Abstract
Extranodal natural killer/T‐cell lymphoma, nasal type (ENKL) is a rare peripheral T‐cell lymphoma that predominantly occurs in Asian and South American populations. The treatment of ENKL has been a challenge for a long time. This study was conducted to compare the clinical efficacy and safety of cisplatin, dexamethasone, gemcitabine, and pegaspargase (DDGP) and methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide (SMILE) regimens for relapsed/refractory ENKL and explore the prognostic factors. From October 2014 to July 2019, 54 patients with relapsed/refractory ENKL who received DDGP or SMILE chemotherapy were retrospectively assessed in this study. Thirty‐one patients received DDGP chemotherapy and 23 patients received SMILE chemotherapy. A higher complete response rate was observed in patients treated with DDGP regimen (61.3% vs. 30.4%, P = 0.025). The DDGP group (95% confidence interval (CI) of 5‐year progression‐free survival (PFS): 24.6–66.2%; 95% CI of 5‐year overall survival (OS): 8.5–91.7%) was also significantly associated with longer 5‐year PFS and 5‐year OS (P = 0.008 for 5‐year PFS, P = 0.023 for 5‐year OS). More serious leucopenia (P = 0.021), neutropenia (P = 0.041), and allergy (P = 0.040) were observed in the SMILE group. Post‐treatment Epstein–Barr virus (EBV)‐DNA status (P = 0.001 for PFS, P = 0.018 for OS) was identified as a significant prognostic factor for PFS and OS in multivariate analysis. The present research suggested that compared with SMILE chemotherapy, DDGP chemotherapy can significantly improve the response and survival of relapsed/refractory ENKL with better tolerance. Post‐treatment EBV‐DNA status was identified as a significant prognostic factor for PFS and OS in relapsed/refractory ENKL.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Extranodal natural killer/T‐cell lymphoma (ENKL) is a rare and distinct entity in the World Health Organization (WHO) classification with poor prognosis. In the latest National Comprehensive Cancer Network (NCCN) guidelines, the cisplatin, dexamethasone, gemcitabine, and pegaspargase (DDGP) chemotherapy is recommended for the first time as an induction regimen for ENKL.
WHAT QUESTION DID THIS STUDY ADDRESS?
The study compared the safety and efficacy of DDGP and methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide (SMILE) regimens for the treatment of relapsed/refractory ENKL, and explored the prognostic factors of relapsed/refractory ENKL.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The results indicate that DDGP chemotherapy resulted in significant improvement in response and survival compared with SMILE chemotherapy for relapsed/refractory ENKL with better tolerance. Post‐treatment Epstein–Barr virus‐DNA status served as an independent prognostic factor for relapsed/refractory ENKL.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
SMILE chemotherapy has been regarded as a main treatment for patients with ENKL for many years. Through the initial comparison of the two regimens, we found that the DDGP regimen may be a better option for patients with relapsed/refractory ENKL.
Extranodal natural killer/T‐cell lymphoma, nasal type (ENKL) is a rare, aggressive and distinct entity characterized by its association with Epstein‐Barr virus (EBV). 1 , 2 ENKL can cause destruction in the involved site because of its highly invasive behavior, which greatly affects patients’ quality of life. In addition, ~ 30–50% patients will relapse after initial treatment within 5 years, and the treatment of relapsed/refractory ENKL remains challenging. 3
Although no standard protocol has been established by the National Comprehensive Cancer Network (NCCN) guidelines, asparaginase‐based regimes, such as methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide (SMILE) regimen are recommended therapeutic options for ENKL. 4 , 5 However, SMILE chemotherapy can lead to serious hematologic toxicity, which may cause related infections or even death. 6 , 7
To explore the possibility of higher efficacy and lower toxicity chemotherapy for ENKL, a novel regimen: cisplatin, dexamethasone, gemcitabine, and pegaspargase(DDGP) regimen was formulated by the Lymphoma Center of the First Affiliated Hospital of Zhengzhou University. In the latest NCCN guidelines, the DDGP chemotherapy is recommended for the first time as an induction regimen for ENKL. 8 , 9
In our previous study, Zhou and colleagues showed that 17 patients with relapsed/refractory ENKL receiving the DDGP regimen as salvage treatment had an overall response rate (ORR) of 88.2%. 10 The DDGP regimen has preliminarily shown efficacy for relapsed/refractory ENKL.
