It is critical to determine how best to prevent SARS-CoV-2 infection in health care workers. Early in the pandemic, a voluntary survey of such workers coupled with serologic testing were used to assess the relative contribution of specific demographic, occupational, residential, and community-level risk factors for SARS-CoV-2 positivity.
Abstract
Background:
Identifying occupational risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among health care workers (HCWs) can improve HCW and patient safety.
Objective:
To quantify demographic, occupational, and community risk factors for SARS-CoV-2 seropositivity among HCWs in a large health care system.
Design:
A logistic regression model was fitted to data from a cross-sectional survey conducted in April to June 2020, linking risk factors for occupational and community exposure to coronavirus disease 2019 (COVID-19) with SARS-CoV-2 seropositivity.
Setting:
A large academic health care system in the Atlanta, Georgia, metropolitan area.
Participants:
Employees and medical staff members elected to participate in SARS-CoV-2 serology testing offered to all HCWs as part of a quality initiative and completed a survey on exposure to COVID-19 and use of personal protective equipment.
Measurements:
Demographic risk factors for COVID-19, residential ZIP code incidence of COVID-19, occupational exposure to HCWs or patients who tested positive on polymerase chain reaction test, and use of personal protective equipment as potential risk factors for infection. The outcome was SARS-CoV-2 seropositivity.
Results:
Adjusted SARS-CoV-2 seropositivity was estimated to be 3.8% (95% CI, 3.4%-4.3%) (positive, n = 582) among the 10 275 HCWs (35% of the Emory Healthcare workforce) who participated in the survey. Community contact with a person known or suspected to have COVID-19 (adjusted odds ratio [aOR], 1.9 [CI, 1.4 to 2.6]; 77 positive persons [10.3%]) and community COVID-19 incidence (aOR, 1.5 [CI, 1.0 to 2.2]) increased the odds of infection. Black individuals were at high risk (aOR, 2.1 [CI, 1.7 to 2.6]; 238 positive persons [8.3%]).
Limitations:
Participation rates were modest and key workplace exposures, including job and infection prevention practices, changed rapidly in the early phases of the pandemic.
Conclusion:
Demographic and community risk factors, including contact with a COVID-19–positive person and Black race, are more strongly associated with SARS-CoV-2 seropositivity among HCWs than is exposure in the workplace.
Primary Funding Source:
Emory COVID-19 Response Collaborative.
Health care workers (HCWs) are presumed to be at high risk for coronavirus disease 2019 (COVID-19) through occupational exposure to infected patients or coworkers. Studies have reported a wide range of seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, among HCWs. This variation has in part been attributed to differential risk for exposure in the community (1). Indeed, recent studies have shown that a substantial number of infections among HCWs could not be traced to occupational exposures (2) and that community exposures were as or more strongly associated with infection (3). Although previous studies have compared seroprevalence in HCWs with that of the general population, few have rigorously considered workplace risk factors alongside community risk factors among HCWs to estimate their relative contribution to overall infection risk (4, 5).
Accounting for the role of community risk, which may be large, is especially important because reports of occupational risk factors for SARS-CoV-2 infection among HCWs have been inconsistent (3–8). Although some studies have shown weak associations between involvement in clinical care, care of patients with COVID-19, and exposure to coworkers with COVID-19 (6–8), others have shown that these are, in fact, risk factors for infection (4, 5). Moreover, previous studies have not accounted for potential participation bias, although they have cited it as a major limitation (1, 2, 4, 7). Adjusting for participation bias while considering both workplace and community risk factors for infection can bring us closer to an accurate understanding of which workplace exposures confer the highest risk for infection for HCWs. This information can inform strategies to protect HCWs as the COVID-19 pandemic continues.
We aimed to quantify occupational, community, and demographic risk factors for SARS-CoV-2 seropositivity among HCWs in a large university-based health care system, adjusting for possible bias due to voluntary participation in testing.
Methods
We analyzed data from Emory Healthcare, which includes 11 hospitals, 250 provider locations, and approximately 25 000 employees and nearly 5000 medical staff members based in the Atlanta, Georgia, metropolitan area.
A voluntary serologic survey was conducted among employees and medical staff members from 19 April through 26 June 2020 to inform process improvement activities. All Emory Healthcare employees and medical staff members received e-mail solicitations offering free antibody testing. These e-mails briefly described the type of test being used, the meaning of positive and negative results, and limitations of the testing. For HCWs who may not routinely check e-mail, such as environmental service workers, department managers were engaged to encourage participation. Testing was offered at 7 strategically located sites (6 at hospitals and 1 at a large outlying clinic) to provide access to the geographically dispersed workforce.
