Abstract
The convergence of artificial intelligence (AI) and precision medicine promises to revolutionize health care. Precision medicine methods identify phenotypes of patients with less‐common responses to treatment or unique healthcare needs. AI leverages sophisticated computation and inference to generate insights, enables the system to reason and learn, and empowers clinician decision making through augmented intelligence. Recent literature suggests that translational research exploring this convergence will help solve the most difficult challenges facing precision medicine, especially those in which nongenomic and genomic determinants, combined with information from patient symptoms, clinical history, and lifestyles, will facilitate personalized diagnosis and prognostication.
In a recent National Academy of Medicine report about the current and future state of artificial intelligence (AI) in health care, the authors noted “unprecedented opportunities” to augment the care of specialists and the assistance that AI provides in combating the realities of being human (including fatigue and inattention) and the risks of machine error. Importantly, the report notes that whereas care must be taken with the use of these technologies, much promise exists. 1 The digitization of health‐related data and the rapid uptake in technology are fueling transformation and progress in the development and use of AI in healthcare. 2 , 3 , 4 However, multimodal data integration, security, federated learning (which requires fundamental advances in areas, such as privacy, large‐scale machine learning, and distributed optimization), model performance, and bias may pose challenges to the use of AI in health care. 5
Three main principles for successful adoption of AI in health care include data and security, analytics and insights, and shared expertise. Data and security equate to full transparency and trust in how AI systems are trained and in the data and knowledge used to train them. As humans and AI systems increasingly work together, it is essential that we trust the output of these systems.
Analytics and insights equate to purpose and people where “augmented intelligence” and “actionable insights” support what humans do, not replace them. AI can combine input from multiple structured and unstructured sources, reason at a semantic level, and use these abilities in computer vision, reading comprehension, conversational systems, and multimodal applications to help health professionals make more informed decisions (e.g., a physician making a diagnosis, a nurse creating a care plan, or a social services agency arranging services for an elderly citizen). Shared expertise equates to our complementary relationship with AI systems, which are trained by and are supporting human professionals, leading to workforce change, which leads to new skills. The ability to create cutting‐edge AI models and build high‐quality business applications requires skilled experts with access to the latest hardware.
A vast amount of untapped data could have a great impact on our health—yet it exists outside medical systems. 6 Our individual health is heavily influenced by our lifestyle, nutrition, our environment, and access to care. These behavioral and social determinants and other exogenous factors can now be tracked and measured by wearables and a range of medical devices. These factors account for about 60% of our determinants of health (behavioral, socio‐economical, physiological, and psychological data), our genes account for about 30%, and last our actual medical history accounts for a mere 10%. 6 Over the course of our lifetimes, we will each generate the equivalent of over 300 million books of personal and health‐related data that could unlock insights to a longer and healthier life. 7
The phenomenon of big data can be described using the five Vs: volume, velocity, variety, veracity, and value. Volume refers to the vast amount of complex and heterogenous data, which makes data sets too large to store and analyze using traditional database technology. Velocity refers to the speed at which new data are generated and moves around. Variety refers to the different types of structured, semistructured, and unstructured data, such as social media conversations and voice recordings. Veracity refers to the certainty, accuracy, relevance, and predictive value of the data. Value refers to the conversion of data into business insights. Whereas the volume, variety, velocity, and veracity of data are contributing to the increasing complexity of data management and workloads—creating a greater need for advanced analytics to discover insights—mobile devices have made technology more consumable, creating user demand for interactive tools for visual analytics.
Big data analytics and AI are increasingly becoming omnipresent across the entire spectrum of health care, including the 5 Ps spanning: payer, provider, policy maker/government, patients, and product manufacturers. Up to 10% of global health care expenditure is due to fraud and abuse and AI‐based tools help mitigate fraud, waste, and abuse in payer programs. 8 Reliable identification of medical coding errors and incorrect claims positively impacts payers, providers, and governments by saving inordinate amounts of money, time, and efforts. 9 As an example, IBM DataProbe, an AI‐based business intelligence tool, was able to detect and recover US $41.5 million in fee‐for‐service payments over a 2‐year period in Medicaid fraud for Iowa Medicaid Enterprise. 10 In the provider space, AI is used for evidence‐based clinical decision support, 11 detection of adverse events, and the usage of electronic health record (EHR) data to predict patients at risk for readmission. 12 Healthcare policymakers and government use AI‐based tools to control and predict infections and outbreaks. An example is FINDER, a machine‐learned model for real‐time detection of foodborne illness using anonymous and aggregated web search and location data. 13 Another example is the integrated data hub and care‐management solution using IBM Connect360 and IBM Watson Care Manager that Sonoma County, California government agencies used to transform health and healthcare for socially disadvantaged groups and other displaced individuals during a time of community‐wide crisis. 14 This solution enabled integration of siloed data and services into a unified citizen status view, identification of clinical and social determinants of health from structured and unstructured sources, construction of algorithms to match clients with services, and streamlining of care coordination during the 2017 and 2019 Sonoma County wildfires. With the advent of the global pandemic coronavirus disease 2019 (COVID‐19) in early 2020, such a model can be used to predict at‐risk populations, and potentially provide additional risk information to clinicians caring for at‐risk patients. 15 The use of AI for patients and life sciences/healthcare products are addressed extensively in the sections that follow.
