Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a newly identified pathogen causing the coronavirus disease 2019 (COVID‐19) pandemic. Hydroxychloroquine (HCQ), an antimalarial and anti‐inflammatory drug, has been shown to inhibit SARS‐CoV‐2 infection in vitro and tested in clinical studies. However, achievement of lung concentrations predicted to have in vivo antiviral efficacy might not be possible with the currently proposed oral dosing regimens. Further, high cumulative doses of HCQ raise concerns of systemic toxicity, including cardiotoxicity. Here, we describe a preclinical study to investigate the pharmacokinetics (PKs) of a novel formulation of liposomal HCQ administered by intratracheal (IT) instillation in Sprague‐Dawley rats. Compared with unformulated HCQ administered intravenously, liposomal HCQ showed higher (~ 30‐fold) lung exposure, longer (~ 2.5‐fold) half‐life in lungs, but lower blood exposure with ~ 20% of peak plasma concentration (Cmax) and 74% of area under the curve from 0 to 72 hours (AUC0–72) and lower heart exposure with 23% of Cmax and 58% of AUC0–24 (normalized for dose). Similar results were observed relative to IT administration of unformulated HCQ. These PKs result in an animal model that demonstrated the proof of concept that inhalable liposomal HCQ may provide clinical benefit and serve as a potential treatment for COVID‐19.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The current dosing regimen of oral hydroxychloroquine (HCQ) has shown limited benefit for patients with coronavirus disease 2019 (COVID‐19). One probable reason could be the low HCQ concentrations in the lungs leading to insufficient antiviral activity against severe acute respiratory syndrome coronavirus 2. However, high doses of oral HCQ may raise safety concerns, including heart rhythm issues.
WHAT QUESTION DID THIS STUDY ADDRESS?
The current preclinical study in a rat model aimed to investigate if an inhalable liposomal HCQ formulation could serve as an alternative strategy for COVID‐19 treatment by providing a more preferable pharmacokinetic profile in the lungs than unformulated HCQ.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Here, a proof‐of‐concept study demonstrated that a novel inhalable liposomal HCQ formulation could provide preferentially higher lung concentrations, reduced systemic exposure, and lower heart distribution compared with unformulated HCQ in a rat model.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
A novel inhalable liposomal HCQ formulation could serve as a promising targeted delivery strategy to prevent or treat COVID‐19 disease by providing in vivo antiviral effect at a significantly lower and safer dose.
Hydroxychloroquine (HCQ), an antimalarial and anti‐inflammatory drug, is inexpensive, safe, and well‐tolerated by most patient populations, including those with chronic diseases or immunocompromised status. HCQ is a weak diprotic base that can pass through the lipid cell membrane and preferentially be sequestered inside acidic cytoplasmic vesicles, such as lysosomes (lysosomotropic). 1 HCQ has shown potential against coronavirus disease 2019 (COVID‐19) in vitro 2 , 3 and is being studied in numerous clinical trials. As of October 16, 2020, there were a total of 258 studies involving HCQ use among 3,611 clinical trials for COVID‐19 registered on clinicaltrials.gov.
However, despite initial indications of HCQ effectiveness in smaller clinical studies, 4 , 5 the benefit of HCQ for patients with COVID‐19 has not been supported by large clinical trials. 6 , 7 One possible reason for the conflicting results is that the optimal dosing regimen of HCQ for treating COVID‐19 remains unclear, and effective in vivo levels of HCQ may not be achievable. The in vitro half‐maximal effective concentration values (0.72–17.31 µM) proposed for optimized dosing regimens are based on extracellular drug concentrations. 2 , 3 Modeling suggested that a higher lung (intracellular) concentration may be required for in vivo antiviral efficacy. 8 The HCQ concentration (6,700 ng/mL) required to clear 100% of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in vitro might not be achievable in lung with the currently proposed oral dosing regimen of 800 mg HCQ sulfate orally daily, followed by a maintenance dose of 400 mg given daily for 4 days. 3 , 9 To achieve an effective antiviral HCQ concentration in the lungs, higher cumulative doses of HCQ would be required, raising concerns of systemic toxicity, including cardiotoxicity. 10
An alternative strategy is needed to bridge the gap between in vitro and clinical use of a potentially effective agent in the context of the COVID‐19 pandemic. Drug delivery directly to the respiratory tracts while minimizing the systemic exposure may be a desirable alternative. 8 As a proof‐of‐concept study, the pharmacokinetics (PKs) of a novel inhalable liposomal HCQ were investigated in a rat model and compared to that of unformulated HCQ through intravenous (IV) or intratracheal (IT) delivery.