Worldwide, there are few reports of comparison between DDGP and SMILE regimens in patients with relapsed/refractory ENKL. Herein, we summarized and analyzed retrospectively 54 cases in our center to supplement and improve DDGP regimen for further clinical practice.
METHODS
Patients
From October 2014 to July 2019, a cohort of 54 patients with relapsed/refractory ENKL was treated with the DDGP or SMILE regimen, at the Department of Medical Oncology, the First Affiliated Hospital of Zhengzhou University. Diagnosis of ENKL was based on clinical features, histopathologic morphology, and immunohistochemistry analysis (CD2+, cytoplasmic CD3ε+, CD43+, CD56+, TIA‐1+, granzyme B+, EBER+, surface CD3−, and CD20−).
Disease evaluation
Pretreatment evaluations included medical history, physical examination, complete blood cell count, serum biochemistry (including hepatic function, renal function, electrolytes, lactate dehydrogenase, β2‐microglobulin, and serum EBV‐DNA levels), bone marrow biopsy, and ultrasonic inspection of superficial lymph nodes, as well as computed tomography scan of the nasal cavity, chest, and abdomen. Positron emission tomography was recommended but was not compulsory. At the end of two and four cycles and treatment completion, the computed tomography scan or positron emission tomography was used to detect residual mass and assess response. The Ann Arbor staging system was used to assess the clinical stage. Performance status (PS) was evaluated on the basis of the Eastern Cooperative Oncology Group (ECOG) scale. International Prognostic Index (IPI) scores and prognostic index of natural killer lymphoma with data for EBV‐DNA were used to determine the classification of risks. 11
Treatment protocol
Thirty‐one patients were treated with the DDGP regimen and 23 patients were treated with the SMILE regimen. The specific details of the DDGP and SMILE regimens are shown in Table 1 . For patients who experienced grade Ⅳ adverse events, doses were reduced accordingly. Patients with neutropenia and thrombocytopenia are given granulocyte colony‐stimulation factor (G‐CSF), recombinant human thrombopoietin (TPO), or interleukin‐11 (IL‐11) as supportive treatment according to NCCN guidelines or instructions: G‐CSF 2–5 μg/kg/day subcutaneously until neutrophil count > 5,000/mm3 (leukocytes > 10,000/mm3) discontinued; TPO 300 μg/kg/day subcutaneously until platelet count > 10 × 109/L or platelet count increase > 5 × 109/L discontinued; IL‐11 25–50 μg/kg/day was used subcutaneously and discontinued until platelet counts returned to normal. The dosage and administration time of G‐CSF, TPO, and IL‐11 were appropriately adjusted according to the intensity of chemotherapy and the degree of myelosuppression in patients.
Table 1.
The DDGP and SMILE regimens
| Agents | Dose | Route | Timing of treatment |
|---|---|---|---|
| DDGP | |||
| Pegaspargase | 2,500 IU/m2 | i.m. | Day 1 |
| Gemcitabine | 800 mg/m2 | i.v. | Days 1 and 8 |
| Cisplatin | 20 mg/m2 | i.v. | Days 1–4 |
| Dexamethasone | 15 mg/m2 | i.v. | Days 1–5 |
| SMILE | |||
| Methotrexate | 2 g/m2 | i.v. (6 hours) | Day 1 |
| Dexamethasone | 40 mg/m2 | i.v. | Days 2–4 |
| Ifosfamide | 1,500 mg/m2 | i.v. | Days 2–4 |
| Mesna | 300 mg/m2 × 3 | i.v. | Days 2–4 |
| Etoposide | 100 mg/m2 | i.v. | Days 2–4 |
| L‐asparaginase | 6,000 U/m2 | i.v. | Days 3–9 |
| or Pegaspargase | 2,500 IU/m2 | i.m. | Day 3 |
Cycles of DDGP and SMILE regimen were repeated every 21 days.
DDGP, cisplatin, dexamethasone, gemcitabine, and pegaspargase; SMILE, methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide.
Response and follow‐up criteria
Revised Cheson’s standard response criteria were adopted to assess treatment response. 12 Complete response (CR) was defined as no evidence of disease and disease‐related symptoms. Partial response (PR) was defined as ≥50% decrease in sum of the product of the diameters of masses and no new lesions. Stable disease was defined as a patient who failed to attain CR or PR but did not fulfill those criteria for progressive disease. Progressive disease was defined as the appearance of new sites or ≥50% increase in sum of the product of the diameter of previous lesions from nadir. The ORR was calculated according to the percentage of CR + PR patients among all patients. Progression‐free survival (PFS) was defined as the time from first dose administration to documentation of disease progression or death. Overall survival (OS) refers to the time interval starting from the day of chemotherapy to death or final follow‐up.
