Abstract
Metformin may act renoprotective prior to kidney transplantation by reducing ischemia‐reperfusion injury (IRI). This study examined whether metformin preconditioning and postconditioning during ex vivo normothermic machine perfusion (NMP) of rat and porcine kidneys affect IRI. In the rat study, saline or 300 mg/kg metformin was administered orally twice on the day before nephrectomy. After 15 minutes of warm ischemia, kidneys were preserved with static cold storage for 24 hours. Thereafter, 90 minutes of NMP was performed with the addition of saline or metformin (30 or 300 mg/L). In the porcine study, after 30 minutes of warm ischemia, kidneys were preserved for 3 hours with oxygenated hypothermic machine perfusion. Subsequently, increasing doses of metformin were added during 4 hours of NMP. Metformin preconditioning of rat kidneys led to decreased injury perfusate biomarkers and reduced proteinuria. Postconditioning of rat kidneys resulted, dose‐dependently, in less tubular cell necrosis and vacuolation. Heat shock protein 70 expression was increased in metformin‐treated porcine kidneys. In all studies, creatinine clearance was not affected. In conclusion, both metformin preconditioning and postconditioning can be done safely and improved rat and porcine kidney quality. Because the effects are minor, it is unknown which strategy might result in improved organ quality after transplantation.
Kidney transplantation is the treatment of choice for patients with end‐stage renal disease. 1 Unfortunately, demand is higher than the availability of organs. 2 This shortage has resulted in increased use of suboptimal quality organs from donation after circulatory death (DCD) donors. 3 Kidneys from DCD donors are more susceptible to ischemia‐reperfusion injury (IRI), which is linked to significantly higher incidences of delayed graft function, affecting both short‐term and long‐term outcome. 4 , 5 IRI is caused by the impairment of blood flow and subsequent reoxygenation, which results in the generation of reactive oxygen species (ROS), initiating a cascade of detrimental cellular responses. 6 , 7 The severity of ischemia correlates strongly with early renal graft failure after kidney transplantation and contributes to increased morbidity. 8 Therefore, warm ischemia (WI) during organ retrieval and implantation, and cold ischemic time during preservation, respectively, should be kept to a minimum. Machine perfusion strategies can play a pivotal role in decreasing IRI by supplying oxygen during ischemic phases in organ donation. Furthermore, it provides a platform to add possible protective agents prior to reperfusion (preconditioning) or during reperfusion (postconditioning), and is a suitable method to assess short‐term renal function and injury. 9 , 10 , 11
The biguanide metformin is the most used oral antihyperglycemic drug to treat patients with type 2 diabetes. The mechanism of action and the pleiotropic effects of metformin primarily results from the inhibition of complex 1 within the mitochondrial respiratory chain. 12 , 13 , 14 Therefore, metformin may attenuate IRI beyond its glucose‐lowering actions, and has been proposed as an organ‐protective agent during circumstances in which IRI occurs, such as transplantation. 15 , 16
The aim of this study was to evaluate potential beneficial effects of preconditioning and postconditioning with metformin on IRI in two different isolated ex vivo normothermic machine perfusion (NMP) models using rat and porcine kidneys.
METHODS
Rat study
Animals
Male Lewis rats weighing 270–300 g were used (Harlan Laboratories, Boxmeer, The Netherlands). The Institutional Animal Care and Use Committee of the University of Groningen approved the study protocol (DEC6708C). The animals received care according to the Dutch Law on Animal Experiments, following the National Institute of Health’s Principles of Laboratory Animal Care.
Experimental design, organ retrieval, and preservation
A total of 31 rats were divided into 6 groups (n = 5–6 per group; Figure 1 ). In all groups, 15 minutes of WI and 24 hours of cold preservation was used to induce ischemic injury. Preconditioning was performed by administering 300 mg/kg metformin (1,1‐dimethylbiguanide hydrochloride; Sigma‐Aldrich, St. Louis, MO) dissolved in saline (0.9% NaCl) or saline alone through oral gavage 12 and 2 hours before nephrectomy. Saline, 30 mg/L metformin or 300 mg/L metformin was added to the perfusate as postconditioning agent. The concentration of 300 mg/kg body weight was chosen, as this dose resulted in serum metformin concentrations that correspond to those found in humans during maintenance metformin therapy. 17
Figure 1.
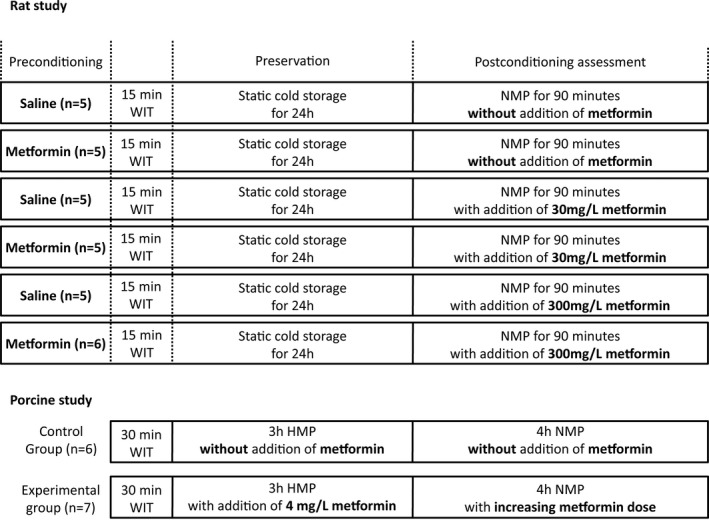
Schematic representation of the experimental groups. Groups contain 5–7 kidneys. HMP, hypothermic machine perfusion; NMP, normothermic machine perfusion; WIT, warm ischemia time.
