Abstract
Objective
The emergence of a novel coronavirus, SARS-CoV-2, and its subsequent spread outside of Wuhan, China, led to the human society experiencing a pandemic of coronavirus disease 2019 (COVID-19). While the development of vaccines and pharmaceutical treatments are ongoing, government authorities in China have implemented unprecedented non-pharmaceutical interventions as primary barriers to curb the spread of the deadly SARS-CoV-2 virus. Although the decline of COVID-19 cases coincided with the implementation of such interventions, we searched for evidence to demonstrate the efficacy of these interventions, since artifactual factors, such as the environment, the pathogen itself, and the phases of epidemic, may also alter the patterns of case development.
Methods
We surveyed common viral respiratory infections that have a similar pattern of transmission, tropism, and clinical manifestation, as COVID-19 under a series of non-pharmaceutical interventions during the current pandemic season. We then compared this data with historical data from previous seasons without such interventions.
Results
Our survey showed that the rates of common respiratory infections, such as influenza and respiratory syncytial virus infections, decreased dramatically from 13.7% (95% CI, 10.82–16.58) and 4.64% (95% CI, 2.88–7.64) in previous years to 0.73% (95% CI, 0.02–1.44) and 0.0%, respectively, in the current season.
Conclusions
Our surveillance provides compelling evidence that non-pharmaceutical interventions are cost-effective ways to curb the spread of contagious agents, and may represent the only practical approach to limit the evolving epidemic until specific vaccines and pharmaceutical treatments are available.
Keywords: SARS-CoV-2, COVID-19, Transmission, Intervention, Respiratory infection
Introduction
The outbreak of novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)-induced disease, COVID-19, spread rapidly from Wuhan, China, in December 2019. This led to China experiencing a major public health emergency with over 83,000 confirmed cases and 4634 deaths as of June 2020 (Rai et al., 2020). Although there are a few newly developed vaccines and treatments, it is conceivable that without some impact on transmission, the virus will continue to circulate, infect, and cause serious disease, in certain segments of the unvaccinated population. This is because variants of SARS-CoV-2 will continue to evolve, even as the majority of the population receive vaccinations. A variety of non-pharmaceutical public health interventions have been employed to stop the surge of COVID-19 cases. These interventions include personal and environmental hygiene, social distancing, travel restrictions, quarantine, and case isolation. The aim of these interventions is to reduce the reproduction number (Rt) of the infection, a fundamental epidemiological quantity that represents the average number of infections generated by each infected case over the course of the infection (t). Approximately one month after the implementation of these interventions, there was a notable downward trend in the number of confirmed new cases. Two months after the implementation of these interventions, there were zero new confirmed indigenous cases in Wuhan (by March 19).
The decline in COVID-19 cases suggests that the implementation of these interventions is potentially effective. European countries, such as the UK have implemented measures that include national lockdowns, border closures, school closures, and international travel bans; these have reduced disease transmission and delayed the epidemic (Flaxman et al., 2020, De Lusignan et al., 2020, Jarvis et al., 2020). Although the spatial spread of infectious diseases has been intensively studied, the efficacy of non-pharmaceutical interventions in preventing the spread of infections remains uncertain. Wuhan’s travel ban was associated with the delayed arrival of COVID-19 in other cities by an estimated 2.91 day; cities that implemented preemptive control measures reported fewer cases (Tian et al., 2020) Cities that suspended intra-city public transport, closed entertainment venues, and banned public gatherings, recorded fewer cases during the first week of the outbreak (Tian et al., 2020). These observations suggested that the interventions were strongly associated with, although not necessarily the cause of, a delay in epidemic growth and a reduction in case numbers during the peak COVID-19 epidemic in China. Artifactual factors, such as the environment, the pathogen itself, and the phases of the epidemic, might also alter the pattern of the epidemic (Pica and Bouvier, 2012, Tang, 2009). At present, there is little evidence to describe the impact of non-pharmaceutical interventions on SARS-CoV-2 transmission. Influenza and respiratory syncytial virus (RSV) are common viral pathogens that cause seasonal respiratory tract infections. Both of these pathogens have transmission pathways, tropism, and clinical manifestations, that are similar to COVID-19 (Chan et al., 2020a, Chan et al., 2020b, Li et al., 2020, Liu et al., 2020). The impact of these interventions on influenza- and RSV-induced acute respiratory infections during the COVID-19 pandemic can act as a surrogate to evaluate their efficacy on SARS-CoV-2 transmission (Ferretti et al., 2020, Cowling et al., 2020).