Toxicity criteria
Adverse reactions were monitored by physical examination, routine blood test, and plasma biochemical tests. They were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 5.0.
Statistical analysis
The clinical and laboratory data, response rate, and adverse effects between DDGP and SMILE groups were compared by χ2 test, Students’ test and Mann–Whitney U test. OS and PFS were estimated using the method of Kaplan–Meier and compared using the log rank test. Prognostic risk factors were estimated with univariate analysis. The Cox proportional hazards regression model was used to estimate the hazard ratio and 95% confidence interval (CI) of significant factors in multivariate analysis. Statistical significance was determined at a level of P < 0.05. SPSS version 21.0 was used for the statistical analysis.
RESULTS
Patients characteristics
Patients characteristics of the DDGP and SMILE groups are shown in Table 2 . The median age was 39 years (range 15–65 years). The ratio of men to women was 2.375:1. Thirty‐eight patients (70.4%) had stage Ⅲ/Ⅳ disease. Systemic B symptoms were present in 20 patients (37.0%). In the DDGP group, one patient (3.2%) was treated with radiation alone as the initial therapy. Among the 30 patients who received chemotherapy as their first‐line therapy, 10 patients (32.3%) were treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) or CHOP‐like regimen, four patients (12.9%) were treated with SMILE regimen, three patients (9.7%) were treated with cisplatin, dexamethasone, and gemcitabine regimen, five patients (16.1%) were treated with etoposide, ifosfamide, dexamethasone, and cisplatin regimen, five patients (16.1%) were treated with etoposide, ifosfamide, cisplatin, and dexamethasone regimen, two patients (6.5%) were treated with l‐asparaginase‐containing regimen, and one patient (3.2%) was treated with gemcitabine, pegaspargase, and oxaliplatin regimen. In the SMILE group, as to first‐line chemotherapy, seven patients (30.4%) were treated with CHOP or CHOP‐like regimen, two patients (8.7%) were treated with the cisplatin, dexamethasone, and gemcitabine regimen, six patients (26.1%) were treated with DDGP regimen, four patients (17.4%) were treated with etoposide, ifosfamide, cisplatin, and dexamethasone regimen, three patients (13.1%) were treated with the etoposide, ifosfamide, dexamethasone, and cisplatin regimen, and one patient (4.3%) was treated with the gemcitabine, pegaspargase, and oxaliplatin regimen.
Table 2.
Patients characteristics of DDGP and SMILE groups
| Characteristic | Number of patients (%) | P value | |
|---|---|---|---|
| DDGP (N = 31) | SMILE (N = 23) | ||
| Age, years | |||
| Median | 39 | 37 | 0.123 |
| Range | 21–65 | 15–61 | |
| Sex | |||
| Male | 22 (71.0%) | 16 (69.6%) | 0.911 |
| Female | 9 (29.0%) | 7 (30.4%) | |
| Disease status | |||
| Relapse | 16 (51.6%) | 15 (65.2%) | 0.317 |
| Refractory | 15 (48.4%) | 8 (34.8%) | |
| Ann Arbor stage | |||
| Ⅰ/Ⅱ | 11 (35.5%) | 5 (21.7%) | 0.274 |
| Ⅲ/Ⅳ | 20 (64.5%) | 18 (78.3%) | |
| B symptoms present | 10 (32.3%) | 10 (43.5%) | 0.399 |
| Elevated β2‐microglobulin | 8 (25.8%) | 8 (34.8%) | 0.475 |
| Elevated LDH | 11 (35.5%) | 13 (56.5%) | 0.124 |
| Pretreatment EBV‐DNA | |||
| Positive | 15 (48.4%) | 9 (39.1%) | 0.498 |
| Negative | 16 (51.6%) | 14 (60.9%) | |
| IPI | |||
| 0–2 | 13 (41.9%) | 8 (34.8%) | 0.594 |
| 3–5 | 18 (58.1%) | 15 (65.2%) | |
| PINK‐E | |||
| 0–2 | 14 (45.2%) | 13 (56.5%) | 0.409 |
| 3–5 | 17 (54.8%) | 10 (43.5%) | |
| ECOG PS | |||
| 0–1 | 19 (61.3%) | 9 (39.1%) | 0.107 |
| 2–4 | 12 (38.7%) | 14 (60.9%) | |
DDGP, cisplatin, dexamethasone, gemcitabine, and pegaspargase; EBV, Epstein–Barr virus; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; LDH, lactate dehydrogenase; PINK‐E, prognostic index of natural killer lymphoma with data for EBV‐DNA; SMILE, methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide.
