Abstract
The aim of this study was to determine the expression of IL‐35 and the lymphatic vessel density (LVD) and microvessel density (MVD) in the pathological tissues from patients with non‐small cell lung cancer (NSCLC) and to analyze their correlation with other common clinical prognostic factors, as well as patients’ overall survival and progression‐free survival. We analyzed the pathological characteristics of 130 patients with NSCLC and determined the IL‐35 expression, MVD, and LVD changes in the pathological tissues by immunohistochemistry. The results showed that IL‐35 expression was significantly correlated with tumor differentiation, lymph node metastasis, T staging, LVD, and MVD (P < 0.05) but was not associated with age, sex, smoking, and other factors. Univariate analysis of risk models showed that age, lymph node metastasis, T stage, and high IL‐35 expression, LVD, and MVD were significantly associated with NSCLC prognosis (P < 0.05), whereas sex, smoking, and high differentiation were not correlated with prognosis. Multivariate analysis of the proportional risk model showed that the IL‐35 expression, lymph node metastasis, high LVD, and high MVD were significantly correlated with NSCLC prognosis (P < 0.05). In conclusion, IL‐35, MVD, and LVD may be independent prognostic markers. In addition, IL‐35 might represent a promising clinical drug target for the treatment of NSCLC.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
IL‐35 expression, lymphatic vessel density (LVD), and microvessel density (MVD) play important roles in the pathogenesis and development of several tumors. However, their roles in non‐small cell lung cancer (NSCLC) remain unclear.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study assessed the relationship of IL‐35 expression, LVD, and MVD with the clinical prognostic factors, overall survival, and progression‐free survival of patients with NSCLC.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The expression of IL‐35 was significantly correlated with tumor differentiation, lymph node metastasis, T staging, LVD, and MVD. In addition, IL‐35 expression, LVD, and MVD were significantly associated with NSCLC prognosis.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
IL‐35, MVD and LVD may be independent prognostic markers, and IL‐35 might represent a promising clinical drug target for the treatment of NSCLC.
Lung cancer is more prevalent in young populations, where it can have a mortality rate as high as 27%. 1 , 2 Despite the progress in the diagnosis and treatment of lung cancer, the 5‐year survival rate remains at only 16%. The highest incidence of lung cancer is seen for non‐small cell lung cancer (NSCLC), which is increasing at an annual rate of 1.63%. Patients with NSCLC show lowered immunity, and especially cellular immunity, which decreases the effective removal of cancer cells in the immune microenvironment and subsequently promotes the proliferation of cancer cells. Therefore, individualized therapy that targets immune responses is urgently needed for NSCLC treatment.
Interleukin‐35 (IL‐35) is a new member of the IL‐12 family. 1 IL‐35 plays an important regulatory role in the process of intracellular infection and inflammation, and its expression is also closely associated with the occurrence and development of auto‐immune diseases and tumors, as well as other clinical diseases. 3 The observation of ectopic IL‐35 expression in various tumor tissues and in the serum of patients with cancer also suggests that IL‐35 is critical for tumorigenesis and cancer development. 1 , 4 , 5 However, few studies have examined the role of IL‐35 in NSCLC.
NSCLC tumors are well‐known to be associated with extensive angiogenesis and lymphangiogenesis. CD34, a marker of hematopoietic stem cells/hematopoietic progenitor cells, is specifically expressed in the endothelial cells of microvessels, making it a useful marker of microvasculature that reflects the tumor microvessel density (MVD). 6 , 7 Similarly, D2‐40 specifically binds to a mucous transmembrane protein on the lymphatic endothelium, thereby distinguishing lymphatic vessels from microvessels for determination of the tumor lymphatic vessel density (LVD). 8 The aim of the present study was to explore the relationship among IL‐35 and MVD and LVD in NSCLC and the clinical significance of this relationship.
METHODS
Clinical data
A total of 130 patients (37 men and 93 women) undergoing NSCLC resection at the Thoracic Surgery Department of Qingdao Municipal Hospital from June 2014 to June 2015 were enrolled in this study. All patients were in follow‐up status. None of the selected patients had undergone any antitumor treatment before the operation. Clinical staging was carried out in accordance with the TNM staging criteria of the 7th edition of the American Joint Committee on Cancer (AJCC). The routine clinical data (age, gender, smoking status, lymph node metastasis, degree of tumor differentiation, T staging, survival data, etc.) of included patients were collected. The research protocol of this study was approved by the Ethics Committee of the Qingdao Municipal Hospital. Informed consent was obtained from all individual participants included in the study.