The procurement of kidneys was performed with minor changes to the DCD procedure, as described previously. 18 In short, rats were anaesthetized with 2–5% isoflurane and laparotomy was performed via a midline incision. Anticoagulation, using 500 IU heparin (Leo Pharma, Ballerup, Denmark), was administered via the dorsal penile vein. After 15 minutes of WI, nephrectomy of the left kidney was performed. The renal artery and ureter were cannulated. The kidney was flushed in situ with 10 mL saline (37°C) and 5 mL 4°C University of Wisconsin (UW) Cold Storage Solution (Bridge to Life, Columbia, SC). Once removed, kidneys were flushed with 5 mL UW once again and stored in UW for 24 hours at 4°C.
Normothermic machine perfusion
The rat NMP method has been described extensively earlier. 18 In brief, NMP was performed for 90 minutes using a roller pump (Ismatec ISM404, Zürich, Switzerland). The perfusion pressure was set at 102 mmHg, controlled at the renal artery. In the control group, the perfusion fluid consisted of 100 mL William’s Medium E supplemented with 30 mmol/L HEPES, 50 g/L albumin, and 7 mmol/L creatinine (all Sigma‐Aldrich). In the experimental groups, either 30 or 300 mg/L metformin was added to the perfusate. The perfusion fluid was oxygenated with 95% oxygen and 5% carbon dioxide with a flow of 0.5 L/min. The temperature of the perfusion fluid was maintained at 37°C using a water bath and heat exchanger (Julabo, Seelbach, Germany). The flow was recorded every 10 minutes, using a calibrated flow sensor (ME1PXN Inline, Transonic Systems, Ithaca, NY). After NMP, biopsies of the kidneys were submerged immediately in 4% formaldehyde or snap‐frozen in liquid nitrogen and subsequently stored at −80°C.
Porcine study
Animals
Kidneys from female Dutch Landrace pigs were collected from an abattoir. The animals were stunned and exsanguinated according to local standard procedures. During exsanguination, approximately 1 L of blood was collected in a container containing 25,000 IU unfractionated heparin (Leo Pharma). Because slaughterhouse waste material was used, no animal ethics committee approval was required.
Experimental design, organ retrieval, and preservation
To induce ischemic injury, 30 minutes of WI was used. After this period, the kidney was flushed with 180 mL saline at 4°C. Immediately after the flush, a cortical biopsy was taken (Invivo, Best, The Netherlands) and stored in 4% buffered formaldehyde. To accommodate transport from the abattoir to the laboratory and as a preservation technique, the renal artery was cannulated and the kidneys were attached to a pulsatile pressure‐controlled hypothermic machine perfusion (HMP) setup (Kidney Assist Transport; Organ Assist, Groningen, The Netherlands). The kidneys were perfused at 4°C using 500 mL UW Machine Perfusion Solution for 3 hours (Bridge to Life, London, UK) with or without the addition of 2 mg metformin, with a mean arterial pressure of 25 mmHg. Oxygen (100%) was supplied to the oxygenator (Hilite LT 1000; Medos Medizintechnik AG, Stolberg, Germany) with a fixed flow rate of 0.1 L/min.
Normothermic machine perfusion
The kidneys were reperfused using an ex vivo NMP setup for 4 hours that was described previously. 11 In our study, increasing doses of metformin or saline were added using an infusion pump. In short, after HMP, the renal artery was cannulated and flushed. The ureter was cannulated for urine collection. Afterward, the kidney was weighed, and a biopsy was taken and stored in 4% buffered formaldehyde for further analysis. Subsequently, the kidneys were placed in an ex vivo pressure‐controlled NMP circuit. NMP was performed with 500 mL leucocyte depleted, (BioR 02 plus; Fresenius Kabi, Bad Homburg, Germany) autologous, oxygenated (carbogen, flow 0.5 L/min) blood for 4 hours at a mean arterial pressure of 80 mmHg. Other compounds added to the perfusion fluid are provided in Table S1 . In the experimental group, metformin dissolved in Ringer’s lactate solution (20 mg/mL; Baxter, Utrecht, The Netherlands) was infused using an infusion pump controlled by custom‐made software in which infusion profiles could be defined (Alaris; CareFusion, Rolle, Switzerland). Every 30 minutes during NMP, the infusion speed was increased according to a prespecified schedule (Table S2 ). This schedule was based on human pharmacokinetic data indicating that metformin clearance is four times higher than the creatinine clearance. 19 In the control group, kidneys were perfused without the addition of metformin. For each experiment, the flow was recorded using the clamp‐on flow probe (ME7PXL; Transonic Systems), which was attached to the tubing close to the renal artery. This flow probe has been calibrated for the tubing used. Every 15 minutes during NMP, urine was collected and replaced with a corresponding volume of Ringer’s lactate solution, which was also recorded. The temperature of the perfusion fluid was maintained at 37°C using an integrated heat exchanger, connected to a water bath (Julabo). Blood and urine samples were taken after 15 and 60 minutes and every following hour for biochemical analyses. When NMP was finished, biopsies were taken and stored in 4% buffered formaldehyde, or snap‐frozen and stored at −80°C for further analysis.
Both studies
Biochemical analyses
Perfusate and urine samples were centrifuged (1,300 g for 10 minutes in the rat model and 1,000 g for 12 minutes in the porcine model, respectively, both at 4°C) and the supernatant was stored at −80°C. Lactate dehydrogenase (LDH), aspartate aminotransferase (ASAT), and creatinine were determined in perfusate, and creatinine in urine, respectively, by the Laboratory Center of the University Medical Center Groningen using standard biochemical analyses. The amount of protein excreted in rat urine was measured using a Pierce BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA), whereas total protein concentration in porcine urine was measured using standard biochemical analyses at the Laboratory Center.