Treatment
The median number of cycles was four (range 2–6 cycles for the DDGP regimen; range 1–6 cycles for the SMILE regimen). In the SMILE group, one patient received one cycle treatment because of severe myelosuppression and sepsis, and died within 2 months. Four cases in the SMILE group and two cases in the DDGP group received two cycles due to tumor progression. In the SMILE group, 14 patients used L‐asparaginase, whereas the other 9 patients used pegaspargase. One patient in the DDGP group underwent autologous hematopoietic stem cell transplantation after achieving interim CR, and the patient is still in remission.
Efficacy and survival
As shown in Table 3 , a higher CR rate (61.3% vs. 30.4%, P = 0.025) was observed among the patients treated with the DDGP regimen, although the ORR (83.9% vs. 60.9%, P = 0.056) showed no significant differences between the two groups. The median follow‐up time was 15.5 months (range 2–62 months) in the entire cohort, and the 5‐year PFS (45.4% vs. 27.6%, P = 0.008) and 5‐year OS (50.1% vs. 32.5%, P = 0.023) in the DDGP group (95% CI of 5‐year PFS: 24.6–66.2%; 95% CI of 5‐year OS: 8.5–91.7%) were significantly better than those in the SMILE group (95% CI of 5‐year PFS: 8.2–47.0%; 95% CI of 5‐year OS: 5.3–59.7%; Figure 1 ).
Table 3.
Response rates of DDGP and SMILE regimens
| Response | Number of patients (%) | P value | |
|---|---|---|---|
| DDGP (N = 31) | SMILE (N = 23) | ||
| CR | 19 (61.3) | 7 (30.4) | 0.025* |
| PR | 7 (22.6) | 7 (30.4) | — |
| SD | 1 (3.2) | 1 (4.3) | — |
| PD | 4 (12.9) | 8 (34.8) | — |
| ORR | 26 (83.9) | 14 (60.9) | 0.056 |
CR, complete response; DDGP, cisplatin, dexamethasone, gemcitabine, and pegaspargase; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; SMILE, methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide.
P < 0.05.
Figure 1.
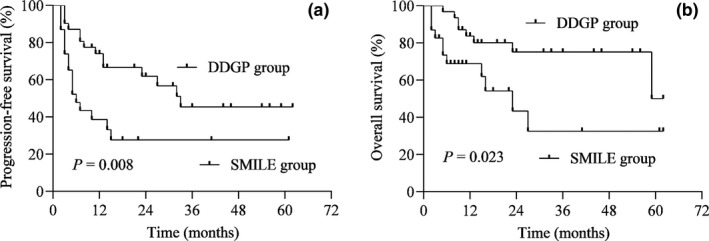
Kaplan–Meier survival curves for all patients with relapsed/refractory extranodal natural killer/T‑cell lymphoma, nasal type (ENKL) treated with cisplatin, dexamethasone, gemcitabine, and pegaspargase (DDGP) or methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide (SMILE) regimen. (a) Progression‐free survival (PFS) is shown for all patients, showing that the DDGP group has a better PFS than the SMILE group (P = 0.008). (b) Overall survival (OS) is shown for all patients, showing that the DDGP group has a better OS than the SMILE group (P = 0.023).
Adverse events
The adverse reactions observed in the patients in the two groups were shown in Table 4 . The primary adverse events included bone marrow suppression, gastrointestinal events, and liver and coagulation dysfunctions, most of which were relieved after active symptomatic treatments. Compared with the SMILE group, the DDGP group had less instances of leukopenia (P = 0.025), neutropenia (P = 0.041), and allergy (P = 0.040). In the SMILE group, one patient discontinued l‐asparaginase because of a grade 3 allergic reaction. Moreover, although sufficient doses of leucovorin had been used for rescue after high‐dose methotrexate treatment, two cases experienced grade 1/2 mucositis in the SMILE group. No allergy, mucositis, or treatment‐related death occurred in the DDGP group.
Table 4.