Specimen analysis
Immunohistochemical staining
Paraffin‐embedded lung tissue sections from each patient were first deparaffinized in xylene and rehydrated in graded ethanol, followed by incubation in Tris‐buffered saline (TBS; 50 mM Tris‐HCl and 150 mM NaCl, pH 7.6) for 10 minutes before antigen retrieval in 1 × antigen decloaker (Biocare). All sections were washed with ddH2O, incubated in TBS, and blocked with TBS containing 10% normal goat serum at room temperature. Tissue sections were further incubated overnight at 4°C with the following primary antibodies: polyclonal antibody to IL‐35 (1:50 dilution, AtaGenix), rat anti‐human CD34 monoclonal antibody (1:100 dilution, Fuzhou Mai New Biotechnology Development, Gulou Fuxhou, Fujian, China), and rat anti‐human D2‐40 monoclonal antibody (1:50 dilution, Beijing Shanshan Jinqiao Biotechnology, Beijing, People’s Republic of China), in 1 × TBS containing 1% normal goat serum, and then immunostained with the secondary antibody. The sections were developed using DAB (ZLI‐9018; ZSGB‐BIO, Beijing, China), which gave a brown positive stain.
Determination of IL‐35, LVD, and MVD levels
A positive result was based on the staining intensity for IL‐35 and the number of positively stained cells. The whole section was first observed at low resolution with a light microscope (× 100). Five nonoverlapping high‐resolution microscopic fields (× 400) were then randomly selected and the average number of positive cells was calculated to give a representative density of macrophages. The median was used to divide the sections into high‐density and low‐density groups for IL‐35. 3 The staining intensity from brown to yellow was used as another positive criterion: brown staining was scored as 3 points; brown‐yellow staining as 2 points; light yellow staining as 1 point; and no stain as 0 points. All the positive cells in the visual field were scored and their average values were taken. The total score ranged from 0 to 9, where a score greater than 4 indicated high expression.
The LVD was measured using the Weidner method. Any endothelial cell or endothelial cell cluster immunostained for CD34 and showing brown cytoplasm and a clear boundary with other surrounding tissues was deemed a single lymphatic vessel. The LVD was derived from the average number of lymphatic vessels in four visual fields. 9
Any light yellow or brown endothelial cell or endothelial cell cluster immunostained for D2‐40 that stood out clearly from the surrounding tumor cells and connective tissue components was deemed a single microvessel. The MVD was the average number of microvessels in four visual fields. 10
Two stochastic pathologists measured IL‐35, LVD, and MVD using a double‐blind method without knowing the patient’s condition. If the difference between the two measurements was > 10%, the results were repeated by another 2 physicians.
Statistical methods
All patients were followed up, with the final follow‐up on December 31, 2015. Results were analyzed using SPSS 18.0 (SPSS, Chicago, IL). The χ2 test was used to analyze the clinical pathological variables. Cox Proportional hazard analysis was used to compare survival rate differences. A value of P < 0.05 was considered statistically significant.
RESULTS
Immunohistochemical staining
Immunohistochemical staining confirmed the expression of IL‐35 and allowed determination of the number of IL‐35–positive cells. We observed brown or yellowish‐brown granules with low‐resolution (× 100) and high‐resolution microscopy (× 400; Figure 1 ).
Figure 1.
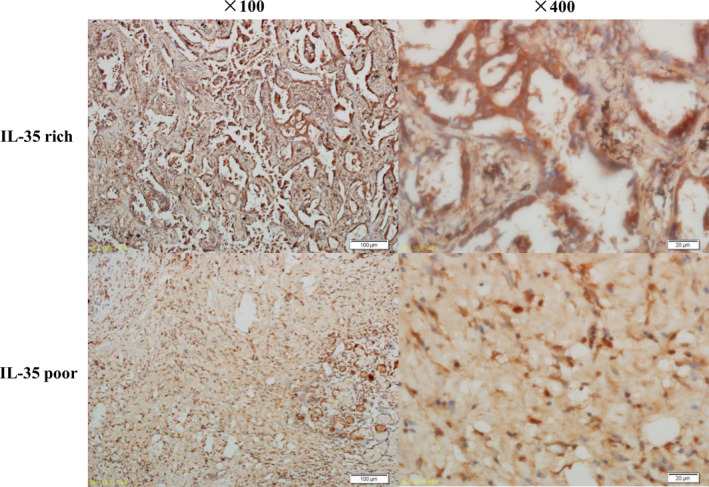
Examination of IL‐35 expression in pathological tissues of patients with non‐small cell lung cancer by immunohistochemistry. The upper panel indicates the IL‐35‐rich group (× 100 and × 400); the lower panel indicates the IL‐35‐poor group (× 100 and × 400).