Population density has an impact on the transmission of pathogens. A previous report described substantial geographical variability in the prevalence of COVID-19, with a higher number of reported cases around densely populated city centers, and lower numbers in less populated coastal areas (Pollán et al., 2020). High population density catalyzes the spread of COVID-19 and has a significant impact on the R0, as this increases the rate of contact (Rocklöv and Sjödin, 2020). One of the key predictions is that the size of the epidemic will increase strongly (and in a non-linear way) with the initial density of a susceptible population (Li et al., 2018). Chengdu is the capital city of Sichuan province located in south-western China with a population of 87.25 million, and is relatively distanced from the epicenter of COVID-19. The greater Chengdu area (including Chengdu and its nearby cities) is one of the most densely populated areas in China; some areas in this region are even overcrowded. The local lifestyle involves social gatherings and entertaining in closed-spaces in teahouses and Mahjong-houses, can easily accelerate the transmission of the pathogen. In addition, a typical subtropical climate provides Chengdu with a unique pattern of transmission for respiratory pathogens (Feng et al., 2012). During the early phase of the pandemic, the number of COVID-19 cases rose quickly but decreased dramatically when interventions were implemented. Nevertheless, there were only 143 confirmed cases and 3 deaths in this area prior to 29 February 2020 (Si et al., 2020). Geographical and population characteristics render Chengdu as an ideal social and environment model for the evaluation of non-pharmaceutical interventions on pathogen transmission. We surveyed influenza and RSV infections under the interventions imposed during the COVID-19 pandemic and compared our data with published surveillance data during the same season from previous years without such interventions. Our study offers a straightforward and reliable evaluation of the overall efficacy of these non-pharmaceutical interventions.
Material and methods
Patient recruitment and sample collection
We recruited all patients who visited the West China Hospital between December 2019 and March 2020 with a fever (≥37.5 °C) and respiratory symptoms. Participation was voluntary and all patients provided written and informed consent. All subjects who were mentally incompetent, or unable to read or write, were assisted by witnesses, parents, or legal guardians. The Institutional Review Board of the West China Hospital approved this study (Clinical trial (device) 2020, No. 4). Oropharyngeal swab (Nanxin Medical Instruments, Guangzhou, China) specimens were collected from each patient following a standard procedure during consultation and were placed immediately into sterile tubes (Nanxin Medical Instruments, Guangzhou) with 3 ml of preservation medium containing Hanks Balanced Salt Solution (HBSS), heat-inactivated fetal bovine serum (FBS), Gentamicin sulfate, and Amphotericin B; samples were then stored at 4 °C and tested within 8 h from the time of collection.
Detection of viral pathogens
Specimens were inactivated in a water bath at 56 °C for 30 min and nucleic acid was extracted from a 200 μl aliquot of sample preparation using TRIZOL (Invitrogen, CA) following a procedure that had been optimized for the in vitro detection of respiratory pathogens. The extracted nucleic acid was quantified, mixed with isothermal amplification buffer, loaded on a microfluidic chip provided in a Respiratory Virus Nucleic Acid Detection Kit (CapitalBio Technology, Chengdu), amplified and analyzed with a RTisochip™ –W Isothermal Microfluidic Chip Analyzer according to the manufacturer’s instructions. This high throughput parallel amplification and detection method can identify the target genes of SARS-CoV-2, influenza A (IVA, including its subtype H1N1 and H3N2), influenza B (IVB), and respiratory syncytial virus (RSV) in one step.
Statistical methods
SPSS 21.0 was used to analyze pathogen prevalence with 95% confidence intervals. The Chi-squared test and Fisher’s exact test were also used for comparisons between groups. The test level was α = 0.05, and P < 0.05 was considered to be statistically significant.