Real‐time quantitative polymerase chain reaction
Real‐time polymerase chain reaction (PCR) was carried out according to standard procedures on the Taqman Applied Biosystems 7900HT Real‐Time PCR system, as described previously. 20 Amplification of gene fragments involved in the regulation of vascular tone (endothelin 1 (EDN‐1, encoding for ET‐1); endothelial nitric oxide synthase (eNOS); and Krüppel‐like factor 2 (KLF‐2)), endothelial activation (Von Willebrand factor (vWF); vascular cell adhesion molecule 1 (VCAM‐1) and IL‐6)), and heat‐shock protein 70 (HSP‐70) was done with primer sets listed in Table S3 . In short, total RNA was extracted from kidney sections using TRIzol (Life Technologies, Gaithersburg, MD). The cDNA obtained from rats was used as an internal reference during PCR to test primer efficiency. Gene expression was normalized with the mean of β‐actin mRNA content. Results were expressed as 2‐ΔΔCT, where the CT value represents the difference between cycle threshold values.
Morphological scoring
Biopsies stored in 4% formaldehyde were embedded in paraffin and were cut into 4 µm slices. Coupes were subsequently stained with periodic acid‐Schiff and scored on proximal tubular cell necrosis (ranging from mild to severe: 1–5), edema (ranging from mild to severe: 1–4), and proximal tubular cell vacuolation (ranging from mild to severe: 1–4). 21 Histopathological assessment was done blinded by two researchers. An independent clinical pathologist with extensive experience in assessing histopathological signs of injury after machine perfusion of rat and porcine organs validated the scores.
Calculations
Intrarenal vascular resistance (IVR) was calculated by dividing the mean arterial pressure by flow (expressed in mmHg/mL min). Creatinine clearance was calculated to estimate glomerular filtration rate using the following equation: [creatinine in urine] * urine flow/[creatinine in perfusate].
Statistical analysis
All data are expressed as mean ± standard error of the mean. When comparing two groups at a single timepoint, differences were assessed using an unpaired Student’s t‐test. Differences for total urine production, morphological scores, and gene expression were tested using analysis of variance. All statistical tests are two‐tailed and P ≤ 0.05 was considered statistically significant. SPSS Statistics version 23 (IBM, Armonk, NY) was used for all analyses. The area under the curve (AUC) was calculated according to the trapezoid rule, and was used to approximate the total creatinine clearance, the total amount of protein excreted in the urine, and the total levels of ASAT and LDH in the perfusate.
RESULTS
Perfusion parameters
The IVR remained constant during NMP in the rat model, without significant differences between the treatment groups (Figure S1 a). No significant differences were found in the porcine kidney groups regarding IVR (Figure S1 b).
Kidney function parameters
No significant differences in total creatinine clearance were observed between all treatment groups in the rat study (Figure 2a ). In the porcine study, total creatinine clearance did not differ between metformin‐treated kidneys and controls (Figure 2b ), although there was a tendency toward lower creatinine clearance in the metformin‐treated group (P = 0.07).
Figure 2.
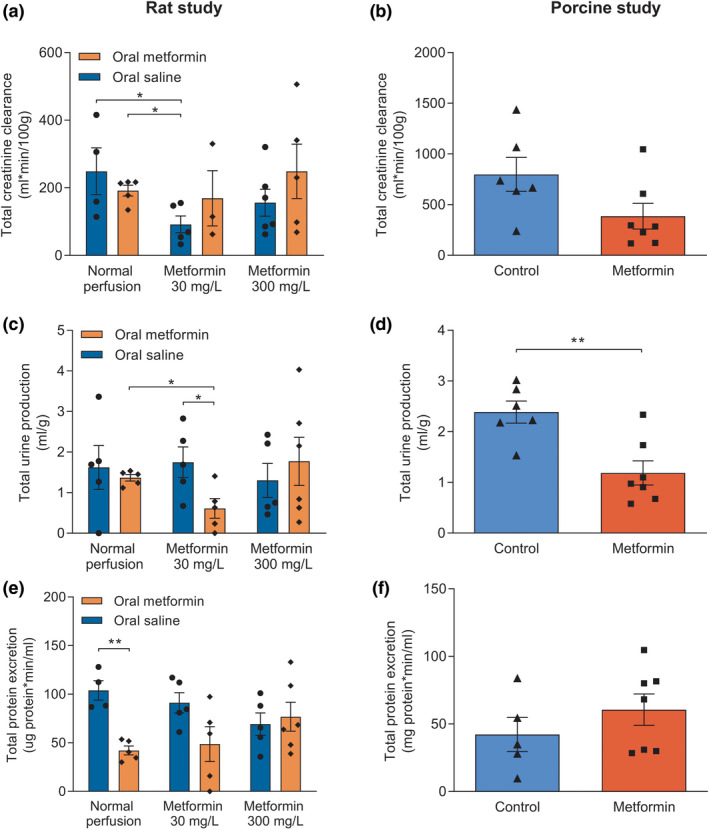
Perfusion and renal function parameters assessed during and after normothermic machine perfusion of rat and porcine kidneys. Total creatinine clearance of rat (a) and porcine (b) kidneys. Total urine production (c, d) and total protein excreted in the urine (e, f) of rat and porcine kidneys, respectively. Data are presented as mean ± standard error of the mean. Groups contain 5–7 kidneys. *P < 0.05 and **P < 0.01.