Analysis of the relationship between the expression of IL‐35 and clinical pathological factors
The correlation analysis between IL‐35 expression and prognostic factors showed a significant association between the expression of IL‐35 in the 130 patients with NSCLC and tumor differentiation, lymph node metastasis, T stage, LVD, and MVD, but not with age, sex, smoking, or other factors (Table 1 ).
Table 1.
The expression of IL‐35 in patients with NSCLC and its correlation with clinical pathological variables
| N total (130) |
IL‐35 expression Poor (69) Rich (61) |
χ 2 | P value | |
|---|---|---|---|---|
| Age | 1.668 | 0.214 | ||
| < 55 | 52 | 24 | 28 | |
| ≥ 55 | 78 | 45 | 33 | |
| Sex | 0.02 | 1.000 | ||
| Men | 37 | 20 | 17 | |
| Women | 93 | 49 | 44 | |
| Smoking | 0.722 | 0.449 | ||
| Yes | 90 | 50 | 40 | |
| No | 40 | 19 | 21 | |
| Differentiation | 6.842 | 0.014 | ||
| Moderate + well | 63 | 26 | 37 | |
| Poor | 67 | 43 | 24 | |
| Lymph node metastasis | 8.436 | 0.005 | ||
| Absence (N0) | 58 | 39 | 19 | |
| Presence (N+) | 72 | 30 | 42 | |
| T stage | 7.000 | 0.03 | ||
| T1 | 61 | 31 | 30 | |
| T2 | 32 | 23 | 9 | |
| T3 | 37 | 15 | 22 | |
| LVD | 6.002 | 0.022 | ||
| Low | 66 | 42 | 24 | |
| High | 64 | 27 | 37 | |
| MVD | 4.494 | 0.037 | ||
| Low | 64 | 40 | 24 | |
| High | 66 | 29 | 37 |
IL‐35, interleukin‐35; LVD, lymphatic vessel density; MVD, microvessel density; NSCLC, non‐small cell lung cancer.
Survival analysis
Univariate analysis of the risk model showed that age, lymph node metastasis, T stage, high expression of IL‐35, high MVD, and high LVD were clearly associated with prognosis, whereas sex, smoking, and differentiation (moderate + well and low) were not significantly correlated with prognosis. The progression‐free survival (PFS) and overall survival (OS) were greatly prolonged in patients with positive lymph node metastasis compared with those with negative lymph node metastasis. The PFS was dramatically increased in patients with increased LVD and MVD, compared with those with reduced LVD and MVD. The number of survivors decreased significantly with prolongation of PFS time. The total survival time of patients was significantly increased in patients with low expression of IL‐35 (Figure 2a ). The OS of patients with high LVD and MVD was significantly reduced compared with that of patients with reduced LVD and MVD (Figure 2b ). In particular, the T1 group had a significantly prolonged PFS and OS compared with the T3 group, but no significant difference was found between the T1 and T2 groups (Table 2 ).
Figure 2.
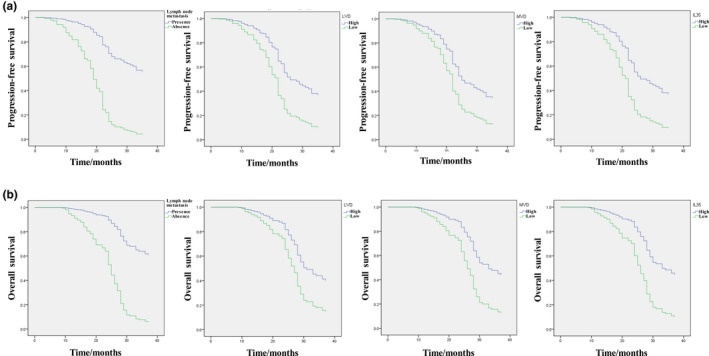
Kaplan–Meier curves of progression‐free survival (a) and overall survival (b) in patients with non‐small cell lung cancer. IL‐35, interleukin‐35; LVD, lymphatic vessel density; MVD, microvessel density.