At the time of registration for the test, HCWs completed a survey describing use of personal protective equipment (PPE) and possible exposure to COVID-19 inside and outside the workplace. If more than 1 survey was completed, only the last survey was included for analysis. These data were combined with employee demographic data. To assess community exposure to COVID-19, we used data from the Georgia Department of Public Health to calculate COVID-19 cumulative incidence by ZIP code and week. As a measure of exposure in their community, we assigned each participant the COVID-19 cumulative incidence in their ZIP code of residence 2 weeks before their test date to account for the lag from the time of infection to seroconversion (Supplement Figure).
The serologic test used to analyze participant samples is an enzyme-linked immunosorbent assay developed at the Emory Medical Laboratory that measures IgG antibody to the receptor binding domain of the SARS-CoV-2 spike protein. This test has received emergency use authorization by the US Food and Drug Administration and has an estimated sensitivity of 97.5% and specificity of 98.0% (9). We estimated both the crude seroprevalence of SARS-CoV-2 among the HCWs in the study as well as an adjusted estimate that accounted for imperfect sensitivity and specificity of the assay.
Statistical Analysis
We fit a logistic regression model to estimate adjusted odds ratios (aORs) between potential risk factors and SARS-CoV-2 seropositivity. Model predictors (Supplement Table 1) included demographic characteristics (age group, sex, race, ethnicity), community exposure (cumulative incidence of COVID-19 by residential ZIP code 2 weeks before testing date; contact with confirmed or suspected COVID-19 cases outside the workplace), and occupational factors (workplace role and location; contact with patients and staff who were COVID-19 positive; use of PPE). Workplace location was self-reported and described the locations where a HCW had spent the most time during the COVID-19 pandemic. Because more than one third (36.4%) of HCWs reported spending equal time in more than 1 location, the workplace location variable was structured as a hierarchy. We ranked locations on the basis of the anticipated risk for encounters with COVID-19–positive patients (Supplement Table 1); locations with a high encounter risk, such as the emergency department and COVID-19–focused units, were ranked highest and those with no patient contact and working from home were ranked lowest. We categorized HCWs into the highest-ranking location where they reported working. To assess associations between a predictor and SARS-CoV-2 positivity, we privileged the magnitude of the point estimate over the bounds of the CIs (that is, we did not rely exclusively on null hypothesis significance testing).
To account for potential selective participation, we used inverse probability of participation weighting to assess whether our results were sensitive to differences between employees who were tested and all employees by age, race, and sex. We used the joint distribution of the age, race, and sex of all employees to weight individuals in the regression analysis, such that demographic groups who were overrepresented among survey participants compared with all employees were downweighted, and vice versa. We calculated aORs by using the weighted data and 95% CIs accounting for the weights.
Two sensitivity analyses were conducted with the aim of accounting for selective participation related to community exposure to COVID-19. In the first, we incorporated cumulative incidence of COVID-19 by residential ZIP code into the inverse probability of participation weighting (in addition to age, race, and sex) and weighted our regression model as described above. In the second, we applied our regression model described above to the subset of participants who reported no known community exposure to COVID-19.
Analyses were conducted in R, version 4.0.2, by using the survey, epiR, boot and zipcode packages. The code for this analysis is available at https://github.com/lopmanlab/emory-hcw-serosurvey.
Informed consent was not obtained from study participants because the serologic testing was initially intended as a quality improvement activity within Emory Healthcare and posed no more than minimal risk to participants. Participants were informed of their test results, and deidentified data were analyzed. This study was approved by the Emory University Institutional Review Board (protocol 00001079).
Role of the Funding Source
This work was funded by the Emory COVID-19 Response Collaborative (Drs. Nelson and Lopman); a National Science Foundation RAPID award (Dr. Lopman); and the National Institute of General Medical Sciences, National Institutes of Health. Dr. Baker received support from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Dr. Roback received funding from The Marcus Foundation. The funding sources had no role in the design or conduct of the trial, interpretation of the data, or preparation of the manuscript.
Results
Among 10 275 participating HCWs (35% of the workforce), the crude SARS-CoV-2 seroprevalence was 5.7% (95% CI, 5.2% to 6.1%) (n = 582). Adjusting for the imperfect sensitivity and specificity of the serology test produced an estimated seroprevalence of 3.8% (CI, 3.4% to 4.3%). A total of 665 participants were missing ZIP code data, leaving 9610 participants available for further analysis.
