Abstract
Background
In this systematic review and meta-analysis, we assessed the association between tricuspid annular plane systolic excursion (TAPSE) measured by echocardiography and mortality in coronavirus disease 2019 (COVID-19).
Methods
We performed a systematic literature search using PubMed, Embase, and Scopus databases with the keywords “COVID-19” OR “SARS-CoV-2” OR “2019-nCoV” AND “Tricuspid annular plane systolic excursion” OR “TAPSE” until January 20, 2021. The main outcome was mortality. The effect estimate was reported as the hazard ratio (HR), which was pooled from the unadjusted and adjusted effect estimates retrieved from the studies included. Mean differences in TAPSE (in mm) between non-survivors and survivors were pooled.
Results
In total, 641 patients from seven studies were included in this systematic review and meta-analysis. TAPSE was lower in non-survivors compared with survivors (mean difference = –3.74 [–5.22, –2.26], p < 0.001; I2: 85.5%, p < 0.001). Each 1 mm decrease in TAPSE was associated with increased mortality (HR = 1.24 [1.18, 1.31], p < 0.001; I2: 0.0%, p = 0.491). In the pooled adjusted model, each 1 mm decrease in TAPSE was associated with increased mortality (HR = 1.21 [1.11, 1.33], p < 0.001; I2: 45.1%, p = 0.156). Meta-regression indicated that the difference in TAPSE between non-survivors and survivors was affected by chronic obstructive pulmonary disease (–0.183, p < 0.001) and pulmonary artery systolic pressure (–0.344, p = 0.039), but not by age (p = 0.668), male gender (p = 0.821), hypertension (p = 0.101), diabetes (p = 0.603), coronary artery disease (p = 0.564), smoking (p = 0.140), and left ventricular ejection fraction (p = 0.452).
Conclusion
Every 1 mm decrease in TAPSE was associated with an increase in mortality of approximately 20%.
PROSPERO ID
CRD42021232194
Keywords: Cardiovascular, Critical care, Echocardiography, Prognosis, TAPSE
Background
Cases of coronavirus disease 2019 (COVID-19) are rising globally, and thus finding reliable predictors of poor outcomes is critical for ensuring the effective use of valuable medical resources (World Health Organization, 2021). The majority of COVID-19 cases exhibit only mild or no symptoms but a small proportion of patients experience severe symptoms and complications, including acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation (DIC), and multiorgan dysfunction (Lim et al., 2020). Some studies suggest that respiratory failure in COVID-19 differs from those in other types of ARDS considering the good tolerance of hypoxemia, preserved pulmonary system compliance, and prominent micro- and macrovascular thrombotic changes in relation to extensive endothelial injury (D’Alto et al., 2020). COVID-19 exhibits unique features but there also appear to be significant similarities in terms of the pulmonary hemodynamics and the effects on cardiac function in COVID-19 pneumonia and non-COVID-19-related ARDS (Stockenhuber et al., 2020).
Several studies have shown that individuals with underlying heart problems are associated with a higher risk of developing severe COVID-19 cases (Pranata et al., 2020a). COVID-19 is associated with myocardial injury and impaired right ventricle (RV) strain, which are independent predictors of a poor prognosis (D’Alto et al., 2020, Wibowo et al., 2021). In addition, the left ventricle (LV) function is usually relatively unaffected in most COVID-19 patients (Moody et al., 2020). Acute cor pulmonale or right heart failure (RHF) is a long-established complication of ARDS in relation to the disease severity and ventilatory strategies associated with permissive hypercapnia and pulmonary hyperinflation (Yonas et al., 2020). Tricuspid annular plane systolic excursion (TAPSE) measures the longitudinal right ventricular function (Aloia et al., 2016). Obtaining TAPSE measurements using echocardiography is simple and time-efficient, and bedside echocardiography systems are readily available in critical care units. Thus, TAPSE measurements may be valuable for diagnosing prognoses in patients with COVID-19. In the present systematic review and meta-analysis, we aimed to assess the association between TAPSE measurements obtained by echocardiography and mortality in COVID-19.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol for this study was registered in PROSPERO (CRD42021232194) (Januar et al., 2021).