AI is not, however, the only data‐driven field impacting health and health care. The field of precision medicine is providing an equal or even greater influence than AI on the direction of health care 16 and has been doing so for more than a decade. 17 Precision medicine aims to personalize care for every individual. This goal requires access to massive amounts of data, such as data collected through the United Kingdom’s UK Biobank and the All of Us project, coupled with a receptive health care ecosystem willing to abandon the conventional approach to care in favor of a more highly individualized strategy. The convergence of these fields will likely accelerate the goals of personalized care and tightly couple AI to healthcare providers for the foreseeable future. In the sections that follow, we will briefly summarize the capabilities of existing AI technology, describe how precision medicine is evolving, and, through a series of examples, demonstrate the potentially transformative effect of AI on the rate and increasing breadth of application for precision medicine.
Artificial intelligence
The past 10 years have seen remarkable growth and acceptance of AI in a variety of domains and in particular by healthcare professionals. AI provides rich opportunities for designing intelligent products, creating novel services, and generating new business models. At the same time, the use of AI can introduce social and ethical challenges to security, privacy, and human rights. 1
AI technologies in medicine exist in many forms, from the purely virtual (e.g., deep‐learning‐based health information management systems and active guidance of physicians in their treatment decisions) to cyber‐physical (e.g., robots used to assist the attending surgeon and targeted nanorobots for drug delivery). 18 The power of AI technologies to recognize sophisticated patterns and hidden structures has enabled many image‐based detection and diagnostic systems in healthcare to perform as well or better than clinicians, in some cases. 19 AI‐enabled clinical decision‐support systems may reduce diagnostic errors, augment intelligence to support decision making, and assist clinicians with EHR data extraction and documentation tasks. 20 Emerging computational improvements in natural language processing (NLP), pattern identification, efficient search, prediction, and bias‐free reasoning will lead to further capabilities in AI that address currently intractable problems. 21 , 22
Advances in the computational capability of AI have prompted concerns that AI technologies will eventually replace physicians. The term “augmented intelligence,” coined by W.R. Ashby in the 1950s, 23 may be a more apt description of the future interplay among data, computation, and healthcare providers and perhaps a better definition for the abbreviation “AI” in healthcare. A version of augmented intelligence, described in the literature in Friedman’s fundamental theorem of biomedical informatics, 24 relates strongly to the role of AI in health care (depicted in Figure 1 ). Consistent with Friedman’s description of augmented intelligence, Langlotz at Stanford stated that “Radiologists who use AI will replace radiologists who don’t.” 25
Figure 1.
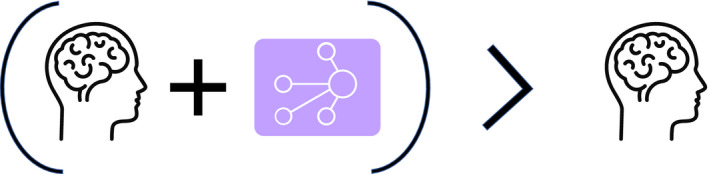
A version of the Friedman’s fundamental theorem of informatics describing the impact of augmented intelligence. “The healthcare system with AI will be better than the healthcare system without it.” AI, artificial intelligence.
An AI system exhibits four main characteristics that allow us to perceive it as cognitive: understanding, reasoning, learning, and empowering. 26 An AI system understands by reading, processing, and interpreting the available structured and unstructured data at enormous scale and volume. An AI system reasons by understanding entities and relationships, drawing connections, proposing hypotheses, deriving inferences, and evaluating evidence that allows it to recognize and interpret the language of health and medicine. An AI system learns from human experts and real‐world cases by collecting feedback, learning from outcomes at all levels and granularities of the system, and continuing to improve over time and experience. An AI system empowers and interacts clinicians and users by providing a more integrated experience in a variety of settings, combining dialog, visualization, collaboration, and delivering previously invisible data and knowledge into actionable insights. In contrast, humans excel at common sense, empathy, morals, and creativity.
Augmenting human capabilities with those provided by AI leads to actionable insights in areas such as oncology, 27 imaging, 28 and primary care. 29 For example, a breast cancer predicting algorithm, trained on 38,444 mammogram images from 9,611 women, was the first to combine imaging and EHR data with associated health records. This algorithm was able to predict biopsy malignancy and differentiate between normal and abnormal screening results. The algorithm can be applied to assess breast cancer at a level comparable to radiologists, as well has having the potential to substantially reduce missed diagnoses of breast cancer. 30 This combined machine‐learning and deep‐learning model trained on a dataset of linked mammograms and health records may assist radiologists in the detection of breast cancer as a second reader.
Precision medicine
The field of precision medicine is similarly experiencing rapid growth. Precision medicine is perhaps best described as a health care movement involving what the National Research Council initially called the development of “a New Taxonomy of human disease based on molecular biology,” or a revolution in health care triggered by knowledge gained from sequencing the human genome. 31 The field has since evolved to recognize how the intersection of multi‐omic data combined with medical history, social/behavioral determinants, and environmental knowledge precisely characterizes health states, disease states, and therapeutic options for affected individuals. 32 For the remainder of this paper, we will use the term precision medicine to describe the health care philosophy and research agenda described above, and the term personalized care to reflect the impact of that philosophy on the individual receiving care.
Precision medicine offers healthcare providers the ability to discover and present information that either validates or alters the trajectory of a medical decision from one that is based on the evidence for the average patient, to one that is based upon individual’s unique characteristics. It facilitates a clinician’s delivery of care personalized for each patient. Precision medicine discovery empowers possibilities that would otherwise have been unrealized.