METHODS
Drugs and reagents
The study drug (liposomal HCQ) was prepared by the Taiwan Liposome Company (TLC), Ltd., Taiwan. The liposomes are composed of dipalmitoylphosphatidylcholine (Lipoid GMBH, Germany) and cholesterol (Carbogen Amcis B.V., The Netherlands). HCQ sulfate was obtained from SCI Pharmtech, Taiwan. The internal standard (primaquine diphosphate and hydroxychloroquine‐d4 sulfate) was purchased from MedChemExpress, USA, and Cayman, USA.
Study design
A total of 52 female Sprague‐Dawley rats (BioLASCO Taiwan) were assigned to 1 of 3 treatment groups: (i) HCQ‐IV: 12 rats received a single dose of 0.590 mg HCQ sulfate per animal via IV injection; (ii) HCQ‐IT: 20 rats received a single dose of 0.590 mg HCQ sulfate per animal via intratracheal (IT) administration; and (iii) liposomal HCQ‐IT: 20 rats received a single dose of 0.284 mg liposomal HCQ sulfate per animal via IT administration. The sampling time points for blood samples were 0.25, 1, 4, 24, and 72 hours postdose and for tissue/organ samples were 0.25, 4, 24, and 72 hours postdose. In this study, inhalable liposomal HCQ was administered through IT instillation to mimic the intended inhaled administration for the clinical setting. All procedures involving animals were performed in the TLC animal facility and in accordance with the ethical guidelines of Institutional Animal Care and Use Committee (IACUC) at TLC, Taiwan (#TLC20IACUC012).
Blood was collected from jugular veins with K2EDTA as the anticoagulant and stored at −80°C. After blood draw, each animal was perfused with K2EDTA/saline solution before lungs and hearts were collected and stored at −80°C.
Bioanalysis and PK calculation
The bioanalytical methods were developed according to the published method 11 with modification. In brief, blood and tissue/organ samples were processed with acetonitrile with 0.1% formic acid for protein precipitation prior to analysis. The concentrations of HCQ were determined by liquid chromatography (Waters ACQUITY UPLC‐I CALSS)‐tandem mass spectrometer (Water Xevo TQS; AB Sciex Triple Quad 5500). The linear range of 3 independent assays were 0.5–500 ng/mL, 0.5–500 ng/mL, and 20–10,000 ng/mL for whole blood, heart, and lung assays, respectively. PK parameters of HCQ were calculated by a noncompartmental method using Phoenix WinNonlin (version 8.0). PK parameters for tissue/organ were calculated using the sparse‐sample option available in Phoenix WinNonlin.
Statistical analysis
Two‐sample t‐tests were used to assess differences in concentration between liposomal HCQ‐IT and HCQ‐IV. P < 0.05 was considered significant.
