Abstract
The recent empirical use of hydroxychloroquine (HCQ) in coronavirus disease 2019 (COVID‐19) revived the interest in its cardiac toxicity, increasingly sidelined over time. We aimed to assess and compare the profile of cardiac adverse drug reactions (CADRs) associated with HCQ before and during COVID‐19. We performed a retrospective comparative observational study using the French Pharmacovigilance network database between 1985 and May 2020 to assess all postmarketing CADRs associated with HCQ notified before COVID‐19 in its approved indications for lupus and rheumatoid arthritis (preCOV), and those concerning its empirical use in COVID‐19 (COV). Eighty‐five CADR in preCOV were compared with 141 CADRs in COV. The most common CADR of preCOV were cardiomyopathies (42.4%) and conduction disorders (28.2%), both statistically more frequent than in COV (P < 0.001). COV notifications significantly highlighted repolarization and ventricular rhythm disorders (78.0%, P < 0.001) as well as sinus bradycardias (14.9%, P = 0.01) as compared with preCOV. Estimated incidence of CADR was significantly higher among patients exposed to off‐label use of HCQ in COVID‐19 (2.9%) than before COVID‐19 in its approved indications (0.01%, P < 0.001). The use of HCQ in COVID‐19 sheds a new light on the spectrum of its cardiac toxicity. This fosters the value of a closer monitoring of all patients treated with HCQ, regardless of its indication, and the importance of an update of its summary of product characteristics.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Hydroxychloroquine (HCQ) is widely prescribed, given its effectiveness in rheumatoid diseases and a purportedly limited toxicity. There has been a surge of cardiac adverse drug reactions (CADRs) notified with HCQ in off‐label use for coronavirus disease 2019 (COVID‐19).
WHAT QUESTION DID THIS STUDY ADDRESS?
How CADRs associated with HCQ in the setting of off‐label use for patients with COVID‐19 shed a new light on its safety in its approved indications?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
CADRs profile reported with HCQ was significantly different in patients with COVID‐19 compared with before. Cardiac monitoring of patients with COVID‐19 permitted early detection of HCQ CADRs.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
These results raise considerations on the importance of this monitoring even in long‐term treated patients, and highlights the need for sustained awareness toward any drugs, even old ones.
Chloroquine (CQ) and its hydroxylated analog hydroxychloroquine (HCQ) had previously proven effectiveness in systemic lupus erythematosus (SLE) 1 and rheumatoid arthritis (RA). 2 They share similar profiles characterized by a wide pharmacological distribution in the body, hence their long half‐life of > 50 days. 3 HCQ disrupts normal lysosomal functions 3 providing an immunomodulatory mechanism. Compared with CQ, HCQ has a lower toxicity, 3 a wider therapeutic index, and no increased risk of infectious or malignant complications.
HCQ has an appealing profile against severe acute respiratory syndrome‐coronavirus 2 compared with CQ given its better benefit‐risk profile. It has therefore been considered as a possible drug of interest in coronavirus disease 2019 (COVID‐19) 4 , 5 on scientific, media, and political levels, often sidelining its potential toxicity. 6 Its empirical use raised a series of concerns on cardiotoxicity and questioned its benefit‐risk ratio. 7 Practitioners having used HCQ for long periods of time in rheumatoid diseases (SLE and RA) progressively dealt with this drug as a harmless one. 8 , 9 , 10 Apart from gastrointestinal symptoms, the expected long‐term complications of HCQ are uncommon retinopathies 11 and myopathies. 12 Nevertheless, HCQ displays multiple ancillary channel blocking properties due particularly to its quinidine ring. 13 It inhibits at relevant concentrations the delayed rectifier potassium channel I K 14 , 15 as well as the sodium I Na, 16 , 17 calcium I Ca, 10 , 18 and funny I f 19 currents. They all modulate different phases of the cardiac action potential with several electrophysiological effects. An array of cardiac adverse events may occur, like rhythm and conduction disorders, as well as repolarization abnormalities, depending upon HCQ titration, individual sensitivity, and risk factors. These cardiac events are being rediscovered in the context of COVID‐19 20 , 21 , 22 and are in contrast with a prevailing feeling of harmlessness.
The aim of our study was to compare notifications of HCQ cardiac adverse drug reactions (CADRs) and their incidences through spontaneous postmarketing reporting before the COVID‐19 period (in SLE and RA) with those reported during the epidemic.
METHODS
Spontaneous notifications
The postmarketing PharmacoVigilance Database (PVD) is implemented by the national network of pharmacovigilance made of 31 regional centers of pharmacovigilance (RCPV). 23 Health professionals have to report all adverse drug reactions (ADRs), especially if serious and/or unexpected, to the National Drug Agency (Agence Nationale de Sécurité du Médicament et des produits de santé (ANSM)) via an RCPV. In turn, the PVD implements the European database EudraVigilance then the World Health Organization (WHO) database Vigibase. Anonymity of patients and notifiers is guaranteed. Given its experience in drug‐induced long QT syndrome, the RCPV of Nice was mandated on March 27, 2020, by ANSM to assess the cases of cardiotoxicity associated with HCQ used against COVID‐19.