Adverse events between DDGP and SMILE groups
| Toxicity | Grade of adverse reaction | P value | |||||
|---|---|---|---|---|---|---|---|
| DDGP (N = 31) | SMILE (N = 23) | ||||||
| Grade | 0 | 1/2 | 3/4 | 0 | 1/2 | 3/4 | |
| Hematologic | |||||||
| Leukopenia | 1 | 12 | 18 | 0 | 3 | 20 | 0.021* |
| Neutropenia | 0 | 10 | 21 | 0 | 2 | 21 | 0.041* |
| Anemia | 3 | 12 | 16 | 1 | 10 | 12 | 0.829 |
| Thrombocytopenia | 2 | 11 | 18 | 2 | 6 | 15 | 0.677 |
| Nonhematologic | |||||||
| Hypofibrinogenemia | 13 | 17 | 1 | 12 | 9 | 2 | 0.637 |
| Prolonged APTT | 18 | 13 | 0 | 16 | 7 | 0 | 0.391 |
| Hyperbilirubinemia | 23 | 7 | 1 | 18 | 3 | 2 | 0.833 |
| ALT elevation | 12 | 19 | 0 | 11 | 7 | 5 | 0.771 |
| AST elevation | 14 | 17 | 0 | 12 | 6 | 5 | 0.734 |
| Creatinine | 29 | 2 | 0 | 20 | 3 | 0 | 0.413 |
| BUN | 29 | 2 | 0 | 20 | 3 | 0 | 0.413 |
| Nausea/vomiting | 0 | 23 | 8 | 0 | 18 | 5 | 0.732 |
| Mucositis | 31 | 0 | 0 | 21 | 2 | 0 | 0.097 |
| Allergy | 31 | 0 | 0 | 20 | 2 | 1 | 0.040* |
ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; DDGP, cisplatin, dexamethasone, gemcitabine, and pegaspargase; SMILE, methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide.
P < 0.05.
Prognostic factors
The correlation between clinical characteristics and survival was evaluated using univariate and multivariate analyses. In univariate analysis (Table 5 ), results demonstrated that stage III/IV (P = 0.007), pretreatment EBV‐DNA positivity (P < 0.001), post‐treatment EBV‐DNA positivity (P < 0.001), high score of IPI (P = 0.017), and poor ECOG PS (P < 0.001) were correlated with a worse OS. In terms of PFS, patients with stage III/IV (P = 0.007), pretreatment EBV‐DNA positivity (P = 0.006), post‐treatment EBV‐DNA positivity (P < 0.001), and poor ECOG PS (P < 0.001) had inferior survival. Therefore, stage, score of ECOG PS, treatment, pretreatment EBV‐DNA status, and post‐treatment EBV‐DNA status were put into the multivariate model. The results showed that post‐treatment EBV‐DNA status (P = 0.001 for PFS, and P = 0.018 for OS) and treatment (P = 0.008 for PFS, and P = 0.040 for OS) were independent factors impacting PFS and OS (Table 6 ).
Table 5.
Univariate analysis of prognostic factors for PFS and OS
| Factors | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age ≥ 60 years | 28.200 (11.219–45.182) | 0.676 | 28.000 (12.968–23.220) | 0.810 |
| Refractory disease | 27.105 (16.829–37.381) | 0.760 | 41.065 (30.193–51.937) | 0.883 |
| B symptoms present | 32.733 (20.102–45.365) | 0.622 | 41.011 (28.993–53.029) | 0.916 |
| ECOG PS (2–4) | 13.054 (8.234–17.875) | < 0.001* | 18.094 (12.968–23.220) | < 0.001* |
| Stage Ⅲ/Ⅳ | 22.628 (14.053–31.204) | 0.007* | 34.176 (24.978–43.375) | 0.007* |
| IPI (3–5) | 24.076 (14.868–33.283) | 0.104 | 33.625 (23.924–43.326) | 0.017* |
| Pretreatment EBV‐DNA positivity | 14.080 (9.140–19.019) | 0.006* | 26.937 (16.233–37.641) | < 0.001* |
| Post‐treatment EBV‐DNA positivity | 29.696 (22.240–37.152) | < 0.001* | 20.282 (6.502–34.063) | < 0.001* |
| Elevated LDH | 27.445 (16.437–38.453) | 0.698 | 36.816 (25.784–47.848) | 0.255 |
| Elevated β2‐microglobulin | 30.406 (19.872–36.780) | 0.866 | 37.667 (25.266–50.067) | 0.455 |
CI, confidence interval; EBV, Epstein‐Barr virus; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; IPI, International Prognostic Index; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression‐free survival.