RESULTS
HCQ pharmacokinetics in the lungs
Upon IT administration of liposomal HCQ, there were significantly higher HCQ concentrations at 0.25 hours (P < 0.001), 4 hours (P < 0.01), 24 hours (P < 0.001), and 72 hours (P < 0.01; Figure 1a ) than with HCQ‐IV and longer apparent half‐life in the lungs compared with those of HCQ by either IV or IT administration (37.5 hours vs. 15.2 hours and 17.7 hours for HCQ‐IV and HCQ‐IT, respectively; Table 1 ). A single dose of 0.284 mg liposomal HCQ‐IT achieved 129.4 μg/g in peak plasma concentration (Cmax) and 4,193.2 h*μg/g in area under the curve from 0 to 72 hours (AUC0–72) and showed overall greater lung exposure with 29‐fold in Cmax and 35‐fold in AUC0–72 compared with HCQ‐IV with normalized dose.
Figure 1.
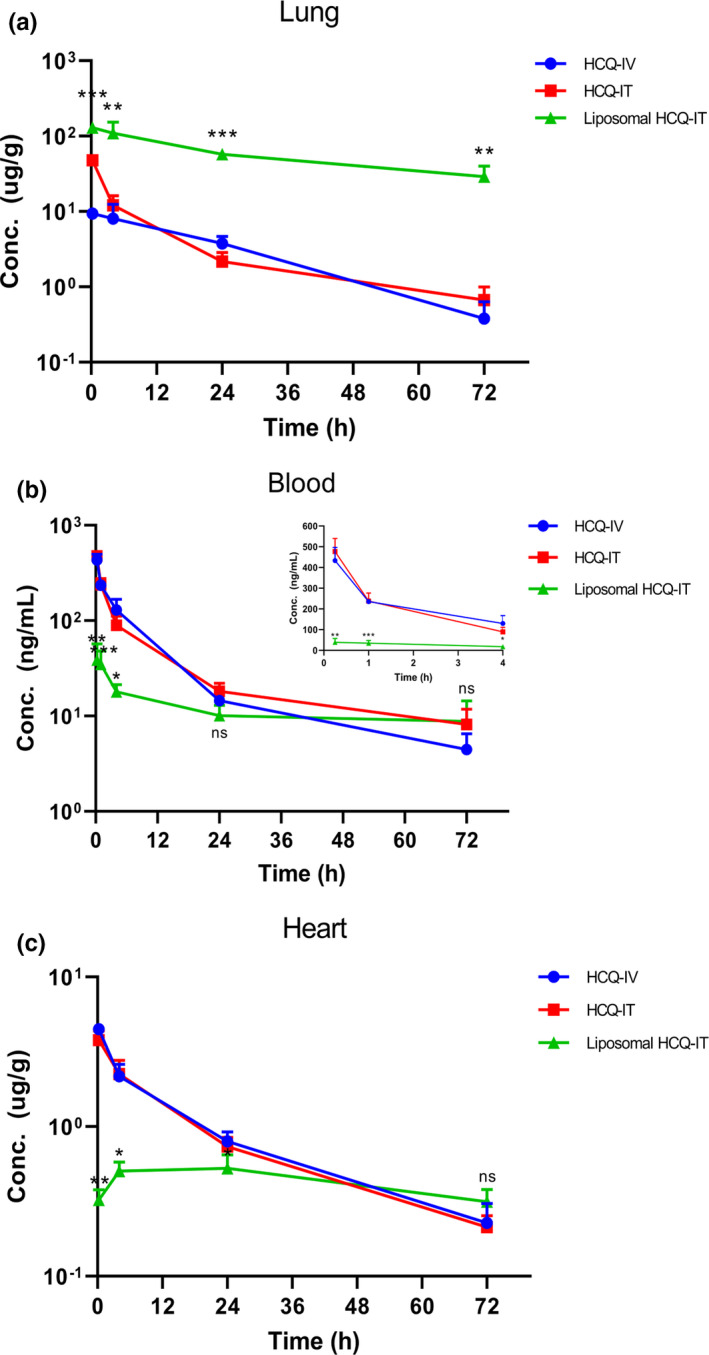
Mean concentration (±SD)—time profiles of hydroxychloroquine (HCQ) after a single administration of HCQ through intravenous (IV) or intratracheal (IT) delivery or liposomal HCQ through IT delivery in rat lungs (a), blood (b), and hearts (c). The inset graph (b) showed mean concentration‐time profiles of HCQ in 0.25–4 hours. *P < 0.05; **P < 0.01; ***P < 0.001; ns P> 0.05 compared with HCQ‐IV.