Queries
All reported postmarketing cases involving any adverse reaction associated with HCQ according to MedDRA terminology (version 23.0) were queried by the ANSM in the PVD. Cases identified were transmitted to the RCPV of Nice and individually screened in order to identify CADRs. For each case, source of reporting, age, sex, therapeutic indication, CADR, time to onset, severity, narrative, final diagnosis, and outcome were collected whenever available. The query was performed on two periods: before onset of the COVID‐19 pandemic, that is between 1985 (creation of the PVD) and December 31, 2019 (defined as the preCOV group), and from March 25, 2020 (date of the first report of HCQ used in COVID‐19), to May 25, 2020, in the setting of its use in COVID‐19 (defined as the COV group). Electrocardiograms (ECGs) were gathered for confirmation of pathological trace and measurement of QT interval.
Assessment
Each case (whether preCOV or COV) was reviewed by two trained residents in clinical pharmacology and pharmacovigilance under the tutelage of a cardiologist specialized in drug‐acquired long QT. Corrected QT intervals (QTc) ≥ 450 ms for men and ≥ 460 ms for women were considered abnormal. 24 QTc ≥ 500 ms and prolongations of QTc (delta QTc) ≥ 60 ms from baseline were deemed serious. 25 Cases poorly documented, or with relevant alternative etiology, irreconcilable chronology, or unconfirmed prolonged QTc upon measurement without other associated CADRs were excluded. For both groups, each CADR was classified into one of the following categories: cardiomyopathies, conduction disorders, repolarization disorders (including isolated long QT), ventricular arrhythmias, and sinus bradycardias. One notification could include several CADRs.
Electrocardiograms
Digitized ECG parameters were measured by standard methods 26 with a digital caliper (Iconico, CardioCalipers). QT intervals were calculated on three consecutive complexes, if possible in lead D2, and corrected according to Bazett and Fridericia formulas. In case of enlarged QRS complex following bundle branch block or electro‐driven rhythm, QT intervals were corrected according to adapted formulae. 27
Incidence estimation of CADRs in PreCOV and COV
For the preCOV group, a 516 mg HCQ defined daily dose provided by the WHO 28 was assumed as a standard. For the COV group, a 200 mg oral t.i.d. for 10 days was agreed corresponding to the treatment scheme mostly adopted, and HCQ cumulative dose per patient amounted to 6,000 mg.
The mean monthly HCQ consumption before COVID‐19 was assessed using the national consumption data. Then, HCQ overconsumption was characterized in March to April 2020, corresponding to its off‐label use in COVID‐19.
Statistics and ethics
Descriptive statistics were expressed as mean ± SD with minimal and maximal (min/max) values according to the model mean ± SD. Percentages were calculated for qualitative data. Pearson’s χ 2 test was used to compare the proportion of each category of CADR, the proportion of symptomatic and asymptomatic CADRs in preCOV and COV, as well as the incidences. Incidence was calculated using Fisher’s exact method (Clopper–Pearson). Results were given as point estimates or 95% confidence intervals. P values < 0.05 were considered as statistically significant. Statistical analysis was performed using R statistical software 3.6.3 (R core team).
This research was approved by the Pharmacovigilance network. All our data originate from the PVD that has been approved by the National Data Protection Agency (Commission nationale de l'informatique et des libertés (CNIL)).
In accordance with European regulation, this observational study did not need approval from an institutional review board/independent ethics committee. 29
RESULTS
The queries from the PVD yielded 250 case‐reports featuring at least one CADR associated with HCQ: 92 in the preCOV group over > 30 years and 158 in the COV group over 2 months (Figure 1 ). Contractile function and conduction disorders were predominantly notified in the preCOV period, whereas repolarization abnormalities and arrhythmia prevailed during COV, as detailed below.
Figure 1.
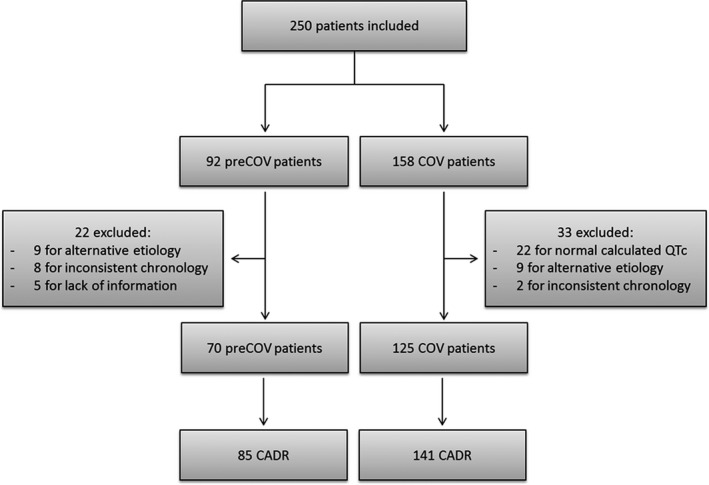
Flowchart. CADR, cardiac adverse drug reaction; COV, coronavirus; QTc, corrected QT.