Eligibility criteria
We included all studies that fulfilled all of the following criteria: 1) observational prospective/retrospective cohort and cross-sectional designs involving COVID-19 patients, 2) TAPSE data provided, and 3) mortality reported. The main outcome was mortality defined as clinically validated death or non-survivor.
We excluded studies that fulfilled at least one of the following criteria: 1) review articles, 2) commentaries, 3) case reports, 4) letters, 5) preprints, 6) conference abstracts, and 7) non-English language.
Search strategy and study selection
We performed a systematic literature search using the PubMed, Embase, and Scopus databases with keywords “COVID-19” OR “SARS-CoV-2” OR “2019-nCoV” AND “Tricuspid annular plane systolic excursion” OR “TAPSE” until January 20, 2021. The PubMed (MEDLINE) search strategy was ((COVID-19) OR (SARS-CoV-2) OR (2019-nCoV)) AND ((Tricuspid annular plane systolic excursion) OR (TAPSE)). Two authors screened the title/abstract of the records after removing duplicates. The full texts of the potentially eligible articles were assessed based on the inclusion and exclusion criteria.
Data extraction
Two authors performed independent data extraction based on the eligible studies to obtain the following data: 1) first author, 2) year of publication, 3) study design, 4) age, 5) male gender, 6) hypertension, 7) diabetes, 8) coronary artery disease (CAD), 9) smoking, 10) chronic obstructive pulmonary disease (COPD), 11) left ventricular ejection fraction (LVEF), 12) pulmonary artery systolic pressure (PASP), and 13) outcome of interest and its effect estimates.
TAPSE refers to measurement of the systolic displacement of the tricuspid lateral annulus using M-mode echocardiography (Li et al., 2020).
The main outcome was mortality. The effect estimate was reported as the hazard ratio (HR), which was pooled from the unadjusted and adjusted effect estimates retrieved from the studies included. Mean differences in TAPSE (in mm) between non-survivors and survivors were pooled. Quality assessments were performed for the studies included using the Newcastle–Ottawa Scale (NOS) by two independent authors. Discrepancies were resolved by discussion.
Statistical analysis
Statistical analyses were performed using STATA 16 (StataCorp. LLC, Texas, US). The mean differences in continuous variables were calculated and their 95% confidence intervals (95% CIs). The unadjusted and adjusted HR values were pooled using restricted-maximum likelihood (REML) random-effects meta-analysis regardless of heterogeneity. The pooled effect estimates were reported as HRs and their corresponding 95% CIs. We considered that p-values indicated statistically significant differences when p < 0.05. The I-squared (I2) and Cochrane Q tests were performed to assess inter-study heterogeneity, where I2 > 50% or p < 0.10 indicated significant heterogeneity. Possible publication bias and small-study effects were assessed using funnel-plot analysis and Egger’s test. REML random effects meta-regressions were performed to assess whether the differences in TAPSE between non-survivors and survivors were affected by age, male gender, hypertension, diabetes, CAD, COPD, smoking, LVEF, and PASP.