Table 1.
PK parameters of HCQ in lungs, blood, and hearts after a single administration of liposomal HCQ or HCQ in rats
| PK parameters |
HCQ‐IV (0.590 mg) (N = 3) |
HCQ‐IT (0.590 mg) (N = 5) |
Liposomal HCQ‐IT (0.284 mg) (N = 5) a |
Liposomal HCQ‐IT to HCQ‐IVb | Liposomal HCQ‐IT to HCQ‐ITb |
|---|---|---|---|---|---|
| Lungs | |||||
| Tmax, hours | 0.25 | 0.25 | 0.25 | — | — |
| Cmax, μg/g | 9.4 | 47.8 | 129.4 | 29 | 5.6 |
| AUC0–72, hour*μg/g | 251.6 | 328.6 | 4193.2 | 35 | 27 |
| t 1/2, hours | 15.2 | 17.7 | 37.5 | — | — |
| Bloodc | |||||
| Tmax, hours | 0.25 | 0.25 | 0.25 | — | — |
| Cmax, ng/mL | 433.4 ± 63.6 | 476.7 ± 63.2 | 42.5 ± 18.5 | 0.20 | 0.19 |
| AUC0–72, hour*ng/mL | 2333.2 ± 247.6 | 2257.0 ± 420.7 | 827.6 ± 286.0 | 0.74 | 0.76 |
| t 1/2, hours | 15.3 ± 2.1 | 22.1 ± 5.7 | 55.2 ± 13.0 | — | — |
| Heart | |||||
| Tmax, hours | 0.25 | 0.25 | 24 | — | — |
| Cmax, μg/g | 4.5 | 3.8 | 0.5 | 0.23 | 0.27 |
| AUC0–24, hour*μg/g | 42.6 | 41.5 | 11.9 | 0.58 | 0.60 |
| AUC0–72, hour*μg/g | 67.2 | 64.2 | 32.1 | 0.99 | 1.04 |
AUC0–24, area under the concentration‐time curve from time zero to 24 hours; AUC0–72, area under the concentration‐time curve from time zero to 72 hours; Cmax, maximum concentration; HCQ, hydroxychloroquine; IT, intratracheal; IV, intravenous; PK, pharmacokinetic; t 1/2, terminal half‐life; Tmax, time to reach maximum concentration.
N = 4 for blood sample at 1 hour timepoint of liposomal HCQ‐IT due to blood coagulation.
Data are presented as dose‐normalized ratios.
Data are presented as mean ± SD, except Tmax are presented as median.
HCQ pharmacokinetics in blood and heart
In contrast to the lungs, liposomal HCQ‐IT showed significantly lower systemic exposure in blood at 0.25 hours (P < 0.01), 1 hour (P < 0.001), and 4 hours (P < 0.05; Figure 1b ) with only around 20% of Cmax and 74% of AUC0–72 after normalizing dose compared with HCQ‐IV. As observed in blood, liposomal HCQ‐IT showed significantly lower concentrations in the heart from 0.25 hours (P < 0.01), 4 hours (P < 0.05), to 24 hours (P < 0.05; Figure 1c ) with only 23% of Cmax and 58% of AUC0–24 with normalized dose compared with HCQ‐IV.
