a given material by dividing the dermal permeability coefficient kp_ssl by the material−water coefficient Kmw (see SI Sections 12 and IX).
Dermal Uptake from the Gas Phase and Airborne Particles.
The transdermal exposure rate Extrans from SVOCs present in the gas phase to dermal capillaries depends on the SVOC gas phase concentration Cg, the overall transdermal permeability coefficient kp_g and the exposed body surface area Aexp (SI Section 13a).161 kp_g can be calculated based on a resistor-in-series model that takes into account the mass transfer coefficient from the bulk gas phase through the boundary layer of the skin as well as the compound-specific permeability coefficient through the stratum corneum/viable epidermis composite.24,150,161 The model has been extended for dynamic conditions by Gong et al.167 and further by Morrison et al.60 Kinetic models are also available that include the impact of clothing and chemical reactions of skin lipids and ozone.81,168 These dynamic models demonstrate that transdermal uptake of SVOCs can be a slow process and that steady-state models might not be appropriate for modeling short-term exposures.
Dermal absorption of SVOCs associated with airborne particles is expected to be much smaller than dermal absorption from the gas phase because particles diffuse much more slowly than gases.161 A model for dermal uptake of SVOCs associated with airborne particles or dust is given in SI Section 13b. From the skin-surface lipids, transdermal uptake could be modeled as for direct dermal contact with a source. SI Section 13c shows a model for the dermal uptake of SVOCs from contaminated indoor surfaces. The equation employs a contact rate and an availability factor, because it can be assumed that not all of the SVOC deposited on the indoor surface is transferred when touched.169 Both parameters, however, are not well-established for SVOCs and further research is necessary.26 Dermal uptake from contact with clothing materials can be modeled using the equation in SI Section 13d. Critical parameters are the SVOC concentration in skin-surface lipids, which depends on the partitioning between clothing and skin-surface lipids and the SVOC concentration in the clothing material, the transdermal permeability coefficient from the skin-surface lipids into dermal capillaries and the exposed skin surface area. The model can be varied in complexity as described by Morrison et al.59 and Cao et al.88 However, because the equation in SI Section 13d assumes equilibrium between the skin-surface lipids and the clothing, it describes a worst-case scenario. In many cases, the duration of contact between clothing and skin is shorter than the time needed to reach equilibrium.31
Inhalation.
For inhalation exposure, the inhalation rate IRinh and the total SVOC concentration in the air (gas plus particle phase SVOCs) are critical parameters (SI Section 14).24 IRinh is well documented in the U.S. EPA Exposure Factors Handbook for different age ranges, activity levels and sexes.170 However, larger particles may be deposited in the respiratory tract and some fraction may be exhaled again or swallowed.24,26,171 Thus, intake, bioaccessibility, and bioavailability fractions should be taken into account for exposure calculations in general.172 The intake fraction is the amount of a compound that enters the human body compared to the amount that has been released from a source.173 Following Wei et al.,171 we define the bioaccessible fraction as the amount of a compound that is released into body fluids and available for absorption, while bioavailability describes the fraction that is able to cross a biological membrane and enter systemic circulation. Assuming intake, bioaccessibility, and bioavailability fractions of one, respectively, will lead to an overestimation of the actual amount being taken up by, and distributed in, the human body. The concept of intake fraction (iF) as described by Shin et al.174 and Fantke et al.175 can be used to provide further insight for including these concepts in exposure estimates for screening purposes, and equally when evaluating exposure to particulate matter indoors.176
Ingestion and Mouthing of a Source.
Nondietary inadvertent ingestion of a source (e.g., shampoo, lotion) and mouthing or licking of a source is particularly relevant for young children. In both cases, the SVOC mass fraction in the source w0, derived from C0, needs to be known. For ingestion, the source intake rate IRsource is the most critical parameter (SI Section 12b). To estimate exposure by mouthing of a source, the mouthed area of the source Amouthing, the migration rate during mouthing MR, the exposure frequency EF, the accessible fraction fA and the exposure duration d are important (SI Section 12c). Studies have been conducted to explore the solubility and leachability of chemicals in saliva from various children’s products, which may affect fA.162,177
Ingestion of Dust, Hand-to-Mouth and Object-to-Mouth Exposure.
Ingestion of dust can occur via hand-to-mouth and object-to-mouth contact, especially for young children.178 Exposure via dust ingestion can be estimated using the dust intake rate IRdust and the weight fraction of SVOCs associated with the dust (SI Section 15a).24,26 As with IRinh, the U.S. EPA Exposure Factors Handbook provides values for IRdust, although these values contain considerable uncertainty.24,179 SVOC bioaccessibility in dust must also be taken into account, as discussed by Raffy et al.180 For calculation of exposure to SVOCs via mouthing of the source, mouthing of hands (hand-to-mouth) or contaminated objects like clothing (object-to-mouth), the frequency of contact events per unit time (EF) and the amount transferred to the mouth during each contact have to be included.24,26 Also, the amount present on the hand or object must be specified along with the fraction of the surface area in contact with the mouth.24,26,181 Huang et al.26 propose a removal efficiency relationship that has been used in a similar form by Isaacs et al.,169 who obtained their relationship by using the Stochastic Human Exposure and Dose Simulation Model for Multimedia, Multipathway Chemicals (SHEDS-MM) to fit available data.
Uncertainty Considerations.
Uncertainty is introduced in the equations at any point where a parameter is being estimated or measured. Generally, measured parameters are preferred to estimated ones, except where generalized estimated parameters might be more representative than parameters measured for one specific situation but applied to another. However, measured parameters have uncertainty associated with the experimental procedures and should only be used in models within the bounds of the experimental data (i.e., temperature and relative humidity). Uncertainty is usually included within the modeling framework as probability distributions of the modeled parameters. The material-phase concentration C0, the octanol/air partition coefficient Koa, and the saturation vapor pressure ps are critical parameters for modeling SVOC emission, transport, and subsequent exposure, because they are commonly used to estimate partition coefficients.31,41,53,182 Dust and particle properties such as the settled dust density ρdust, the particle density ρpart, and the organic content of dust fom_dust and of particles fom_part are also necessary to describe SVOC distribution between different phases indoors.182,183 Dust and particle properties vary greatly among different indoor environments (e.g., due to smoking, pets, occupant habits) and modeling cannot always account for the broad range of individual settings, but has to rely on averages based on measurements or target specific scenarios. Salthammer and Schripp183 reviewed the results of several dust sampling campaigns and concluded that an organic content of 35% and a density of 1 g cm−3 appear to be reasonable parameters. Other frequently used values can be found in Weschler and Nazaroff.43 The uncertainty associated with any of the parameters used in the models (e.g., C0, Koa, ps, ρdust, ρpart, fom_dust, fom_part) propagates further when using them to obtain other modeling parameters.
C0 can be measured with chemical extraction methods and GC-MS analysis.184,185 Suspect-screening analysis and non-targeted analysis of chemicals in products can further expand our knowledge of product composition.186 Databases such as the U.S. EPA’s CPDat, which is part of the CompTox Chemistry Dashboard, provide additional information on product composition and reference values that can be used for modeling.187,188
In contrast to C0, ps and Koa are often poorly known for SVOCs, particularly at room temperature and for less volatile compounds, which makes calculating partition coefficients challenging.183 Salthammer and Schripp reviewed the available literature and reported that values of ps for one SVOC can span several orders of magnitude for different measurement techniques.183,189 They estimated that uncertainty can be expressed as ps ± 0.95·ps, following a normal distribution.183 O’Meara et al.190 provide a quantitative assessment of state-of-the-art ps estimation methods for VOCs, SVOCs, and low volatility organic compounds (LVOCs). Aside from a statistical evaluation, they discuss restrictions in the coverage of different elements and functional groups for the representation of organic structures, as well as the treatments of the temperature dependence. O’Meara et al. further provide insight into the impact of combinations of different normal boiling point and vapor pressure estimation methods on predicted SOA mass concentrations based on a large number of compounds in their test set, indicating that the best vapor pressure estimation methods do not necessarily result in the best predictions of SOA mass concentrations. In the absence of reliable measurements and when the set of functional groups of the system components allow its application, the EVAPORATION model by Compernolle et al.191 is the recommended ps estimation method.
Koa can be determined experimentally,192 but many Koa values found in the literature have been derived from the air/water partition coefficient Kaw, the octanol/water partition coefficient Kow and/or Henry’s law constant H, which introduces additional uncertainty.183,193,194 When Koa is obtained from Kaw and Kow, the uncertainty of Koa can be calculated as the combined uncertainty of the parameters used to obtain Koa. For example, if Koa = KowRT/H is used, the uncertainty of Koa is the combined uncertainty of Kow and H.183 Numerous algorithms and quantitative structure−property relationship (QSPR) approaches have been developed for the prediction of chemical properties.195 These are available via tools like SPARC, EPI Suite, LSER, and COSMOTHERM to estimate Koa and ps, as well as online tools such as UManSysProp.107,183,196,197 However, the results differ depending on the selected algorithm and, in case of QSPR approaches, on the quality of the input data and data training sets. Moreover, associated uncertainties are difficult to quantify.193 Alternatively, Koa can be determined from linear free energy relationships as described by Schwarzenbach et al. 198
Kp calculated using Koa can be quite different from Kp calculated using ps.193 Additional uncertainty might arise from the sigmoidal shape of the equation predicting the fraction of a component partitioned to the particle phase, especially for compounds of intermediate volatility (10−6 Pa < ps < 10−2 Pa). Small changes in ps, Kow, and H (and thus Koa), and in the particle concentration result in large differences in the concentration ratio between gas and particle phase.183,193 In those cases, it is not possible to predict reliably if an SVOC is predominantly in the gas or in the particle phase. Additional difficulty arises from the humidity-dependent potential of liquid−liquid phase separation in multicomponent aerosol particles or films, affecting the equilibrium partitioning of SVOCs.155 Generally, the range of error of both experimentally derived and calculated values for Kp is within 1 order of magnitude, but can be much greater, particularly for polar compounds.107,199
y0 is a critical parameter to describe emission and can be measured directly.49,54 Furthermore, y0 can also be derived from correlations with C0 for certain SVOCs.53,56,105 Similarly, regressions for different classes of SVOCs partitioning to certain types of sink materials have been established to estimate Ks.40,137,200 However, these correlations also depend on ps and have similar challenges to those discussed above.
When operating with parameter distributions, Monte Carlo simulations are helpful to quantify how uncertainties propagate to derived parameters. Wei et al.182 reviewed published data for Kp and Kdust and described the distributions of log10Kp and log10Kdust for 72 SVOCs that can be used as references for Monte Carlo simulations. They also developed an empirical linear relationship between log10Kp and log10Kdust, which may serve to estimate Kdust based on Kp or vice versa, if no other sources are available.182 Additionally, modeling parameters, especially partition coefficients, are influenced by indoor environmental factors, for example, temperature, humidity, ventilation, size fractions of airborne particles, and potential biodegradation of SVOCs.25,52,106,130,132 These relationships require further research, but utilizing parameter distributions as model inputs provide some sense of the uncertainty associated with their estimation.