Results
The prevalence of viral respiratory infections
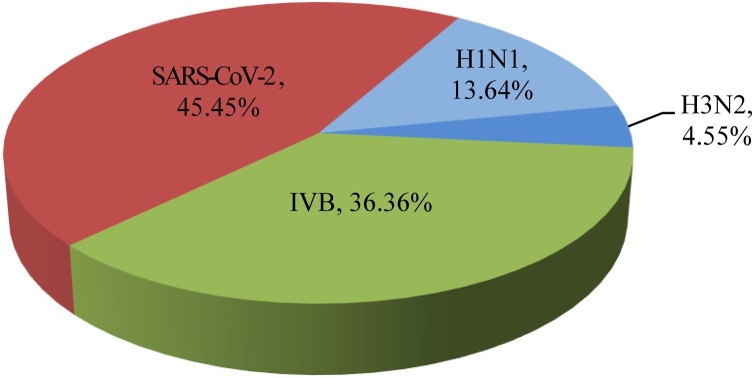
Of the 546 swab specimens collected between December 2019 and March 2020, 22 were positive for viral pathogens. The positive rate was 4.03% (95% CI: 2.38–5.68). Of these 22 positive specimens, four were positive for IVA (0.73%; 95% CI: 0.02–1.44), eight were positive for IVB (1.47%; 95% CI: 0.47–1.47), zero were positive for RSV, and ten were positive for SARS-CoV-2 (1.83%; 95% CI: 0.71–2.94). Of the IVA infections, three were positive for H1N1 (0.55%; 95% CI: −0.07–1.17) and one was positive for H3N2 (0.18%; 95% CI: −0.18–0.54). When compared to the surveys conducted in 2006–2016 (Zhou et al., 2019) and 2016–2018 (Xiang et al., 2019, Zhang et al., 2017), the total infection rate, and the infection rates for IVA and IVB, including subtypes, had decreased dramatically. When compared to the survey in 2010–2011, the infection rate for RSV infection had also decreased (Table 1 ). In our present survey, SARS-CoV-2 was responsible for a larger proportion of infections than influenza A and influenza B, although there was no statistically significant difference when comparing the frequencies of the pathogens detected (χ2 = 2.649, P > 0.05, Figure 1 ).
Table 1.
Prevalence of viral respiratory pathogens.
| Surveillance period | Specimen, (No.) | Positive, (No.) | Frequency, (%) | No. (%) Frequency of pathogens positives |
||||
|---|---|---|---|---|---|---|---|---|
| IVA |
IVB | RSV | SARS-CoV-2 | |||||
| H1N1 | H3N2 | |||||||
| December 2019 to March 2020 | 546 | 22 | 4.03 | 3 (0.55) | 1 (0.18) | 8 (1.47) | 0 (0.0) | 10 (1.83) |
| May 2006 to April 2016 (Zhou et al., 2019)* | 10,981 | 2516 | 22.91 | 862 (7.85)a | 803 (7.30) | 736 (6.70) | NA | NA |
| January 2016 to December 2018 (Xiang et al., 2019)* | 925 | 127 | 13.93 | 38 (4.11) | 37 (4.22) | 50 (5.41) | NA | NA |
| July 2010 to April 2011 (Pan et al., 2011)* | 302 | 72 | 23.84 | NA | NA | NA | 14 (4.64) | NA |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
Surveillance data quoted from references.
H1N1 data were from survey of October 2009 to April 2016.
Figure 1.
The proportions of pathogens in samples that were positive for respiratory viruses. Pie chart showing the proportions of each pathogen in the 22 positive samples. The subtypes of influenza A (H1N1 and H3N2) are shown individually.