Advances in precision medicine manifest into tangible benefits, such as early detection of disease 33 and designing personalized treatments are becoming more commonplace in health care. 34 The power of precision medicine to personalize care is enabled by several data collection and analytics technologies. In particular, the convergence of high‐throughput genotyping and global adoption of EHRs gives scientists an unprecedented opportunity to derive new phenotypes from real‐world clinical and biomarker data. These phenotypes, combined with knowledge from the EHR, may validate the need for additional treatments or may improve diagnoses of disease variants.
Perhaps the most well‐studied impact of precision medicine on health care today is genotype‐guided treatment. Clinicians have used genotype information as a guideline to help determine the correct dose of warfarin. 35 The Clinical Pharmacogenetics Implementation Consortium published genotype‐based drug guidelines to help clinicians optimize drug therapies with genetic test results. 36 Genomic profiling of tumors can inform targeted therapy plans for patients with breast or lung cancer. 34 Precision medicine, integrated into healthcare, has the potential to yield more precise diagnoses, predict disease risk before symptoms occur, and design customized treatment plans that maximize safety and efficiency. The trend toward enabling the use of precision medicine by establishing data repositories is not restricted to the United States; examples from Biobanks in many countries, such as the UK Biobank, 37 BioBank Japan, 38 and Australian Genomics Health Alliance 39 demonstrate the power of changing attitudes toward precision medicine on a global scale.
Although there is much promise for AI and precision medicine, more work still needs to be done to test, validate, and change treatment practices. Researchers face challenges of adopting unified data formats (e.g., Fast Healthcare Interoperability Resources), obtaining sufficient and high quality labeled data for training algorithms, and addressing regulatory, privacy, and sociocultural requirements.
Future Synergies Between AI and Precision Medicine
AI and precision medicine are converging to assist in solving the most complex problems in personalized care. Figure 2 depicts five examples of personalized healthcare dogma that are inherently challenging but potentially amenable to progress using AI. 40 , 41 , 42
Figure 2.
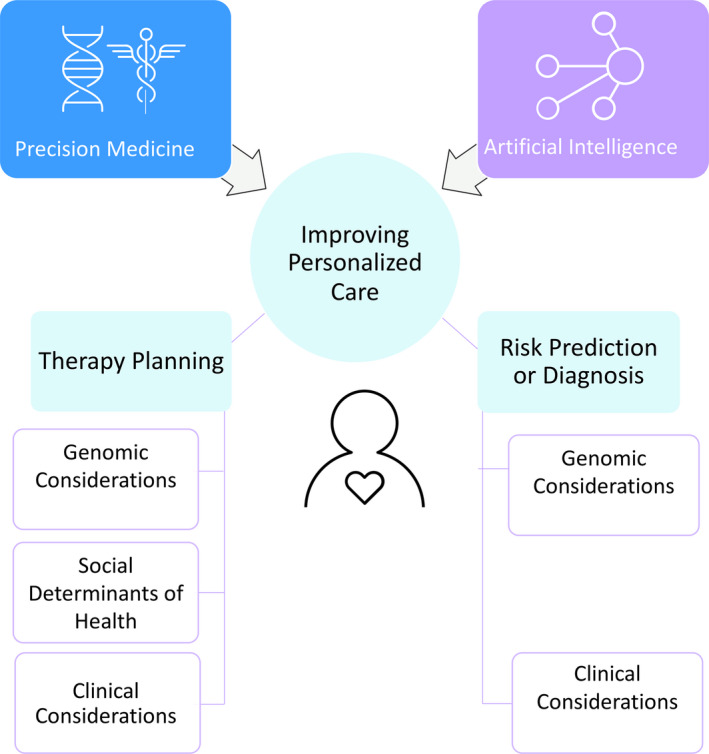
Dimensions of synergy between AI and precision medicine. Both precision medicine and artificial intelligence (AI) techniques impact the goal of personalizing care in five ways: therapy planning using clincal, genomic or social and behavioral determinants of health, and risk prediction/diagnosis, using genomic or other variables.
Genomic considerations in therapy planning: Patients with pharmacogenomically actionable variants may require altered prescribing or dosing
Genome‐informed prescribing is perhaps one of the first areas to demonstrate the power of precision medicine at scale. 43 However, the ability to make real‐time recommendations hinges on developing machine‐learning algorithms to predict which patients are likely to need a medication for which genomic information. The key to personalizing medications and dosages is to genotype those patients before that information is needed. 44
This use case was among the earliest examples of the convergence between AI and precision medicine, as AI techniques have proven useful for efficient and high‐throughput genome interpretation. 45 As noted recently by Zou and colleagues, 46 deep learning has been used to combine knowledge from the scientific literature with findings from sequencing to propose 3D protein configurations, identify transcription start sites, model regulatory elements, and predict gene expression from genotype data. These interpretations are foundational to identifying links among genomic variation and disease presentation, therapeutic success, and prognosis.