Limitations and Conditions of Model Application.
As discussed above, the current limitation of this framework is model parametrization. Availability and quality of data for input parameters impact significantly the confidence in modeling outcomes and thus constitute a priority for further research, for exmple, through integration of existing data sets, QSAR and QSPR approaches, and expert opinions to decrease uncertainties and improve the predictive power of models. It is often not possible or feasible to measure parameters directly, thus they have to be estimated or obtained from databases. The implementation of the proposed framework has to include ways to access databases like the U.S. EPA CompTox Chemistry Dashboard, including CPDat for information on C0, and other platforms that contain data derived from HT screening approaches or QSARs/QSPRs that can serve as input for mechanistic models.103,166,188,201,202 Parameter estimation approaches vary greatly in their accuracy, and thus different forms of uncertainty are introduced, which have to be addressed appropriately.199 The SI provides information on approaches to estimate important parameters together with ranges of applicability and uncertainty, if available. However, the comparison of estimated parameters used in exposure models with empirical data is an important component of model evaluation.
The modular structure of the framework allows aspects of exposure modeling to chemicals released indoors to be included or excluded. The framework could, for example, be extended to include VOCs or other groups of chemicals, such as per- and polyfluoroalkyl substances (PFAS), by including additional processes and parameters. It has to be noted that the models provided with this framework have been derived for neutral SVOCs and are not directly applicable to ionic and ionizable species, which includes many PFAS.203 Some modules are better understood than others, making it tempting to ignore those that might introduce complexity. However, even if some parts of the framework are not addressed quantitatively, they should be part of the broader discussion of the resulting exposure estimates so that they can be placed in the right context.
In addition to chemical-related model parameters, those parameters describing specific exposure scenarios may also be unknown or highly uncertain. Whenever possible, variability has to be taken into account with respect to exposed populations and occupant characteristics,204,205 occupant behaviors,9,64,146,206 and indoor environmental settings.207,208 Applying Monte Carlo-based approaches to include parameter distributions and addressing certain activities to identify high exposure situations should be part of the application of the framework. It has been shown that cooking and cleaning activities greatly enhance the levels of SVOCs in indoor environments compared to background levels.9,65,206 The possibility of aggregate exposures due to certain occupant behaviors and potential consequences for exposed individuals should thus be incorporated in the exposure assessment.209 When assigning parameter distributions, one needs to be mindful that much of the indoor literature is based on data representative of midsocio-economic status (SES) households. However, low SES individuals can be particularly vulnerable to higher exposures resulting from poor quality housing and higher SVOC emissions.35,205 Although the models are fundamentally “neutral”, their parametrization may be biased toward mid-SES situations that do not capture indoor exposures of vulnerable populations in low SES communities.210,211
Concepts of bioaccessibility and bioavailability, as discussed for example by Wei et al.171 for inhalation exposure and by Raffy et al.180 for dust ingestion, should be considered in the calculations when estimating intake rates based on the models presented in the SI. SVOC bioaccessibility and bioavailability can span a wide range, depending on the pathway, the specific substance, and many other influencing factors.171,180 If bioaccessibility and bioavailability factors are not taken into account, the resulting intake rates might overestimate exposure. For higher-complexity modeling purposes, exposure estimates derived with this framework should be combined with a mechanistic model describing, for example, the fate of gas and particle phase species in the human respiratory tract, possibly followed by the application of physiologically based pharmacokinetic (PBPK) models and similar strategies to quantitatively predict concentrations in plasma or target tissues, as has been done, for example, in the PROTEX model.2,66,68,171,212,213
Additional uncertainty may be introduced when evaluating SVOC sources that are not in direct contact with the indoor environment, such as those present in the building envelope (e.g., rim joists, vapor barriers, and building insulation). Strongly varying temperatures and infiltration paths into the indoor environment may significantly affect the infiltration rate of SVOCs from the building envelope into the indoor environment.
THE IMPORTANCE OF MECHANISTIC MODELING FOR SOLVING KEY CHALLENGES
Mechanistic models have been identified as a critical element for addressing key challenges in exposure modeling.21,25,77,78 Mechanistically consistent SECs are the basis of the proposed framework and allow modelers and users to identify relevant exposure pathways quantitatively, including assessments of uncertainty and parameter sensitivity. For modeling these pathways, mechanistic models with different levels of complexity are available and can be applied to derive exposure estimates, but gaps remain. Specific mechanistic model development needs for each SECs are listed in Table 1.
Table 1.
Mechanistic Model Development Needs for Each Source Emission Category (SEC)
| source emission category (SEC) | model development needs |
|---|---|
| solid, soft and frequent contact | • estimation of y0 based on chemical structure, polymer characteristics and physicochemical properties for a broad range of SVOCs |
| applied liquid | • dynamic pulse emission model, slow release to the gas phase |
| sprayed liquid | • dynamic pulse emission model, pressure release to the gas phase |
| • dynamic pulse emission model for release of airborne particles | |
| high temperature | • dynamic pulse emission model for emission into the gas phase |
| • dynamic pulse emission model for release of airborne particles | |
| • inclusion of chemical reactions and reaction products |
Factors influencing the complexity of modeling SVOC emission, transport and exposure in indoor environments include the following:
Assumption of quasi-instantaneous equilibrium or of dynamic, kinetically limited conditions;
Consideration of the influence of indoor environmental factors on model parameters, especially on partition coefficients (e.g., temperature, humidity, ventilation, size fractions of airborne particles, and source loading factor), and respective uncertainties;
Consideration of indoor chemistry, its effect on partitioning behavior, reactivity and subsequent exposure, and SVOC transformation products resulting from oxidation, hydrolysis, and other reactions; and
Influence of occupancy (e.g., in terms of surface soiling, dust resuspension, cleaning habits, and other occupant behavior) and occupant characteristics (e.g., age, sex, socio-economic status, variability of exposure factors) on exposure estimates.
Modeling of complex scenarios within indoor environments is a challenging task. There will always be a need to make assumptions, accept uncertainty, and adapt to new knowledge. However, modeling affords the opportunity to incorporate state-of-the art mechanistic understanding, evaluate the sensitivity to assumptions and data gaps, and to quantify uncertainty. As such, advancing exposure modeling approaches to appropriately reflect real-world conditions will increase transparency and confidence in exposure analyses and the resulting risk management decisions.
IMPLICATIONS AND RECOMMENDATIONS
The proposed framework integrates the current state of knowledge of SVOC exposure in indoor environments to advance chemical exposure assessment. Exposure science plays a crucial role in a fully integrated system of chemical risk assessment to inform and prioritize higher tier toxicity testing, describe and rank risks, and develop and evaluate models.214–221 The driving context for development of this framework was to facilitate efficient integration of exposure estimates derived using this framework with toxicity data for different end points and toxicokinetic mechanisms to accelerate chemical risk prioritization.21,222,223 As an example of such an approach, Shin et al.224 proposed a strategy to combine exposure estimates based on intake rates with toxicity data derived from ToxCast in vitro bioactivity assays, deriving bioactivity quotients (BQ) for HT risk ranking and prioritization. Another example can be found in Li et al.,225 who used a dimensionless risk assessment factor (RAF), that is, the ratio of actual emission or application rate to a critical emission or application rate, to rank chemicals. The choice of metric depends on the respective research question and on the requirements of the risk assessment strategy.
More broadly, the proposed framework can be employed to inform chemical management decision contexts beyond rapid risk prioritization.6,226 We envision this mechanistic exposure modeling framework to be developed and used as a community model available to better leverage ongoing research and inform chemical management decisions. Such a platform would enable further evaluation of the proposed framework through a continuous improvement process. This includes consideration of additional SVOC modeling and parameter estimation approaches, and of new empirical data as they become available. A common platform would also allow rapid and simple optimization of different components, depending on the user’s needs and the knowledge available. As work progresses, the source categories can be adjusted, VOC and aerosol emissions can be added, and indoor chemistry can be included. The Community Multiscale Air Quality (CMAQ) model227–229 could serve as an example of how the framework can be developed, with open-source contributions from researchers worldwide and participation of relevant stakeholders.
Finally, understanding the health effects of exposures to chemicals has important implications for public health and also for more sustainable design and production of building materials and other indoor articles and products, which is especially relevant in a circular economy context.230 Currently, chemical management authorities, regulators, and policy makers focus on evaluating risks from exposures to individual or groups of chemicals based on the properties of those chemicals. This approach rarely enables policy makers to link increased risk of public health outcomes to chemical management actions. As a result, chemical managers are challenged to anticipate impacts of chemical use and focus resources on addressing the most pressing concerns. A public health perspective, as described by Gwinn et al.,231 starts with the health outcome of concern and incorporates multiple data streams to inform preventative policy decisions. The goal is to extend the scope of the considerations that support chemical management decisions and advance the tools for integrating this more complex information. Our framework can support this approach by informing design of exposure studies, facilitating interpretation of exposure data (i.e., chemical occurrence, biomonitoring and other exposomic measurements), and enabling risk assessments for environmental epidemiology studies. The proposed framework may therefore serve as the foundation for developing an open-source community model to better support collaborative research with improved access for application by stakeholders to advance effective chemical management decisions and protect public health.
Supplementary Material
ACKNOWLEDGMENTS
This work was initially funded by the National Toxicology Program of the United States Department of Health and Human Services, and we thank Dr. John Bucher for support and guidance. We are grateful to the Alfred P. Sloan Foundation for providing funding to the Modeling Consortium for Chemistry of Indoor Environments (MOCCIE) 2 (G-2019-12306). We also acknowledge the Scientific and Technical Center for Building (CSTB) and the University of La Rochelle, both in France, for providing sabbatical support for J.C.L., which further catalyzed the initiative, and the Department of Building Science at Tsinghua University for hosting the kick-off workshop. We also thank and acknowledge the signatories who support the framework. A full list of signatories is provided in the SI.
Footnotes
The authors declare no competing financial interest.
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the United States Environmental Protection Agency (U.S. EPA) or any other agency. Certain commercial equipment, instruments, materials or software are identified in this paper in order to specify the exposure model adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology or the U.S. EPA, nor is it intended to imply that the materials, software, or equipment identified are necessarily the best available for the purpose.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.0c02329.
Nomenclature, list of consensus points, mechanistic modeling framework to predict exposure to SVOCs (Figure S1), emission and transport modeling equations, exposure modeling equations, estimation approaches for model parameters, comparison of Koa values (Figure S2), decision tree for exposure mechanism selection based on the source emission category (Figure S3), and list of signatories who support the proposed framework (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.est.0c02329
Contributor Information
Clara M. A. Eichler, Department of Civil and Environmental Engineering, Virginia Tech, Blacksburg, Virginia 24060, United States; Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
Elaine A. Cohen Hubal, Office of Research and Development, U.S. EPA, Research Triangle Park, North Carolina 27711, United States.