The prevalence of viral respiratory infections across different age groups
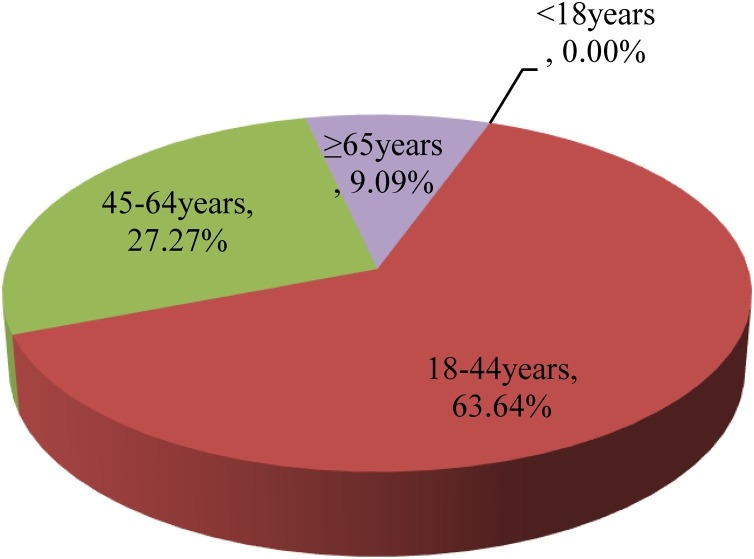
We classified specimens into four groups based on age. Group <18 represents a population of subjects who were pre-school and school age; Group 18–45 represents a population of working adults with the largest extent of work-load and family duties; Group 45−64 represents those with stable and secure work; and Group ≥65 represents the most senior population of retirees. We did not detect viral respiratory pathogens in group <18. Group 18–44 had the highest rate of infections (5.19%) and accounted for 63.64% of all viral-positive patients; this was followed by group 45−64 which had a 3.92% infection rate and accounted for 27.27% of all viral-positive patients. The ≥65 group had a 1.94% infection rate and accounted for 9.09% of all viral-positive patients (Table 2 and Figure 2 ). When considering the different pathogens, group 18−44 were positive for IVB (2.96%) and SARS-CoV-2 (2.22%); group 45−64 were positive for IVA (2.96%, all H1N1) and SARS-CoV-2 (1.96%); and group ≥65 was positive for IVA (2.96%, including H1N1 0.97% and H3N2 0.97%) and SARS-CoV-2 (0.97%). All groups were positive for SARS-CoV-2, although the types of influenza were slightly different, except for group <18. There was no statistical difference in terms of the infection rates when comparing the age groups above 18 years (χ2 = 1.994, P > 0.05).
Table 2.
Prevalence of viral respiratory pathogens in age groups.
| Age group | Specimen, (No.) | Positive, (No.) | Frequency, (%) | No. (%) Frequency of pathogens positives |
||||
|---|---|---|---|---|---|---|---|---|
| IVA |
||||||||
| H1N1 | H3N2 | IVB | RSV | SARS-CoV-2 | ||||
| <18 | 20 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0 |
| 18−44 | 270 | 14 | 5.19 | 0 | 0 | 8 (2.96) | 0 | 6 (2.22) |
| 45−64 | 153 | 6 | 3.92 | 3 (1.96) | 0 | 0 | 0 | 3 (1.96) |
| ≥65 | 103 | 2 | 1.94 | 0 | 1 (0.97) | 0 | 0 | 1 (0.97) |
Figure 2.
The proportions of viral-positive patients in different age groups. Pie chart showing the proportion of the 22 positive patients across each age group.
The prevalence of viral respiratory infections by gender
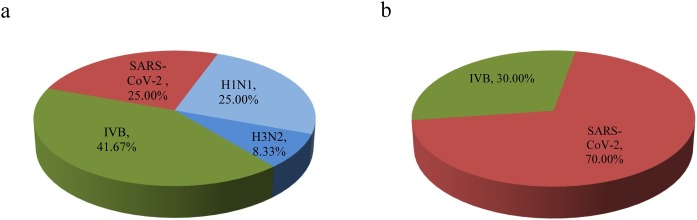
Our specimen pool consisted of 297 samples from males and 249 samples from females. We found that both genders had similar positive rates for viral pathogens (4.04% male, 95% CI: 1.81–6.27; 4.02% female, 95% CI: 1.59–6.45; χ2 = 0.000, P > 0.05). Of those infected, males showed a greater diversity of pathogens than females, including 33.33% IVA (25.00% H1N1 and 8.33% H3N2), 41.67% IVB, and 25.00% SARS-CoV-2; this compared to 30.00% IVB and 70.00% SARS-CoV-2 in females (Table 3 and Figure 3 ).There were no significant differences among the pathogens detected in male and female patients (male, χ2 = 0.507, P > 0.05; female, χ2 = 1.633, P > 0.05).
