Article puplished on Clinical and Translational Science (2020) 13, 57–66; https://doi.org/10.1111/cts.12678 (first published on Clinical and Translational Science 13(1), July2019, https://doi.org/10.1111/cts.12678) and entitled:
First‐in‐Human, Healthy Volunteers' Integrated Protocol of ETC‐206, an Oral MNK 1/2 Kinase Inhibitor Oncology Drug
Vincenzo Teneggi1, Veronica Novotny‐Diermayr1, Lay Hoon Lee1, Maryam Yasin1, Pauline Yeo1, Kantharaj Ethirajulu1, Sylvia Bong Hwa Gan1, Stephanie E. Blanchard1, Ranjani Nellore1, Dhananjay N. Umrani1, Roberto Gomeni2, Darren Lim Wan Teck 3, Greg Li3, Qing Shu Lu4, Yang Cao4, and Alex Matter1,5; 1D3 (Drug Discovery and Development), A*STAR, Singapore; 2Pharmacometrica, La Fouillade (France);3 SingHealth Investigational Medicine Unit, Singapore Health Services, Singapore; 4Singapore Clinical Research Institute, Singapore; 5Experimental Therapeutics Centre, A*STAR, Singapore.
We noticed that the above article carries few inaccuracies in the layout of Figure 4 and in the text of the related legend. Although the findings do not affect the overall meaning of the paper and its conclusions, still some rephrasing is deserved. We report in the table below the details of the changes related to Figure 4 and its legend, as well as the related changes in the text of the article. Moreover, in order to make the article more accurate and readable, we report below also some other minor inaccuracies we identified with the related amendments. For each amendment, a brief rationale is provided.
| Section | Paragraph | Page | Line(s) or Figure(s) or Table(s) | Original text | Amended text |
Action (and rationale) |
|---|---|---|---|---|---|---|
| INTRODUCTION | / | 58 |
Lines 19 to 24 |
ETC‐206 has been shown to inhibit the phosphorylation of eIF4E in vitro and in vivo.on tumor and surrogate tissues (i.e., hair follicles (HFs) and plasma peripheral blood mononuclear cells (PBMCs), and to decrease plasma levels of pro‐inflammatory cytokines, chemokines, and growth factors in vivo.30 | ETC‐206 has been shown to inhibit the phosphorylation of eIF4E in vitro and in vivo, on tumor30. | Some text was deleted (to more accurately reflect the content of the cited reference #30). |
| METHODS | PD assessments | 59 |
Lines 27 to 28 |
The levels of relative phosphorylation of eIF4E (p‐eIF4E) in blood (PBMCs), … | The levels of relative p‐eIF4E in blood (PBMCs), …. | Some text was reworded (to be technically more accurate) |
| Statistical Analysis | 60 |
Lines 15 to 18 |
…and the correlation between the AUC of ETC‐206 concentrations and the AUC of p‐eIF4E levels in PBMCs was conducted using SAS version 9.4. | …and the correlation analysis between the AUC of ETC‐206 concentrations and the AUC of relative p‐eIF4E levels in PBMCs was conducted by Pharmacometrica using SAS (v9.4 ‐ Institute, North Carolina, USA). | Some text was reworded or added (to be technically more accurate) | |
| RESULTS | PDs | 60 |
Lines 57 to 59 |
… (b) the correlation of ETC‐206 partial AUC (0–4 hour) with p‐eIF4E partial AUC (0–4 hour) and…. | …(b) the relationship between ETC‐206 partial plasma concentration AUC (0‐4 hr) with relative p‐eIF4E partial AUC (0‐4hr) and…. | Some text was reworded (to be technically more accurate) |
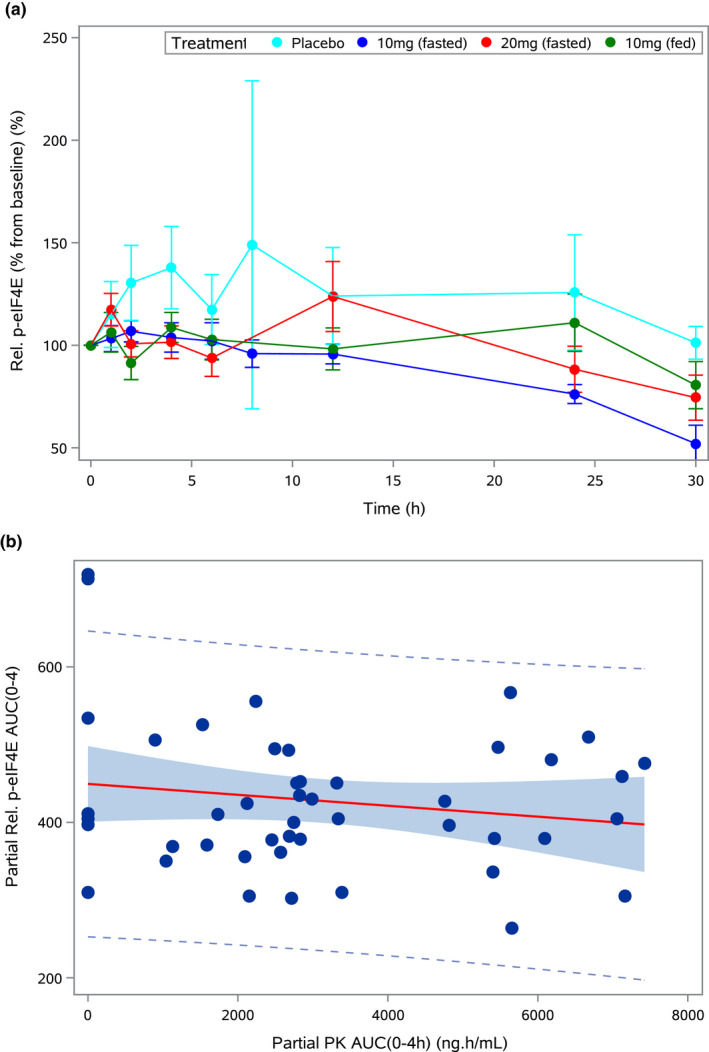
| DISCUSSION | PDs | 62 | Figure 4 | See in the original paper | See revised Figure 4 (v1.3.3.) | The layout of the figure 4 was revised (to align the last sampling values with the 30 h time point (4a); to improve the quality of the caption (4a‐4b)) |