The majority (71%) of participants were tested in May 2020 (Supplement Table 2). More than three quarters of participants were female (77.6%), with female and male participants having similar unadjusted seropositivity (5.7% and 5.9%, respectively). Black HCWs were notably underrepresented in the serologic survey, comprising 30% of those who volunteered for antibody testing and 49% of the workforce. Prior confirmed COVID-19 infection was reported for 133 participants (1.4%). Forty-four percent (245 of 555) of seropositive HCWs reported no fever or COVID-19–like symptoms since 1 February 2020.
Contact with a person known or suspected to have COVID-19 outside the workplace (aOR, 1.9 [CI,1.4 to 2.6]) and race were most strongly associated with seropositivity (Table). Black (aOR, 2.1 [CI, 1.7 to 2.6]) and multiracial (aOR,1.7 [CI, 0.8 to 3.6]) HCWs had higher odds of infection than White HCWs. Higher residential ZIP code incidence of COVID-19 (aOR, 1.5 [CI, 1.0 to 2.2]) was also associated with seropositivity.
Table. Association of Demographic, Community, and Occupational Risk Factors With Seropositivity in Unadjusted and Adjusted Analyses.
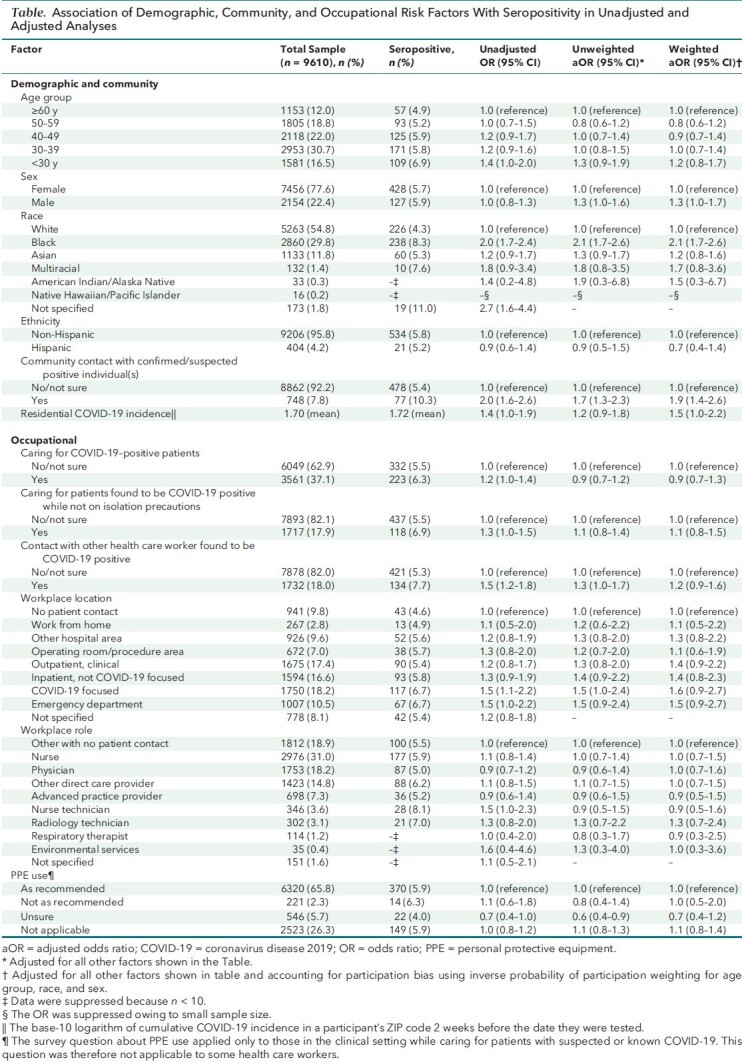
In the workplace, participants who reported close contact with a COVID-19–positive HCW had increased odds of seropositivity (aOR, 1.2 [CI, 0.9 to 1.6]). Although HCWs who reported caring for patients with COVID-19 did not have increased odds of seropositivity (aOR, 0.9 [CI, 0.7 to 1.3]), working in clinical locations, such as inpatient non–COVID-19–focused areas (aOR, 1.4 [CI, 0.8 to 2.3]), the emergency department (aOR, 1.5 [CI, 0.9 to 2.7]), or COVID-19–focused units (aOR, 1.6 [CI, 0.9 to 2.7]), was associated with higher odds of seropositivity. Respiratory therapists (aOR, 0.9 [CI, 0.3 to 2.5]) and those who work in the operating room or procedure areas (aOR, 1.1 [CI, 0.6 to 1.9]) were not at increased risk for being seropositive. Differences in seropositivity based on HCW role were generally present in the unadjusted but not the adjusted models. Accounting for participation bias did not result in appreciably different estimated associations (Table).