DISCUSSION
In this rat PKs study, a significantly higher exposure of HCQ with sustained release profile in the lungs was observed by targeted delivery of inhalable liposomal HCQ, suggesting a potential treatment delivery option for COVID‐19 pulmonary disease. Fan et al. and others have suggested that significantly higher lung (intracellular) concentrations relative to the in vitro half‐maximal effective concentration would be required to achieve in vivo antiviral efficacy SARS‐CoV‐2. 8 , 9 In addition, it has been proposed that the prediction of in vivo efficacy should be driven primarily by high lung HCQ concentrations for treatment of viral pneumonia instead of HCQ blood exposure. 9 Due to the indication that current dosing for HCQ is unlikely to inhibit the COVID‐19 virus, the US Food and Drug Administration (FDA) recently revoked the emergency use authorization to use HCQ to treat COVID‐19 in certain hospitalized patients, and the World Health Organization (WHO) also suspended the HCQ arm of the COVID‐19 Solidarity Trial. Here, our findings supported the feasibility of alternative targeted delivery of HCQ to the lungs, achieving potentially efficacious antiviral levels while minimizing systemic exposure.
The aerosolized delivery of therapeutic drugs to the lower respiratory tract has been applied for the treatment of various lung infectious and inflammatory disorders. For example, aerosolized HCQ was tested in clinical trials for asthma. 12 Moreover, ARIKAYCE, an inhalable liposomal amikacin, has been approved to treat lung infections with Mycobacterium avium complex. 13 As shown in this proof‐of‐concept study, the inhalable liposomal HCQ did increase exposure in the lungs with extended residence time, while reducing systemic exposure and heart distribution, compared with systemically administered HCQ. Notably, the significantly increased lung exposure (at normalized dose) suggested a relatively lower dose regimen of inhalable liposomal HCQ than oral regimens of HCQ tablets with loading dose initiation 14 could be explored and evaluated as a potential alternative strategy for COVID‐19 treatment in future clinical studies.
One of the well‐known side effects of HCQ is cardiotoxicity, including abnormal heart rhythms, such as corrected QT interval prolongation and ventricular tachycardia, a dangerously rapid heart rate.15 Although the comparable dose‐normalized AUC0–72 in the heart was observed for liposomal HCQ‐IT and HCQ‐IV in the current study, there was no aberrant heart tissue observed grossly. Moreover, in a separate study with multiple‐dose IT administration of liposomal HCQ (0.30 mg) for 7 consecutive days, there was no histopathological change observed in rat heart tissues by hematoxylin and eosin staining (data not shown). The HCQ exposure in the heart with inhalable liposomal HCQ is expected to be much lower than with the conventional oral HCQ regimen used in the current clinical setting, given that a much lower dose would be required through inhalation. Nonetheless, considering the known cardiotoxicity potential of HCQ, electrocardiogram monitoring for cardiac safety assessment would be warranted in future clinical trials of inhalable liposomal HCQ.
Although there is no commercially available aerosolized formulation of HCQ, a recent empirical study of inhaled HCQ aerosols at 4 mg per day over 1 week found it was well‐tolerated without significant adverse events. 16 Further, aerosolized HCQ has been proposed to be delivered directly to the lungs for early treatment or prophylaxis of COVID‐19 at a lower dose (20 mg daily), which has been demonstrated to be well‐tolerated for up to 21 days of dosing. 17 This further supports the rationale of applying inhalable liposomal HCQ with direct lung targeting in COVID‐19 infection/disease prevention and treatment.
A limitation of the current study is that the PKs of liposomal HCQ were evaluated in a rat model using IT instillation to mimic the intended inhalation administration. The typical lung deposition efficiency with inhaled aerosols is very likely lower than that of the IT instilled microsprayed‐droplets. Furthermore, aerosol‐generating procedures, such as nebulization, should be performed cautiously in patients with known or suspected COVID‐19 given that SARS‐CoV‐2 is highly contagious through the respiratory route. 18 Therefore, we have developed a disposable closed‐loop system connected to the nebulizer to maximize the targeted delivery of inhaled liposomal HCQ while minimize the spreading of aerosols and contamination of the air and environment (data not shown).