P < 0.05.
Table 6.
Multivariate analysis of prognostic factors for PFS and OS
| Factors | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| ECOG PS (2–4) | 2.185 (0.778–6.131) | 0.138 | 3.945 (0.851–18.295) | 0.080 |
| Stage Ⅲ/Ⅳ | 1.201 (0.351–4.112) | 0.770 | 1.581 (0.159–15.697) | 0.696 |
| Treatment (SMILE) | 3.085 (1.334–7.136) | 0.008* | 2.860 (1.050–7.787) | 0.040* |
| Pre‐treatment EBV‐DNA positivity | 1.101 (0.376–3.221) | 0.861 | 1.785 (0.407–7.817) | 0.442 |
| Post‐treatment EBV‐DNA positivity | 5.233 (1.958–13.984) | 0.001* | 4.197 (1.285–13.714) | 0.018* |
CI, confidence interval; EBV, Epstein‐Barr virus; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival; SMILE, methotrexate, dexamethasone, ifosfamide, L‐asparaginase, and etoposide.
P < 0.05.
DISCUSSION
In recent years, the cure and survival rates of ENKL have been improved with the investigation and implication of asparaginase. In a multicenter prospective study conducted by the Asia Lymphoma Study Group, 43 patients with newly diagnosed stage IV and 44 patients with refractory/relapsed ENKL were treated with the SMILE regimen, and the results showed an overall CR of 66%. 13 In another retrospective study, 20 patients with advanced‐stage or refractory/relapsed ENKL were treated with the SMILE regimen and 45% of patients achieved CR. 14 In the present study, the CR and PR rates observed in the SMILE group were 30.4%, respectively. There are several differences from previous researches. First, the proportion of patients with poor‐risk was high (stage Ⅲ/Ⅳ disease: 78.3%, IPI of 3–5: 65.2%) in this study; second, more than half of patients had poor performance status (ECOG PS of 2–4: 60.9%) in our research, which may have a negative effect on the clinical outcomes.
Currently, there are few studies comparing the clinical efficacy and safety of the DDGP and SMILE regimens in patients with ENKL. Li and colleagues conducted a multicenter clinical trial analyzing the effectiveness and toxicity of DDGP and SMILE chemotherapies in patients with newly diagnosed, advanced‐stage ENKL, and showed that patients in the DDGP group had higher CR rate (71% vs. 29%, P = 0.005) and ORR (95% vs. 67%, P = 0.018) than those in the SMILE group. 15 Our study further expanded the sample size to include 54 patients with relapsed/refractory ENKL. The results showed that there were significant differences in CR rates (61.3% and 30.4%, P = 0.025), 5‐year PFS (P = 0.008), and 5‐year OS (P = 0.023) between the two groups. This study further confirmed that DDGP chemotherapy resulted in significant improvement in clinical outcomes compared with SMILE chemotherapy for relapsed/refractory ENKL. In the present study, although there was no statistical difference in ORR between the two groups, the ORR in the DDGP group was generally consistent with that in previous studies. 16 , 17 The difference in clinical outcomes between this study and Li’s study may be related to two key factors. First, our study only included relapsed/refractory patients, which suggested that different patient groups were selected in the two studies; second, all patients had received at least one prior treatment protocol in the present study, which means that the chemosensitivity of these patients was relatively limited.
In a phase Ⅳ study of the use of the DDGP regimen to treat patients with newly diagnosed, advanced‐stage ENKL, the analysis showed that common grade 3/4 hematological adverse events were leukopenia (58.3%), neutropenia (75.0%), and thrombocytopenia (45.8%). The main nonhematology toxicities were prolonged activated partial thromboplastin time (50.0%) and hypofibrinogenemia (58.3%). 18 In this study, the risk of leucopenia (P = 0.021) and neutropenia (P = 0.041) was lower in the DDGP group than in the SMILE group. In addition, three cases in the SMILE group developed allergy (P = 0.040) by l‐asparaginase. Studies have confirmed that pegaspargase is less immunogenic than l‐asparaginase and has a longer half‐life. 19 , 20 Common side effects related to pegaspargase include liver dysfunction, coagulation dysfunction, hypoalbuminemia, and hypertriglyceridemia. 21 , 22 This study showed that only one patient experienced grade 3/4 hypofibrinogenemia in the DDGP group. Liver dysfunction caused by pegaspargase was grade 1/2 and could be well controlled with supportive treatments. It has been reported that pegaspargase can induce pancreatitis and venous thrombosis, 23 but no such adverse events occurred in the present study.