Table 2.
Univariate analysis of 3 year OS and PFS in patients with NSCLC (n = 130)
| OS | PFS | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | ||||
| ≥ 55 | 0.491 (0.310−0.775) | 0.002 | 0.503 (0.322−0.785) | 0.002 |
| < 55 | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Sex | ||||
| Men | 0.767 (0.473−1.246) | 0.284 | 1.277 (0.794−2.052) | 0.313 |
| Women | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Smoking | ||||
| Yes | 1.090 (0.684−1.737) | 0.717 | 1.035 (0.652−1.644) | 0.884 |
| No | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Lymph node metastasis | ||||
| Presence (N1 + N2) | 0.243 (0.150−0.395) | 0.000 | 0.249 (0.156−0.398) | 0.000 |
| Absence (N0) | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Differentiation | ||||
| Moderate + well | 0.749 (0.488−1.151) | 0.187 | 0.707 (0.464−1.080) | 0.109 |
| Low | 1.000 (Ref.) | 1.000 (Ref.) | ||
| T stage | ||||
| T3 | 1.806 (1.088−2.996) | 0.022 | 1.703 (1.034−2.803) | 0.036 |
| T2 | 1.448 (0.863−2.428) | 0.160 | 1.336 (0.803−2.222) | 0.265 |
| T1 | 1.000 (Ref.) | 1.000 (Ref.) | ||
| T1 | 0.554 (0.334−0.919) | 0.022 | 0.587 (0.357−0.967) | 0.036 |
| T2 | 0.802 (0.460−1.398) | 0.430 | 0.785 (0.450−1.368) | 0.392 |
| T3 | 1.000 (Ref.) | 1.000 (Ref.) | ||
| LVD | ||||
| High | 0.452 (0.293−0.696) | 0.000 | 0.473 (0.310−0.724) | 0.001 |
| Low | 1.000 (Ref.) | 1.000 (Ref.) | ||
| MVD | ||||
| High | 0.457 (0.296−0.706) | 0.000 | 1.965 (1.286−3.003) | 0.002 |
| Low | 1.000 (Ref.) | 1.000 (Ref.) | ||
| IL‐35 | ||||
| High | 0.257 (0.163−0.405) | 0.000 | 0.279 (0.179−0.435) | 0.000 |
| Low | 1.000 (Ref.) | 1.000 (Ref.) | ||
CI, confidence interval; hr, hazard ratio; IL‐35, interleukin‐35; LVD, lymphatic vessel density; MVD, microvessel density; NSCLC, non‐small cell lung cancer; OS, overall survival; PFS, progression‐free survival.
The multivariate analysis of the proportional risk model showed that the IL‐35 expression, lymph node metastasis, high LVD, and high MVD were significantly correlated with the prognosis (Table 1 ). The OS was longer in the T1 group than in the T2 group, but no significant difference was found between the T1 and T3 groups or between the T2 and T3 groups (Table 3 ). No differences were found in PFS among the T1, T2, and T3 groups (Table 3 ).
Table 3.
Multivariate analysis of 3‐year OS and PFS in patients with NSCLC
| OS | PFS | |||
|---|---|---|---|---|
| hr (95% CI) | P | hr (95% CI) | P | |
| Age | ||||
| ≥ 55 | 0.599 (0.321−1.118) | 0.108 | 0.581 (0.318−1.063) | 0.078 |
| < 55 | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Gender | ||||
| Men | 0.600 (0.296−1.215) | 0.156 | 0.639 (0.317−1.286) | 0.209 |
| Women | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Smoking | ||||
| Yes | 0.968 (0.418−2.245) | 0.940 | 0.910 (0.399−2.076) | 0.823 |
| No | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Differentiation | ||||
| Moderate + well | 1.182 (0.686−2.035) | 0.547 | 1.044 (0.614−1.774) | 0.874 |
| Low | 1.000 (Ref.) | 1.000 (Ref.) | ||
| Lymph node metastasis | ||||
| Presence (N1 + N2) | 0.173 (0.097−0.310) | 0.000 | 0.181 (0.103−0.319) | 0.000 |
| Absence (N0) | 1.000 (Ref.) | 1.000 (Ref.) | ||
| T stage | ||||
| T3 | 1.398 (0.706−2.768) | 0.336 | 1.352 (0.692−2.642) | 0.378 |
| T2 | 2.187 (1.201−3.981) | 0.011 | 1.756 (0.975−3.165) | 0.061 |
| T1 | 1.000 (Ref.) | 1.000 (Ref.) | ||
| T1 | 0.715 (0.361−1.416) | 0.336 | 0.740 (0.379−1.446) | 0.378 |
| T2 | 1.564 (0.712−3.434) | 0.265 | 1.299 (0.601−2.811) | 0.506 |
| T3 | 1.000 (Ref.) | 1.000 (Ref.) | ||
| LVD | ||||
| High | 0.478 (0.250−0.912) | 0.025 | 0.431 (0.232−0.800) | 0.008 |
| Low | 1.000 (Ref.) | 1.000 (Ref.) | ||
| MVD | ||||
| High | 0.397 (0.210−0.753) | 0.005 | 0.513 (0.284−0.928) | 0.027 |
| Low | 1.000 (Ref.) | 1.000 (Ref.) | ||
| IL‐35 | ||||
| High | 0.351 (0.186−0.663) | 0.001 | 0.415 (0.224−0.766) | 0.005 |
| Low | 1.000 (Ref.) | 1.000 (Ref.) | ||
CI, confidence interval; IL‐35, interleukin‐35; LVD, lymphatic vessel density; MVD, microvessel density; NSCLC, non‐small cell lung cancer; OS, overall survival; PFS, progression‐free survival.