In medulloblastoma, the emergence of discrete molecular subgroups of the disease following AI‐mediated analysis of hundreds of exomes, has facilitated the administration of the right treatment, at the right dosage, to the right cohort of pediatric patients. 47 Although conventional treatment of this disease involved multimodal treatment, including surgery, chemotherapy, and whole brain radiation, precision genomics has enabled treatment of the “wingless” tumor subgroup, which is more common in children, with chemotherapy alone—obviating the need for radiation. 48 Avoiding radiotherapy is particularly impactful for mitigating potential neurocognitive sequelae and secondary cancers from whole‐brain radiation among disease survivors. 49 , 50
The initial successful paradigm of AI in imaging recognition has also given rise to radiogenomics. Radiogenomics, as a novel precision medicine research field, focuses on establishing associations between cancer imaging features and gene expression to predict a patient’s risk of developing toxicity following radiotherapy. 51 , 52 , 53 For example, Chang et al. proposed a framework of multiple residual convolutional neural networks to noninvasively predict isocitrate dehydrogenase genotype in grades II–IV glioma using multi‐institutional magnetic resonance imaging datasets. Besides, AI has been used in discovering radiogenomic associations in breast cancer, 52 liver cancer, 54 and colorectal cancer. 53 Currently, limited data availability remains the most formidable challenge for AI radiogenomics. 51
Knowing the response to therapy can help clinicians choose the right treatment plan. AI demonstrates potential applications in this area. For example, McDonald et al. trained a support vector machine using patients’ gene expression data to predict their response to chemotherapy. Their data show encouraging outcomes across multiple drugs. 55 Sadanandam et al. proposed approaches of discovering patterns in gene sequences or molecular signatures that are associated with better outcomes following nontraditional treatment. Their findings may assist clinicians in selecting a treatment that is most likely to be effective. 56 Although tremendous progress has been made using AI techniques and genomics to predict treatment outcome, more prospective and retrospective clinical research and clinical studies still need to be conducted to generate the data that can then train the algorithms.
Environmental considerations in therapy planning: A patient’s zip code should not impact care quality and availability
Incorporating environmental considerations into management plans require sufficient personal and environmental information, which may affect a patient’s risk for a poor outcome, knowledge about care alternatives, and conditions under which each alternative may be optimal.
One such example has been the challenge of identifying homelessness in some patients. 57 , 58 , 59 These patients may require care in varying locations over a short period, requiring frequent reassessments of patient demographic data. Related issues, such as transportation, providing medications that require refrigeration, or using diagnostic modalities that require electricity (for monitoring), need to be modified accordingly.
Another environmental consideration is the availability of expertise in remote locations, including the availability of trained professionals at the point of need. AI has provided numerous examples of augmenting diagnostic capabilities in resource‐poor locations, which may translate into better patient classification and therefore more personalized therapy planning. Examples include the use of deep learning to identify patients with malaria 60 and cervical cancer, 61 as well as predicting infectious disease outbreaks, 62 environmental toxin exposure, 63 and allergen load. 64
Clinical considerations in therapy planning: Co‐morbidities are always in play and AI can assist stratification
Finally, in addition to genomic considerations and social determinants of health, clinical factors are imperative to successful therapy planning. Age, co‐morbidities, and organ function in particular predicate treatment considerations and AI has emerged as a central pillar in stratifying patients for therapy. In one study, machine learning classifiers were used to analyze 30 co‐morbidities to identify critically ill patients who will require prolonged mechanical ventilation and tracheostomy placement. 65 Other studies have used AI algorithms to analyze bedside monitored adverse events and other clinical parameters to predict organ dysfunction and failure. 66 , 67
Genomic considerations in risk prediction or diagnosis: Patients with genome‐validated risk for disease may warrant different preventive care strategies
Actress Angelina Jolie’s response to her inheritance of the BRCA gene illustrates the potential impact of more advanced genomic information on disease risk and prevention options. 68 This case is not novel; the case of Woodie Guthrie and Huntington’s disease disclosed a similar conundrum for health care. 69 Although the ethics of genetic testing without a clear cure continues to be debated, the broad availability of genetic information offered by next‐generation sequencing and direct‐to‐consumer testing renders personalized prevention and management of serious diseases a reality. 70
Cardiovascular medicine is an area with a long history of embracing predictive modeling to assess patient risk. 71 Recent work has uncovered methods to predict heart failure 72 and other serious cardiac events in asymptomatic individuals. 73 When combined with personalized prevention strategies, 74 , 75 these models may positively impact disease incidence and sequela. Complex diseases, such as cardiovascular disease, often involve the interplay among gender, genetic, lifestyle, and environmental factors. Integrating these attributes requires attention to the heterogeneity of the data. 76 AI approaches that excel at discovering complex relationships among a large number of factors provide such opportunities. A study from Vanderbilt demonstrated early examples of combining EHR and genetic data, with positive results in cardiovascular disease prediction. 77 AI‐enabled recognition of phenotype features through EHR or images and matching those features with genetic variants may allow faster genetic disease diagnosis. 78 For example, accurate and fast diagnosis for seriously ill infants that have a suspected genetic disease can be attained by using rapid whole‐genome sequencing and NLP‐enabled automated phenotyping. 79
Nongenomic considerations in risk prediction or diagnosis: Patients with altered speech or gait patterns might be at risk for depression
Automated speech analytics have benefited from improvements in the technical performance of NLP, understanding, and generation. Automated speech analytics may provide indicators for assessment and detection of early‐stage dementia, minor cognitive impairment, Parkinson’s disease, and other mental disorders. 80 , 81 , 82 , 83 Efforts also are underway to detect changes in mental health using smartphone sensors. 84
AI‐assisted monitoring may also be used in real‐time to assess the risk of intrapartum stress during labor, guiding the decision of cesarean section vs. normal vaginal deliveries, in an effort to decrease perinatal complications and stillbirths. 85 This exemplifies real‐time AI‐assisted monitoring of streaming data to reduce manual error associated with human interpretation of cardiotocography data during childbirth.