Table 3.
Prevalence of viral respiratory pathogens in gender groups.
| Sex | Specimen, (No.) | Positive, (No.) | Frequency, (%) | No. (%) Frequency of pathogens positives |
||||
|---|---|---|---|---|---|---|---|---|
| IVA |
||||||||
| H1N1 | H3N2 | IVB | RSV | SARS-CoV-2 | ||||
| Male | 297 | 12 | 4.04 (54.54) | 3 (1.01) | 1 (0.34) | 5 (1.68) | 0 | 3 (1.01) |
| Female | 249 | 10 | 4.02 (45.45) | 0 | 0 | 3 (1.20) | 0 | 7 (2.81) |
Figure 3.
The proportions of respiratory viral pathogens in positive samples. Pie chart a and b show the proportion of each pathogen in the 12 positive male samples and 10 positive female samples respectively. The subtypes of influenza A (H1N1 and H3N2) are shown individually.
Discussion
Our survey revealed a dramatic decline in the prevalence of influenzas and RSV during the COVID-19 pandemic under the implementation of interventions when compared with data from previous seasons without these interventions (Pan et al., 2011). Similar declines in viral respiratory infections were also observed during the SARS epidemic in 2003 (Lo et al., 2005), and in the prevalence of influenza during the 2019–2020 season in Hong Kong, as based on published surveillance data (Chan et al., 2020a, Chan et al., 2020b).
The decline in the number of cases of common viral respiratory infections is a consequence of public health interventions implemented during the COVID-19 pandemic. Previous surveillance of viral respiratory infections has shown that the rate of influenza infections remained consistent during epidemic seasons from 2010 to 2015 in the greater Chengdu area (Zhang et al., 2017, Yang et al., 2011, Cao et al., 2014). Other surveys reported a similar epidemic of influenza in the Mianyang area nearby Chengdu between 2010–2018 (Wen et al., 2020). The implementation of quarantine and case isolation led to the locking down of etiological sources. Restrictions on transportation, particularly long-distance travel, prevented the spread of the pathogen from the epicenter to naïve populations. This, in turn, reduced the formation of new epicenters. Personal and environmental hygiene, social distancing, and the closure of public facilities reduced personal contact with potential pathogen-carriers, limited virus transmission, constrained viral reproduction, and prevented the infection of new hosts. All of these measures created different layers of a barrier to hamper the chain of virus transmission, not only for SARS-CoV-2 but also for other respiratory pathogens. Our survey provides, to the best of our knowledge, direct evidence for the first time that these non-pharmaceutical interventions are the most cost-effective way to halt transmission, reduce the Rt value, and control the epidemic of contagious diseases before or even after a vaccine and specific treatments are available.
Our survey suggests that the contraction and transmission of pathogens is highly associated with social activity. People who are 18–44 years-of-age are the most active socially, and responsible for the largest extent of workload and family duties; consequently, these groups are associated with a higher infection rate than the other groups. Subjects of pre- and school age experienced restricted social activity because of school closures, stay-home orders, and online learning. Senior subjects experienced reduced levels of social activity due to limited public transportation, and the stress of underlying health conditions. This is in line with other reports stating that people with higher rates of social contact are more likely to contract infections (Mossong et al., 2008). Males and females showed no differences in the rates of viral infections in our survey; this concurred with previous reports (Pollán et al., 2020, Huang et al., 2020). However, males may experience more social activity and personal contacts; consequently, males showed more diversity in terms of pathogenic infection when compared to females. Male patients had been equally positive for infections arising from IVA (including both H1N1, N3N2), IVB, and SARS-CoV-2. In contrast, female patients were only positive for IVB and SARS-CoV-2. Thus, social distancing is one of the key measures with which to reduce the transmission of pathogens.