Ying Xu, Department of Building Science, Tsinghua University, Beijing 100084, China.
Jianping Cao, School of Environmental Science and Engineering, Sun Yat-sen University, Guangzhou, Guangdong 510006, China.
Chenyang Bi, Department of Civil and Environmental Engineering, Virginia Tech, Blacksburg, Virginia 24060, United States.
Charles J. Weschler, Environmental and Occupational Health Sciences Institute, Rutgers University, Piscataway, New Jersey 08854, United States; International Centre for Indoor Environment and Energy, Department of Civil Engineering, Technical University of Denmark, Lyngby 2800, Denmark.
Tunga Salthammer, Fraunhofer WKI, Department of Material Analysis and Indoor Chemistry, Braunschweig 38108, Germany.
Glenn C. Morrison, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
Antti Joonas Koivisto, Institute for Atmospheric and Earth System Research (INAR), University of Helsinki, Helsinki 00014, Finland.
Yinping Zhang, Department of Building Science, Tsinghua University, Beijing 100084, China.
Corinne Mandin, University of Paris-Est, Scientific and Technical Center for Building (CSTB), French Indoor Air Quality Observatory (OQAI), Champs sur Marne 77447, France.
Wenjuan Wei, University of Paris-Est, Scientific and Technical Center for Building (CSTB), French Indoor Air Quality Observatory (OQAI), Champs sur Marne 77447, France.
Patrice Blondeau, Laboratoire des Sciences de l’Ingénieur pour l’Environnement - LaSIE, Université de La Rochelle, La Rochelle 77447, France.
Dustin Poppendieck, Engineering Lab, National Institute of Standards and Technology, Gaithersburg, Maryland 20899, United States.
Xiaoyu Liu, Office of Research and Development, U.S. EPA, Research Triangle Park, North Carolina 27711, United States.
Christiaan J. E. Delmaar, National Institute for Public Health and the Environment, Center for Safety of Substances and Products, Bilthoven 3720, The Netherlands
Peter Fantke, Quantitative Sustainability Assessment, Department of Technology, Management and Economics, Technical University of Denmark, Kgs. Lyngby 2800, Denmark.
Olivier Jolliet, Department of Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, Michigan 48109, United States.
Hyeong-Moo Shin, Department of Earth and Environmental Sciences, University of Texas at Arlington, Arlington, Texas 76019, United States.
Miriam L. Diamond, Department of Earth Sciences, University of Toronto, Toronto, Ontario M5S 3B1, Canada.
Manabu Shiraiwa, Department of Chemistry, University of California, Irvine, California 92697, United States.
Andreas Zuend, Department of Atmospheric and Oceanic Sciences, McGill University, Montreal, Quebec H3A0B9, Canada.
Philip K. Hopke, Center for Air Resources Engineering and Science, Clarkson University, Potsdam, New York 13699-5708, United States; Department of Public Health Sciences, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642, United States.
Natalie von Goetz, Federal Office of Public Health, Berne 3003, Switzerland.
Markku Kulmala, Institute for Atmospheric and Earth System Research (INAR), University of Helsinki, Helsinki 00014, Finland.
John C. Little, Department of Civil and Environmental Engineering, Virginia Tech, Blacksburg, Virginia 24060, United States.
REFERENCES
- (1).Bolinius DJ; Sobek A; Löf MF; Undeman E. Evaluating the consumption of chemical products and articles as proxies for diffuse emissions to the environment. Environmental Science: Processes & Impacts 2018, 20, 1427–1440. [DOI] [PubMed] [Google Scholar]
- (2).Cohen Hubal EA; Wetmore BA; Wambaugh JF; El-Masri H; Sobus JR; Bahadori T Advancing internal exposure and physiologically-based toxicokinetic modeling for 21st-century risk assessments. J. Exposure Sci. Environ. Epidemiol. 2019, 29, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kavlock RJ; Bahadori T; Barton-Maclaren TS; Gwinn MR; Rasenberg M; Thomas RS Accelerating the Pace of Chemical Risk Assessment. Chem. Res. Toxicol. 2018, 31, 287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Pellizzari ED; Woodruff TJ; Boyles RR; Kannan K; Beamer PI; Buckley JP; Wang A; Zhu Y; Bennett DH Identifying and Prioritizing Chemicals with Uncertain Burden of Exposure: Opportunities for Biomonitoring and Health-Related Research. Environ. Health Perspect. 2019, 127 (12), 126001 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Cowan-Ellsberry C; Zaleski RT; Qian H; Greggs W; Jensen E, Perspectives on advancing consumer product exposure models. J. Exposure Sci. Environ. Epidemiol. 2020, 30856. [DOI] [PubMed] [Google Scholar]
- (6).Eichler CMA; Cohen Hubal EA; Little JC Assessing Human Exposure to Chemicals in Materials, Products and Articles: The International Risk Management Landscape for Phthalates. Environ. Sci. Technol. 2019, 53 (23), 13583–13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Morrison GC; Carslaw N; Waring MS Editorial: A modeling enterprise for chemistry of indoor environments (CIE). Indoor Air 2017, 27 (6), 1033–1038. [DOI] [PubMed] [Google Scholar]
- (8).Li D; Suh S Health risks of chemicals in consumer products: A review. Environ. Int. 2019, 123, 580–587. [DOI] [PubMed] [Google Scholar]
- (9).Kristensen K; Lunderberg DM; Liu Y; Misztal PK; Tian Y; Arata C; Nazaroff WW; Goldstein AH Sources and dynamics of semivolatile organic compounds in a single-family residence in northern California. Indoor Air 2019, 29, 645–655. [DOI] [PubMed] [Google Scholar]
- (10).Lucattini L; Poma G; Covaci A; de Boer J; Lamoree MH; Leonards PEG A review of semi-volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere 2018, 201, 466–482. [DOI] [PubMed] [Google Scholar]
- (11).Guo Y; Kannan K A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol. 2013, 47 (24), 14442–14449. [DOI] [PubMed] [Google Scholar]
- (12).Nicolas CI; Mansouri K; Phillips KA; Grulke CM; Richard AM; Williams AJ; Rabinowitz J; Isaacs KK; Yau A; Wambaugh JF Rapid experimental measurements of physicochemical properties to inform models and testing. Sci. Total Environ. 2018, 636, 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Phillips KA; Wambaugh JF; Grulke CM; Dionisio KL; Isaacs KK High-throughput screening of chemicals as functional substitutes using structure-based classification models. Green Chem. 2017, 19 (4), 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Moreau M; Leonard J; Phillips KA; Campbell J; Pendse SN; Nicolas C; Phillips M; Yoon M; Tan Y-M; Smith S; Pudukodu H; Isaacs K; Clewell H Using exposure prediction tools to link exposure and dosimetry for risk-based decisions: A case study with phthalates. Chemosphere 2017, 184, 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wambaugh JF; Wang A; Dionisio KL; Frame A; Egeghy P; Judson RS; Woodrow Setzer R High Throughput Heuristics for Prioritizing Human Exposure to Environmental Chemicals. Environ. Sci. Technol. 2014, 48, 12760–12767. [DOI] [PubMed] [Google Scholar]
- (16).Russo DP; Strickland J; Karmaus AL; Wang W; Shende S; Hartung T; Aleksunes LM; Zhu H Nonanimal Models for Acute Toxicity Evaluations: Applying Data-Driven Profiling and Read-Across. Environ. Health Perspect. 2019, 127 (4), 047001–1–047001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Richard AM; Judson RS; Houck KA; Grulke CM; Volarath P; Thillainadarajah I; Yang C; Rathman J; Martin MT; Wambaugh JF; Knudsen TB; Kancherla J; Mansouri K; Patlewicz G; Williams AJ; Little SB; Crofton KM; Thomas RS ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem. Res. Toxicol. 2016, 29, 1225–1251. [DOI] [PubMed] [Google Scholar]
- (18).Ernstoff AS; Fantke P; Huang L; Jolliet O High-throughput migration modelling for estimating exposure to chemicals in food packaging in screening and prioritization tools. Food Chem. Toxicol. 2017, 109 (1), 428–438. [DOI] [PubMed] [Google Scholar]
- (19).Koivisto AJ; Kling KA; Hänninen O; Jayjock M; Löndahl J; Wierzbicka A; Fonseca AS; Uhrbrand K; Boor BE; Jiménez AS; Hämeri K; Dal Maso M; Arnold SF; Jensen KA; Viana M; Morawska L; Hussein T Source specific exposure and risk assessment for indoor aerosols. Sci. Total Environ. 2019, 668, 13–24. [DOI] [PubMed] [Google Scholar]
- (20).Dionisio KL; Frame AM; Goldsmith M-R; Wambaugh JF; Liddell A; Cathey T; Smith D; Vail J; Ernstoff AS; Fantke P; Jolliet O; Judson RS Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicology Reports 2015, 2, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Thomas RS; Bahadori T; Buckley TJ; Cowden J; Deisenroth C; Dionisio KL; Frithsen JB; Grulke CM; Gwinn MRJAH; Higuchi M; Houck KA; Hughes MF; Hunter ES; Isaacs KK; Judson RS; Knudsen TB; Lambert JC; Linnenbrink M; Martin TM; Newton SR; Padilla S; Patlewicz G; Paul-Friedman K; Phillips KA; Richard AM; Sams A; Shafer TJ; Woodrow Setzer R; Shah I; Simmons JE; Simmons SO; Singh A; Sobus JR; Strynar M; Swank A; Tornero-Velez R; Ulrich EM; Villeneuve DL; Wambaugh JF; Wetmore BA; Williams AJ The next generation blueprint of computational toxicology at the U.S. Environmental Protection Agency. Toxicol. Sci. 2019, 169 (2), 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wood MD; Plourde K; Larkin S; Egeghy PP; Williams AJ; Zemba V; Linkov I; Vallero DA, Advances on a Decision Analytic Approach to Exposure-Based Chemical Prioritization. Risk Anal. 2020, 4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Cherrie JW; Fransman W; Heussen GAH; Koppisch D; Jensen KA Exposure Models for REACH and Occupational Safety and Health Regulations. Int. J. Environ. Res. Public Health 2020, 17 (383), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Salthammer T; Zhang Y; Mo J; Koch HM; Weschler CJ Assessing human exposure to organic pollutants in the indoor environment. Angew. Chem., Int. Ed. 2018, 57 (38), 12228–12263. [DOI] [PubMed] [Google Scholar]
- (25).Liang Y; Bi C; Wang X; Xu Y A general mechanistic model for predicting the fate and transport of phthalates in indoor environments. Indoor Air 2019, 29, 55–69. [DOI] [PubMed] [Google Scholar]