AI is also being used in the detection and characterization of polyps in colonoscopy. 86 Wider adaptation of AI during endoscopy may lead to a higher rate of benign adenoma detection and reduction of cost and risk for unwarranted polypectomy. 87 AI‐mediated image analysis aimed at improving disease risk prediction and diagnosis will likely continue to increase in use for detection of diabetic retinopathy 88 and metastasis in cancer, 89 as well as for identification of benign melanoma. 90 AI‐based image analysis has become a part of a direct‐to‐consumer diagnostic tool for anemia as well. 91
The widespread use of home monitoring and wearable devices has long been accompanied by the expectation that collected data could help detect disease at an earlier state. Indeed, these advances have fueled new, noninvasive, wearable applications for monitoring and detecting specific health conditions, such as diabetes, epilepsy, pain management, Parkinson’s disease, cardiovascular disease, sleep disorders, and obesity. 92 Digital biomarkers are expected to facilitate remote disease monitoring outside of the physical confines of a hospital setting and can support decentralized clinical trials. 93 Wearable tools that provide continuous multidimensional measurements of preselected biomarkers would enable the detection of minimum residual disease and monitor disease progression. 94 In the field of cancer care, evolving technology using wearable devices continuously analyzes circulating tumor cells to screen for relapsed disease. 95
Ongoing Challenges Using AI in Precision Medicine
We have observed increasing efforts to implement AI in precision medicine to perform tasks such as disease diagnosis, predicting risk, and treatment response. Although most of these studies showed promising experimental results, how AI improved health care is not fully demonstrated. In reality, the success of transforming an AI system to a real‐world application not only depends on the accuracy but also relies on the capability of working accurately in a reliable, safe, and generalizable manner. 5 For example, the difference among institutions in coding definitions, report formats, or cohort diversity, may result in a model trained using one‐site data to not work well in another site (https://www.bmj.com/content/368/bmj.l6927). Here, we highlighted three main challenges that would impact the success of transitions to real‐world healthcare.
Fairness and bias. The health data can be biased while building and processing the dataset (e.g., a lack of diverse sampling, missing values, and imputation methods; https://datasociety.net/library/fairness‐in‐precision‐medicine/). An AI model trained on the data might amplify the bias and make nonfavorable decisions toward a particular group of people characterized by age, gender, race, geographic, or economic level. Such unconscious bias may harm clinical applicability and health quality. Thus, it is crucial to detect and mitigate the bias in data and models. Some potential solutions include improving the diversity of the data, such as the All of Us program that aimed to recruit participants with diverse backgrounds. AI communities also proposed several techniques to fight against bias (https://arxiv.org/abs/1908.09635). IBM has developed an online toolkit (AI Fairness 360) that implemented a comprehensive set of fairness metrics to help researchers examine the bias among datasets and models, and algorithms to mitigate bias in classifiers (https://doi.org/10.1147/JRD.2019.2942287). However, fairness and protected attributes are closely related to the domain context and applications. More work is needed in biomedical research to define and explore the fairness and bias in AI models trained with historical patient data. To address the challenge, a collaborative effort that involves the AI and biomedical community is needed.
Socio‐environmental factors. The environmental factors and workflows where the AI model would be deployed may impact model performance and clinical efficacy. A recent prospective study carried out by Google Health evaluated an AI system for screening diabetic retinopathy in a real clinical environment. The AI system was developed to augment diabetic retinopathy screening by providing in‐time assessment, before this the process may take several weeks. Despite a specialist‐level accuracy (> 90% sensitivity and specificity) achieved on retrospective patient data; however, the system has undergone unexpected challenges when applied to Thailand clinics (https://doi.org/10.1145/3313831.3376718). For example, the variety of conditions and workflows in clinics impaired the quality of the images that did not meet the system'’ high standards, resulting in a high rejection rate of images. The unstable internet connection restricted the processing speed of the AI models and caused a longer waiting time for the patients. Travel and travel costs may deter participants from remaining in the study. Such prospective studies highlighted the importance of validating the AI models in the clinical environment and considering an iteration loop—that collects users’ feedback as new input for learning and system improvement 96 before applying the AI system widely. Of note, in healthcare, obtaining such feedback would take a long time at a high cost. It may take a longer time to evaluate a therapy’s effect and associated long‐term health outcomes than what is required to validate whether a product is appealing to a customer. There is a need to explore other ways to facilitate creation of high‐performing AI systems, for example, generating synthetic data that carries similar distributions and variances as the real‐world data, or leveraging a simulated environment. Early examples by groups, such as Baowaly and colleagues, 89 demonstrate much promise, but more AI research efforts are needed.
Data safety and privacy. Data is crucial to an AI‐driven system. As AI and precision medicine are converging, data (e.g., genomics, medical history, behaviors, and social data that covers peoples’ daily lives) will be increasingly collected and integrated. Individuals’ concerns for data privacy are closely related to trust when they use AI‐enabled services. Building a safe and well‐controlled ecosystem for data storage, management, and sharing is essential, requiring new technology adoptions, and collaborations, as well as the creation of new regulations and business models.
Discussion
The training of AI methods and validation of AI models using large data sets prior to applying the methods to personal data may address many of the challenges facing precision medicine today. The cited examples reinforce the importance of another potential use of augmented intelligence, namely that of the role of technology in the hands of consumers to help communicate “just‐in‐time” risk or as an agent of behavior change. Although most studies to date are small and the data are limited, the ability to identify at‐risk patients will translate into personalized care when identification is combined with strategies to notify and intervene. Researchers are actively pursuing the use of mobile apps, wearables, voice assistants, and other technology to create person‐specific interfaces to intelligent systems. A review of these approaches is beyond the scope of this paper.