PreCOV patients
Of 92 preCOV patients, 70 were retained (Figure 1 ) and 22 were excluded from the assessment. The mean age was 55 (± 16) years and 52 patients (74.3%) were women. Fourteen of 70 patients (20.0%) had at least 2 concomitant CADRs. A total of 85 different CADRs were described (Figure 2 ) with 56 (65.9%) being symptomatic (Figure 3 ). Sixty‐one of 70 reports (87.1%) were classified as serious with 5 fatal outcomes (7.1%).
Figure 2.
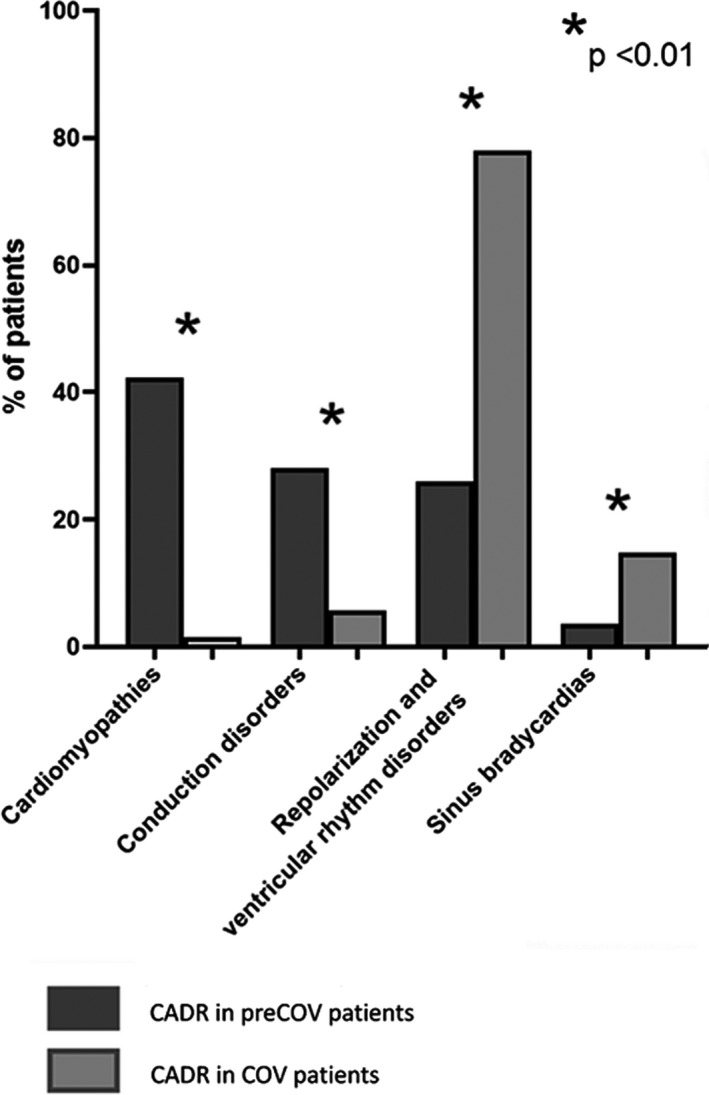
Proportion of cardiac adverse drug reactions in preCOV compared to COV. CADR, cardiac adverse drug reactions; COV, coronavirus.
Figure 3.
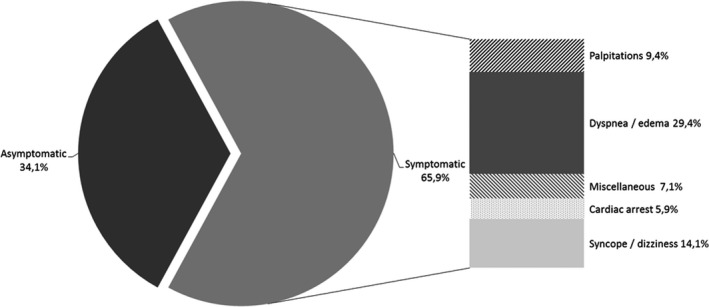
Initial presentation of cardiac adverse drug reactions in preCOV patients. COV, coronavirus.
Alteration of the cardiac function
There were 36 CADRs of cardiomyopathies (42.4%). Median time to onset was 18.5 months (7.5–90.0). Thirty‐five (> 97%) reports were serious, including two deaths. A value for left ventricular ejection fraction (LVEF) was available for 28 patients and 22 (78.6%) presented an LVEF < 40%. Outcome after HCQ withdrawal was reported for 16 patients: heart failure was reversible in 9 patients (56.3%) with a median time to recovery of 14 weeks (3–16) and outcome was deleterious in 7 patients (43.8%).