Results
Baseline characteristics
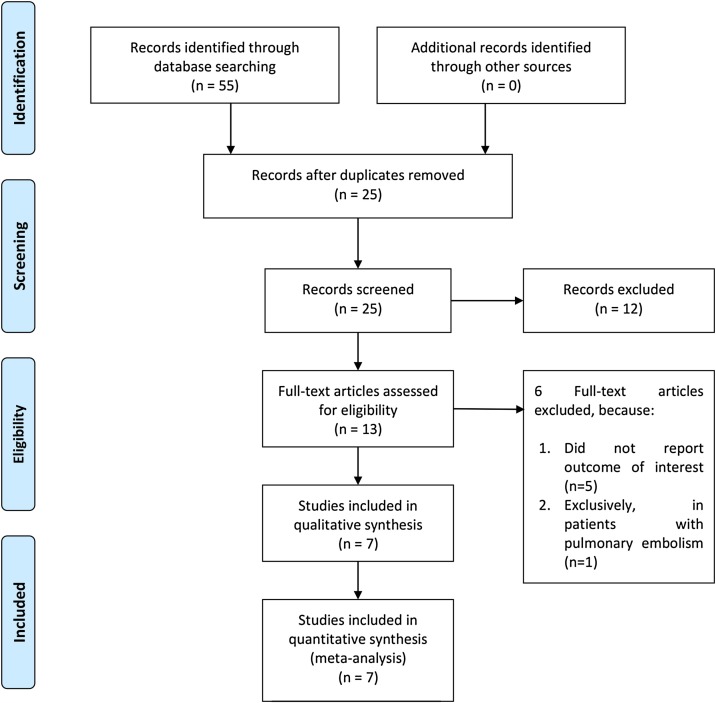
In total, 641 patients from seven studies were included in this systematic review and meta-analysis [Figure 1 , Table 1 ] (Bursi et al., 2020, D’Alto et al., 2020, Lassen et al., 2020, Li et al., 2020, Liu et al., 2020, Sattarzadeh Badkoubeh et al., 2021, Stockenhuber et al., 2020). Table 1 shows the baseline characteristics of the studies included and the risk of bias assessment using the NOS.
Figure 1.
PRISMA flowchart.
Table 1.
Baseline characteristics of the included studies.
| Authors | Design | Sample | Mean age (years) | Male (%) | Hypertension (%) | Diabetes (%) | CAD (%) | Smoking (%) | COPD (%) | LVEF (%) | PASP (mmHg) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Badkoubeh, 2020 | CS | 86 | 58.8 | 60.5 | 41.9 | 33.7 | 24.4 | 3.5 | 3.5 | 48.2 | 28.0 | 5 |
| Bursi, 2020 | RC | 49 | 65.7 | 63.3 | 49 | 18.4 | 22.4 | 20.4 | 12.2 | 53 | 33 | 7 |
| D’Alto, 2020 | PC | 94 | 63.6 | 74.5 | 67.0 | 17.0 | 18.1 | 16.0 | 29.8 | 59.5 | 33.2 | 9 |
| Lasssen, 2020 | PC | 214 | 68.9 | 54.7 | 57 | 25.5 | 15.9 | 6.2 | 15 | 57.6 | NR | 9 |
| Li, 2020 | RC | 120 | 61 | 48 | 40 | 11.7 | 9.2 | 5 | 5 | 63.4 | 31 | 8 |
| Liu, 2020 | RC | 43 | 64.5 | 51.2 | 44.2 | 27.9 | 11.63 | 37.2 | NR | 62.5 | 39.8 | 9 |
| Stockenhuber, 2020 | PC | 35 | 72 | 79 | 53 | 35 | NR | 6 | 9 | 61 | NR | 7 |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CS, cross-sectional; LVEF, left ventricular ejection fraction; NOS, Newcastle Ottawa Scale; PASP, pulmoary artery systolic pressure; PC, prospective cohort; RC, retrospective cohort.