Summary
Active research in both AI and precision medicine is demonstrating a future where health‐related tasks of both medical professionals and consumers are augmented with highly personalized medical diagnostic and therapeutic information. The synergy between these two forces and their impact on the healthcare system aligns with the ultimate goal of prevention and early detection of diseases affecting the individual, which could ultimately decrease the disease burden for the public at large, and, therefore, the cost of preventable health care for all.
Funding
This work was funded by a partnership between IBM Watson Health and Vanderbilt University Medical Center.
Conflict of Interest
Drs. Weeraratne, Rhee, and Snowdon are employed by IBM Watson Health. All other authors declared no competing interests for this work.
Acknowledgment
The authors thank Karlis Draulis for his assistance with the figures.
References
- 1. Matheny, M. E. et al AI in Health Care: The Hope, the Hype, the Promise, the Peril (National Academy of Medicine, Washington, DC, 2019). [Google Scholar]
- 2. Topol, E. J. High‐performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56 (2019). [DOI] [PubMed] [Google Scholar]
- 3. Hashimoto, D.A. et al Artificial intelligence in surgery: promises and perils. Ann. Surg. 268, 70–76 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayo, R.C. & Leung, J. Artificial intelligence and deep learning ‐ radiology's next frontier? Clin. Imaging 49, 87–88 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Wang, F. & Preininger, A. AI in health: state of the art, challenges, and future directions. Yearb. Med. Inform. 28, 16–26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGinnis, J.M. et al The case for more active policy attention to health promotion. Health Aff. (Millwood) 21, 78–93 (2002). [DOI] [PubMed] [Google Scholar]
- 7. IBM and Partners to Transform Personal Health with Watson and Open Cloud. <https://www‐03.ibm.com/press/us/en/pressrelease/46580.wss> 2015.
- 8. Joudaki, H. et al Improving fraud and abuse detection in general physician claims: a data mining study. Int. J. Health Policy Manag. 5, 165–172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davenport, T. & Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 6, 94–98 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iowa Medicaid Enterprise: State Medicaid program recovers millions in fraudulent and wasteful claims. Case Studies 2019 (cited March 27, 2020). <https://www.ibm.com/case‐studies/iowa‐medicaid‐enterprise‐watson‐health>.
- 11. Patel, N.M. et al Enhancing next‐generation sequencing‐guided cancer care through cognitive computing. Oncologist 23, 179–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajkomar, A. et al Scalable and accurate deep learning with electronic health records. NPJ Digit. Med. 1, 18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sadilek, A. et al Machine‐learned epidemiology: real‐time detection of foodborne illness at scale. NPJ Digit. Med. 1, 36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Snowdon, J.L. et al Empowering caseworkers to better serve the most vulnerable with a cloud‐based care management solution. Appl. Clin. Inform. 11, 617–621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaishya, R. et al Artificial intelligence (AI) applications for COVID‐19 pandemic. Diabetes Metab. Syndr. 14, 337–339 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aronson, S.J. & Rehm, H.L. Building the foundation for genomics in precision medicine. Nature 526, 336–342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson, J.L. et al Randomized trial of genotype‐guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 116, 2563–2570 (2007). [DOI] [PubMed] [Google Scholar]
- 18. Hamet, P. & Tremblay, J. Artificial intelligence in medicine. Metabolism 69S, S36–S40 (2017). [DOI] [PubMed] [Google Scholar]
- 19. Topol, E.J. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again (Basic Books, New York, NY, 2019). [Google Scholar]
- 20. Davenport, H.T. et al Using AI to improve electronic health records. Harvard Bus. Rev. 12, 1–6 (2018). [Google Scholar]
- 21. Biamonte, J. et al Quantum machine learning. Nature 549, 195–202 (2017). [DOI] [PubMed] [Google Scholar]
- 22. Gil, D. et al Research insights: coming soon to your business ‐‐ quantum computing: five strategies to prepare for the paradigm‐shifting technology. <https://www.ibm.com/downloads/cas/OV1V0NLX> (2018).
- 23. Ashby, W.R. An Introduction to Cybernetics (Chapman & Hall Ltd., London, UK, 1957). [Google Scholar]
- 24. Friedman, C.P. A "fundamental theorem" of biomedical informatics. J. Am. Med. Inform. Assoc. 16, 169–170 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanford University . Rads who use AI will replace rads who don’t. Center for Artificial Intelligence in Medicine & Imaging 2017 <https://aimi.stanford.edu/about/news/rsna‐2017‐rads‐who‐useai‐will‐replace‐rads‐who‐don‐t>.