Conduction disorders
There were 24 CADRs of conduction disorders (28.2%). Median time to onset was 5 years (7–10). The clinical presentations were syncope in five patients (20.8%) and dizziness in five patients (20.8%). Amidst the 24 conduction disorders, 14 (58.3%) were high‐grade atrioventricular blocks, 9 were bundle branch blocks (37.5%), and 1 (4.2%) was a sinoatrial block. One patient (4.2%) died from extreme bradycardia and complete atrioventricular block. Thirteen of 24 patients (54.2%) required permanent implantation of a pacemaker and 7 (29.2%) had concomitant cardiomyopathies.
Repolarization and ventricular rhythm disorders
There were 22 CADRs of repolarization disorders and ventricular arrhythmias (25.9%). Median time to onset was 10 weeks (4–365). Fifteen patients (68.2%) presented with various symptoms whereas seven (31.8%) were asymptomatic. The notified final diagnosis was ventricular tachycardia or cardiac arrest in 11 patients (50%, leading to electric cardioversion in 2 patients), ventricular extrasystoles in 7 patients (31.8%), and isolated QTc prolongations in 4 patients (18.2%). Two patients died of ventricular arrhythmia.
Sinus bradycardias
There were 3 CADRs of sinus bradycardia (3.5%) all reversible after HCQ discontinuation, including 1 case ≤ 40 bpm. Two of three patients (66.7%) had concomitant QT prolongation.
COV patients
Among 158 patients with COV, 33 were excluded from the analysis (Figure 1 ). The 125 remaining patients yielded 141 CADRs (Figure 2 ), the majority of which was asymptomatic (126, 88.7%; Figure 4 ). This represents a significant difference from the preCOV group (P < 0.001). The patients’ mean age was 65 (± 14) years and 91 patients (72.8%) were men. Most reports (112/125, 89.6%) were serious and 4 (3.2%) had a fatal outcome. The other CADR led to HCQ discontinuation. Sixteen of 125 patients presented with at least 2 concomitant CADRs.
Figure 4.
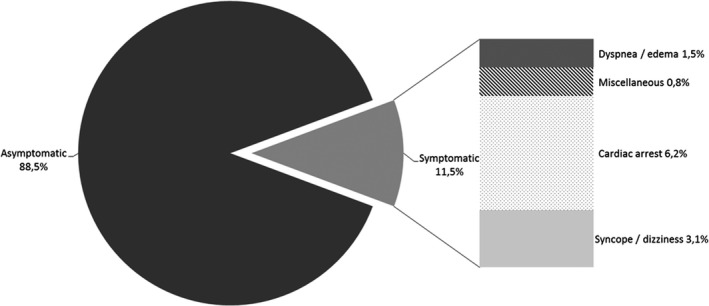
Initial presentation of cardiac adverse drug reactions in patients with coronavirus (COV).
Repolarization and ventricular rhythm disorders
There were 110 CADRs of repolarization and ventricular rhythm disorders (78.0%), which is significantly higher than in preCOV (P < 0.001). Median time to onset was 3 days (interquartile range (IQR): 2–5). Fourteen patients (12.7%) presented with symptomatic CADRs whereas 97 (88.2%) remained asymptomatic. The final diagnosis was polymorphic or monomorphic ventricular tachycardia, ventricular fibrillation, or cardiac arrest in 19 patients (17.3%) leading to electrical cardioversion in 2 cases, ventricular extrasystoles in 6 patients (5.4%), and isolated QTc prolongations in 85 patients (77.3%). When available, QTc value was ≥ 500 ms in 39 of 93 patients (41.9%) and delta QTc was ≥ 60 ms in 17 of 93 patients (18.3%). Four patients died from ventricular arrhythmia.
Sinus bradycardias
There were 21 CADRs of sinus bradycardias (14.9%), which is significantly higher than preCOV (P = 0.01). Heart rate was ≤ 40 bpm in 10 patients (47.6%). Median time to onset was 2 days (IQR: 1–4). Two patients were symptomatic (hemodynamic instability). Twelve of 21 patients (57.1%) presented concomitant repolarization disorders and ventricular arrhythmias.
Conduction disorders
There were 8 CADRs of conduction disorders (5.7%), which is significantly lower than in preCOV (P < 0.001). Median time to onset was 3 days (IQR: 2.5–3.5). Disorders included one (12.5%) high‐degree block, five (62.5%) bundle branch blocks, and two (25%) first‐degree atrioventricular blocks, all of them asymptomatic. None of them required pacemaker implantation.