Metformin‐pretreated rats of whom kidneys were perfused with 30 mg/L metformin had a significantly lower urine production compared with rats pretreated with metformin without subsequent perfusion with metformin (P = 0.02), and rats pretreated with oral saline whose kidneys were postconditioned with 30 mg/L metformin (P = 0.04). Postconditioning with 300 mg/L metformin did not yield any significant difference in urine production, irrespective of preconditioning conditions. Compared with controls, total urine production in porcine kidneys was significantly lower in metformin‐treated kidneys (P = 0.004; Figure 2c,d ).
In the rat study, total protein excretion was lower in metformin‐preconditioned kidneys without subsequent perfusion with metformin than the control group (P = 0.001). No differences in total protein excretion were observed between all other experimental groups (Figure 2e ).
During NMP of porcine kidneys, no significant differences in urinary protein excretion were found between the metformin and control group (Figure 2f ).
Injury markers
Metformin‐preconditioned rats whose kidneys were not perfused with metformin had lower LDH release than controls (P = 0.005). Metformin‐preconditioned rats whose kidneys subsequently were perfused with 30 mg/L metformin had lower LDH release than controls (P = 0.01) and had a lower LDH release than saline‐preconditioned rats whose kidneys were perfused with 30 mg/L metformin (P = 0.04). Perfusion with 300 mg/L metformin did not result in decreased LDH release (Figure 3a ). Total LDH release during NMP did not differ between metformin‐treated porcine kidneys and controls (Figure 3b ).
Figure 3.
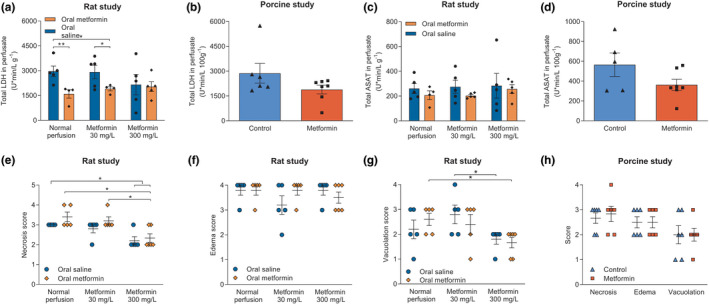
Injury markers in perfusion fluid and morphological injury in tissue after normothermic machine perfusion. Total markers of injury were calculated as the area under the curve. Total amount of lactate dehydrogenase (LDH) (a, b) and aspartate aminotransferase (ASAT) (c, d) in the perfusate. Tissue was scored on proximal tubular cell necrosis (ranging from mild to severe: 1–5), edema (ranging from mild to severe: 1–4), and proximal tubular cell vacuolation (ranging from mild to severe: 1–4) in the rat study, respectively (e–g). Signs of ischemia reperfusion injury in porcine kidneys were scored (h). Data are presented as mean ± standard error of the mean. Groups contain 5–7 kidneys. *P < 0.05, **P < 0.01, compared with all other groups.
Both preconditioning and postconditioning with metformin was not associated with a significant difference in the total amount of released ASAT in the rat study (Figure 3c ). Likewise, no differences between metformin‐treated kidneys and controls in ASAT levels were found in porcine kidneys (Figure 3d ).
Morphological signs of ischemia reperfusion injury
Compared with controls, tubular necrosis was significantly reduced in kidneys that were perfused with 300 mg/L metformin, independent of saline (P = 0.02) or metformin (P = 0.02) preconditioning. Metformin‐preconditioned rats whose kidneys were perfused with 300 mg/L metformin had less tubular necrosis compared with metformin‐preconditioned rats without subsequent metformin perfusion (P = 0.01) and metformin‐preconditioned rats of whose kidneys were perfused with 30 mg/L metformin (P = 0.02; Figure 3e ).
Proximal tubular cell vacuolation was significantly reduced in saline‐preconditioned rats whose kidneys were perfused with 300 mg/L metformin compared with saline‐preconditioned rats whose kidneys were perfused with 30 mg/L metformin (P = 0.05). Vacuolation was also significantly lower in metformin‐preconditioned kidneys perfused with 300 mg/L metformin compared with metformin‐preconditioned kidneys without metformin perfusion (P = 0.02) (Figure 3g ). No statistical differences regarding edema formation were seen in the rat study (Figure 3f ). No differences in morphological signs of IRI were observed at the end of NMP in porcine kidneys (Figure 3h ).
Gene expression
Relative mRNA expression of genes involved in the regulation of the vascular tone was evaluated in both rat and porcine kidneys. Compared with controls, the expression of EDN‐1 was significantly decreased in rat kidneys perfused with 300 mg/L metformin, regardless of preconditioning with saline (P = 0.002) or metformin (P = 0.005; Figure 4a ). Compared with saline‐pretreated rats whose kidneys were perfused with 30 mg/L metformin, eNOS expression was significantly decreased in controls (P = 0.04) and saline‐preconditioned rats whose kidneys were perfused with 300 mg/L metformin (P = 0.01; Figure 4b ). No differences regarding EDN‐1 or eNOS expression were found in the porcine kidney study (Figure 4h ).
Figure 4.