TAPSE and mortality
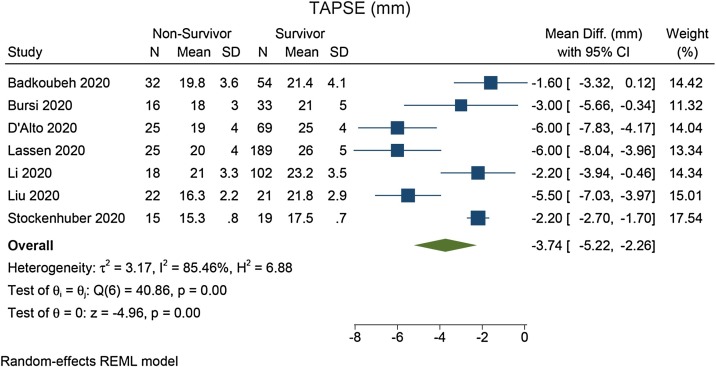
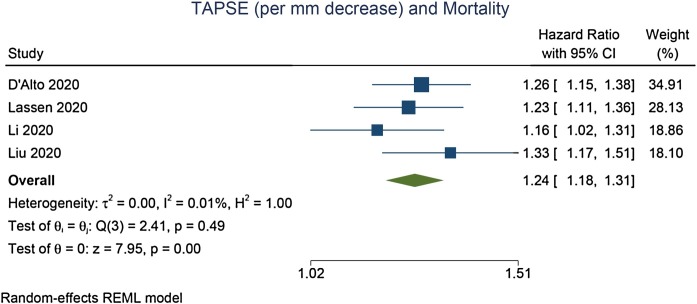
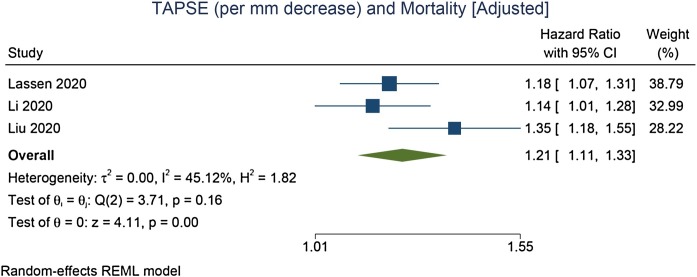
TAPSE was lower in non-survivors compared with survivors (mean difference = –3.74 [–5.22, –2.26], p < 0.001; I2: 85.5%, p < 0.001) [Figure 2 ]. Each 1 mm decrease in TAPSE was associated with increased mortality (HR = 1.24 [1.18, 1.31], p < 0.001; I2: 0.0%, p = 0.491) [Figure 3 ]. In the pooled adjusted model, each 1 mm decrease in TAPSE was associated with increased mortality (HR = 1.21 [1.11, 1.33], p < 0.001; I2: 45.1%, p = 0.156) [Figure 4 ].
Figure 2.
Mean difference in TAPSE between non-survivors and survivors.
Figure 3.
Association between each 1 mm decrease in TAPSE and mortality [unadjusted model].
Figure 4.
Association between each 1 mm decrease in TAPSE and mortality [adjusted model].
Meta-regression
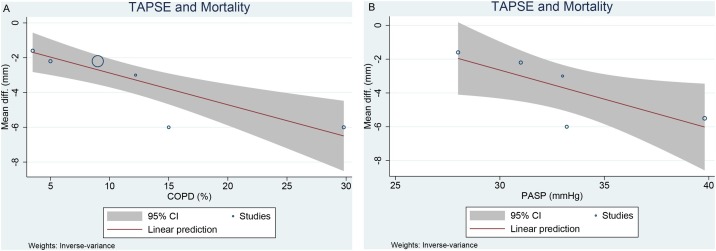
Meta-regression indicated that the differences in TAPSE between non-survivors and survivors were affected by COPD (–0.183, p < 0.001) [Figure 5 A] and PASP (–0.344, p = 0.039) [Figure 5B], but not by age (p = 0.668), male gender (p = 0.821), hypertension (p = 0.101), diabetes (p = 0.603), CAD (p = 0.564), smoking (p = 0.140), and LVEF (p = 0.452).
Figure 5.
Meta-regression analysis: effects of COPD (A) and PASP (B) on the mean difference in TAPSE between non-survivors and survivors.