The sensitivity analysis in which cumulative incidence of COVID-19 by residential ZIP code was incorporated into the inverse probability of participation weighting resulted in little change in point estimates compared with the primary analysis, with 3 notable exceptions: 1) a decrease in the aOR for HCWs in COVID-19–focused areas (aOR, 1.2 [CI, 0.7 to 2.3]); 2) a decrease in the aOR for HCWs in the emergency department (aOR, 1.1 [CI, 0.6 to 2.1]); and 3) an increase in the aOR for HCWs in environmental services, though with a wide CI (aOR, 2.5 [CI, 0.6 to 9.5]). In contrast, the sensitivity analysis restricting the model to those with no known exposure to COVID-19 in the community resulted in an increase in the aOR for HCWs in COVID-19–focused areas (aOR, 1.9 [CI, 1.1 to 3.3]) and the emergency department (aOR, 1.8 [CI, 1.0 to 3.2]).
Discussion
In this large serologic testing effort of U.S. HCWs in a university-based health care system, we found an adjusted overall SARS-CoV-2 seroprevalence of 3.8% (CI, 3.4% to 4.3%) after the initial surge of the epidemic. This rate is lower than the overall 6.0% seroprevalence reported from 13 U.S. academic medical centers and another study reporting 14% seroprevalence among HCWs in New York City, neither of which adjusted for test specificity (1, 4). The percentage of seropositive HCWs who reported no past COVID-19–like illness was also similar to past estimates of asymptomatic SARS-CoV-2 infections (1).
We found that community and demographic factors-—contact with a confirmed or suspected COVID-19–positive case and Black race—were generally stronger predictors of seropositivity than occupational factors. Notably, racial disparities, now well documented in the general population (10–12), persist in HCWs (1, 13) after accounting for other risk factors including job role, underscoring the fundamental racial inequities that have become a hallmark of the COVID-19 pandemic. We partially adjusted for community risk by including ZIP code–level COVID-19 incidence in our model, but we cannot account for more proximal factors that may have contributed to higher risk for infection among Black HCWs, including higher likelihood of exposure at home or use of public transportation. Ultimately, these structural risk factors are tied to entrenched, systemic social processes that underlie many individual and population health disparities (14, 15).
We found few strong risk factors for infection in the workplace. Persons who reported caring for patients with COVID-19 and those working in procedure areas where aerosol-generating procedures are routine were not more likely to be seropositive, supporting the efficacy of PPE practices in caring for patients known to have COVID-19. Working in clinical areas was associated with increased odds of seropositivity, although the aORs were imprecise. We were unable to determine whether risk associated with workplace location was specifically from patient exposure, including contact with unsuspected COVID-19 patients, or from coworkers. Risk from contact with HCWs later found to be COVID-19 positive could reflect transmission during presymptomatic or asymptomatic infection before universal use of masks, or transmission in settings where masks were not worn. In our hospital system, contact tracing identified staff eating together in break rooms as a risk factor for transmission. Of note, the workplace factors included in our model were limited to those available from the survey questionnaire. Misclassification and excluding unknown or unmeasured exposures may bias our estimates of the association between workplace factors and SARS-CoV-2 seropositivity, though it is difficult to know the direction of this bias.
Although there appeared to be a difference in seropositivity based on workplace role in the unadjusted models, aORs were similar for all workplace roles in the adjusted model, highlighting the importance of considering demographic factors in assessing seroprevalence risk. Other studies that did not control for demographic or other risk factors outside of the workplace have reported different seroprevalence rates depending on job role (4, 5); these results need to be interpreted with caution. Although most of the risk may originate from the community, workplace risk cannot be ignored. In this cross-sectional analysis including time periods before and after universal masking, we were unable to assess the effect of infection prevention policies in reducing infection risk among HCWs. Going forward, studies should investigate the role of specific exposures contributing to infection risk, including risk from HCW-to-HCW exposure, and the efficacy of interventions to prevent transmission. It is critical to know whether the current level of interventions, including screening for asymptomatic viral shedding, PPE practices, and efforts to prevent HCW-to-HCW transmission, have substantially reduced or eliminated the workplace risk identified in this and other studies.