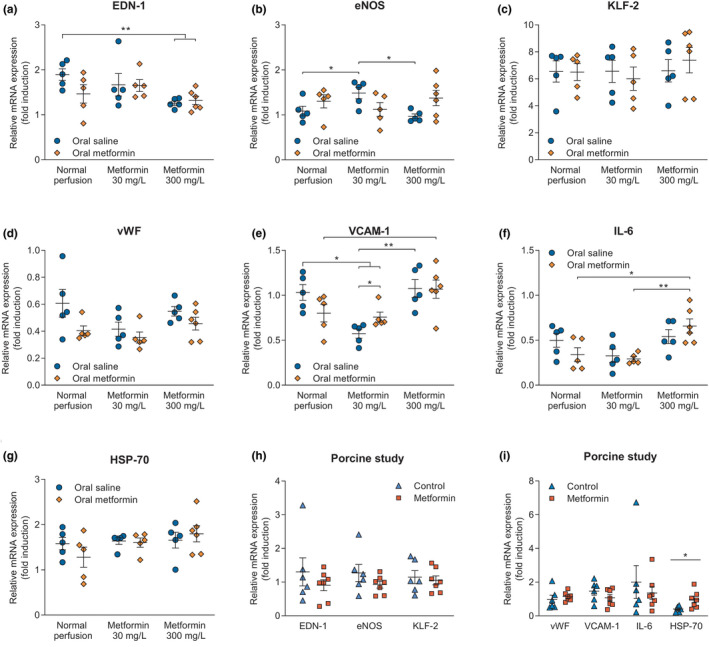
Relative mRNA expression of genes involved in the regulation of vascular tone, including vasoconstriction (endothelin 1 (EDN‐1)), vasodilatation (endothelial nitric oxide synthase (eNOS)), and laminar flow shear stress (Krüppel‐like factor 2 (KLF‐2)) in both the rat (respectively, a–c) and porcine study (h). Genes involved in the activation of the endothelium, playing a role in platelet (von Willebrand factor (vWF)) and leukocyte adhesion (vascular cell adhesion molecule 1 (VCAM‐1)), and inflammatory processes (IL‐6) were assessed in rat (respectively, d–f) and porcine kidneys (i). Heat‐shock protein 70 (HSP‐70) expression was evaluated as well in both rat and porcine kidneys (respectively, g, i). Data are presented as mean ± standard error of the mean. Groups contain 5–7 kidneys. *P < 0.05, **P < 0.01.
KLF‐2 expression was not different between groups in both the rat and porcine kidney study (Figure 4c, h).
Moreover, genes involved in endothelial activation were evaluated. The vWF expression did not differ between treatment groups in both rat and porcine kidneys (Figure 4d,i ). Compared with controls, gene expression of VCAM‐1 was significantly decreased in rat kidneys perfused with 30 mg/L metformin irrespective of preconditioning with saline (P = 0.002) or metformin (P = 0.03). Saline‐pretreated rats whose kidneys were perfused with 30 mg/L metformin had lower VCAM‐1 expression than metformin‐preconditioned rats whose kidneys were also perfused with 30 mg/L metformin (P = 0.04). Saline‐preconditioned rats whose kidneys were perfused with 300 mg/L metformin had significantly higher VCAM‐1 expression than saline‐preconditioned rats whose kidneys were perfused with 30 mg/L metformin (P = 0.002). On the other hand, metformin‐preconditioned rats whose kidneys were perfused with 300 mg/L metformin had significantly higher VCAM‐1 expression than controls (P = 0.03; Figure 4e ).
Compared with metformin‐preconditioned rats whose kidneys were perfused with 300 mg/L metformin, IL‐6 gene expression was decreased in metformin‐preconditioned rats whose kidneys were not perfused with metformin (P = 0.03), and metformin‐preconditioned rats whose kidneys were perfused with 30 mg/L metformin (P = 0.004; Figure 4f ).
HSP‐70 expression in rat kidneys was not different between treatment groups (Figure 4g ). Expression of vWF, VCAM‐1, and IL‐6 was not significantly different between experimental groups within the porcine kidney study (Figure 4i ). However, HSP‐70 was upregulated in metformin‐treated porcine kidneys (P = 0.03; Figure 4l ).
DISCUSSION
The aim of this study was to evaluate potential benefits of metformin preconditioning and postconditioning on IRI in two distinct ex vivo NMP models using rat and pig kidneys. We found that, in terms of renal function, metformin preconditioning was associated with significantly lower proteinuria, representing a favorable effect on glomerular integrity. 22 In terms of cellular injury, significantly lower LDH values were observed in preconditioned rat kidneys, whereas postconditioning was not associated with differences in this biomarker. Interestingly, postconditioning with 300 mg/L metformin resulted in less tubular cell necrosis and vacuolation. Furthermore, metformin preconditioning and postconditioning resulted in gene expression upregulation of genes encoding for endothelial activation and inflammation in rats and a significant upregulation of HSP‐70 in metformin‐perfused porcine kidneys. The results from this study are indicative that metformin has indeed beneficial effects on renal integrity, albeit these effects are minimal and not conclusive. Therefore, we did not find a clear answer on whether and in which modality metformin can be used as a renoprotective strategy to reduce IRI.
To increase the robustness of our findings and to reduce potential interspecies effects, two methodological distinct ex vivo experimental NMP models were used in our study. Because the models differed in so many ways, we do not believe the results can be compared directly, but should be interpreted as two separate albeit complementary studies. However, we found beneficial effects of metformin in the rat study, whereas no apparent differences were found in the porcine experiments. This might be explained by differences in experimental design. Metformin was administered to rats as a preconditioning agent, whereas metformin was only added to porcine kidneys during HMP and NMP as a postconditioning agent. Because porcine kidneys were obtained from the abattoir, we were not able to perform any preconditioning actions. Second, metformin was given at the start of NMP in the rat study, whereas porcine kidneys were exposed to increasing doses over time. Third, both studies used organs from different sexes. Therefore, potential gender effects could have arisen, as previous studies showed differences in effects of brain death on IRI and microcirculation between sexes. 23 , 24 Moreover, even sex‐dependent differences in the anticancer effects of metformin have been reported. 25 Whether this is also the case with metformin used in transplantation settings is worth elucidating.
Remarkably, the administration of metformin in concentrations that are associated with toxicity in humans was not associated with cellular injury in both our ex vivo models. This is an important finding because metformin can accumulate in patients with renal insufficiency and who subsequently can develop severe lactic acidosis. 19 , 26 Our study shows that administration of metformin in high doses does not induce cellular injury and, therefore, could be safely used in ex vivo machine perfusion setups.