A number of studies have demonstrated a close correlation in patients with ENKL between clinical outcomes and plasma EBV‐DNA levels. 24 , 25 Kwong and colleagues showed that patients with negative pretreatment EBV‐DNA had higher PFS (P = 0.002) and OS (P < 0.001) rates than patients with positive pretreatment EBV‐DNA. 26 In this study, multivariate analysis showed that post‐treatment EBV‐DNA positivity correlated with inferior PFS (P = 0.001) and OS (P = 0.018), suggesting that post‐treatment EBV‐DNA positivity can predict early relapse and poor prognosis for patients with relapsed/refractory ENKL. In addition, treatment was an independent prognostic factor in multivariate analysis (P = 0.008 for PFS, and P = 0.040 for OS), which indicates that for patients with relapsed/refractory ENKL, DDGP chemotherapy can significantly improve disease control compared with SMILE chemotherapy.
In conclusion, the DDGP regimen had better efficacy and lower toxicity than the SMILE regimen and may be a potential option for patients with relapsed/refractory ENKL. However, this is a retrospective study with a relatively limited sample size. Multicenter, prospective, randomized, and large sample clinical trials will be required to validate the efficacy and safety of the DDGP regimen in the treatment of relapsed/refractory ENKL.
At present, immunotherapy is a quite active research field for the treatment of relapsed/refractory oncology. 27 , 28 The DDGP regimen combined with immunotherapy, such as checkpoint inhibitors, may bring another breakthrough in the treatment of relapsed/refractory ENKL, and further research is warranted to validate this new medical combination.
Funding
This study was supported in part by National Natural Science Foundation of China (81700187).
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
X.W., J.H., M.D., M.D., L.Z., J.W., Z.S., X.L., L.Z., L.L., X.W., X.F., G.W., Q.C., M.Z., and X.Z. wrote the manuscript. X.W. and J.H. designed the research. X.W., J.H., M.D., M.D., L.Z., J.W., and Z.S. performed the research. X.W. and J.H. analyzed the data. X.W., J.H., G.W., Q.C., M.Z., and X.Z. contributed new reagents/analytical tools.
Acknowledgments
The authors acknowledge the contributions of the Departments of Medical Oncology and Pathology for study collaboration, and thank National Natural Science Foundation of China for partial financial support.
References
- 1. Cai, Q. et al Epstein‐Barr virus‐positive natural killer/T‐cell lymphoma. Front. Oncol. 9, 386 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu, Z.L. et al The clinical utility of circulating Epstein‐Barr virus DNA concentrations in NK/T‐cell lymphoma: a meta‐analysis. Dis. Markers 2018, 1961058 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang, L. et al Lymphopenia during routine follow‐up may predict relapse in patients with extranodal NK/T cell lymphoma. Tumour Biol. 36, 1747–1753 (2015). [DOI] [PubMed] [Google Scholar]
- 4. Makita, S. & Tobinai, K. Clinical features and current optimal management of natural killer/T‐cell lymphoma. Hematol. Oncol. Clin. North Am. 31, 239–253 (2017). [DOI] [PubMed] [Google Scholar]
- 5. Suzuki, R. NK/T cell lymphoma: updates in therapy. Curr. Hematol. Malig. Rep. 13, 7–12 (2018). [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi, M. et al Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T‐cell lymphoma, nasal type: the NK‐Cell Tumor Study Group study. J. Clin. Oncol. 29, 4410–4416 (2011). [DOI] [PubMed] [Google Scholar]
- 7. Yamaguchi, M. , Suzuki, R. & Oguchi, M. Advances in the treatment of extranodal NK/T‐cell lymphoma, nasal type. Blood 131, 2528–2540 (2018). [DOI] [PubMed] [Google Scholar]
- 8. Wang, X. et al Efficacy and survival in newly diagnosed advanced extranodal natural killer/T‐cell lymphoma: a randomized, controlled, multicenter and open‐labeled study with DDGP regimen versus SMILE regimen [abstract]. Blood 134, Abstract 463 (2019). [Google Scholar]
- 9. National Comprehensive Cancer Network (NCCN) . Clinical Practice Guidelines in Oncology. T‐Cell Lymphomas, Version 1. 2020. <https://www.nccn.org/professionals/physician‐gls/f_guidelines.asp>.