- (26).Huang L; Ernstoff AS; Fantke P; Csiszar SA; Jolliet O A review of models for near-field exposure pathways of chemicals in consumer products. Sci. Total Environ. 2017, 574, 1182–1208. [DOI] [PubMed] [Google Scholar]
- (27).Mao Y-F; Li Z; Zhang Y; He Y-L; Tao W-Q A review of mass-transfer models and mechanistic studies of semi-volatile organic compounds in indoor environments. Indoor Built Environ. 2018, 27 (10), 1307–1321. [Google Scholar]
- (28).ASTM, Standard Guide for Selecting Volatile Organic Compounds (VOCs) and Semi-Volatile Organic Compounds (SVOCs) Emission Testing Methods to Determine Emission Parameters for Modeling of Indoor Environments In ASTM D8141–17; ASTM International: West Conshohocken, PA, 2017. [Google Scholar]
- (29).WHO. Indoor Air Quality: Organic Pollutants; World Health Organization: Copenhagen, NL, 1989. [Google Scholar]
- (30).ISO, 16000–6 Indoor air -- Part 6: Determination of volatile organic compounds in indoor and test chamber air by active sampling on Tenax TA sorbent, thermal desorption and gas chromatography using MS or MS-FID. In 13.040.20 - Ambient Atmospheres; International Organization for Standardization, 2011. [Google Scholar]
- (31).Weschler CJ; Nazaroff WW Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42 (40), 9018–9040. [Google Scholar]
- (32).Donahue NM; Kroll JH; Pandis SN; Robinson AL A two-dimensional volatility basis set - Part 2: Diagnostics of organic-aerosol evolution. Atmos. Chem. Phys. 2012, 12, 615–634. [Google Scholar]
- (33).van t’Erve TJ; Rosen EM; Barrett ES; Nguyen RHN; Sathyanarayana S; Milne GL; Calafat AM; Swan SH; Ferguson KK Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ. Sci. Technol. 2019, 53, 3258–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Liu W; Huang C; Li BZ; Zhao Z; Xu Y; Deng Q; Zhang X; Qian H; Sun Y; Qu F; Wang L; Lin JZ; Lu C; Wang H; Wang J; Cai J; Zhang J; Sun CJ; Mo J; Weschler LB; Norbäck D; Sundell J; Zhang Y Household renovation before and during pregnancy in relation to preterm birth and low birthweight in China. Indoor Air 2019, 29, 202–214. [DOI] [PubMed] [Google Scholar]
- (35).Bi C; Maestre JP; Li H; Zhang G; Givehchi R; Mahdavi A; Kinney KA; Siegel J; Horner SD; Xu Y Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low-income homes: Association with season, building characteristics, and childhood asthma. Environ. Int. 2018, 121, 916–930. [DOI] [PubMed] [Google Scholar]
- (36).Lu X; Xu X; Lin Y; Zhang Y; Huo X Phthalate exposure as a risk factor for hypertension. Environ. Sci. Pollut. Res. 2018, 25, 20550–20561. [DOI] [PubMed] [Google Scholar]
- (37).Little JC; Weschler CJ; Nazaroff WW; Liu Z; Cohen Hubal EA Rapid Methods to Estimate Potential Exposure to Semivolatile Organic Compounds in the Indoor Environment. Environ. Sci. Technol. 2012, 46 (20), 11171–11178. [DOI] [PubMed] [Google Scholar]
- (38).Melymuk L; Bohlin-Nizzetto P; Vojta S; Krátká M; Kukucka P; Audy O; Pribylová P; Klánová J Distribution of legacy and emerging semivolatile organic compounds in five indoor matrices in a residential environment. Chemosphere 2016, 153, 179–186. [DOI] [PubMed] [Google Scholar]
- (39).Bennett DH; Furtaw EJ Fugacity-Based Indoor Residential Pesticide Fate Model. Environ. Sci. Technol. 2004, 38 (7), 2142–2152. [DOI] [PubMed] [Google Scholar]
- (40).Xu Y; Little JC Predicting emissions of SVOCs from polymeric materials and their interaction with airborne particles. Environ. Sci. Technol. 2006, 40 (2), 456–461. [DOI] [PubMed] [Google Scholar]
- (41).Weschler CJ; Salthammer T; Fromme H Partitioning of phthalates among the gas phase, airborne particles and settled dust in indoor environments. Atmos. Environ. 2008, 42, 1449–1460. [Google Scholar]
- (42).Shin HM; McKone TE; Tulve NS; Clifton MS; Bennett DH Indoor Residence Times of Semivolatile Organic Compounds: Model Estimation and Field Evaluation. Environ. Sci. Technol. 2013, 47 (2), 859–867. [DOI] [PubMed] [Google Scholar]
- (43).Weschler CJ; Nazaroff WW SVOC partitioning between the gas phase and settled dust indoors. Atmos. Environ. 2010, 44 (30), 3609–3620. [Google Scholar]
- (44).Liu C; Zhao B; Zhang Y The influence of aerosol dynamics on indoor exposure to airborne DEHP. Atmos. Environ. 2010, 44 (16), 1952–1959. [Google Scholar]
- (45).Clausen PA; Liu Z; Kofoed-Sørensen V; Little JC; Wolkoff P Influence of Air Flow Rate on Emission of DEHP from Vinyl Flooring: Measurements and CFD Simulation. Atmos. Environ. 2010, 44, 2760–2766. [Google Scholar]
- (46).Clausen PA; Liu Z; Kofoed-Sørensen V; Little JC; Wolkoff P The Influence of Temperature on the Emission of Di(2 ethylhexyl)phthalate (DEHP) from PVC Flooring in the Emission Cell FLEC. Environ. Sci. Technol. 2012, 46 (2), 909–915. [DOI] [PubMed] [Google Scholar]
- (47).Shin HM; McKone TE; Nishioka MG; Fallin MD; Croen LA; Hertz-Picciotto I; Newschaffer CJ; Bennett DH Determining Source Strength of Semivolatile Organic Compounds using Measured Concentrations in Indoor Dust. Indoor Air 2014, 24, 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Zhang X; Diamond ML; Ibarra C; Harrad S Multimedia Modeling of Polybrominated Diphenyl Ether Emissions and Fate Indoors. Environ. Sci. Technol. 2009, 42, 2845–2850. [DOI] [PubMed] [Google Scholar]
- (49).Wu Y; Xie M; Cox SS; Marr LC; Little JC Simple method to measure the gas-phase SVOC concentration adjacent to a material surface. Indoor Air 2016, 26 (6), 903–912. [DOI] [PubMed] [Google Scholar]
- (50).Wu Y; Eichler CMA; Chen S; Little JC Simple Method To Measure the Vapor Pressure of Phthalates and Their Alternatives. Environ. Sci. Technol. 2016, 50, 10082–10088. [DOI] [PubMed] [Google Scholar]
- (51).Wu Y; Eichler CMA; Leng W; Cox SS; Marr LC; Little JC Adsorption of Phthalates on Impervious Indoor Surfaces. Environ. Sci. Technol. 2017, 51 (5), 2907–2913. [DOI] [PubMed] [Google Scholar]
- (52).Wu Y; Eichler CMA; Cao J; Benning JL; Olson A; Chen S; Liu C; Vejerano EP; Marr LC; Little JC Particle/gas partitioning of phthalates to organic and inorganic airborne particles in the indoor environment. Environ. Sci. Technol. 2018, 52 (6), 3583–3590. [DOI] [PubMed] [Google Scholar]
- (53).Eichler CMA; Wu Y; Cao J; Shi S; Little JC Equilibrium relationship between SVOCs in PVC products and the air in contact with the product. Environ. Sci. Technol. 2018, 52 (5), 2918–2925. [DOI] [PubMed] [Google Scholar]
- (54).Liang Y; Xu Y Improved method for measuring and characterizing phthalate emissions from building materials and its application to exposure assessment. Environ. Sci. Technol. 2014, 48 (8), 4475–4484. [DOI] [PubMed] [Google Scholar]
- (55).Liang Y; Xu Y Emission of Phthalates and Phthalate Alternatives from Vinyl Flooring and Crib Mattress Covers: The Influence of Temperature. Environ. Sci. Technol. 2014, 48 (24), 14228–14237. [DOI] [PubMed] [Google Scholar]
- (56).Liang Y; Liu X; Allen MR Measurements of Parameters Controlling the Emissions of Organophosphate Flame Retardants in Indoor Environments. Environ. Sci. Technol. 2018, 52 (10), 5821–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Cao J; Weschler CJ; Luo J; Zhang Y Cm-History Method, a Novel Approach to Simultaneously Measure Source and Sink Parameters Important for Estimating Indoor Exposures to Phthalates. Environ. Sci. Technol. 2016, 50 (2), 825–834. [DOI] [PubMed] [Google Scholar]
- (58).Morrison GC; Weschler CJ; Bekö G; [Google Scholar]; Koch HM; Salthammer T; Schripp T; Toftum J; Clausen G Role of clothing in both accelerating and impeding dermal absorption of airborne SVOCs. J. Exposure Sci. Environ. Epidemiol. 2016, 26, 113–118. [DOI] [PubMed] [Google Scholar]
- (59).Morrison GC; Weschler CJ; Bekö G. Dermal uptake of phthalates from clothing: Comparison of model to human participant results. Indoor Air 2017, 27 (3), 642–649. [DOI] [PubMed] [Google Scholar]
- (60).Morrison GC; Weschler CJ; Bekö G. Dermal uptake directly from air under transient conditions: advances in modeling and comparisons with experimental results for human subjects. Indoor Air 2016, 26 (6), 913–924. [DOI] [PubMed] [Google Scholar]
- (61).Weschler CJ; Bekö G; Koch HM; Salthammer T; Schripp T; Toftum J; Clausen G Transdermal uptake of diethyl phthalate and di(n-butyl) phthalate directly from air: Experimental verification. Environ. Health Perspect. 2015, 123 (10), 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Weschler CJ; Nazaroff WW Growth of organic films on indoor surfaces. Indoor Air 2017, 27 (6), 1101–1112. [DOI] [PubMed] [Google Scholar]
- (63).Eichler CMA; Cao J; Isaacman-VanWertz G; Little JC Modeling the Formation and Growth of Organic Films on Indoor Surfaces. Indoor Air 2019, 29, 17–29. [DOI] [PubMed] [Google Scholar]
- (64).Kvasnicka J; Cohen Hubal E; Ladan J; Zhang X; Diamond ML, Transient Multimedia Model for Investigating the Influence of Indoor Human Activities on Exposure to SVOCs. Environ. Sci. Technol. 2020, 54, 10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Uhde E; Varol D; Mull B; Salthammer T, Distribution of five SVOCs in a model room: effect of vacuuming and air cleaning measures. Environ. Sci.: Processes Impacts 2019, 211353. [DOI] [PubMed] [Google Scholar]
- (66).Li L; Arnot JA; Wania F Revisiting the Contributions of Far- and Near-Field Routes to Aggregate Human Exposure to Polychlorinated Biphenyls (PCBs). Environ. Sci. Technol. 2018, 52 (12), 6974–6984. [DOI] [PubMed] [Google Scholar]
- (67).Li L; Arnot JA; Wania F Towards a systematic understanding of the dynamic fate of polychlorinated biphenyls in indoor, urban and rural environments. Environ. Int 2018, 117, 57–68. [DOI] [PubMed] [Google Scholar]
- (68).Li L; Hoang C; Arnot JA; Wania F Clarifying Temporal Trend Variability in Human Biomonitoring of Polybrominated Diphenyl Ethers through Mechanistic Modeling. Environ. Sci. Technol. 2019, 54 (1), 166–175. [DOI] [PubMed] [Google Scholar]