Herd immunity is a strategy that is commonly used to halt the transmission of pathogens during the early phase and to provide protection to individuals, such as infants and seniors, who respond poorly to immunization or cannot receive vaccine inoculation for various reasons. Herd immunity can be reached when about 70–90% of a population becomes immune to a pathogen either through infection and recovery or by vaccination. When herd immunity is reached, the pathogen is less likely to spread to naïve people who are not immune because there are simply not enough carriers or hosts for replication. However, with such high levels of mortality, herd immunity against the SARS-CoV-2 cannot be reasonably anticipated without a significant number of deaths, even if herd immunity can be established. In the case of influenza, we did not observe significant levels of herd immunity. Our data show that IVA and IVB had almost the same prevalence as SARS-CoV-2 under the same non-pharmaceutical interventions, although both types of influenza are seasonal and circulate annually, and there are vaccines available. Prior to being proven effective, relying on the establishment of herd immunity through an excessive number of infections could be risky. It is still unclear whether COVID-19 survivors have immunity, and a third of infections determined by serology were reported to be asymptomatic (Pica and Bouvier, 2012). Data has shown that 40% of asymptomatic and 13% of symptomatic patients had undetectable levels of antibodies just 8 weeks after recovery (Long et al., 2020). Therefore, keeping up with non-pharmaceutical interventions is necessary until a vaccine and specific treatments become available, or herd immunity is proven to be effective.
Our study has several limitations that need to be considered. Under the stress of the pandemic, patients might self-medicate at home, thus reducing the overall number of outpatients and emergency visits. However, those visiting the hospital were more likely to be severely affected patients. In addition, we only surveyed a small proportion of viral respiratory infections, including IVA (including H1N1 and H3N2 subtype), IVB, RSV, and SARS-CoV-2 infection. The prevalence of other common respiratory infections, not only viral but also bacterial, also needs to be estimated in order to acquire a full spectrum of common respiratory infections under the implementation of non-pharmaceutical intervention. Furthermore, the assays used for historical data and the current survey are slightly different, although the procedure for sample collection was standardized. These factors may exert some impact when comparing our data to previous surveys, although we believe that our isothermal amplification chip assay for viral pathogens is more sensitive than the assays used in previous publications.
In conclusion, non-pharmaceutical interventions represent a cost-effective approach to curb the spread of contagious agents and may be the only practical approach to limit the evolving epidemic of infections until vaccines and pharmaceutical treatments become widely available.
Conflict of interest
CapitalBio Technology is the manufacture of Respiratory Virus Nucleic Acid Detection Kit and RTisochip™ –W Isothermal Microfluidic Chip Analyzer. XX and DL are employees of CapitalBio Technology. All other authors have no competing interests
Funding
This study was supported by West China Hospital (HX-2019-nCoV-017), Sichuan Science and Technology Program (2020YFS0008), and the National Natural Science Foundation (81671551).
Individual contributions
DL and JL contributed to study concept and design; LC coordinated the recruitment of volunteers; QY, XX, XG, TC, and CH contributed to data acquisition; QY and JL contributed to data analysis; QY, JL, and DL contributed to initial drafting of the manuscript. All authors contributed to data interpretation and critically revised the manuscript.
Ethics committee approval
This study received appropriate approval from an ethics committee: Clinical trial (device) 2020, No. 4; Clinical Trial Committee, West China Hospital of Sichuan University.
Acknowledgments
We thank Chengdu CapitalBio Medical Laboratory and Department of Clinical Research, West China Hospital, West China School of Medicine, Sichuan University for their invaluable contribution to this study.