Previously, it is reported that preconditioning with metformin can reduce IRI. 27 , 28 , 29 Protective effects of metformin were observed during cerebral ischemia and subsequent reperfusion in rats. 29 , 30 In murine myocardial infarction models, administration of metformin before ischemia or during reperfusion was associated with decreased infarction size. 28 , 31 However, these results could not be reproduced in a swine model. 32 Metformin has also been clinically studied as a preconditioning agent to reduce IRI. However, neither beneficial effects on myocardial injury nor other IRI‐reducing effects of metformin preconditioning were found in these randomized clinical trials. 33 , 34 Postconditioning with metformin has also been assessed clinically, in which no effects of metformin treatment initiated after myocardial infarction were observed on renal function 35 or left ventricular function. 36 In previous experiments performed by our group, metformin preconditioning of donor rat livers was able to improve hepatobiliary function during NMP and orthotopic liver transplantation thereafter. 37
The exact mechanism of action of metformin is unknown, but mild inhibition of complex 1 within the mitochondrial electron transport chain seems to play a pivotal role. 12 , 13 , 14 Moreover, metformin is thought to affect endothelial function through several distinct pathways, including regulation of nitric oxide production, inhibition of apoptosis, and alteration of the cellular energy state with subsequent activation of kinase pathways. 38 , 39 , 40 In line with previous experiments, 39 , 41 postconditioning with 300 mg/L metformin decreases expression of ET‐1. Endothelin 1 has proinflammatory effects, stimulates ROS production, and modifies the glomerular barrier. 42 Moreover, elevated ET‐1 is associated with vascular and kidney injury. 42 , 43 Increased expression of eNOS was found in rat kidneys perfused with a low dose of metformin, which is associated with decreased IRI and oxidative stress. 38 , 44 , 45 However, no differences in KLF‐2, a marker of fluid laminar shear stress, or in vascular resistance itself, were observed. Expression of VCAM‐1 correlates with severe structural damage of renal parenchyma, 46 and has previously been reported to be less abundant in the metformin‐treated kidneys. 47 Moreover, expression of VCAM‐1, also involved in leukocyte adhesion, can be induced by ROS in endothelial cells during inflammation. 48 Indeed, we found reduced VCAM‐1 expression in kidneys perfused with 30 mg/L metformin. Summarizing, the findings in gene expression suggest that endothelial activation was moderately affected by exposure to metformin.
In our study, we mainly observed a protective effect of preconditioning with metformin, implying that metformin needs to be administered before the ischemic insult. Therefore, a donor intervention should be considered in a transplantation setting. Diminishing organ injury by changing donor management has been proposed and is performed sparsely by others. 49 , 50 However, the logistical and ethical issues concerning donor‐intervention research hamper performing randomized controlled trials. 49 Furthermore, the proposed intervention should not negatively affect other organs that are supposed to be transplanted. Therefore, for now, the only likely translational use would be ex vivo postconditioning with metformin. Machine perfusion modalities are increasingly used and are offering an ideal platform for pharmacological interventions, like metformin. As postconditioning with metformin showed some modest beneficial effects in our study, it might be tested as a renoprotective agent in future research. However, our results do not necessarily advocate the use of metformin as a pharmacological intervention prior to kidney transplantation, and we suggest it can be considered to be tested in combination with other potential treatments.
A limitation reducing the clinical translational aspect of our study is the lack of a full kidney transplantation after NMP, which should be the direction for further research in this field and our study may, therefore, be considered as a step toward a transplantation experiment.
Another limitation of our study was that we did not measure urinary markers of acute injury, like kidney injury molecule 1. However, we did measure indicators of renal injury, like LDH and ASAT, that only showed minor beneficial effects of metformin treatment. Future research should comprehend these markers when examining effects of metformin on renal IRI.
In conclusion, although modest, metformin preconditioning prior to NMP was associated with reduced IRI possibly by protecting renal integrity, whereas postconditioning with metformin was associated with reduced tubular cell necrosis and vacuolation, as well as improved endothelial gene expression. Importantly, no safety signals or potentially irreversible damage was observed when exposed to in vivo toxic metformin concentrations, indicating that it seems to be safe to use these concentrations of metformin during machine perfusion. Our study indicates that both metformin preconditioning and postconditioning improved rat and porcine kidney quality. However, these effects were minor and it remains unclear whether metformin can be used as renoprotective strategy during machine perfusion before renal transplantation.
Funding
This work was externally funded by a European Commission Seventh Framework Programme grant (No. 305934) to H.G.D.L. and Stichting De Cock‐Hadders project grant (No. 2018‐36) to R.A.P.
Conflicts of Interests
All authors declared no competing interests for this work.
Author Contributions
T.M.H., L.H.V., and R.A.P. wrote the manuscript. R.A.P., L.H.V., A.C.W., D.J.T., M.W.N., and H.G.D.L. designed the research. T.M.H., L.H.V., R.A.P., N.D.V., and P.J.O. performed the research. T.M.H., N.D.V., and P.J.O. analyzed the data.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Ischemia‐reperfusion injury (IRI) negatively affects outcomes of kidney transplantation, whereas metformin has been proposed to attenuate IRI beyond its glucose‐lowering actions. Machine perfusion provides a platform to add protective agents, like metformin, prior to or during reperfusion to decrease IRI. Previous data showed that metformin preconditioning was able to improve hepatobiliary function during normothermic machine perfusion and subsequent transplantation of rat livers.
WHAT QUESTION DID THIS STUDY ADDRESS?