- 26. Kelly, J.E. & Hamm, S. Smart Machines: IBM's Watson and the Era of Cognitive Computing (Columbia Business School Publishing, New York, NY, 2013). [Google Scholar]
- 27. McNamara, D.M. et al Differential impact of cognitive computing augmented by real world evidence on novice and expert oncologists. Cancer Med. 8, 6578–6584 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller, D.D. & Brown, E.W. How cognitive machines can augment medical imaging. AJR Am. J. Roentgenol. 212, 9–14 (2019). [DOI] [PubMed] [Google Scholar]
- 29. Lin, S.Y. et al Ten ways artificial intelligence will transform primary care. J. Gen. Intern. Med. 34, 1626–1630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akselrod‐Ballin, A. et al Predicting breast cancer by applying deep learning to linked health records and mammograms. Radiology 292, 331–342 (2019). [DOI] [PubMed] [Google Scholar]
- 31. National Research Council . Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease (The National Academies Press, Washington, DC, 2011). [PubMed] [Google Scholar]
- 32. Ziegelstein, R.C. Personomics and precision medicine. Trans. Am. Clin. Climatol. Assoc. 128, 160–168 (2017). [PMC free article] [PubMed] [Google Scholar]
- 33. van der Schee, M. et al Breath biopsy for early detection and precision medicine in cancer. Ecancermedicalscience 12, ed84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartmaier, R.J. et al High‐throughput genomic profiling of adult solid tumors reveals novel insights into cancer pathogenesis. Cancer Res. 77, 2464–2475 (2017). [DOI] [PubMed] [Google Scholar]
- 35. Jorgensen, A.L. et al Implementation of genotype‐guided dosing of warfarin with point‐of‐care genetic testing in three UK clinics: a matched cohort study. BMC Med. 17, 76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caudle, K.E. et al Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr. Drug Metab. 15, 209–217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bycroft, C. et al The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagai, A. et al Overview of the BioBank Japan Project: study design and profile. J. Epidemiol. 27(3S), S2–S8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stark, Z. et al Australian genomics: a federated model for integrating genomics into healthcare. Am. J. Hum. Genet. 105, 7–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arnar, D.O. & Palsson, R. Precision medicine and advancing clinical care: insights from iceland. JAMA Intern. Med. 179, 139–140 (2019). [DOI] [PubMed] [Google Scholar]
- 41. Pulley, J.M. et al Accelerating precision drug development and drug repurposing by leveraging human genetics. Assay Drug Dev. Technol. 15, 113–119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gottesman, O. et al The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet. Med. 15, 761–771 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pulley, J.M. et al Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin. Pharmacol. Ther. 92, 87–95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schildcrout, J.S. et al A prognostic model based on readily available clinical data enriched a pre‐emptive pharmacogenetic testing program. J. Clin. Epidemiol. 72, 107–115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deep learning for genomics. Nat. Genet. 51, 1 (2019). [DOI] [PubMed] [Google Scholar]
- 46. Zou, J. et al A primer on deep learning in genomics. Nat. Genet. 51, 12–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kagechika, H. et al Retinobenzoic acids. 2. Structure‐activity relationships of chalcone‐4‐carboxylic acids and flavone‐4'‐carboxylic acids. J. Med. Chem. 32, 834–840 (1989). [DOI] [PubMed] [Google Scholar]
- 48. Sengupta, S. et al The evolution of medulloblastoma therapy to personalized medicine. F1000Res. 6, 490 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoang, D.H. et al Cognitive disorders in pediatric medulloblastoma: what neuroimaging has to offer. J. Neurosurg. Pediatr. 14, 136–144 (2014). [DOI] [PubMed] [Google Scholar]
- 50. Sherif, R.S. et al The risk of secondary cancer in pediatric medulloblastoma patients due to three‐dimensional conformal radiotherapy and intensity‐modulated radiotherapy. Indian J. Cancer 55, 372–376 (2018). [DOI] [PubMed] [Google Scholar]
- 51. Trivizakis, E. et al Artificial intelligence radiogenomics for advancing precision and effectiveness in oncologic care (Review). Int. J. Oncol. 57, 43–53 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu, Z. et al Deep learning for identifying radiogenomic associations in breast cancer. Comput. Biol. Med. 109, 85–90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bibault, J.E. et al Deep Learning and Radiomics predict complete response after neo‐adjuvant chemoradiation for locally advanced rectal cancer. Sci. Rep. 8, 12611 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trivizakis, E. et al Extending 2‐D convolutional neural networks to 3‐D for advancing deep learning cancer classification with application to MRI liver tumor differentiation. IEEE J. Biomed. Health Inform. 23, 923–930 (2019). [DOI] [PubMed] [Google Scholar]
- 55. Huang, C. et al Machine learning predicts individual cancer patient responses to therapeutic drugs with high accuracy. Sci. Rep. 8, 16444 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Poudel, P. et al Heterocellular gene signatures reveal luminal‐A breast cancer heterogeneity and differential therapeutic responses. NPJ Breast Cancer 5, 21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Biederman, D.J. et al Identifying patients experiencing homelessness in an electronic health record and assessing qualification for medical respite: a five‐year retrospective review. J. Health Care Poor Underserved 30, 297–309 (2019). [DOI] [PubMed] [Google Scholar]
- 58. Brown, M. et al Applying a time‐patterned typology of homelessness among individuals with mental illness. Am. J. Community Psychol. 59, 306–315 (2017). [DOI] [PubMed] [Google Scholar]
- 59. Roy, L. et al Occupation‐based practices and homelessness: a scoping review. Can. J. Occup. Ther. 84, 98–110 (2017). [DOI] [PubMed] [Google Scholar]
- 60. Poostchi, M. et al Image analysis and machine learning for detecting malaria. Transl. Res. 194, 36–55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. William, W. et al A review of image analysis and machine learning techniques for automated cervical cancer screening from pap‐smear images. Comput. Methods Program Biomed. 164, 15–22 (2018). [DOI] [PubMed] [Google Scholar]