- (69).Prevedouros K; Jones KC; Sweetman AJ Estimation of the Production, Consumption, and Atmospheric Emissions of Pentabrominated Diphenyl Ether in Europe between 1970 and 2000. Environ. Sci. Technol. 2004, 38 (12), 3224–3231. [DOI] [PubMed] [Google Scholar]
- (70).Serrano SE; Braun J; Trasande L; Dills R; Sathyanarayana S Phthalates and diet: a review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Wormuth M; Scheringer M; Vollenweider M; Hungerbühler K What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26 (3), 803–824. [DOI] [PubMed] [Google Scholar]
- (72).Phthalate Esters; Springer-Verlag: Berlin, Heidelberg, 2003. [Google Scholar]
- (73).Poma G; Glynn A; Malarvannan G; Covaci A; Darnerud PO Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food Chem. Toxicol. 2017, 100, 1–7. [DOI] [PubMed] [Google Scholar]
- (74).Yuan B; Strid A; Darnerud PO; de Wit CA; Nyström J; Bergman Å Chlorinated paraffins leaking from hand blenders can lead to significant human exposures. Environ. Int. 2017, 109, 73–80. [DOI] [PubMed] [Google Scholar]
- (75).Rudel RA; Gray JM; Engel CL; Rawsthorne TW; Dodson RE; Ackerman JM; Rizzo J; Nudelman JL; Brody JG Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ. Health Perspect. 2011, 119 (7), 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Li L; Arnot JA; Wania F, How are Humans Exposed to Organic Chemicals Released to Indoor Air? Environ. Sci. Technol. 2019, 53191127611284. [DOI] [PubMed] [Google Scholar]
- (77).Krewski D; Andersen ME; Tyshenko MG; Krishnan K; Hartung T; Boekelheide K; Wambaugh JF; Jones D; Whelan M; Thomas R; Yauk C; Barton-Maclaren T; Cote I Toxicity testing in the 21st century: progress in the past decade and future perspectives. Arch. Toxicol. 2020, 94, 1–58. [DOI] [PubMed] [Google Scholar]
- (78).Wei W; Ramalho O; Malingre L; Sivanantham S; Little JC; Mandin C Machine learning and statistical models for predicting indoor air quality. Indoor Air 2019, 29, 704–726. [DOI] [PubMed] [Google Scholar]
- (79).Licina D; Morrison GC; Bekö G; Weschler CJ; Nazaroff WW Clothing-Mediated Exposures to Chemicals and Particles. Environ. Sci. Technol. 2019, 53 (10), 5559–5575. [DOI] [PubMed] [Google Scholar]
- (80).Bekö G; Morrison G; Weschler CJ; Koch HM; Pälmke C; Salthammer T; Schripp T; Eftekhari A; Toftum J; Clausen G Dermal uptake of nicotine from air and clothing: Experimental verification. Indoor Air 2018, 28, 247–257. [DOI] [PubMed] [Google Scholar]
- (81).Lakey PSJ; Morrison GC; Won Y; Parry KM; von Domaros M; Tobias DJ; Rim D; Shiraiwa M The impact of clothing on ozone and squalene ozonolysis products in indoor environments. Communications Chemistry 2019, 2 (56), 1–8. [Google Scholar]
- (82).Cao J; Mo J; Sun Z; Zhang Y Indoor particle age, a new concept for improving the accuracy of estimating indoor airborne SVOC concentrations, and applications. Build. Environ. 2018, 136, 88–97. [Google Scholar]
- (83).Mackay D; Celsie AKD; Parnis JM Kinetic Delay in Partitioning and Parallel Particle Pathways: Underappreciated Aspects of Environmental Transport. Environ. Sci. Technol. 2019, 53 (1), 234–241. [DOI] [PubMed] [Google Scholar]
- (84).Fortenberry C; Walker M; Dang A; Loka A; Date G; Karolina Cysneiros de Carvalho, K.; Morrison, G.; Williams, B. Analysis of indoor particles and gases and their evolution with natural ventilation. Indoor Air 2019, 29, 761–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Xu Y; Liu Z; Park J; Clausen PA; Benning JL; Little JC Measuring and Predicting the Emission Rate of Phthalate Plasticizer from Vinyl Flooring in a Specially-Designed Chamber. Environ. Sci. Technol. 2012, 46 (22), 12534–12541. [DOI] [PubMed] [Google Scholar]
- (86).Pei J; Yin Y; Cao J; Sun Y; Liu J; Zhang Y Time dependence of characteristic parameter for semi-volatile organic compounds (SVOCs) emitted from indoor materials. Build. Environ. 2017, 125, 339–347. [Google Scholar]
- (87).Zhang Y; Xiong J; Mo J; Gong M; Cao J Understanding and controlling airborne organic compounds in the indoor environment: mass transfer analysis and applications. Indoor Air 2016, 26, 39–60. [DOI] [PubMed] [Google Scholar]
- (88).Cao J; Zhang X; Zhang Y Predicting Dermal Exposure to Gas-Phase Semivolatile Organic Compounds (SVOCs): A Further Study of SVOC Mass Transfer between Clothing and Skin Surface Lipids. Environ. Sci. Technol. 2018, 52, 4676–4683. [DOI] [PubMed] [Google Scholar]
- (89).Wang L; Chen Q Evaluation of some assumptions used in multizone airflow network models. Build. Environ. 2008, 43 (10), 1671–1677. [Google Scholar]
- (90).Boor BE; Spilak MP; Laverge J; Novoselac A; Xu Y Human exposure to indoor air pollutants in sleep microenvironments: A literature review. Build. Environ. 2017, 125, 528–555. [Google Scholar]
- (91).Wang P; Wang SL; Fan CQ Atmospheric distribution of particulate- and gas-phase phthalic esters (PAEs) in a Metropolitan City, Nanjing, East China. Chemosphere 2008, 72 (10), 1567–1572. [DOI] [PubMed] [Google Scholar]
- (92).Teil MJ; Blanchard M; Chevreuil M Atmospheric fate of phthalate esters in an urban area (Paris-France). Sci. Total Environ. 2006, 354 (2–3), 212–223. [DOI] [PubMed] [Google Scholar]
- (93).Rudel RA; Perovich LJ Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009, 43, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).McKone TE; Castorina R; Harnly ME; Kuwabara Y; Eskenazi B; Bradman A Merging Models and Biomonitoring Data to Characterize Sources and Pathways of Human Exposure to Organophosphorus Pesticides in the Salinas Valley of California. Environ. Sci. Technol. 2007, 41 (9), 3233–3240. [DOI] [PubMed] [Google Scholar]
- (95).Hoffman K; Hammel SC; Phillips AL; Lorenzo AM; Chen A; Calafat AM; Ye X; Webster TF; Stapleton HM Biomarkers of exposure to SVOCs in children and their demographic associations: The TESIE Study. Environ. Int. 2018, 119, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).OECD. Internationally Harmonised Functional, Product and Article Use Categories, 2017. [Google Scholar]
- (97).Isaacs KK; Dionisio K; Phillips K; Bevington C; Egeghy P; Price PS Establishing a system of consumer product use categories to support rapid modeling of human exposure. J. Exposure Sci. Environ. Epidemiol. 2020, 30, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).ECHA Chapter R.12: Use description; European Chemicals Agency, 2015. [Google Scholar]
- (99).Shu H; Jönsson BAG; Gennings C; Lindh CH; Nånberg W; Bornehag C-G PVC flooring at home and uptake of phthalates in pregnant women. Indoor Air 2019, 29, 43–54. [DOI] [PubMed] [Google Scholar]
- (100).Seifert B; Ullrich D Methodologies for evaluating sources of volatile organic chemicals (VOC) in homes. Atmos. Environ. 1987, 21 (2), 395–404. [Google Scholar]
- (101).Liu X; Guo Z; Krebs KA; Greenwell DJ; Roache NF; Stinson RA; Nardin JA; Pope RH Laboratory Study of PCB Transport from Primary Sources to Settled Dust. Chemosphere 2016, 149, 62–69. [DOI] [PubMed] [Google Scholar]
- (102).Rauert C.; Harrad S. Mass transfer of PBDEs from plastic TV casing to indoor dust via three migration pathways — test chamber investigation. Sci. Total Environ. 2015, 536, 568–574. [DOI] [PubMed] [Google Scholar]
- (103).Huang L; Jolliet O A quantitative structure-property relationship (QSPR) for estimating solid material-air partition coefficients of organic compounds. Indoor Air 2019, 29, 79–88. [DOI] [PubMed] [Google Scholar]
- (104).Huang L; Jolliet O A parsimonious model for the release of volatile organic compounds (VOCs) encapsulated in products. Atmos. Environ. 2016, 127, 223–235. [Google Scholar]
- (105).Addington CK; Phillips KA; Isaacs KK Estimation of the Emission Characteristics of SVOCs from Household Articles Using Group Contribution Methods. Environ. Sci. Technol. 2019, 54, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Wei W; Ramalho O; Mandin C A long-term dynamic model for predicting the concentration of semivolatile organic compounds in indoor environments: Application to phthalates. Build. Environ. 2019, 148, 11–19. [Google Scholar]
- (107).Salthammer T; Goss K-U Predicting the Gas/Particle Distribution of SVOCs in the Indoor Environment Using Poly Parameter Linear Free Energy Relationships. Environ. Sci. Technol. 2019, 53, 2491–2499. [DOI] [PubMed] [Google Scholar]
- (108).Abdallah MA-E; Harrad S Dermal contact with furniture fabrics is a significant pathway of human exposure to brominated flame retardants. Environ. Int. 2018, 118, 26–33. [DOI] [PubMed] [Google Scholar]
- (109).Boor BE; Liang Y; Crain NE; Järnström H; Novoselac A; Xu Y Identification of Phthalate and Alternative Plasticizers, Flame Retardants, and Unreacted Isocyanates in Infant Crib Mattress Covers and Foam. Environ. Sci. Technol. Lett. 2015, 2, 89–94. [Google Scholar]
- (110).Yang T; He Z; Zhang S; Tong L; Cao J; Xiong J Emissions of DEHP from vehicle cabin materials: parameter determination, impact factors and exposure analysis. Environmental Science: Processes & Impacts 2019, 21, 1323–1333. [DOI] [PubMed] [Google Scholar]
- (111).Saini A; Okeme JO; Mark Parnis J; McQueen RH; Diamond ML From air to clothing: characterizing the accumulation of semi-volatile organic compounds to fabrics in indoor environments. Indoor Air 2017, 27, 631–641. [DOI] [PubMed] [Google Scholar]
- (112).Yang C; Harris SA; Jantunen LM; Siddique S; Kubwabo C; Tsirlin D; Latifovic L; Fraser B; St-Jean M; De La Campa R; You H; Kulka R; Diamond ML Are cell phones an indicator of personal exposure to organophosphate flame retardants and plasticizers? Environ. Int. 2019, 122, 104–116. [DOI] [PubMed] [Google Scholar]
- (113).Pelletier M; Bonvallot N; Ramalho O; Blanchard O; Mercier F; Mandin C; Le Bot B; Glorennec P Dermal absorption of semivolatile organic compounds from the gas phase: Sensitivity of exposure assessment by steady state modeling to key parameters. Environ. Int. 2017, 102, 106–113. [DOI] [PubMed] [Google Scholar]