Publication bias
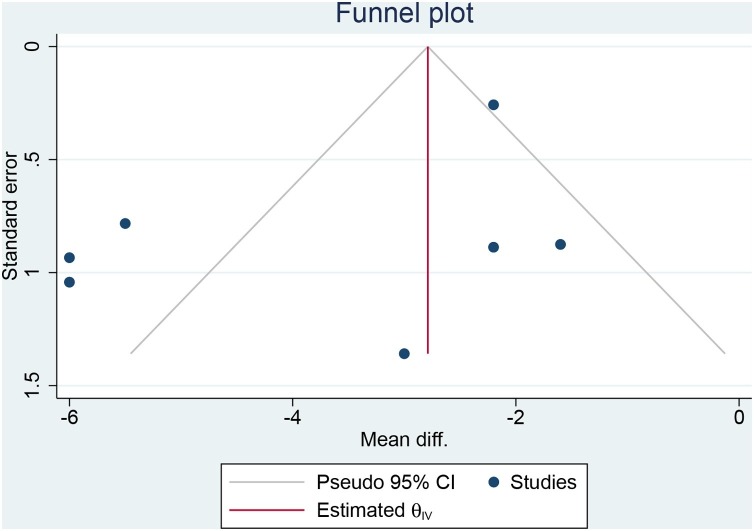
The funnel plot was asymmetrical [Figure 6 ]. Egger’s test demonstrated no indication of small-study effects (p = 0.497).
Figure 6.
Funnel-plot analysis.
Discussion
COVID-19 non-survivors had lower TAPSE measurements compared with survivors. Every 1 mm decrease in TAPSE was associated with an increase in mortality of approximately 20%.
Several potential confounders have been shown to be associated with mortality in COVID-19 and these variables may also affect echocardiographic parameters (Huang et al., 2020a; Pranata et al., 2021a, 2021b; Pranata et al., 2020b, 2020c, 2020d). Thus, regression analyses were performed in order to analyze whether these variables might have affected the mean difference in TAPSE between non-survivors and survivors. Meta-regression analysis indicated that the difference in TAPSE between non-survivors and survivors was reduced by the presence of COPD and a higher PASP. Right ventricular function and PASP might be altered in patients with COPD, thereby explaining the narrower margin between non-survivors and survivors. However, age, male gender, hypertension, diabetes, CAD, smoking, and LVEF did not influence the association.
Cardiac involvement in COVID-19 patients is generally detected by increased troponin levels with or without electrocardiogram (ECG) changes and it can be associated with symptoms of chest pain and heart failure (Stockenhuber et al., 2020). Elevated high-sensitivity cardiac troponin (HScTn) was quite prevalent in patients admitted with severe COVID-19, which can probably be explained by RV rather than LV injury. HScTn is a powerful prognostic marker in COVID-19 with multiple potential causes, including myocarditis, coronary microvascular ischemia, stress cardiomyopathy, and tachycardiomyopathy (Moody et al., 2020).
Patients with proven myocardial damage are advised to undergo echocardiography as initial cardiac imaging, which appears to substantially alter clinical management in one-third of patients (Stockenhuber et al., 2020). Likely echocardiography findings are: (1) swollen heart mostly due to pericardial effusion characterized by excessive cardiac weight, thickened biventricular walls, and a subsequent increase in biventricular mass; (2) hemodynamic disorder reflected by reduced cardiac output and increased LV filling pressure; and (3) functional impairment most commonly observed as RV systolic dysfunction rather than LV, with pulmonary hypertension and RV enlargement (Liu et al., 2020, Sattarzadeh Badkoubeh et al., 2021).