- 62. Chae, S. et al Predicting infectious disease using deep learning and big data. Int. J. Environ. Res. Public Health 15, 1596 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qian, F. et al Direct prediction of the toxic gas diffusion rule in a real environment based on LSTM. Int. J. Environ. Res. Public Health 16, 2133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zewdie, G.K. et al Applying deep neural networks and ensemble machine learning methods to forecast airborne ambrosia pollen. Int. J. Environ. Res. Public Health 16, 1992 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Parreco, J. et al Using artificial intelligence to predict prolonged mechanical ventilation and tracheostomy placement. J. Surg. Res. 228, 179–187 (2018). [DOI] [PubMed] [Google Scholar]
- 66. Qiu, Q. , et al Development and validation of three machine‐learning models for predicting multiple organ failure in moderately severe and severe acute pancreatitis. BMC Gastroenterol. 19, 118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Silva, A. et al Rating organ failure via adverse events using data mining in the intensive care unit. Artif. Intell. Med. 43, 179–193 (2008). [DOI] [PubMed] [Google Scholar]
- 68. Dunlop, K. et al In the wake of Angelina ‐ managing a family history of breast cancer. Aust. Fam. Physician 43, 76–78 (2014). [PubMed] [Google Scholar]
- 69. Wexler, A. Huntington's disease ‐ a brief historical perspective. J. Huntingtons Dis. 1, 3–4 (2012). [DOI] [PubMed] [Google Scholar]
- 70. Low, S.K. et al Breast cancer: the translation of big genomic data to cancer precision medicine. Cancer Sci. 109, 497–506 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Krittanawong, C. et al Artificial intelligence in precision cardiovascular medicine. J. Am. Coll. Cardiol. 69, 2657–2664 (2017). [DOI] [PubMed] [Google Scholar]
- 72. Maragatham, G. & Devi, S. LSTM model for prediction of heart failure in big data. J. Med. Syst. 43, 111 (2019). [DOI] [PubMed] [Google Scholar]
- 73. Ambale‐Venkatesh, B. et al Cardiovascular event prediction by machine learning: the multi‐ethnic study of atherosclerosis. Circ. Res. 121, 1092–1101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schwalm, J.D. et al Resource effective strategies to prevent and treat cardiovascular disease. Circulation 133, 742–755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. August, G.J. & Gewirtz, A. Moving toward a precision‐based, personalized framework for prevention science: introduction to the special issue. Prev. Sci. 20, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tannenbaum, C. et al Sex and gender analysis improves science and engineering. Nature 575, 137–146 (2019). [DOI] [PubMed] [Google Scholar]
- 77. Zhao, J. et al Learning from longitudinal data in electronic health record and genetic data to improve cardiovascular event prediction. Sci. Rep. 9, 717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gurovich, Y. et al Identifying facial phenotypes of genetic disorders using deep learning. Nat. Med. 25, 60–64 (2019). [DOI] [PubMed] [Google Scholar]
- 79. Clark, M.M. et al Diagnosis of genetic diseases in seriously ill children by rapid whole‐genome sequencing and automated phenotyping and interpretation. Sci. Transl. Med. 11, eaat6177 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Konig, A. et al Automatic speech analysis for the assessment of patients with predementia and Alzheimer's disease. Alzheimers Dement. (Amst.). 1, 112–124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Garcia, A.M. et al How language flows when movements don't: an automated analysis of spontaneous discourse in Parkinson's disease. Brain Lang. 162, 19–28 (2016). [DOI] [PubMed] [Google Scholar]
- 82. Konig, A. et al Use of speech analyses within a mobile application for the assessment of cognitive impairment in elderly people. Curr. Alzheimer Res. 15, 120–129 (2018). [DOI] [PubMed] [Google Scholar]
- 83. Corcoran, C.M. et al Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry 17, 67–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ben‐Zeev, D. et al Next‐generation psychiatric assessment: Using smartphone sensors to monitor behavior and mental health. Psychiatr. Rehabil. J. 38, 218–226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fergus, P. et al Classification of caesarean section and normal vaginal deliveries using foetal heart rate signals and advanced machine learning algorithms. Biomed. Eng. Online 16, 89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kudo, S.E. et al Artificial intelligence and colonoscopy: current status and future perspectives. Dig. Endosc. 31, 363–371 (2019). [DOI] [PubMed] [Google Scholar]
- 87. Mori, Y. et al Simultaneous detection and characterization of diminutive polyps with the use of artificial intelligence during colonoscopy. VideoGIE. 4, 7–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ting, D.S.W. et al Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 318, 2211–2223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ehteshami Bejnordi, B. et al Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 318, 2199–2210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Han, S.S. et al Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J. Invest. Dermatol. 138, 1529–1538 (2018). [DOI] [PubMed] [Google Scholar]
- 91. Mannino, R.G. et al Smartphone app for non‐invasive detection of anemia using only patient‐sourced photos. Nat. Commun. 9, 4924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Piwek, L. et al The rise of consumer health wearables: promises and barriers. PLoS Med. 13, e1001953 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Coravos, A. et al Developing and adopting safe and effective digital biomarkers to improve patient outcomes. NPJ Digit. Med. 2, 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gold, M. et al Digital technologies as biomarkers, clinical outcomes assessment, and recruitment tools in Alzheimer's disease clinical trials. Alzheimers Dement. (NY) 4, 234–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kim, T.H. et al A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat. Commun. 10, 1478 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Abramoff, M.D. et al Pivotal trial of an autonomous AI‐based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit. Med. 1, 39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