- (114).Garrido JA; Parthasarathy S; Moschet C; Young TM; McKone TE; Bennett DH Exposure Assessment For Air-To-Skin Uptake of Semivolatile Organic Compounds (SVOCs) Indoors. Environ. Sci. Technol. 2019, 53 (3), 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Shin H-M; McKone TE; Bennett DH Model framework for integrating multiple exposure pathways to chemicals in household cleaning products. Indoor Air 2017, 27, 829–839. [DOI] [PubMed] [Google Scholar]
- (116).Jahnke JC; Hornbuckle KC PCB Emissions from Paint Colorants. Environ. Sci. Technol. 2019, 53 (9), 5187–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Hu D; Hornbuckle KC Inadvertent Polychlorinated Biphenyls in Commercial Paint Pigments. Environ. Sci. Technol. 2010, 44 (8), 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).USEPA. Wall Paint Exposure Model (WPEM): Version 3.2 User’s Guide; US Environmental Protection Agency: Washington, DC, 2001. [Google Scholar]
- (119).Daniels W; Lee S; Miller A EPA’s Exposure Assessment Tools and Models. Appl. Occup. Environ. Hyg. 2003, 18 (2), 82–86. [DOI] [PubMed] [Google Scholar]
- (120).Glytsos T; Ondráček J; Džubová L; Kopanakis I; Lazaridis M Characterization of particulate matter concentrations during controlled indoor activities. Atmos. Environ. 2010, 44 (12), 1539–1549. [Google Scholar]
- (121).Weschler CJ; Carslaw N Indoor Chemistry. Environ. Sci. Technol. 2018, 52 (5), 2419–2428. [DOI] [PubMed] [Google Scholar]
- (122).Wallace LA; Ott WRCJW; Lai ACK Desorption of SVOCs from Heated Surfaces in the Form of Ultrafine Particles. Environ. Sci. Technol. 2017, 51 (3), 1140–1146. [DOI] [PubMed] [Google Scholar]
- (123).Shi S; Zhao B Estimating indoor semi-volatile organic compounds (SVOCs) associated with settled dust by an integrated kinetic model accounting for aerosol dynamics. Atmos. Environ. 2015, 107, 52–61. [Google Scholar]
- (124).Shi S; Zhao B Comparison of the predicted concentration of outdoor originated indoor polycyclic aromatic hydrocarbons between a kinetic partition model and a linear instantaneous model for gas-particle partition. Atmos. Environ. 2012, 59, 93–101. [Google Scholar]
- (125).Eriksson AC; Andersen C; Krais AM; Nøjgaard JK; Clausen PA; Gudmundsson A; Wierzbicka A; Pagels J Influence of Airborne Particles’ Chemical Composition on SVOC Uptake from PVC Flooring - Time-Resolved Analysis with Aerosol Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 85–91. [DOI] [PubMed] [Google Scholar]
- (126).Zhou X; Luo C; Liu K; Wang X A novel model for predicting the semivolatile organic compound partition coefficient of multicomponent airborne particles. Build. Environ. 2020, 167 (106446), 1–9. [Google Scholar]
- (127).Corsi RL; Siegel JA; Chiang C Particle Resuspension During the Use of Vacuum Cleaners on Residential Carpet. J. Occup. Environ. Hyg. 2008, 5 (4), 232–238. [DOI] [PubMed] [Google Scholar]
- (128).Clausen PA; Hansen V; Gunnarsen L; Afshari P; Wolkoff P Emission of Di-2-ethylhexyl Phthalate from PVC Flooring into Air and Uptake in Dust: Emission and Sorption Experiments in FLEC and CLIMPAQ. Environ. Sci. Technol. 2004, 38 (9), 2531–2537. [DOI] [PubMed] [Google Scholar]
- (129).Schripp T; Fauck C; Salthammer T Chamber studies on mass-transfer of di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DnBP) from emission sources into house dust. Atmos. Environ. 2010, 44 (24), 2840–2845. [Google Scholar]
- (130).Bope A; Haines SR; Hegarty B; Weschler CJ; Peccia J; Dannemiller KC, Degradation of phthalate esters in floor dust at elevated relative humidity. Environ. Sci.: Processes Impacts 2019211268. [DOI] [PubMed] [Google Scholar]
- (131).Wang C; Collins DB; Arata C; Goldstein AH; Mattila JM; Farmer DK; Ampollini L; DeCarlo PF; Novoselac A; Vance ME; Nazaroff WW; Abbatt JPD Surface reservoirs dominate dynamic gas-surface partitioning of many indoor air constituents. Science Advances 2020, 6 (8), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Wei W; Mandin C; Ramalho O Influence of indoor environmental factors on mass transfer parameters and concentrations of semi-volatile organic compounds. Chemosphere 2018, 195, 223–235. [DOI] [PubMed] [Google Scholar]
- (133).Bi C; Liang Y; Xu Y Fate and transport of phthalates in indoor environments and the influence of temperature: A case study in a test house. Environ. Sci. Technol. 2015, 49 (16), 9674–9681. [DOI] [PubMed] [Google Scholar]
- (134).Vernier M; Audy O; Vojta S; Bečanová J; Romanak K; Krátká M.; Kukučka P.; Okeme JO.; Saini A.; Diamond ML.; Klánová J. Brominated flame retardants in the indoor environment — Comparative study of indoor contamination from three countries. Environ. Int. 2016, 84, 150–160. [DOI] [PubMed] [Google Scholar]
- (135).Vykoukalová M; Vernier M; Vojta S; Melymuk L; Bečanová J; Romanak K; Prokeš R; Okeme JO; Saini A; Diamond ML; Klánová J Organophosphate esters flame retardants in the indoor environment. Environ. Int. 2017, 106, 97–104. [DOI] [PubMed] [Google Scholar]
- (136).Schwartz-Narbonne H; Donaldson DJ Water uptake by indoor surface films. Sci. Rep. 2019, 9 (11089), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Liang Y; Liu X; Allen MR Measuring and modeling surface sorption dynamics of organophosphate flame retardants on impervious surfaces. Chemosphere 2018, 193 (754–762), 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Zhang X; Arnot JA; Wania F Model for Screening-Level Assessment of Near-Field Human Exposure to Neutral Organic Chemicals Released Indoors. Environ. Sci. Technol. 2014, 48 (20), 12312–12319. [DOI] [PubMed] [Google Scholar]
- (139).Saini A; Rauert C; Simpson MJ; Harrad S; Diamond ML Characterizing the sorption of polybrominated diphenyl ethers (PBDEs) to cotton and polyester fabrics under controlled conditions. Sci. Total Environ. 2016, 563–564, 99–107. [DOI] [PubMed] [Google Scholar]
- (140).Saini A; Thaysen C; Jantunen L; McQueen RH; Diamond ML From Clothing to Laundry Water: Investigating the Fate of Phthalates, Brominated Flame Retardants, and Organophosphate Esters. Environ. Sci. Technol. 2016, 50, 9289–9297. [DOI] [PubMed] [Google Scholar]
- (141).Morrison GC; Li H; Mishra S; Buechlein M Airborne phthalate partitioning to cotton clothing. Atmos. Environ. 2015, 115, 149–152. [Google Scholar]
- (142).Morrison G; Andersen HV; Gunnarsen L; Voral D; Uhde E; Kolarik B Partitioning of PCBs from air to clothing materials in a Danish apartment. Indoor Air 2018, 28 (1), 188–197. [DOI] [PubMed] [Google Scholar]
- (143).Weschler CJ Chemistry in indoor environments: 20 years of research. Indoor Air 2011, 21, 205–218. [DOI] [PubMed] [Google Scholar]
- (144).Wang C; Waring MS Secondary organic aerosol formation initiated from reactions between ozone and surface-sorbed squalene. Atmos. Environ. 2014, 84, 222–229. [DOI] [PubMed] [Google Scholar]
- (145).Abbatt JPD; Wang C The atmospheric chemistry of indoor environments. Environmental Science: Processes & Impacts 2020, 22, 25–48. [DOI] [PubMed] [Google Scholar]
- (146).Mattila JM; Lakey PSJ; Shiraiwa M; Wang C; Abbatt JPD; Arata C; Goldstein AH; Ampollini L; Katz EF; DeCarlo PF; Zhou S; Kahan TF; Cardoso-Saldaña FJ; Hildebrandt Ruiz L; Abeleira A; Boedicker EK; Vance ME; Farmer DK Multiphase Chemistry Controls Inorganic Chlorinated and Nitrogenated Compounds in Indoor Air during Bleach Cleaning. Environ. Sci. Technol. 2020, 54, 1730–1739. [DOI] [PubMed] [Google Scholar]
- (147).Wang C; Collins DB; Abbatt JPD Indoor Illumination of Terpenes and Bleach Emissions Leads to Particle Formation and Growth. Environ. Sci. Technol. 2019, 53 (20), 11792–11800. [DOI] [PubMed] [Google Scholar]
- (148).Nazaroff WW; Weschler CJ, Indoor acids and bases. Indoor Air 2020, 30559 [DOI] [PubMed] [Google Scholar]
- (149).Weschler CJ Roles of the human occupant in indoor chemistry. Indoor Air 2016, 26, 6–24. [DOI] [PubMed] [Google Scholar]
- (150).Weschler CJ; Nazaroff WW SVOC exposure indoors: fresh look at dermal pathways. Indoor Air 2012, 22 (5), 356–377. [DOI] [PubMed] [Google Scholar]
- (151).Shiraiwa M; Carslaw N; Tobias DJ; Waring MS; Rim D; Morrison G; Lakey PSJ; Kruza M; von Domaros M; Cummings BE; Won Y Modelling Consortium for Chemistry of Indoor Environments (MOCCIE): Integrating chemical processes from molecular to room scales. Environmental Science: Processes & Impacts 2019, 21, 1240–1254. [DOI] [PubMed] [Google Scholar]
- (152).Liu X; Mason M; Krebs K; Sparks L Full-Scale Chamber Investigation and Simulation of Air Freshener Emissions in the Presence of Ozone. Environ. Sci. Technol. 2004, 38 (10), 2802–2812. [DOI] [PubMed] [Google Scholar]
- (153).Mendez M; Blond N; Blondeau P; Schoemaecker C; Hauglustaine DA Assessment of the impact of oxidation processes on indoor air pollution using the new time-resolved INCA-Indoor model. Atmos. Environ. 2015, 122, 521–530. [Google Scholar]
- (154).Cummings BE; Waring MS Predicting the importance of oxidative aging on indoor organic aerosol concentrations using the two-dimensional volatility basis set (2D-VBS). Indoor Air 2019, 29, 616–629. [DOI] [PubMed] [Google Scholar]
- (155).Zuend A; Seinfeld JH Modeling the gas-particle partitioning of secondary organic aerosol: the importance of liquid-liquid phase separation. Atmos. Chem. Phys. 2012, 12, 3857–3882. [Google Scholar]
- (156).Springs M; Wells JR; Morrison GC Reaction rates of ozone and terpenes adsorbed to model indoor surfaces. Indoor Air 2011, 21 (4), 319–327. [DOI] [PubMed] [Google Scholar]
- (157).Sleiman M; Gundel LA; Pankow JF; Ill PJ; Singer BC; Destaillats H Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (15), 6676–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Fang Y; Lakey PSJ; Riahi S; McDonald AT; Shrestha M; Tobias DJ; Shiraiwa M; Grassian VH A molecular picture of surface interactions of organic compounds on prevalent indoor surfaces: limonene adsorption on SiO2. Chemical Science 2019, 10, 2906–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Zhou S; Hwang BCH; Lakey PSJ; Zuend A; Abbatt JPD; Shiraiwa M Multiphase reactivity of polycyclic aromatic hydrocarbons is driven by phase separation and diffusion limitations. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (24), 11658–11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Wei W; Mandin C; Ramalho O Reactivity of Semivolatile Organic Compounds with Hydroxyl Radicals, Nitrate Radicals, and Ozone in Indoor Air. Int. J. Chem. Kinet. 2017, 49 (7), 506–521. [Google Scholar]