Dilated RV is defined as an RV basal diameter measured >41 mm, and RV systolic dysfunction is defined as a fractional area change <35% or TAPSE < 17 mm. Decreased RV systolic function is an independent predictor of all-cause death with an almost twofold increase in mortality hazard (Moody et al., 2020). Reduced RV function measured by echocardiography as an absolute RV longitudinal strain (RVLS) <20% was significantly correlated with increased mortality. Hence, measuring RVLS in patients with a clinical suspicion of heart failure or a finding of elevated troponin levels may effectively forecast clinical outcomes and determine whether high-level intervention is required (Stockenhuber et al., 2020, Wibowo et al., 2021). Several studies found that RV strain and TAPSE were associated with higher severity and mortality (Bursi et al., 2020, D’Alto et al., 2020, Lassen et al., 2020, Li et al., 2020, Liu et al., 2020, Sattarzadeh Badkoubeh et al., 2021, Stockenhuber et al., 2020). LV function is usually preserved or hyperdynamic in COVID-19 patients (Moody et al., 2020) but if it is affected, this may be secondary to RV volume and excess pressure due to ventricular interdependence. Direct cardiac complications have been observed as acute myocardial damage, myocarditis, and Takotsubo cardiomyopathy (Lassen et al., 2020).
Increasing evidence of DIC and venous thromboembolism characterized by elevated D-dimer in severe and critically ill COVID-19 patients suggests that RV injury may be secondary to pulmonary thrombosis. Widespread small pulmonary arteriolar fibrin thrombi and widespread alveolar capillary thrombi specific to COVID-19 compared with influenza cases support the hypothesis that RV dilatation is partly due to pressure overload (Huang et al., 2020b, Moody et al., 2020, Stockenhuber et al., 2020). TAPSE was shown to be associated with the occurrence of pulmonary embolism in patients with COVID-19 (Scudiero et al., 2021). Myocardial injury and hyperinflammation in COVID-19-induced cytokine storm could be an additional cause of ARDS-related acute RHF (Lim et al., 2020, Yonas et al., 2020). In non-COVID-19-related ARDS, poor RV function identified by echocardiography was shown to be a predictor of patient deterioration and poor overall outcome (Stockenhuber et al., 2020). A non-English study was excluded but it indicated that TAPSE was not correlated with mortality, although only five non-survivors were included (Calderón-Esquivel et al., 2021).
Clinical implications
The use of echocardiography for early assessment of RV function in patients with clinical suspicion or evidence of heart problems can provide valuable insights for clinical care. Echocardiographic parameters should be considered as important for cardiac screening in COVID-19 patients, especially TAPSE measurement, PASP, and pericardial effusion (Bursi et al., 2020, Sattarzadeh Badkoubeh et al., 2021). We found that RV dysfunction characterized by lower TAPSE may explain the ultimate mechanism that is directly or indirectly associated with a poor prognosis in COVID-19. Every 1 mm decrease in TAPSE was associated with an increase in mortality of approximately 20%. It would be useful to combine these echocardiographic parameters for obtaining prognoses in COVID-19 patients.
Limitations
Most of the studies were retrospective, which is a potential source of bias. The available data did not satisfy the requirement for performing a diagnostic test meta-analysis, which would be useful for determining the post-test probability of mortality in patients with TAPSE below a specific cut-off point if reported. Meta-regression analysis was limited to several commonly reported comorbidities.
Conclusion
Lower TAPSE was associated with mortality in patients with COVID-19.
Conflict of interest
None.
Funding
None.
Ethical approval
Not applicable.
Informed consent
Not applicable
Data availability
Data are available on reasonable request.
CRediT authorship contribution statement
Januar Wibawa Martha: Conceptualization, Investigation, Writing - review & editing, Supervision. Raymond Pranata: Conceptualization, Methodology, Software, Data curation, Formal analysis, Investigation, Validation, Writing - original draft, Writing - review & editing. Arief Wibowo: Investigation, Writing - original draft. Michael Anthonius Lim: Data curation, Investigation, Writing - original draft.