Alteration of cardiac contractile function
There were two CADRs of heart failure (1.4%), which is significantly lower than in preCOV (P < 0.001). Time to onset was 1 and 3 days, respectively. LVEF was available for 1 patient and was ≤ 40%. Outcome was deleterious for one patient and unknown for the other.
Incidences
Before COVID‐19
In its approved indications, HCQ is a long‐term treatment and its consumption remained steady over the years. In 2019, an estimated total of 36,689 patients were exposed to HCQ, with only 4 cases of CADRs notified to the PVD during that period. This yields an estimated preCOV incidence for HCQ‐induced CADRs of 0.011% (95% Confidence Interval: 0.003 to 0.028).
During COVID‐19
The use of HCQ surged in March and April 2020. Considering the 25,926 g of HCQ used for COVID‐19, the number of patients exposed to HCQ was estimated to be 4,321. CADRs associated with HCQ in COV amounted to 125 reports, yielding an estimated COV incidence for HCQ‐induced CADRs of 2.892% (95% Confidence Interval: 2.414 to 3.437), which is significantly different from the estimated preCOV incidence for HCQ‐induced CADRs (P < 0.001).
DISCUSSION
This study provides the first exhaustive comparison of HCQ‐induced CADRs before and during COVID‐19 and the evolution in its perception. There was an ~ 290‐fold incidence increase in CADRs notified for patients with COV compared with preCOV patients.
Possible reasons for the different incidences
Impact of COVID‐19 on cardiac function has already been described, such as virus‐induced inflammation of cardiac tissues, 30 contributing to this difference.
Moreover, under‐notification of adverse reactions may be higher in office‐based medicine than in a hospital setting. This difference also highlights the prominent role of monitoring in detecting CADRs at an early stage, as demonstrated by numerous asymptomatic yet identified cases in the COV group.
Over time, HCQ has progressively been considered harmless from a cardiac point of view for several reasons. First, physicians seldom suspect a long‐term treatment when a new ADR occurs. 31 The relative lack of past spontaneous notifications, together with few references to cardiotoxicity in its Summary of Product Characteristics, confers to HCQ the reputation of a rather innocuous drug, further contributing to the vicious circle of under‐notification. Cases of HCQ‐induced cardiotoxicity had been published in the past, but imperfectly described, forsaking their mention in monitoring guidelines. Furthermore, HCQ‐treated patients for rheumatoid diseases frequently have complex comorbidities, including cardiac involvement of their underlying disease favoring the attribution of CADRs to the latter. Possible “disease‐drug” interactions 32 may have been neglected as for long‐term cardiomyopathies. These patients also bear the frequent cardiovascular toxicity of their concomitant immunosuppressive treatment. 33
Most preCOV reportings were triggered by the severity of cardiotoxic events, reflecting under‐reporting, whereas COV reportings also included a consistent amount of mild to moderate CADRs. 22 Despite the lack of data on the efficacy of HCQ in the treatment of COVID‐19, an understandable appeal for guidance and therapeutic options led to its prescription. Health authorities 14 , 34 and learned societies 35 , 36 spread information and warnings about potential ADRs associated with the use of HCQ, 37 prompting clinicians to duly notify adverse reactions.
Indeed, HCQ was already listed as the fifth cause of drug‐induced cardiomyopathy reported to MedWatch, the American US Food and Drug Administration’s (FDA) adverse event reporting system. HCQ was also the fifth drug with the highest proportional reporting ratio, just behind drugs known for their cardiovascular toxicity, such as digoxin or trastuzumab. 38
Profile of cardiac toxicity
The preCOV and COV profiles of cardiotoxicity identified from our study pertain to the same array of HCQ ancillary properties.
HCQ blocks the delayed rectifier current (I K), particularly its rapid component I Kr, hence prolongs the duration of repolarization. 39 , 40 This phenomenon creates an arrhythmogenic substrate and bears an increased risk for torsades de pointes, a life‐threatening polymorphic ventricular tachycardia typically triggered by the occurrence of early after‐depolarizations in a setting of repolarization heterogeneity. 14 , 41
The quinidine ring is known to be deleterious to the cardiac conduction and contractile function. 13 Inhibition of sodium channels confers to HCQ a negative inotropic effect 16 , 17 and may also lead to iatrogenic cardiomyopathies. 10 , 18 On a long‐term basis, septal infiltration of metabolic wastes 32 from lysosomal dysfunction may be toxic toward conductive tissue and cardiomyocytes as well. 9 , 32 , 42 , 43 , 44
HCQ reduces the hyperpolarization‐activated funny current I f, which plays a crucial role in the pacemaker cells of the sinoatrial node. This “ivabradine‐like” mechanism together with I Na inhibition enhances chronotropic negative tendency and thus the occurrence of bradycardia. 19 Some authors suggest the use HCQ as a treatment of atrial fibrillation. 45
Differences were nevertheless observed in the groups: mostly symptomatic CADRs with cardiomyopathies and conduction disorders in preCOV and mostly repolarization disorders, subsequent ventricular arrhythmias, and sinus bradycardias in COV.