References
- Cao W.P., Zhang Y.L., Yue Y. Influenza monitoring in a sentinel hospital of Chengdu city from 2011 to 2013. J Tro Dis Paras. 2014;12(03):146–148. doi: 10.3969/j.issn.1672-2302.2014.03.007. [DOI] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Lee P.W., Chan C.Y., Lam K.B.H., Ho P.L. Monitoring respiratory infections in covid-19 epidemics. BMJ. 2020;369:m1628. doi: 10.1136/bmj.m1628. [DOI] [PubMed] [Google Scholar]
- Cowling B.J., Perera R.A.P.M., Fang V.J., Chu D.K.W., Hui A.P.W., Yeung A.P.C. Maternal antibodies against influenza in cord blood and protection against laboratory-confirmed influenza in infants. Clin Infect Dis. 2020;71(7):1741–1748. doi: 10.1093/cid/ciz1058. 2019. [DOI] [PubMed] [Google Scholar]
- De Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20(9):1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Shay D.K., Jiang Y., Zhou H., Chen X., Zheng Y. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003–2008. Bull World Health Organ. 2012;90(4):279B–288B. doi: 10.2471/BLT.11.096958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti L., Wymant C., Kendall M., Zhao L., Nurtay A., Abeler-Dörner L. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368(6491) doi: 10.1126/science.abb6936. eabb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman S., Mishra S., Gandy A., Unwin H.J.T., Mellan T.A., Coupland H. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584(7820):257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. medRxiv. 2020;11(1):4704. doi: 10.1101/2020.04.14.20065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis C.I., Van Zandvoort K., Gimma A., Prem K., CMMID COVID-19 working group, Klepac P. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18(1):124. doi: 10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Ruiqi, Richmond Peter, Roehner Bertrand M. Effect of population density on epidemics. Physica A. 2018;510:713–724. doi: 10.1016/j.physa.2018.07.025. [DOI] [Google Scholar]
- Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo J.Y., Tsang T.H., Leung Y.H., Yeung E.Y., Wu T., Lim W.W. Respiratory infections during SARS outbreak, Hong Kong, 2003. Emerg Infect Dis. 2005;11(11):1738–1741. doi: 10.3201/eid1111.050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Mossong J., Hens N., Jit M., Beutels P., Auranen K., Mikolajczyk R. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M., Li T.S., Liu L., Yang H.P., Yin Q.J., Guo H. Viral infections of fever respiratory syndrome patients in Chengdu. J Prev Med Inf. 2011;27(11):861–864. [Google Scholar]
- Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Curr Opin Virol. 2012;2(1):90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai N.K., Ashok A., Akondi B.R. Consequences of chemical impact of disinfectants: safe preventive measures against COVID-19. Crit Rev Toxicol. 2020;50(6):513–520. doi: 10.1080/10408444.2020.1790499. [DOI] [PubMed] [Google Scholar]
- Rocklöv J., Sjödin H. High population densities catalyse the spread of COVID-19. J Travel Med. 2020;27(3) doi: 10.1093/jtm/taaa038. taaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y., Zhao Z., Chen R., Zhong H., Liu T., Wang M. Epidemiological surveillance of common respiratory viruses in patients with suspected COVID-19 in Southwest China. BMC Infect Dis. 2020;20(1):688. doi: 10.1186/s12879-020-05392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W. The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface. 2009;6 Suppl 6(Suppl 6):S737–S746. doi: 10.1098/rsif.2009.0227.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Liu Y., Li Y., Wu C.H., Chen B., Kraemer M.U.G. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368(6491):638–642. doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Chen G., Yang X.R., Wang X.J., Li Y.Q. Results of influenza surveillance in Mianyang City from 2010 to 2018. Occup Health. 2020;36(08):1075–1078. doi: 10.13329/j.cnki.zyyjk.2020.0248. [DOI] [Google Scholar]
- Xiang L.J., Gu M.Q., Xie M., Zheng L. A comparative analysis of clinical characteristics of 127 inpatients with different subtypes of influenza confirmed at a surveillance outpost hospital in Chengdu from 2016 to 2018. West China Med J. 2019;34(3):295–298. doi: 10.7507/1002-0179.201901165. [DOI] [Google Scholar]
- Yang H.P., Pan M., Huang T., Liu L., Liu L. Analysis of influenza surveillance in Sichuan from 2010–2011. Mod Prev Med. 2011;38(24):5128–5130. [Google Scholar]
- Zhang X.C., Chen Z.H., Huang W.W., Bai Y., Chen Z.H. Surveillance of influenza-like illness in Chengdu City in 2015 and its evaluation. Pract Prev Med. 2017;24(02):156–159. doi: 10.3969/j.issn.1006-3110.2017.02,008. [DOI] [Google Scholar]
- Zhou L., Yang H., Kuang Y., Li T., Xu J., Li S. Temporal patterns of influenza A subtypes and B lineages across age in a subtropical city, during pre-pandemic, pandemic, and post-pandemic seasons. BMC Infect Dis. 2019;19(1):89. doi: 10.1186/s12879-019-3807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]