References
- Aloia E., Cameli M., D’Ascenzi F., Sciaccaluga C., Mondillo S. TAPSE: an old but useful tool in different diseases. Int J Cardiol. 2016;225:177–183. doi: 10.1016/j.ijcard.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Bursi F., Santangelo G., Sansalone D., Valli F., Vella A.M., Toriello F. Prognostic utility of quantitative offline 2D-echocardiography in hospitalized patients with COVID-19 disease. Echocardiography. 2020;37:2029–2039. doi: 10.1111/echo.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Esquivel N., Vázquez-Flores A.D., González-Chon O., García-Briones A., Gutiérrez-Villaseñor A.O., Romero-González J.P. Correlación de variables ecocardiográficas y biomarcadores en pacientes graves con COVID-19. Cir Cir. 2021;89(1):57–62. doi: 10.24875/ciru.20000900. [DOI] [PubMed] [Google Scholar]
- D’Alto M., Marra A.M., Severino S., Salzano A., Romeo E., De Rosa R. Right ventricular-arterial uncoupling independently predicts survival in COVID-19 ARDS. Crit Care. 2020;24:1–10. doi: 10.1186/s13054-020-03385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januar Martha, Raymond Pranata, Michael Lim. Tricuspid annular plane systolic excursion (TAPSE) measured by echocardiography and mortality in COVID-19: a systematic review and meta-analysis. PROSPERO 2021 CRD42021232194. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021232194. [DOI] [PMC free article] [PubMed]
- Lassen M.C.H., Skaarup K.G., Lind J.N., Alhakak A.S., Sengeløv M., Nielsen A.B. Echocardiographic abnormalities and predictors of mortality in hospitalized COVID-19 patients: the ECHOVID-19 study. ESC Hear Fail. 2020;7:4189–4197. doi: 10.1002/ehf2.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li H., Zhu S., Xie Y., Wang B., He L. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. 2020;7 doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xie J., Gao P., Tian R., Qian H., Guo F. Swollen heart in COVID-19 patients who progress to critical illness: a perspective from echo-cardiologists. ESC Hear Fail. 2020;7:3621–3632. doi: 10.1002/ehf2.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W.E., Mahmoud-Elsayed H.M., Senior J., Gul U., Khan-Kheil A.M., Horne S. Impact of right ventricular dysfunction on mortality in patients hospitalized with COVID-19, according to race. CJC Open. 2020;3:91–100. doi: 10.1016/j.cjco.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis: clinical frailty scale in COVID-19. Arch Gerontol Geriatr. 2021;93(1):1–6. doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. JRAAS - J Renin-Angiotensin-Aldosterone Syst. 2020;21(2):1–11. doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. 2021;14(7):1–10. doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Soeroto A.Y., Huang I., Lim M.A., Santoso P., Permana H. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. 2020;24:838–843. doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- Pranata R., Supriyadi R., Huang I., Permana H., Lim M.A., Yonas E. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2020;14(1):1–9. doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattarzadeh Badkoubeh R., Khoshavi M., Laleh far V., Mehrakizadeh A., Eslami M., Salahshour F. Imaging data in COVID-19 patients: focused on echocardiographic findings. Int J Cardiovasc Imaging. 2021;16(1):1–8. doi: 10.1007/s10554-020-02148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero F., Silverio A., Di Maio M., Russo V., Citro R., Personeni D. Pulmonary embolism in COVID-19 patients: prevalence, predictors and clinical outcome. Thromb Res. 2021;198:34–39. doi: 10.1016/j.thromres.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockenhuber A., Vrettos A., Androschuck V., George M., Robertson C., Bowers N. A pilot study on right ventricular longitudinal strain as a predictor of outcome in COVID-19 patients with evidence of cardiac involvement. Echocardiography. 2020:1–8. doi: 10.1111/echo.14966. [DOI] [PubMed] [Google Scholar]
- Wibowo A., Pranata R., Astuti A., Tiksnadi B.B., Martanto E., Martha J.W. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: a systematic review and meta-analysis. J Intensive Care. 2021;9:9. doi: 10.1186/s40560-020-00519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2021. Weekly epidemiological update - 5 January 2021. Geneva. [Google Scholar]
- Yonas E., Alwi I., Pranata R., Huang I., Lim M.A., Gutierrez E.J. Effect of heart failure on the outcome of COVID-19 — a meta analysis and systematic review. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.07.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.