In this study, potential beneficial effects of preconditioning and postconditioning with metformin on IRI were evaluated in different isolated ex vivo normothermic machine perfusion models using rat and porcine kidneys.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We show that both metformin preconditioning and postconditioning reduced rat and porcine kidney injury, but that the improvements were minor. It remains unknown whether addition of metformin during machine perfusion results in improved organ quality after transplantation. Moreover, we show that the use of in vivo toxic concentrations of metformin during machine perfusion was not associated with cellular damage, indicating that it seems to be safe to use these concentrations during ex vivo perfusion of kidneys.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Being widely implemented, machine perfusion is an ideal platform for pharmacological interventions to diminish or prevent IRI, and to test therapeutics in isolated ex vivo organs. As the effects of metformin were minor, it remains unclear whether it should be used as a renoprotective agent prior to transplantation. Our results do not necessarily advocate the use of metformin as an intervention prior to kidney transplantation, but future research is required to investigate whether metformin in combination with other potential treatments might be beneficial.
Supporting information
Figure S1‐S2‐Table S1‐S3
Acknowledgments
The authors greatly acknowledge the technical assistance provided by D.N. van Heereveld, N. Tiddens, J. Zheng, T. Eertman, P. Mahboub, J.E. van Zanden, N.M. Jager, A. Brat, and J. Wiersema‐Buist (all Department of Surgery, University Medical Center Groningen, Groningen, The Netherlands).
References
- 1. Abecassis, M. et al Kidney transplantation as primary therapy for end‐stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 3, 471–480 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Girlanda, R. Deceased organ donation for transplantation: challenges and opportunities. World J. Transplant. 6, 451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moers, C. , Leuvenink, H.G.D. & Ploeg, R.J. Non‐heart beating organ donation: overview and future perspectives. Transpl. Int. 20, 567–575 (2007). [DOI] [PubMed] [Google Scholar]
- 4. Ponticelli, C. Ischaemia‐reperfusion injury: a major protagonist in kidney transplantation. Nephrol. Dial. Transplant. 29, 1134–1140 (2014). [DOI] [PubMed] [Google Scholar]
- 5. Siedlecki, A. , Irish, W. & Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transplant. 11, 2279–2296 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malek, M. & Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 4, 20–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carden, D.L. & Granger, D.N. Pathophysiology of ischaemia‐reperfusion injury. J. Pathol. 190, 255–266 (2000). [DOI] [PubMed] [Google Scholar]
- 8. Zhao, H. , Alam, A. , Soo, A.P. , George, A.J.T. & Ma, D. Ischemia‐reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine 28, 31–42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sedigh, A. et al Modifying the vessel walls in porcine kidneys during machine perfusion. J. Surg. Res. 191, 455–462 (2014). [DOI] [PubMed] [Google Scholar]
- 10. Maassen, H. et al Hydrogen sulphide‐induced hypometabolism in human‐sized porcine kidneys. PLoS One 14, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venema, L.H. et al Effects of oxygen during long‐term hypothermic machine perfusion in a porcine model of kidney donation after circulatory death. Transplantation 103, 2057–2064 (2019). [DOI] [PubMed] [Google Scholar]
- 12. Rena, G. , Hardie, D.G. & Pearson, E.R. The mechanisms of action of metformin. Diabetologia 60, 1577–1585 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owen, M.R. , Doran, E. & Halestrap, A.P. Complex 1 of the mitochondrial respiratory. Chain. 614, 607–614 (2000). [PMC free article] [PubMed] [Google Scholar]
- 14. El‐Mir, M.‐Y. et al Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I *. J. Biol. Chem. 275, 223–228 (2000). [DOI] [PubMed] [Google Scholar]
- 15. De Broe, M.E. , Kajbaf, F. & Lalau, J.‐D. Renoprotective effects of metformin. Nephron 138, 261–274 (2017). [DOI] [PubMed] [Google Scholar]
- 16. Mohsin, A.A. et al Mitochondrial complex I inhibition by metformin limits reperfusion injury. J. Pharmacol. Exp. Ther. 369, 282–290 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rouru, J. , Huupponen, R. , Santti, E. & Koulu, M. Effect of subchronic metformin treatment on macronutrient selection in genetically obese Zucker rats. Pharmacol. Toxicol. 72, 300–303 (1993). [DOI] [PubMed] [Google Scholar]
- 18. van den Eijnden, M.M. et al Effect of brain death and non‐heart‐beating kidney donation on renal function and injury: an assessment in the isolated perfused rat kidney. Exp. Clin. Transplant. 1, 85–95 (2003). [PubMed] [Google Scholar]
- 19. Graham, G.G. et al Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 50, 81–98 (2011). [DOI] [PubMed] [Google Scholar]
- 20. Rebolledo, R.A. et al Slow induction of brain death leads to decreased renal function and increased hepatic apoptosis in rats. J. Transl. Med. 14, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dittrich, S. et al Influence of cold storage on renal ischemia reperfusion injury after non‐heart‐beating donor explantation. Nephron Exp. Nephrol. 96, 97–102 (2004). [DOI] [PubMed] [Google Scholar]
- 22. Menon, M.C. , Chuang, P.Y. & He, C.J. The glomerular filtration barrier: components and crosstalk. Int. J. Nephrol. 2012, 1–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferreira, S.G. et al Differential effects of brain death on rat microcirculation and intestinal inflammation: female versus male. Inflammation 41, 1488–1497 (2018). [DOI] [PubMed] [Google Scholar]