Hypotheses for the observed differences in CADRs
Patients with COVID‐19 treated with HCQ are monitored as recommended by recent specific guidelines. 35 , 36 PreCOV patients were not usually monitored, thus asymptomatic CADRs were likely to remain undiagnosed. This probably explains the severity of CADRs reported in preCOV patients and their serious long‐lasting consequences, such as cardiomyopathies and pacemaker implantation. HCQ chronic intake in preCOV patients results in high cumulated doses that can lead to metabolic‐induced chronic cardiac toxicity.
COVID‐19 exposes to additional risk factors for delayed repolarization and its consequences, such as COVID‐19‐induced hypokalemia, myocarditis, 46 hypomagnesemia, fever, and inflammatory QTc prolongation. 47 The combined use in different trials of HCQ with other QT‐prolonging drugs, such as azithromycin, 22 , 41 represents a further increase in cardiotoxicity. Thereby, asymptomatic repolarization and ventricular rhythm disorders were more frequently observed in COV compared with preCOV.
If cardiac monitoring during treatment with HCQ in an acute hospital setting, such as the COVID‐19 epidemic, is strongly recommended, and this should be also true to a lesser extent for patients treated chronically with HCQ for approved indications and prone to QTc prolongation and arrhythmia in immunodepressed contexts. 48
Strengths and limits of the study
This retrospective analysis relies on spontaneous notifications that have inherent qualitative virtues 23 , 49 but need cautious interpretations because the real incidence of HCQ‐induced CADRs is likely underestimated. Surveillance bias may have occurred.
In adddition, patients in the preCOV and COV groups differ in therapeutic indications, duration of treatment, and concomitant prescriptions, hence comorbidities, which all may explain the different profiles of cardiac toxicity that we observed.
In spite of its obvious limitations, this study provides a rationale on cardiac risk of HCQ based on spontaneous safety reporting and through contextualization according to pharmacological properties, history, indications, and clinical objectivity. It clearly points out that widespread opinion on HCQ cardiac innocuity is overstated.
CONCLUSIONS
The perception of HCQ being an innocuous drug finds its historical explanations in a relative lack of ADR notifications, due to its long‐term use prior to COVID‐19 that trivializes its prescription and blunts the recognition of ADRs. An insufficiently explicit Summary of Product Characteristics may also hinder adequate consideration of cardiotoxicity.
At the onset of COVID‐19 epidemic and its widespread use in an acute context, a sharp increase of spontaneous reporting of HCQ toxicity, notably CADRs, was observed. Such an acute situation represents a unique opportunity to remind us of the pharmacological properties of HCQ and shed a new light on its cardiac safety profile.
Our results foster warnings before initiating a treatment with HCQ in patients, regardless of its indication. Each patient should benefit from baseline and iterative monitoring, whether on a short or long‐term basis. 44 Besides a thorough clinical examination, this should include the respect of its contraindications and precautions of use, regular ECG monitoring, and HCQ therapeutic drug monitoring when available. 43 , 50 If not prevented, ADRs should be suspected as early as possible and notified even during long‐term use. This should be true for any purportedly safe drug.
Funding
No funding was received for this work.
Conflict of Interest
All authors declared no competing interests for this work.
Author Contributions
S.R., A.G., E.V.O., J.M., F.R., and M.‐D.D. wrote the manuscript. S.R., A.G., F.R., and M.‐D.D. designed the research. S.R., A.G., A.F., D.V., F.R., and M.‐D.D. performed the research. S.R., A.G., A.F., and D.V. analyzed the data.
Disclaimer
The opinions expressed in this study are those of the authors and do not represent the views of the Agence Nationale de Sécurité du Médicament et des produits de santé (ANSM).
Acknowledgment
The authors thank Arne Hessenbruch (PhD, lecturer at the Massachusetts Institute of Technology) for proofreading and useful comments. He received no compensation for this work.
References
- 1. Fanouriakis, A. et al 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745 (2019). [DOI] [PubMed] [Google Scholar]
- 2. Smolen, J.S. et al EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79, 685–699 (2020). [DOI] [PubMed] [Google Scholar]
- 3. Schrezenmeier, E. & Dorner, T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 16, 155–166 (2020). [DOI] [PubMed] [Google Scholar]
- 4. Yao, X. et al In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin. Infect. Dis. 71, 732–739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia‐Cremades, M. et al Optimizing hydroxychloroquine dosing for patients with COVID‐19: an integrative modeling approach for effective drug repurposing. Clin. Pharmacol. Ther. 108, 253–263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bump, P. The rise and fall of Trump’s obsession with hydroxychloroquine (The Washington Post, Washington, DC, 2020). [Google Scholar]
- 7. OMS . WHO Director‐General's opening remarks at the media briefing on COVID‐19 ‐ May 25, 2020 <https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐‐‐25‐may‐2020> (2020). Accessed May 29, 2020.