- (161).Weschler CJ; Nazaroff WW Dermal Uptake of Organic Vapors Commonly Found in Indoor Air. Environ. Sci. Technol. 2014, 48, 1230–1237. [DOI] [PubMed] [Google Scholar]
- (162).CPSC Review of Exposure Data and Assessments for Select Dialkyl Ortho-Phthalates; U.S. Consumer Product Safety Commission: 2010. [Google Scholar]
- (163).Delmaar JE; Bokkers BGH; ter Burg W; van Engelen JGM First tier modeling of consumer dermal exposure to substances in consumerarticles under REACH: A quantitative evaluation of the ECETOC TRAfor consumers tool. Regul. Toxicol. Pharmacol. 2013, 65, 79–86. [DOI] [PubMed] [Google Scholar]
- (164).ECHA Chapter R.15: Consumer exposure assessment. In European Chemicals Agency: 2016. [Google Scholar]
- (165).Giovanoulis G; Alves A; Papadopoulou E; Cousins AP; Schütze A; Koch HM; Haug LS; Covaci A; Magnér J; Voorspoels S Evaluation of exposure to phthalate esters and DINCH in urine and nails from a Norwegian study population. Environ. Res. 2016, 151, 80–90. [DOI] [PubMed] [Google Scholar]
- (166).Huang L; Jolliet O A combined quantitative property-property relationship (QPPR) for estimating packaging-food and solid material-water partition coefficients of organic compounds. Sci. Total Environ. 2019, 658, 493–500. [DOI] [PubMed] [Google Scholar]
- (167).Gong M; Zhang Y; Weschler CJ Predicting dermal absorption of gas-phase chemicals: transient model development, evaluation, and application. Indoor Air 2014, 24, 292–306. [DOI] [PubMed] [Google Scholar]
- (168).Lakey PSJ; Wisthaler A; Berkemeier T; Mikoviny T; Pöschl U; Shiraiwa M Chemical kinetics of multiphase reactions between ozone and human skin lipids: Implications for indoor air quality and health effects. Indoor Air 2017, 27, 816–828. [DOI] [PubMed] [Google Scholar]
- (169).Isaacs KK; Glen WG; Egeghy P; Goldsmith M-R; Smith L; Vallero D; Brooks R; Grulke CM; Özkaynak H SHEDS-HT: An Integrated Probabilistic Exposure Model for Prioritizing Exposures to Chemicals with Near-Field and Dietary Sources. Environ. Sci. Technol. 2014, 48 (21), 12750–12759. [DOI] [PubMed] [Google Scholar]
- (170).USEPA, Chapter 5 - Soil and Dust Ingestion In Exposure Factors Handbook, Update of 2011 ed.; US Environmental Protection Agency, 2017. [Google Scholar]
- (171).Wei W; Bonvallot N; Gustafsson Å; Raffy G; Glorennec P; Krais A; Ramalho O; Le Bot B; Mandin C Bioaccessibility and bioavailability of environmental semi-volatile organic compounds via inhalation: A review of methods and models. Environ. Int. 2018, 113, 202–213. [DOI] [PubMed] [Google Scholar]
- (172).Xie S-Y; Lao J-Y; Wu C-C; Bao L-J; Zeng EY In vitro inhalation bioaccessibility for particle-bound hydrophobic organic chemicals: Method development, effects of particle size and hydrophobicity, and risk assessment. Environ. Int. 2018, 120, 295–303. [DOI] [PubMed] [Google Scholar]
- (173).Nazaroff WW Inhalation intake fraction of pollutants from episodic indoor emissions. Build. Environ. 2008, 43, 269–277. [Google Scholar]
- (174).Shin H-M; McKone TE; Bennett DH Intake Fraction for the Indoor Environment: A Tool for Prioritizing Indoor Chemical Sources. Environ. Sci. Technol. 2012, 46, 10063–10072. [DOI] [PubMed] [Google Scholar]
- (175).Fantke P; Huang L; Overcash M; Griffing E; Jolliet O Life cycle based alternatives assessment (LCAA) for chemical substitution. Green Chem. 2020, 22, 6008–6024. [Google Scholar]
- (176).Hodas N; Loh M; Shin H-M; Li D; Bennett D; McKone TE; Jolliet O; Weschler CJ; Jantunen L; Lioy P; Fantke P Indoor inhalation intake fractions of fine particulate matter: review of influencing factors. Indoor Air 2016, 26 (6), 836–856. [DOI] [PubMed] [Google Scholar]
- (177).Ionas AC; Ulevicus J; Ballesteros Gómez A; Brandsma SH; Leonards PEG; van de Bor M; Covaci A Children’s exposure to polybrominated diphenyl ethers (PBDEs) through mouthing toys. Environ. Int. 2016, 87, 101–107. [DOI] [PubMed] [Google Scholar]
- (178).von Lindern I; Spalinger S; Stifelman ML; Stanek LW; Bartrem C Estimating Children’s Soil/Dust Ingestion Rates through Retrospective Analyses of Blood Lead Biomonitoring from the Bunker Hill Superfund Site in Idaho. Environ. Health Perspect. 2016, 124 (9), 1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Wilson R; Jones-Otazo H; Petrovic S; Mitchell I; Bonvalot Y; Willams D; Richardson M Revisiting Dust and Soil Ingestion Rates Based on Hand-to-Mouth Transfer. Hum. Ecol. Risk Assess. 2013, 19 (1), 158–188. [Google Scholar]
- (180).Raffy G; Mercier F; Glorennec P; Mandin C; Le Bot B Oral bioaccessibility of semi-volatile organic compounds (SVOCs) in settled dust: A review of measurement methods, data and influencing factors. J. Hazard. Mater. 2018, 352, 215–227. [DOI] [PubMed] [Google Scholar]
- (181).Cohen Hubal EA; Nishioka MG; Ivancic WA; Morara M; Egeghy PP Comparing Surface Residue Transfer Efficiencies to Hands using Polar and Nonpolar Fluorescent Tracers. Environ. Sci. Technol. 2008, 42 (3), 934–939. [DOI] [PubMed] [Google Scholar]
- (182).Wei W; Mandin C; Blanchard O; Mercier F; Pelletier M; Le Bot B; Glorennec P; Ramalho O Distributions of the particle/gas and dust/gas partition coefficients for seventy-two semi-volatile organic compounds in indoor environment. Chemosphere 2016, 153, 212–219. [DOI] [PubMed] [Google Scholar]
- (183).Salthammer T; Schripp T Application of the Junge- and Pankow-equation for estimating indoor gas/particle distribution and exposure to SVOCs. Atmos. Environ. 2015, 106, 467–476. [Google Scholar]
- (184).Cox SS; Little JC; Hodgson AT Predicting the Emission Rate of Volatile Organic Compounds from Vinyl Flooring. Environ. Sci. Technol. 2002, 36 (4), 709–714. [DOI] [PubMed] [Google Scholar]
- (185).ASTM. Standard Practice for Determination of Monomeric Plasticizers in Poly (Vinyl Chloride) (PVC) by Gas Chromatography. In ASTM D7083–16; ASTM International: West Conshohocken, PA, 2016. [Google Scholar]
- (186).Phillips KA; Yau A; Favela KA; Isaacs KK; McEachran A; Grulke C; Richard AM; Williams AJ; Sobus JR; Thomas RS; Wambaugh JF Suspect Screening Analysis of Chemicals in Consumer Products. Environ. Sci. Technol. 2018, 52 (5), 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Goldsmith M-R; Grulke CM; Brooks RD; Transue TR; Tan YM; Frame A; Egeghy PP; Edwards R; Chang DT; Tornero-Velez R; Isaacs KK; Wang A; Johnson J; Holm K; Reich M; Mitchell J; Vallero DA; Phillips L; Phillips M; Wambaugh JF; Judson RS; Buckley TJ; Dary CC Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem. Toxicol. 2014, 65, 269–279. [DOI] [PubMed] [Google Scholar]
- (188).Dionisio KL; Phillips K; Price PS; Grulke CM; Williams A; Biryol D; Hong T; Isaacs KK Data Descriptor: The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Scientific Data 2018, 5 (180125), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Zhang X; Sühring R; [Google Scholar]; Serodio D; Bonnell M; Sundin N; Diamond ML Novel flame retardants: Estimating the physical-chemical properties and environmental fate of 94 halogenated and organophosphate PBDE replacements. Chemosphere 2016, 144, 2401–2407. [DOI] [PubMed] [Google Scholar]
- (190).O’Meara S; Booth AM; Barley MH; Topping D; McFiggans G An assessment of vapour pressure estimation methods. Phys. Chem. Chem. Phys. 2014, 16 (36), 19453–19469. [DOI] [PubMed] [Google Scholar]
- (191).Compernolle S; Ceulemans K; Müller JF. Evaporation: A New Vapour Pressure Estimation Method for Organic Molecules Including Non-Additivity and Intramolecular Interactions. Atmos. Chem. Phys. 2011, 11 (18), 9431–9450. [Google Scholar]
- (192).Okeme JO; Rodgers TFM; Parnis JM; Diamond ML; Bidleman TF; Jantunen LM Gas Chromatographic Estimation of Vapor Pressures and Octanol-Air Partition Coefficients of Semivolatile Organic Compounds of Emerging Concern. J. Chem. Eng. Data 2020, 65 (5), 2467–2675. [Google Scholar]
- (193).Schossler P; Schripp T; Salthammer T; Bahadir M Beyond phthalates: Gas phase concentrations and modeled gas/particle distribution of modern plasticizers. Sci. Total Environ. 2011, 409 (19), 4031–4038. [DOI] [PubMed] [Google Scholar]
- (194).Cousins I; Mackay D Correlating the physical-chemical properties of phthalate esters using the ‘three solubility’ approach. Chemosphere 2000, 41 (9), 1389–1399. [DOI] [PubMed] [Google Scholar]
- (195).Boethling RS; Mackay D Handbook of Property Estimation Methods for Chemicals: Environmental and Health Sciences; CRC Press: Boca Raton, FL, 2000. [Google Scholar]
- (196).Topping D; Barley M; Bane MK; Higham N; Aumont B; Dingle N; McFiggans G UManSysProp v1.0: an online and open-source facility for molecular property prediction and atmospheric aerosol calculations. Geosci. Model Dev. 2016, 9, 899–914. [Google Scholar]
- (197).Klamt A; Eckert F; Arlt W COSMO-RS: An Alternative to Simulation for Calculating Thermodynamic Properties of Liquid Mixtures. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 101–122. [DOI] [PubMed] [Google Scholar]
- (198).Schwarzenbach RP; Gschwend PM; Imboden DM Environmental Organic Chemistry; John Wiley & Sons: Hoboken, NJ, 2017. [Google Scholar]
- (199).Okeme JO; Rodgers TFM; Jantunen LM; Diamond ML Examining the Gas-Particle Partitioning of Organophosphate Esters: How Reliable Are Air Measurements? Environ. Sci. Technol. 2018, 52 (23), 13834–13844. [DOI] [PubMed] [Google Scholar]
- (200).Xu Y; Cohen Hubal EA; Clausen PA; Little JC Predicting Residential Exposure to Phthalate Plasticizer Emitted from Vinyl Flooring: A Mechanistic Analysis. Environ. Sci. Technol. 2009, 43 (7), 2374–2380. [DOI] [PubMed] [Google Scholar]
- (201).USEPA Toxicity Estimation Software Tool (TEST). https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test (accessed 2020/6/30).