Our study has limitations. First, serologic testing was voluntary, which may introduce bias if groups more likely to participate were also more (or less) likely to be seropositive. We partially adjusted for participation using demographic characteristics of HCWs overall. This adjustment at least partially accounts for poor representation of Black HCWs, in whom seroprevalence was higher than the population average, among those who volunteered for testing. However, other factors related to infection risk may have influenced participation. Community incidence of COVID-19 is one factor in particular that might influence participation; we attempted to adjust for this potential bias within the constraints of our data (Supplement Tables 3 and 4) but obtained inconsistent results. Relatedly, the low response rate for the survey may bias the results and limit their generalizability to the target population of HCWs.
Second, a test with imperfect specificity in a population where seroprevalence is low will result in some false-positive findings. We accounted for test specificity in our seroprevelance estimate, but not in the multivariate analysis. Because we do not expect specificity to vary by any of the exposures we examined, this may be expected to bias our estimated aORs toward the null.
Third, we could not account for rapidly evolving infection prevention practices early in the pandemic and social behavior inside or outside the workplace. Fourth, although there may have been differences in risk factors by facility, we could not adjust for this factor because of the large number (99) of locations represented. However, infection prevention policies for SARS-CoV-2 were typically implemented at the health system level, and their effects are expected to be largely consistent across locations.
Finally, the large influx of patients with COVID-19 caused major shifts in care delivery and personnel deployment. Many HCWs worked in multiple locations and even different roles, making classification of these factors in our analysis challenging. Our results may not be generalizable to HCWs in different settings or in different stages of the pandemic.
In conclusion, using a model incorporating demographic, community, and occupational risk factors for infection, we quantified community and occupational risk for SARS-CoV-2 seropositivity in HCWs. We found that the largest predictors of risk related to community exposure; ongoing efforts to keep the health care workforce safe should emphasize risk mitigation in and outside the workplace. After adjustment for many community and occupational risk factors, race remains a critical marker of infection risk. Future seroprevalence studies of HCWs need to account for these community and demographic factors.
Supplementary Material
Footnotes
This article was published at Annals.org on 29 January 2021.
* Drs. Baker and Nelson contributed equally.
References
- 1. Self WH , Tenforde MW , Stubblefield WB , et al; CDC COVID-19 Response Team. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network—13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221-1226. [PMID: ] doi: 10.15585/mmwr.mm6935e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kluytmans-van den Bergh MFQ, Buiting AGM, Pas SD, et al.. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3:e209673. [PMID: ] doi: 10.1001/jamanetworkopen.2020.9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ran L , Chen X , Wang Y , et al. Risk factors of healthcare workers with coronavirus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020;71:2218-2221. [PMID: ] doi: 10.1093/cid/ciaa287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moscola J , Sembajwe G , Jarrett M , et al; Northwell Health COVID-19 Research Consortium. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324:893-895. [PMID: ] doi: 10.1001/jama.2020.14765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iversen K , Bundgaard H , Hasselbalch RB , et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401-1408. [PMID: ] doi: 10.1016/S1473-3099(20)30589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai X , Wang M , Qin C , et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3:e209666. [PMID: ] doi: 10.1001/jamanetworkopen.2020.9666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steensels D , Oris E , Coninx L , et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195-197. [PMID: ] doi: 10.1001/jama.2020.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leeds JS , Raviprakash V , Jacques T , et al. Risk factors for detection of SARS-CoV-2 in healthcare workers during April 2020 in a UK hospital testing programme. EClinicalMedicine. 2020;26:100513. [PMID: ] doi: 10.1016/j.eclinm.2020.100513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suthar MS , Zimmerman MG , Kauffman RC , et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020;1:100040. [PMID: ] doi: 10.1016/j.xcrm.2020.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore JT , Ricaldi JN , Rose CE , et al; COVID-19 State, Tribal, Local, and Territorial Response Team. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5-18, 2020—22 states, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1122-1126. [PMID: ] doi: 10.15585/mmwr.mm6933e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adhikari S , Pantaleo NP , Feldman JM , et al. Assessment of community-level disparities in coronavirus disease 2019 (COVID-19) infections and deaths in large US metropolitan areas. JAMA Netw Open. 2020;3:e2016938. [PMID: ] doi: 10.1001/jamanetworkopen.2020.16938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Webb Hooper M, Nápoles AM, Pérez-Stable EJ.. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466-2467. [PMID: ] doi: 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nguyen LH , Drew DA , Graham MS , et al; COronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475-e483. [PMID: ] doi: 10.1016/S2468-2667(20)30164-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arah OA . On the relationship between individual and population health. Med Health Care Philos. 2009;12:235-44. [PMID: ] doi: 10.1007/s11019-008-9173-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annual Review of Sociology. 2015;41:311-30. doi:10.1146/annurev-soc-073014-112305
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