- 8. Tselios, K. et al Antimalarial‐induced cardiomyopathy in systemic lupus erythematosus: as rare as considered? J. Rheumatol. 46, 391–396 (2019). [DOI] [PubMed] [Google Scholar]
- 9. Tonnesmann, E. , Kandolf, R. & Lewalter, T. Chloroquine cardiomyopathy ‐ a review of the literature. Immunopharmacol. Immunotoxicol. 35, 434–442 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Chatre, C. , Roubille, F. , Vernhet, H. , Jorgensen, C. & Pers, Y.M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 41, 919–931 (2018). [DOI] [PubMed] [Google Scholar]
- 11. Melles, R.B. & Marmor, M.F. The risk of toxic retinopathy in patients on long‐term hydroxychloroquine therapy. JAMA Ophthalmol. 132, 1453–1460 (2014). [DOI] [PubMed] [Google Scholar]
- 12. Ruiz‐Irastorza, G. , Ramos‐Casals, M. , Brito‐Zeron, P. & Khamashta, M.A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann. Rheum. Dis. 69, 20–28 (2010). [DOI] [PubMed] [Google Scholar]
- 13. Smith, E.R. & Klein‐Schwartz, W. Are 1–2 dangerous? Chloroquine and hydroxychloroquine exposure in toddlers. J. Emerg. Med. 28, 437–443 (2005). [DOI] [PubMed] [Google Scholar]
- 14. Sanchez‐Chapula, J.A. , Salinas‐Stefanon, E. , Torres‐Jacome, J. , Benavides‐Haro, D.E. & Navarro‐Polanco, R.A. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J. Pharmacol. Exp. Ther. 297, 437–445 (2001). [PubMed] [Google Scholar]
- 15. de Olano, J. , Howland, M.A. , Su, M.K. , Hoffman, R.S. & Biary, R. Toxicokinetics of hydroxychloroquine following a massive overdose. Am. J. Emerg. Med. 37, 2264.e5–2264.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 16. Honerjager, P. The contribution of Na channel block to the negative inotropic effect of antiarrhythmic drugs. Basic Res. Cardiol. 81(suppl. 1), 33–37 (1986). [DOI] [PubMed] [Google Scholar]
- 17. Honerjager, P. , Loibl, E. , Steidl, I. , Schonsteiner, G. & Ulm, K. Negative inotropic effects of tetrodotoxin and seven class 1 antiarrhythmic drugs in relation to sodium channel blockade. Naunyn Schmiedebergs Arch. Pharmacol. 332, 184–195 (1986). [DOI] [PubMed] [Google Scholar]
- 18. White, N.J. Cardiotoxicity of antimalarial drugs. Lancet Infect. Dis. 7, 549–558 (2007). [DOI] [PubMed] [Google Scholar]
- 19. Capel, R.A. et al Hydroxychloroquine reduces heart rate by modulating the hyperpolarization‐activated current IF: novel electrophysiological insights and therapeutic potential. Heart Rhythm 12, 2186–2194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chorin, E. et al The QT interval in patients with COVID‐19 treated with hydroxychloroquine and azithromycin. Nat. Med. 26, 808–809 (2020). [DOI] [PubMed] [Google Scholar]
- 21. Mercuro, N.J. et al Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 5, 1036–1041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gérard, A. et al “Off‐label” use of hydroxychloroquine, azithromycin, lopinavir‐ritonavir and chloroquine in COVID‐19: a survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers. Therapie 75, 371–379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bihan, K. , Lebrun‐Vignes, B. , Funck‐Brentano, C. & Salem, J.E. Uses of pharmacovigilance databases: an overview. Therapie 10.1016/j.therap.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 24. Rautaharju, P.M. et al AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 53, 982–991 (2009). [DOI] [PubMed] [Google Scholar]
- 25. Drew, B.J. et al Prevention of torsade de pointes in hospital settings. Circulation 121, 1047–1060 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vink, A.S. et al Determination and Interpretation of the QT Interval. Circulation 138, 2345–2358 (2018). [DOI] [PubMed] [Google Scholar]
- 27. Rautaharju, P.M. , Zhang, Z.M. , Prineas, R. & Heiss, G. Assessment of prolonged QT and JT intervals in ventricular conduction defects. Am. J. Cardiol. 93, 1017–1021 (2004). [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization (WHO) . WHOCC ATC/DDD Hydroxychloroquine <https://www.whocc.no/atc_ddd_index/?code=P01BA02> (2019). Accessed May 27, 2020.