DISCUSSION
Lung cancer ranks highest in incidence among cancers in the world, and NSCLC accounts for 80–85% of all lung cancers, with a high risk of malignancy and poor prognosis. The prognosis of patients with NSCLC is frustrating, with a 1‐year median survival time and a < 20% 5‐year survival rate. Currently, few studies have examined the relationship between IL‐35 expression and NSCLC. Our findings have confirmed a correlation of IL‐35 expression with high LVD and MVD and with the prognosis of NSCLC, and provide further evidence for an association of IL‐35 expression in pathological NSCLC tissues with patients’ OS and PFS. 11
IL‐35 is a new member of the IL‐12 family. 12 Studies have shown that IL‐35 can mediate immunosuppression through single‐strand signaling, but the induction of IL‐35 requires a complete IL‐35 receptor. STAT1 and STAT4 are involved in the signal transduction of IL‐35. IL‐35 is mainly secreted by regulatory T cells, which can inhibit the function of effector T cells, regulate T cells, and inhibit Th17 cells to achieve anti‐inflammatory effects. 13 , 14 IL‐35 may be widely involved in the immune response process in vivo and has become a research hotspot in cytokine research, as it provides a new perspective in the biological treatment of infectious diseases, autoimmune diseases, and tumors. 3 , 15 , 16 , 17 , 18 , 19
Growing evidence now supports a role for IL‐35 in tumor occurrence and development. For example, the level of plasma IL‐35 was significantly higher in patients with NSCLC than in healthy controls (21.37 ± 11.55 pg/mL vs. 10.09 ± 5.32 pg/mL). Moreover, the level of plasma IL‐35 in these patients was significantly correlated with TNM stage and lymph node metastasis, indicating that IL‐35 is an independent prognostic indicator of NSCLC. However, the expression of IL‐35 in pathological lung tissues has not been examined.
In the present study, immunohistochemistry staining confirmed the expression of IL‐35 and the increase in LVD and MVD in 130 patients with NSCLC. The correlation between IL‐35 expression and prognostic factors showed that this expression was significantly correlated with tumor differentiation, lymph node metastasis, T stage, and increased LVD and MVD, but not with age, sex, smoking, and other factors. Univariate analysis of risk model showed that age, lymph node metastasis, T stage, high LVD and MVD, and high IL‐35 expression were significantly associated with prognosis, but not with sex, smoking, and high differentiation. Multivariate analysis of the proportional risk model showed that the IL‐35 expression, lymph node metastasis, high LVD, and high MVD were significantly associated with the prognosis. Univariate analysis showed a significantly prolonged PFS and OS in the T1 vs. the T3 group, but no significant difference was discovered between T1 and T2 groups. Multivariate analysis showed that OS was longer in T1 than in T2, but no significant difference was found between T1 and T3 groups or in the T2 and T3 groups. Based on these findings, lymph node metastasis and high IL‐35 expression, high LVD, and high MVD might be important prognostic factors that determine the effect of the T stage on OS.