- (202).Cronin MTD (Q)SARs to predict environmental toxicities: current status and future needs . Environmental Science: Processes & Impacts 2017, 19, 213–220. [DOI] [PubMed] [Google Scholar]
- (203).Eichler CMA; Little JC A Framework to Model Exposure to Per- and Polyfluoroalkyl Substances in Indoor Environments. Environmental Science: Processes & Impacts 2020, 22 (3), 500–511. [DOI] [PubMed] [Google Scholar]
- (204).Ring CL; Pearce RG; Woodrow Setzer R; Wetmore BA; Wambaugh JF Identifying populations sensitive to environmental chemicals by simulating toxicokinetic variability. Environ. Int. 2017, 106, 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Wan Y; Diamond ML; Siegel JA Elevated Concentrations of Semivolatile Organic Compounds in Social Housing Multiunit Residential Building Apartments. Environ. Sci. Technol. Lett. 2020, 7 (3), 191–197. [Google Scholar]
- (206).Farmer DK; Vance ME; Abbatt JPD; Abeleira A; Alves MR; Arata C; Boedicker E; Grassian VH; Hildebrandt Ruiz L; Jimenez JL; Kahan TF; Katz EF; Mattila JM; Nazaroff WW; Novoselac A; O’Brien RE; Or VW; Patel S; Sankhyan S; Stevens PS; Tian Y; Wade M; Wang C; Zhou S; Zhou Y, Overview of HOMEChem: House Observations of Microbial and Environmental Chemistry. Environ. Sci.: Processes Impacts 2019, Advance Article. DOI: 10.1039/C9EM00228F [DOI] [PubMed] [Google Scholar]
- (207).Jayjock M; Havics AA Residential inter-zonal ventilation rates for exposure modeling. J. Occup. Environ. Hyg. 2018, 15 (5), 376–388. [DOI] [PubMed] [Google Scholar]
- (208).Manuja A; Ritchie J; Buch K; Wu Y; Eichler CMA; Little JC; Marr LC Total surface area in indoor environments. Environmental Science: Processes & Impacts 2019, 21, 1384–1392. [DOI] [PubMed] [Google Scholar]
- (209).Avery AM; Waring MS; DeCarlo PF Human occupant contribution to secondary aerosol mass in the indoor environment . Environmental Science: Processes & Impacts 2019, 21 (8), 1301–1312. [DOI] [PubMed] [Google Scholar]
- (210).Cushing L; Morello-Frosch R; Wander M; Pastor M The Haves, the Have-Nots, and the Health of Everyone: The Relationship Between Social Inequality and Environmental Quality. Annu. Rev. Public Health 2015, 36, 193–209. [DOI] [PubMed] [Google Scholar]
- (211).Solomon GM; Morello-Frosch R; Zeise L; Faust JB Cumulative Environmental Impacts: Science and Policy to Protect Communities. Annu. Rev. Public Health 2016, 37, 83–96. [DOI] [PubMed] [Google Scholar]
- (212).Ciffroy P; Alfonso B; Altenpohl A; Banjac Z; Bierkens J; Brochot C; Critto A; De Wilde T; Fait G; Fierens T; Garratt J; Giubilato E; Johansson E; Radomyski A; Reschwann K; Suciu N; Tanaka T; Verdonck F Modelling the exposure to chemicals for risk assessment: a comprehensive library of multimedia and PBPK models for integration, prediction, uncertainty and sensitivity analysis - the MERLIN-Expo tool. Sci. Total Environ. 2016, 568, 770–784. [DOI] [PubMed] [Google Scholar]
- (213).Suciu N; Tediosi A; Ciffroy P; Altenpohl A; Brochot C; Verdonck F; Ferrari F; Giubilato E; Capri E; Fait G Potential for MERLIN-Expo, an advanced tool for higher tier exposure assessment, within the EU chemical legislative frameworks. Sci. Total Environ. 2016, 562, 474–479. [DOI] [PubMed] [Google Scholar]
- (214).Sheldon LS; Cohen Hubal EA Exposure as Part of a Systems Approach for Assessing Risk. Environ. Health Perspect. 2009, 117 (8), 1181–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).NRC. Toxicity Testing in the 21st Century: A Vision and a Strategy; Washington, DC, 2007. [Google Scholar]
- (216).NRC. Exposure Science in the 21st Century: A Vision and a Strategy; Washington, DC, 2012. [Google Scholar]
- (217).NASEM. Using 21st Century Science to Improve Risk-Related Evaluations; Washington, DC, 2017. [PubMed] [Google Scholar]
- (218).Ring CL; Arnot JA; Bennett DH; Egeghy PP; Fantke P; Huang L; Isaacs KK; Jolliet O; Phillips KA; Price PS; Shin H-M; Westgate JN; Setzer RW; Wambaugh JF Consensus Modeling of Median Chemical Intake for the U.S. Population Based on Predictions of Exposure Pathways. Environ. Sci. Technol. 2019, 53, 719–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Fantke P; Aylward L; Bare J; Chiu WA; Dodson R; Dwyer R; Ernstoff A; Howard B; Jantunen L; Jolliet O; Judson R; Kirchhübel N; Li D; Miller A; Paoli G; Price P; Rhomberg L; Shen H; Shin H-M; Teeguarden J; Vallero D; Wambaugh J; Wetmore BA; Zaleski R; McKone TE Advancements in life cycle human exposure and toxicity characterization. Environ. Health Perspect. 2018, 126 (12), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Fantke P; von Goetz N; Schluter U; Bessems J; Connolly A; Dudzina T; Ahrens A; Bridges J; Coggins MA; Conrad A; Hanninen O; Heinemeyer G; Kephalopoulos S; McLachlan M; Meijster T; Poulsen V; Rother D; Vermeire T; Viegas S; Vlaanderen J; Jeddi MZ; Bruinen de Bruin Y Building a European exposure science strategy. J. Exposure Sci. Environ. Epidemiol. 2020, 30, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Gangwal S; Reif DM; Mosher S; Egeghy PP; Wambaugh JF; Judson RS; Cohen Hubal EA Incorporating Exposure Information into the Toxicological Prioritization Index Decision Support Framework. Sci. Total Environ. 2012, 435–436, 316–325. [DOI] [PubMed] [Google Scholar]
- (222).USEPA Toxicity ForeCaster (ToxCast) Data. https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data (accessed 2020/6/20).
- (223).USEPA Toxicity Forecasting. https://www.epa.gov/chemical-research/toxicity-forecasting (accessed 2020/7/2).
- (224).Shin H-M; Ernstoff A; Arnot JA; Wetmore BA; Csiszar SA; Fantke P; Zhang X; McKone TE; Jolliet O; Bennett DH Risk-Based High-Throughput Chemical Screening and Prioritization using Exposure Models and in Vitro Bioactivity Assays. Environ. Sci. Technol. 2015, 49, 6760–6771. [DOI] [PubMed] [Google Scholar]
- (225).Li L; Westgate JN; Hughes L; Zhang X; Givehchi B; Toose L; Armitage JM; Wania F; Egeghy P; Arnot JA A Model for Risk-Based Screening and Prioritization of Human Exposure to Chemicals from Near-Field Sources. Environ. Sci. Technol. 2018, 52 (24), 14235–14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Meyer DE; Bailin SC; Vallero D; Egeghy PP; Liu SV; Cohen Hubal EA Enhancing life cycle chemical exposure assessment through ontology modeling. Sci. Total Environ. 2020, 712 (136263), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Byun D; Schere KL Review of the Governing Equations, Computational Algorithms, and Other Components of the Models-3 Community Multiscale Air Quality (CMAQ) Modeling System. Appl. Mech. Rev. 2006, 59 (2), 51–77. [Google Scholar]
- (228).USEPA CMAQ: The Community Multiscale Air Quality Modeling System. https://www.epa.gov/cmaq (accessed 2020/4/1).
- (229).CMAS CMAQ. https://www.cmascenter.org/cmaq/ (accessed 2020/4/1).
- (230).Fantke P; Illner N Goods that are good enough: Introducing an absolute sustainability perspective for managing chemicals in consumer products. Current Opinion in Green and Sustainable Chemistry 2019, 15, 91–97. [Google Scholar]
- (231).Gwinn MR; Axelrad DA; Bahadori T; Bussard D; Cascio WE; Deener K; Dix D; Thomas RS; Kavlock RJ; Burke TA Chemical Risk Assessment: Traditional vs Public Health Perspectives. Am. J. Public Health 2017, 107 (7), 1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.