- 24. Simão, R.R. et al Sex differences on solid organ histological characteristics after brain death. Acta Cir. Bras. 31, 278–285 (2016). [DOI] [PubMed] [Google Scholar]
- 25. Park, J.W. et al Sex‐dependent difference in the effect of metformin on colorectal cancer‐specific mortality of diabetic colorectal cancer patients. World J. Gastroenterol. 23, 5196–5205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lalau, J.D. , Arnouts, P. , Sharif, A. & De Broe, M.E. Metformin and other antidiabetic agents in renal failure patients. Kidney Int. 87, 308–322 (2015). [DOI] [PubMed] [Google Scholar]
- 27. Ye, Y. , Perez‐Polo, J.R. , Aguilar, D. & Birnbaum, Y. The potential effects of anti‐diabetic medications on myocardial ischemia‐reperfusion injury. Basic Res. Cardiol. 106, 925–952 (2011). [DOI] [PubMed] [Google Scholar]
- 28. Messaoudi, S.E. , Rongen, G.A. , De Boer, R.A. & Riksen, N.P. The cardioprotective effects of metformin. Curr. Opin. Lipidol. 22, 445–453 (2011). [DOI] [PubMed] [Google Scholar]
- 29. Karimipour, M. , Zarghani, S.S. , Milani, M.M. & Soraya, H. Pre‐treatment with metformin in comparison with post‐treatment reduces cerebral ischemia reperfusion induced injuries in rats. Bull. Emerg. Trauma 6, 115–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seo‐Mayer, P.W. et al Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am. J. Physiol. Physiol. 301, F1346–F1357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yin, M. et al Metformin improves cardiac function in a nondiabetic rat model of post‐MI heart failure. Am. J. Physiol. Heart Circ. Physiol. 301, H459–H468 (2011). [DOI] [PubMed] [Google Scholar]
- 32. Techiryan, G. , Weil, B.R. , Palka, B.A. & Canty, J.M. Effect of intracoronary metformin on myocardial infarct size in swine. Circ. Res. 123, 986–995 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Messaoudi, S. et al Impact of metformin on endothelial ischemia‐reperfusion injury in humans in vivo: a prospective randomized open, blinded‐endpoint study. PLoS One 9, e96062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El Messaoudi, S. et al Effect of metformin pretreatment on myocardial injury during coronary artery bypass surgery in patients without diabetes (MetCAB): a double‐blind, randomised controlled trial. Lancet Diabetes Endocrinol. 3, 615–623 (2015). [DOI] [PubMed] [Google Scholar]
- 35. Posma, R.A. et al Effect of metformin on renal function after primary percutaneous coronary intervention in patients without diabetes presenting with ST‐elevation myocardial infarction: data from the GIPS‐III trial. Cardiovasc. Drugs Ther. 29, 451–459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lexis, C.P.H. et al Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS‐III randomized clinical trial. JAMA 311, 1526–1535 (2014). [DOI] [PubMed] [Google Scholar]
- 37. Westerkamp, A.C. et al Metformin preconditioning improves hepatobiliary function and reduces injury in a rat model of normothermic machine perfusion and orthotopic transplantation. Transplantation. https://doi.org/ 10.1097/tp.0000000000003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Triggle, C.R. & Ding, H. Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium. Acta Physiol. 219, 138–151 (2017). [DOI] [PubMed] [Google Scholar]
- 39. Chen, H. , Li, J. , Yang, O. , Kong, J. & Lin, G. Effect of metformin on insulin‐resistant endothelial cell function. Oncol. Lett. 9, 1149–1153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nafisa, A. et al Endothelial function and dysfunction: impact of metformin. Pharmacol. Ther. 192, 150–162 (2018). [DOI] [PubMed] [Google Scholar]
- 41. Diamantis‐Kandarakis, E. , Spina, G. , Kouli, C. & Migdalis, I. Increased endothelin‐1 levels in women with polycystic metformin therapy. J. Clin. Endocrinol. Metab. 86, 4666–4673 (2001). [DOI] [PubMed] [Google Scholar]
- 42. Miguel, C.D. , Speed, J.S. , Kasztan, M. , Gohar, E.Y. & Pollock, M. Endothelin‐1 and the kidney: new perspectives and recent findings. Curr. Opin. Nephrol. Hypertens. 25, 35–41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coelho, S.C. et al Three‐month endothelial human endothelin‐1 overexpression causes blood pressure elevation and vascular and kidney injury. Hypertension 71, 208–216 (2018). [DOI] [PubMed] [Google Scholar]
- 44. Ozturk, H. , Cetinkaya, A. , Duzcu, S.E. , Tekce, B.K. & Ozturk, H. Carvacrol attenuates histopathogic and functional impairments induced by bilateral renal ischemia/reperfusion in rats. Biomed. Pharmacother. 98, 656–661 (2018). [DOI] [PubMed] [Google Scholar]
- 45. Goligorsky, M.S. , Brodsky, S.V. & Noiri, E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 61, 855–861 (2002). [DOI] [PubMed] [Google Scholar]
- 46. Hauser, I.A. , Riess, R. , Hausknecht, B. , Thüringer, H. & Sterzel, R.B. Expression of cell adhesion molecules in primary renal disease and renal allograft rejection. Nephrol. Dial. Transplant. 12, 1122–1131 (1997). [DOI] [PubMed] [Google Scholar]
- 47. De Jager, J. et al Effects of short‐term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo‐controlled trial. J. Intern. Med. 257, 100–109 (2005). [DOI] [PubMed] [Google Scholar]
- 48. Cook‐Mills, J.M. , Marchese, M.E. & Abdala‐Valencia, H. Vascular cell adhesion molecule‐1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 15, 1607–1638 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Niemann, C.U. et al Therapeutic hypothermia in deceased organ donors and kidney‐graft function. N. Engl. J. Med. 373, 405–414 (2015). [DOI] [PubMed] [Google Scholar]
- 50. Schnuelle, P. et al Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA 302, 1067–1075 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S2‐Table S1‐S3