In conclusion, the expression of IL‐35 was significantly associated with the level of tumor differentiation, lymph node metastasis, T stage, LVD, and MVD. IL‐35 expression and high LVD and MVD, and lymph node metastasis are independent prognostic markers, and IL‐35 may represent an important clinical target for the treatment of NSCLC.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
Authors Contributions
T.L.Z., J.N., and X.J.L. wrote the manuscript. L.C. and C.Y.G. designed the research. T.L.Z., J.N., X.J.L., Z.L.H., N.D., K.G., and Y.L. performed the research. T.L.Z., J.N., and X.J.L. analyzed the data. T.L.Z., J.N., and X.J.L. contributed new reagents/analytical tools.
Contributor Information
Ling Chen, Email: Chenling56789@163.com.
Chengye Guo, Email: Guochengye321@163.com.
References
- 1. Siegel, R. , Ma, J. , Zou, Z. & Jemal, A. Cancer statistics, 2014. CA: Cancer J. Clin. 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- 2. Zhao, J. , Han, Y. , Li, J. , Chai, R. & Bai, C. Prognostic value of KRAS/TP53/PIK3CA in non‐small cell lung cancer. Oncol. Lett. 17, 3233–3340 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang, Y. et al IL‐35 recombinant protein reverses inflammatory bowel disease and psoriasis through regulation of inflammatory cytokines and immune cells. J. Cell Mol. Med. 22, 1014–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng, H. et al Cancer survival in China, 2003–2005: a population‐based study. Int. J. Cancer 136, 1921–1930 (2015). [DOI] [PubMed] [Google Scholar]
- 5. Moreira, A.L. & Thornton, R.H. Personalized medicine for non–small‐cell lung cancer: implications of recent advances in tissue acquisition for molecular and histologic testing. Clin. Lung Cancer 13, 334–339 (2012). [DOI] [PubMed] [Google Scholar]
- 6. Trebeschi, S. et al Predicting response to cancer immunotherapy using non‐invasive radiomic biomarkers. Ann. Oncol. 30, 998–1004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rafei, H , El‐Bahesh, E , Finianos, A , Nassereddine, S & Tabbara, I. Immune‐based therapies for non‐small cell lung cancer. Anticancer Res. 37, 377–387 (2017). [DOI] [PubMed] [Google Scholar]
- 8. Hahn, H.P. , Shahsafaei, A. & Odze, R.D. Vascular and lymphatic properties of the superficial and deep lamina propria in Barrett esophagus. Am. J. Surg. Pathol. 32, 1454–1461 (2008). [DOI] [PubMed] [Google Scholar]
- 9. Sundov, Z. et al Prognostic value of MVD, LVD and vascular invasion in lymph node‐negative colon cancer. Hepatogastroenterology 60, 432–438 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Chen, L. et al Clinical significance of cancer‐associated fibroblasts and their correlation with microvessel and lymphatic vessel density in lung adenocarcinoma. J. Clin. Lab. Anal. 33, e22832 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu, X. et al Elevated plasma interleukin‐35 levels predict poor prognosis in patients with non‐small cell lung cancer. Tumor Biol. 36, 2651–2656 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Vignali, D.A. & Kuchroo, V.K. IL‐12 family cytokines: immunological playmakers. Nat. Immunol. 13, 722 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collison, L.W. et al IL‐35‐mediated induction of a potent regulatory T cell population. Nat. Immunol. 11, 1093 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long, J. et al IL‐35 over‐expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem. Biophys. Res. Comm. 430, 364–369 (2013). [DOI] [PubMed] [Google Scholar]
- 15. Hu, W. , Liu, Y. & Chen, J. Concurrent gene alterations with EGFR mutation and treatment efficacy of EGFR‐TKIs in Chinese patients with non‐small cell lung cancer. Oncotarget 8, 25046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korpanty, G.J. , Graham, D.M. , Vincent, M.D. & Leighl, N.B. Biomarkers that currently affect clinical practice in lung cancer: EGFR, ALK, MET, ROS‐1, and KRAS. Front. Oncol. 4, 204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deben, C. et al Deep sequencing of the TP53 gene reveals a potential risk allele for non–small cell lung cancer and supports the negative prognostic value of TP53 variants. Tumor Biol. 39, 1010428317694327 (2017). [DOI] [PubMed] [Google Scholar]
- 18. VanderLaan, P.A. et al Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: correlation with clinical outcomes. Lung Cancer (Amsterdam, Netherlands) 106, 17–21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mascaux, C. et al The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta‐analysis. Br. J. Cancer 92, 131 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]