- 29. European Parliament . Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. <https://ec.europa.eu/health/sites/health/files/files/eudralex/vol‐1/dir_2001_20/dir_2001_20_en.pdf> (2001). [PubMed]
- 30. Madjid, M. , Safavi‐Naeini, P. , Solomon, S.D. & Vardeny, O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 5, 831–840 (2020). [DOI] [PubMed] [Google Scholar]
- 31. Karimi, G. , Star, K. , Norén, G.N. & Hägg, S. The impact of duration of treatment on reported time‐to‐onset in spontaneous reporting systems for pharmacovigilance. PLoS One 8, e68938‐e (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yogasundaram, H. et al Hydroxychloroquine‐induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can. J. Cardiol. 30, 1706–1715 (2014). [DOI] [PubMed] [Google Scholar]
- 33. Miller, L.W. Cardiovascular toxicities of immunosuppressive agents. Am. J. Transplant. 2, 807–818 (2002). [DOI] [PubMed] [Google Scholar]
- 34. European Medicines Agency (EMA) . COVID‐19: reminder of risk of serious side effects with chloroquine and hydroxychloroquine. April 23, 2020. <https://www.ema.europa.eu/en/news/covid‐19‐reminder‐risk‐serious‐side‐effects‐chloroquine‐hydroxychloroquine>. Accessed April 27, 2020.
- 35. Funck‐Brentano, C. , Salem, J.E. , Nguyen, L.S. , Drici, M.D. & Roden, D.M. Response to the editorial "COVID‐19 in patients with cardiovascular diseases": COVID‐19 treatment with hydroxychloroquine or chloroquine and azithromycin: a potential risk of Torsades de Pointes. Arch. Cardiovasc. Dis. 113, 367–368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roden, D.M. , Harrington, R.A. , Poppas, A. & Russo, A.M. Considerations for drug interactions on QTc in exploratory COVID‐19 (coronavirus disease 2019) treatment. J. Am. Coll. Cardiol. 010, e906–e907 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mehra, M.R. , Desai, S.S. , Ruschitzka, F. & Patel, A.N. Retraction‐Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: a multinational registry analysis. Lancet 395, 1820 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sumpter, M.D. , Tatro, L.S. , Stoecker, W.V. & Rader, R.K. Evidence for risk of cardiomyopathy with hydroxychloroquine. Lupus 21, 1594–1596 (2012). [DOI] [PubMed] [Google Scholar]
- 39. Roden, D.M. & Drici, M.D. Drug‐induced sudden death In Sudden Cardiac Death: A Handbook for Clinical Practice (eds. Priori, S.G. , & Zipes, D.P. ) 177–188 (Wiley‐Blackwell, Hoboken, NJ, 2005). [Google Scholar]
- 40. Drici, M.D. & Barhanin, J. Cardiac K+ channels and drug‐acquired long QT syndrome. Therapie 55, 185–193 (2000). [PubMed] [Google Scholar]
- 41. Nguyen, L.S. et al Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the world health organization pharmacovigilance database. Circulation 142, 303–305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frustaci, A. , Morgante, E. , Antuzzi, D. , Russo, M.A. & Chimenti, C. Inhibition of cardiomyocyte lysosomal activity in hydroxychloroquine cardiomyopathy. Int. J. Cardiol. 157, 117–119 (2012). [DOI] [PubMed] [Google Scholar]
- 43. Saussine, A. et al Chloroquine cardiotoxicity in long‐term lupus therapy in two patients. Ann. Dermatol. Venereol. 136, 530–535 (2009). [DOI] [PubMed] [Google Scholar]
- 44. Tselios, K. , Deeb, M. , Gladman, D.D. , Harvey, P. & Urowitz, M.B. Antimalarial‐induced cardiomyopathy: a systematic review of the literature. Lupus 27, 591–599 (2018). [DOI] [PubMed] [Google Scholar]
- 45. Yue, L. Effect of hydroxychloroquine on atrial fibrillation recurrence <https://clinicaltrials.gov/ct2/show/NCT03592823> (2020). Accessed May 15, 2020.
- 46. American College of Cardiology . COVID‐19 clinical guidance for the cardiovascular care team <https://www.acc.org/~/media/665AFA1E710B4B3293138D14BE8D1213.pdf> (2020). Accessed May 15, 2020.
- 47. Xu, C.Y. et al Combined treatment of tocilizumab and chloroquine on severe COVID‐19: a case report. QJM 113, 569–572 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kang, I. & Park, S.H. Infectious complications in SLE after immunosuppressive therapies. Curr. Opin. Rheumatol. 15, 528–534 (2003). [DOI] [PubMed] [Google Scholar]
- 49. Grandvuillemin, A. , Fresse, A. , Cholle, C. , Yamani, S. & Dautriche, A. Adverse drug reactions of hydroxychloroquine: analysis of French pre‐pandemic SARS‐CoV2 pharmacovigilance data. Therapie 75, 385–387 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandhu, V.K. & Weisman, M.H. Hydroxychloroquine ‐ how much is too much? J. Rheumatol. 46, 340–342 (2019). [DOI] [PubMed] [Google Scholar]