- 10. Zhou, Z. et al Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann. Hematol. 93, 1889–1994 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Kim, S.J. et al A prognostic index for natural killer cell lymphoma after non‐anthracycline‐based treatment: a multicentre, retrospective analysis. Lancet Oncol. 17, 389–400 (2016). [DOI] [PubMed] [Google Scholar]
- 12. Cheson, B.D. et al Revised response criteria for malignant lymphoma. J. Clin. Oncol. 25, 579–586 (2007). [DOI] [PubMed] [Google Scholar]
- 13. Kwong, Y.L. et al SMILE for natural killer/T‐cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 120, 2973–2980 (2012). [DOI] [PubMed] [Google Scholar]
- 14. Yang, L. et al Retrospective study of modified SMILE chemotherapy for advanced‐stage, relapsed, or refractory extranodal natural killer (NK)/T cell lymphoma, nasal type. Med. Oncol. 30, 720 (2013). [DOI] [PubMed] [Google Scholar]
- 15. Li, X. et al DDGP versus SMILE in newly diagnosed advanced natural killer/T‐cell lymphoma: a randomized controlled, multicenter, open‐label study in China. Clin. Cancer Res. 22, 5223–5228 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Zhao, Q. et al Clinical efficacy of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) in the initial treatment of advanced stage (stage III‐IV) extranodal NK/T‐cell lymphoma, and its correlation with Epstein‐Barr virus. Cancer Manag. Res. 11, 3555–3564 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang, L. et al The DDGP (cisplatin, dexamethasone, gemcitabine, and pegaspargase) regimen for treatment of extranodal natural killer (NK)/T‐cell lymphoma, nasal type. Oncotarget 7, 58396–58404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang, L. et al Efficacy and safety of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) regimen in newly diagnosed, advanced‐stage extranodal natural killer/T‐cell lymphoma: interim analysis of a phase 4 study NCT01501149. Oncotarget 7, 55721–55731 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim, H.J. et al Comparison of native Escherichia coli L‐Asparaginase versus pegylated asparaginase, in combination with ifosfamide, methotrexate, etoposide, and prednisolone, in extranodal NK/T‐cell lymphoma, nasal type. Cancer Res. Treat. 5, 670–680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tong, W.H. et al A prospective study on drug monitoring of PEG‐asparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood 123, 2026–2033 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu, P.P. et al A phase II study of methotrexate, etoposide, dexamethasone and pegaspargase sandwiched with radiotherapy in the treatment of newly diagnosed, stage IE to IIE extranodal natural‐killer/T‐cell lymphoma, nasal type. EBioMedicine 25, 41–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang, R. et al A phase 2 study of methotrexate, etoposide, dexamethasone, and pegaspargase chemotherapy for newly diagnosed, relapsed, or refractory extranodal natural killer/T‐cell lymphoma, nasal type: a multicenter trial in Northwest China. Hematol. Oncol. 35, 619–629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang, J.H. et al Analysis of the efficacy and safety of a combined gemcitabine, oxaliplatin and pegaspargase regimen for NK/T‐cell lymphoma. Oncotarget 7, 35412–35422 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang, Z.Y. et al Clinical implications of plasma Epstein‐Barr virus DNA in early‐stage extranodal nasal‐type NK/T‐cell lymphoma patients receiving primary radiotherapy. Blood 120, 2003–2010 (2012). [DOI] [PubMed] [Google Scholar]
- 25. Hohaus, S. et al The viral load of Epstein‐Barr virus (EBV) DNA in peripheral blood predicts for biological and clinical characteristics in Hodgkin lymphoma. Clin. Cancer Res. 17, 2885–2892 (2011). [DOI] [PubMed] [Google Scholar]
- 26. Kwong, Y.L. et al Quantification of circulating Epstein‐Barr virus DNA in NK/T‐cell lymphoma treated with the SMILE protocol: diagnostic and prognostic significance. Leukemia 28, 865–870 (2014). [DOI] [PubMed] [Google Scholar]
- 27. Ji, Y. et al Challenges and opportunities in dose finding in oncology and immuno‐oncology. Clin. Transl. Sci. 11, 345–351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merryman, R.W. et al Checkpoint blockade in Hodgkin and non‐Hodgkin lymphoma. Blood Adv. 1, 2643–2654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