In conclusion, this study in a rat model demonstrated the desirable PKs of inhalable liposomal HCQ in vivo. It supports the working hypothesis that inhalable liposomal HCQ might serve as a potential treatment option to deliver HCQ directly to the lungs for COVID‐19 pulmonary disease with less frequent dosing and at a relatively lower dose.
Funding
This study was supported by Taiwan Liposome Company, Ltd., Taiwan.
Conflict of Interest
T.T.T., T.J.W., Y.C.T., H.T.W., A.M.W., and S.F.S. are employees of Taiwan Liposome Company, Ltd., Taipei, Taiwan. All other authors declared no competing interests for this work.
Author Contributions
T.T.T., T.J.W., A.M.W., and Y.C.C. wrote the manuscript. T.J.W., Y.C.T., H.T.W., and S.F.S. designed the research. T.J.W. and Y.C.T. performed the research. T.J.W., H.D.W., Y.C.T., and H.T.W. analyzed the data.
Acknowledgments
The authors thank Dr. George Spencer‐Green and Dr. Carl Brown for their critical review and suggestions for the manuscript. We also thank Ting‐Yu Cheng for formulation development, staff in the Department of Pharmacokinetics, Taiwan Liposome Company for laboratory support, and staff in the Nonclinical Department, Taiwan Liposome Company for support with the animal model.
References
- 1. Solitro, A.R. & MacKeigan, J.P. Leaving the lysosome behind: novel developments in autophagy inhibition. Future Med. Chem. 8, 73–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu, J. et al Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 6, 16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yao, X. et al In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin. Infect. Dis. 71, 732–739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gautret, P. et al Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int. J. Antimicrob. Agents 56, 105949 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Chen, Z. et al Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. medRxiv. 1101/2020.03.22.20040758. [Google Scholar]
- 6. Boulware, D.R. et al A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid‐19. N. Engl. J. Med. 383, 517–525 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavalcanti, A.B. et al Hydroxychloroquine with or without azithromycin in mild‐to‐moderate Covid‐19. N. Engl. J. Med. 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan, J. et al Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID‐19 patients. Clin. Infect. Dis. 10.1093/cid/ciaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnold, S.L.M. & Buckner, F. Hydroxychloroquine for treatment of SARS‐CoV‐2 infection? Improving our confidence in a model‐based approach to dose selection. Clin. Transl. Sci. 10.1111/cts.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schrezenmeier, E. & Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 16, 155–166 (2020). [DOI] [PubMed] [Google Scholar]
- 11. Chhonker, Y.S. , Sleightholm, R.L. , Li, J. , Oupický, D. & Murry, D.J. Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC‐ESI‐MS/MS: an application for pharmacokinetic studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1072, 320–327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dayton, F. et al Development of an inhaled hydroxychloroquine sulfate product using the AERx system to treat asthma. Respir. Drug Deliv. 429–432 (2006). [Google Scholar]
- 13. Shirley, M. Amikacin liposome inhalation suspension: a review in mycobacterium avium complex lung disease. Drugs 79, 555–562 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lê, M.P. et al Rationale of a loading dose initiation for hydroxychloroquine treatment in COVID‐19 infection in the DisCoVeRy trial. J. Antimicrob. Chemother. 75, 2376–2380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration (FDA) . FDA cautions against use of hydroxychloroquine or chloroquine for COVID‐19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. FDA Drug Safety Communication <https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐cautions‐against‐use‐hydroxychloroquine‐or‐chloroquine‐covid‐19‐outside‐hospital‐setting‐or> (2020).
- 16. Klimke, A. , Hefner, G. , Will, B. & Voss, U. Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS‐CoV‐2 infection. Med. Hypotheses 142, 109783 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kavanagh, O. et al Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID‐19. Med. Hypotheses 143, 110110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention (CDC) . National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID‐19) Pandemic<https://www.cdc.gov/coronavirus/2019‐ncov/hcp/infection‐control‐recommendations.html>.