| Legend | Figure 4 Relative phosphorylation of eIF4E (p‐eIF4E) mean time course levels in peripheral blood mononuclear cells (PBMCs); correlation of ETC‐206 partial area under curve (AUC) with p‐eIF4E partial AUC. (a) Mean relative p‐eIF4E levels in PBMCs (± SD) for placebo and at the doses of 10 mg (fasted), 20 mg (fasted), and 10 mg (fed). (b) Correlation between the partial AUC (0–4 hour) of the PK concentration‐time curve and the partial AUC (0–4 hour) of the relative p‐eIF4E levels in PBMCs (P = 0.00566). The solid red line represents the regression line, the shaded area represents the 95% confidence limits, and the dotted lines identify the 95% prediction limits. AUC, area under the concentration‐time curve; PK, pharmacokinetic | Figure 4 Relative phospho‐eIF4E (p‐eIF4E) levels compared to baseline in peripheral blood mononuclear cells (PBMCs); relationship of ETC‐206 plasma concentration partial area under curve (AUC 0‐4h) with relative p‐eIF4E levels partial AUC (0‐4h). ‐ (a) ‐ Mean relative p‐eIF4E levels in PBMCs (± SD) for placebo and at the doses of 10 mg (fasted), 20 mg (fasted) and 10 mg (fed). The SDs values for the measurements at 8 h (for placebo) and at 30 h (for 10 mg fasted) were based on only two subjects (b) – Relationship between the partial AUC (0‐4h) of the PK concentration‐time curve and the partial AUC (0‐4h) of the relative p‐eIF4E levels in PBMCs (Pearson’s r: ‐0.16587, P = 0.2706). The solid red line represents the regression line, the shaded area represents the 95% Confidence Intervals, and the dotted lines identify the 95% prediction limits. AUC, area under the concentration‐time curve; PK, pharmacokinetic. | Some text was reworded (to provide clarifications about the measurements at 8 h and 30 h;. to correct the original ‘P’ value and add the Pearson’s ‘r’) | |||
| 64 | Line 15 | The p‐eIF4E and the regulation of cytokines, chemokines, and… | The phosphorylation of eIF4E (p‐eIF4E) and the regulation of cytokines, chemokines, and… | Some text was added (to be technically more accurate) | ||
|
Lines 23 to 39 |
The assessment of the effect of SDs of ETC‐206 on relative p‐eIF4E levels showed a large variability (see Figure 4). Nevertheless, a signal of the treatment effect with respect to the placebo response was observed (Figure 4a) in the initial part of the time course of the relative p‐eIF4E levels in PBMCs (i.e., ≤4 hours postdose). To better assess the magnitude of this signal and the potential relationship with the ETC‐206 plasma levels, the partial area under the ETC‐206 plasma levels (AUC PK (0–4 hour)) and the partial area under the relative p‐eIF4E levels (AUC rel. p‐eIF4E (0–4 hour)) have been computed (Figure 4b). A statistically significant correlation (P = 0.00566) has been found between these two measures, indicating that as the ETC‐206 plasma levels increase, the relative p‐eIF4E levels decrease. This finding may indicate the presence of an exposure–response relationship between ETC‐206 and a target biomarker and deserves further investigations in coming studies. |
Looking at the mean relative p‐eIF4E levels a large variability for placebo‐treated subjects was observed, which is partially explained due to the low sample numbers (see Figure 4). To better explore the relationship of the relative p‐eIF4E levels in PBMCs with the ETC‐206 plasma levels, the partial area under the ETC‐206 plasma levels (AUC) and the partial area under the relative p‐eIF4E levels (AUC rel. p‐eIF4E) during the first 4 h post‐dose, have been computed (Figure 4b). Despite of the lack of statistical significance, the negative slope of the regression analysis represented a signal of treatment effect. This signal should be carefully considered given the limited measurements available and needs to be confirmed in further experiment properly designed and powered. |
Some text was reworded (to better describe the variability of the observations and improve the the phrasing around the treatment effect) |
|||
| Line 40 | .. and skin, or on plasma cytokines, chemokines, and growth factors… | .. and skin, or statistically significant effects on plasma cytokines, chemokines and growth factors… | Some text was added (to be technically more accurate | |||
| CONCLUSIOS | / | 64 | Line 50 | .. PD signals could be detected and… | .. potential PD signals could be detected and.. | Some wording added (to better align with changes made in the legend of Figure 4). |
|
Table S5 Methods for Pharmacodynamic Testing |
Supplementary Material | / | / | See in the original paper | See revised Tale S5 (v1.3) |
Some text reworded (to clarify methods and add a missing timepoint) |
Figure 4.
Supporting information
Table S5
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S5


